Abstract
Peroxisomes are multifunctional organelles, well known for their role in cellular lipid homeostasis. Their importance is highlighted by the life-threatening diseases caused by peroxisomal dysfunction. Importantly, most patients suffering from peroxisomal biogenesis disorders, even those with a milder disease course, present with a number of ocular symptoms, including retinopathy. Patients with a selective defect in either peroxisomal α- or β-oxidation or ether lipid synthesis also suffer from vision problems. In this review, we thoroughly discuss the ophthalmological pathology in peroxisomal disorder patients and, where possible, the corresponding animal models, with a special emphasis on the retina. In addition, we attempt to link the observed retinal phenotype to the underlying biochemical alterations. It appears that the retinal pathology is highly variable and the lack of histopathological descriptions in patients hampers the translation of the findings in the mouse models. Furthermore, it becomes clear that there are still large gaps in the current knowledge on the contribution of the different metabolic disturbances to the retinopathy, but branched chain fatty acid accumulation and impaired retinal PUFA homeostasis are likely important factors.
1. Introduction
Peroxisomes are ubiquitous cellular organelles that host a number of exclusive anabolic and catabolic lipid conversions besides other metabolic and non-metabolic functions. This includes the α-oxidation of phytanic acid, the β-oxidation of various substrates and ether lipid synthesis [1,2].
Optimal peroxisomal function is of pivotal importance for human health, as highlighted by a set of inherited peroxisomal diseases. This group of rare metabolic diseases can be categorized into peroxisomal biogenesis disorders and single enzyme deficiencies. The clinical presentation strongly varies and depends on the involved metabolic pathways and the type of mutation [3]. Interestingly, almost all peroxisomal disorder patients, even those with a mild clinical presentation, develop an ocular phenotype, which often includes retinopathy [4].
Roughly, the retina is subdivided into the neural retina and the retinal pigment epithelium (RPE). The neural retina consists of six distinct layers containing six large groups of specialized cell types: photoreceptors, interneurons (horizontal, amacrine and bipolar cells), ganglion cells, and Müller cells, which are a type of glial cell [5].
The photoreceptors are the light sensitive neurons of the retina. There are two types, rods and cones, that differ in spatial distribution and function. In the human retina, cones are concentrated in the macula, with the highest density in its center (i.e., the fovea), enable day and colour vision and establish high visual acuity. Rods are more prominent in the periphery and are necessary for night and peripheral vision. Both cell types exhibit a similar peculiar morphology and are built up of five main compartments: the outer and inner segment that are connected via the connecting cilium, the cell body containing the nucleus, the inner fiber and synaptic terminal [6].
The photoreceptor outer segment (POS) is a form of modified primary cilium that is filled with hundreds of lipid-rich disc membranes, which contain the light sensitive visual pigments. As elaborated on in sections below, the fatty acid composition is unusual and might require peroxisomal function for their synthesis and/or degradation. The inner segment is important for the synthesis of POS components that are subsequently delivered via the connecting cilium. Lastly, the inner fiber is the axon of the photoreceptor, which makes a synapse with interneurons. The latter relay to the ganglion cells, whose axons form the optic nerve that will transfer the visual signal to the brain [5,7].
The RPE is a monolayer of postmitotic and highly polarized cells that exert an array of functions, with the primary goal to maintain healthy photoreceptors [8,9]. One important characteristic of RPE cells is that they are connected by tight junctions, establishing the outer blood–retina barrier, and they act as important distributors of nutrients and waste products. RPE cells are also essential players in the visual cycle, i.e., the re-isomerization and recycling of 11-cis-retinal. In addition, they are involved in the phagocytosis of damaged POS that are shed on a daily basis by photoreceptors. The building blocks are either recycled towards the photoreceptors or degraded. Of note, in the human retina, each RPE cell supports approximately 45 photoreceptors and it takes about 10 days to replace an entire outer segment. This demonstrates that RPE cells are exposed to high amounts of proteins and lipids originating from the POS and, therefore, have to be very active metabolically in order to process the huge amount of cellular material. Hence, it is conceivable that lipid metabolism constitutes an important activity of these cells [8,9]. Finally, it is important to mention that the energy metabolism of photoreceptors and RPE cells is strongly intertwined in a so-called metabolic ecosystem [10].
In this review, we first discuss the distribution of peroxisomes in the retina. Next, we summarize the ophthalmological pathologies in the different peroxisomal disorders in humans and, where applicable, in animal models, with a special focus on the retinal symptoms. Subsequently, the potential role of deregulated lipids as a cause of retinopathy will be scrutinized.
2. The Location of Peroxisomes in the Retina
Although peroxisomes reside in all eukaryotic cells, with the exception of red blood cells, their abundance, content and size strongly vary between cell types and according to the environmental conditions [11]. Decades ago, diaminobenzidine (DAB) histochemistry was the golden standard to visualize peroxisomes. This technique detects the peroxidatic activity of catalase, a peroxisomal enzyme involved in redox metabolism. In different species, catalase-positive peroxisomes were detected in the RPE and to a lesser extent in the neural retina, observations that were later confirmed by immunohistochemical stainings (IHC) for catalase [12,13,14,15,16,17].
Only recently, more systematic studies were performed to elucidate the distribution of peroxisomes [18]. IHC and immunoblotting for peroxins (such as PEX14, PEX1 and PEX6), which are essential for peroxisome biogenesis and can be considered as housekeeping peroxisomal proteins, revealed their presence in the RPE, photoreceptor inner segments, interneurons and ganglion cells (Figure 1) [18,19,20,21,22]. Transcripts of the central enzyme of peroxisomal β-oxidation, Multifunctional protein 2 (MFP2) showed a similar spatial distribution compared to PEX14 immunoreactivity (Figure 1) [23]. In contrast, other proteins involved in peroxisomal lipid metabolism were differentially expressed between the RPE and neural retina, whereby some were more enriched in the RPE and others in the neural retina [18]. One striking example is the expression pattern of the ATP-binding cassette type D (ABCD) transporters that couple ATP hydrolysis to substrate translocation over the peroxisomal membrane, prior to its degradation. There are three types of ABCD transporters that have different substrate specificities. Although the specificity is not absolute, ABCD1 (adrenoleukodystrophy protein, ALDP) preferentially imports VLCFAs, ABCD2 (adrenoleukodystrophy related protein, ALDRP) transfers PUFAs, whereas ABCD3 (peroxisomal membrane protein 70, PMP70) handles dicarboxylic acids, 2-methyl branched chain fatty acids and bile acid intermediates [24]. ABCD1 was equally expressed in the RPE and neural retina, while ABCD2 and ABCD3 were more abundant in the RPE, suggesting that peroxisomal β-oxidation handles specific substrates in the different retinal cell types [18].
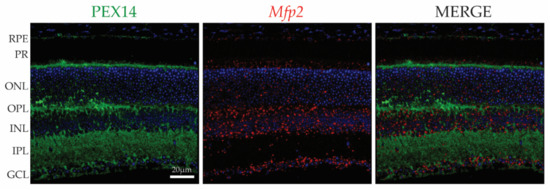
Figure 1.
Spatial distribution of PEX14 protein and Mfp2 mRNA in the mouse retina. IHC for PEX14 (green) and RNAscope® in situ hybridization for Mfp2 (red) reveals the presence of peroxisomes in the RPE, photoreceptors, interneurons and ganglion cells. Nuclei were counterstained with Hoechst (blue). PEX14, peroxin 14; Mfp2, multifunctional protein 2; RPE, retinal pigment epithelium; PR, photoreceptor layer; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Interestingly, it was reported that peroxisome abundance (visualized by DAB histochemistry coupled to electron microscopy) in the RPE followed the diurnal changes in POS phagocytosis, with higher peroxisome numbers at the burst of phagocytosis [25]. However, these observations were recently refuted as PEX14 and ABCD3 protein levels were relatively constant throughout the day [22]. Still, in the latter report, increased catalase activity coinciding with POS phagocytosis was shown, but a clear link remains to be established.
Altogether, these findings suggest that peroxisomes exert different functions in the inner and outer retina. However, their exact role in retinal homeostasis and integrity has only recently gained more attention.
3. Zellweger Spectrum Disorders
Peroxisome biogenesis involves the assembly of peroxisomal membranes and maintenance of functional peroxisomes. The latter includes the import of the matrix proteins, containing a peroxisomal targeting signal (PTS1 or PTS2), from the cytosol. Peroxisome biogenesis requires the close collaboration of the so-called peroxins, encoded by one of fourteen PEX genes in mammals. Defective peroxisomal biogenesis results in an array of clinical disease presentations, described as Zellweger spectrum disorders (ZSD), in which the import of both PTS1 and PTS2 proteins is defective, and rhizomelic chondrodysplasia punctata (RCDP) type 1 and 5, in which only PTS2 protein import is hampered [3,26].
To date, mutations in thirteen PEX genes are known to cause ZSD, with PEX1 and PEX6 mutations being the most prevalent [26,27]. The disease severity depends on the type of mutation and, hence, on the residual function of the corresponding peroxin. Patients suffering from the most severe phenotype of the ZSD, i.e., Zellweger syndrome (also known as cerebro-hepato-renal syndrome), present with general multi-organ failure and mostly die within the first year of life [26].
3.1. Retinopathy: A Recurrent Phenomenon
The diverse clinical image of ZSD patients often entails a number of ocular symptoms, even in patients surviving into adulthood. These symptoms include optic atrophy, glaucoma, cataract and nystagmus. In addition, retinopathy is also frequently observed, with abnormal retinal pigmentation, retinal degeneration (i.e., loss of retinal cells) and impaired ERG responses [4,26,28,29,30]. The pigmentary retinopathy has a varied clinical presentation, including retinitis pigmentosa [26]. In the latter, rods are damaged first, resulting in night blindness and peripheral vision loss, followed by loss of cones, leading to complete blindness. The typical fundoscopic image includes retinal vessel attenuation, optic disc pallor and abnormal retinal pigmentation (i.e., either hypopigmentation due to RPE atrophy or hyperpigmentation with bone spicule-shaped deposits), starting in the periphery (cf. rods first affected) and progressing towards the macula (cf. secondary cone damage) [31]. Furthermore, the diverse retinal pathology is underscored by the fact that several patients presenting with a disease phenotype of Leber congenital amaurosis [32,33,34] and Usher syndrome [20,35,36,37], two well-known diseases that are characterized by pigmentary retinopathy, have been re-diagnosed as suffering from ZSD.
Interestingly, the ophthalmological symptoms of patients suffering from Heimler syndrome, the mildest ZSD and caused by mutations in the PEX1, PEX6 or PEX26 gene, are confined to the retina. The pathology varies from abnormal retinal pigmentation to macular dystrophy. Other symptoms are sensorineural hearing loss, enamel hypoplasia and nail abnormalities (beau’s lines) [38].
Furthermore, it is remarkable that patients with mild mutations in other PEX genes, i.e., PEX2, PEX10 and PEX12, primarily present with an early-onset cerebellar ataxia phenotype in the absence of retinopathy. Notably, the corresponding peroxins are part of the same complex in the peroxisomal membrane and it is to date unresolved why defects in these peroxins result in a distinct clinical phenotype [26,39].
3.2. Histopathology
There are only a few old histopathological descriptions of the retinas of severe Zellweger syndrome patients, reporting RPE atrophy, bi-leaflet inclusions in the RPE, photoreceptor outer and inner segment degeneration, photoreceptor loss, atrophy of the ganglion cells and optic nerve. In addition, macrophage infiltration in the retina was observed [40,41].
Besides a fundoscopic evaluation, the development of the non-invasive technique optical coherence tomography (OCT) has greatly improved the diagnosis of inherited retinal diseases and allows the in vivo assessment of the retinal structure [42]. Fundoscopy of a 20-month-old patient, with a slightly less severe phenotype compared to Zellweger syndrome, revealed normal retinal vessels and optic nerve, but the macula appeared dull without a foveal light reflex and there was abnormal retinal pigmentation in a leopard-spot pattern in the midperiphery. OCT detected severe outer retinal atrophy in both the fovea and the areas with pigmentary changes. In addition, in the latter, hyperreflective spots on the RPE and bulging into the POS layer were observed. Of note, this patient also displayed severely reduced dark- and light-adapted ERG responses, indicating impaired rod and cone function, which is in accordance with the observed structural anomalies [29].
The retinal structure of two mild PEX1 patients was described. These patients were initially suspected to suffer from Usher syndrome [37] and Leber congenital amaurosis [33]. The alleged Usher syndrome patient developed neurosensory hearing loss at the age of 3 months and night-blindness at the age of 9 years. Fundoscopy and OCT revealed RPE and macular dystrophy complicated by macular cystoid oedema. Dark-adapted ERG responses were strongly impaired while light-adapted ERG was only mildly affected [37]. The retinal pathology of the re-diagnosed Leber congenital amaurosis patient appeared more complicated, as the macula and periphery were affected, while the perifoveal region was not. Fundoscopy and OCT showed extensive RPE atrophy in the affected regions and severe foveal thinning, respectively. In addition, no ERG responses were detectable [33].
Finally, several reports described the OCT findings in Heimler syndrome patients. Outer retinal pathology, mostly complicated by cystoid macular oedema, was frequently observed, involving photoreceptor abnormalities (e.g., POS disruption and loss of the junction between the photoreceptor outer and inner segments), photoreceptor loss and RPE atrophy [21,43,44,45,46].
Together, these pathological investigations indicate that loss of peroxisome function induces photoreceptor degeneration, but also the RPE and the inner retina are affected.
3.3. Insights form the Pex1 Knock-In Mouse Model
While several mouse models for ZSD have been developed, only the retinal phenotype of the Pex1 knock-in mouse, homozygous for the most common mild human PEX1 mutation (G843D), was reported [19,47].
Adult mice displayed reduced visual acuity. However, the onset of the pathology occurred already in the juvenile stage, involving both photoreceptors and interneurons. Although no photoreceptor loss was detected, rods and cones displayed distinct abnormalities. At the age of 2 weeks, the photoreceptor outer and inner segments were decreased in length, but this normalized by 3 weeks of age. In addition, rod function, measured via ERG (i.e., scotopic a-wave), was decreased in the juvenile mice, then normalized at the age of 4 weeks and subsequently gradually declined. It should be noted that the Pex1 knock-in mice are severely growth impaired compared to age matched controls during the lactation period, possibly also resulting in a delay in photoreceptor maturation.
Cone function (i.e., photopic b-wave) was already impaired at the age of 2 weeks and remained affected, which is in contrast to rods. Moreover, IHC for cone arrestin, labelling cones from the POS to the synaptic terminals, showed loss of outer and inner segments and of connections with the interneurons. These morphological changes were detected at the age of 6 weeks with no further information at earlier time points. At the ultrastructural level, photoreceptors, presumably rods, of 32-week-old mice showed normal POS, but disorganized inner segments. The exact consequence of the latter is unclear, since the main function of the inner segments is to synthesize the outer segments.
ERG analyses also revealed impaired function of bipolar cells (i.e., reduced scotopic b-wave) from the juvenile period into adulthood. Interestingly, the functional deficits preceded the morphological defects, which were only shown in 32-week-old mice via IHC for protein kinase C alpha (PKCα). Markers of other interneurons seemed to be unaltered. The ongoing photoreceptor stress might induce an adaptive response in the underlying retinal cells, which is part of a process called retinal remodelling [48]. On the other hand, in view of the abundance of peroxisomes in interneurons, a primary defect caused by peroxisomal dysfunction in these cells cannot be excluded and requires further research.
Finally, transmission of the visual signal to the brain was also affected, as shown via measurements of the visual evoked potentials. This could coincide with the retinal phenotype, but the optic nerve and visual cortex were not investigated and defects at these levels cannot be ruled out.
Hence, it appears that some of the retinal pathologies observed in peroxisome biogenesis disorders are recapitulated in the Pex1 knock-in mice, including photoreceptor dysfunction, but, in contrast to the patients, anomalies of the RPE were not reported in the mice.
4. Single Enzyme Deficiencies
In peroxisomal biogenesis disorders, all metabolic pathways are dysfunctional, obscuring the cause of the retinal pathology. More information on the metabolic origin can be obtained by examining peroxisomal single enzyme deficiencies in which only one metabolic path is defective. An overview of the metabolic abnormalities and the ocular pathology in peroxisomal biogenesis disorders and single enzyme deficiencies is provided in Table 1.

Table 1.
Overview of the biochemical abnormalities and ocular phenotypes in peroxisomal biogenesis disorders and single enzyme deficiencies.
4.1. Defects in α-Oxidation
4.1.1. Retinitis Pigmentosa in Refsum Disease
Patients with a defect in peroxisomal α-oxidation are diagnosed with (adult) Refsum disease. To date, mutations in two genes have been identified to lie at the basis of the disease. The most frequent cause is a mutation in the gene encoding phytanoyl-CoA hydroxylase (PHYH) that catalyses the first reaction of the α-oxidation pathway. Mild mutations in the PEX7 gene are the second cause. The corresponding peroxin is dedicated to the import of PTS2-containing proteins, i.e., alkylglycerone phosphate synthase (AGPS), PHYH and acetyl-CoA acyltransferase (ACAA1) [3,49]. Although in this case, the disease is in principle a peroxisomal biogenesis disorder, mild mutations lead to the impaired function of PHYH, while plasmalogen levels are either near normal or sufficient to prevent associated pathologies. Hence, the phenotype corresponds to a selective defect in peroxisomal α-oxidation [86,87].
Clinically, retinitis pigmentosa is considered as the cardinal symptom, besides the polyneuropathy. Other frequently observed ophthalmological symptoms are miosis, attenuated pupillary light responses, iris atrophy, and cataract. Biochemically, patients are characterized by phytanic acid accumulation [49]. This long chain branched fatty acid is catabolized by peroxisomes via α-oxidation to produce pristanic acid, which in turn undergoes peroxisomal β-oxidation (Figure 2) [2]. Phytanic acid is a metabolic by-product of chlorophyll, produced by the intestinal flora of ruminants, and the main sources are the dietary uptake from dairy products and the meat of those animals [49].
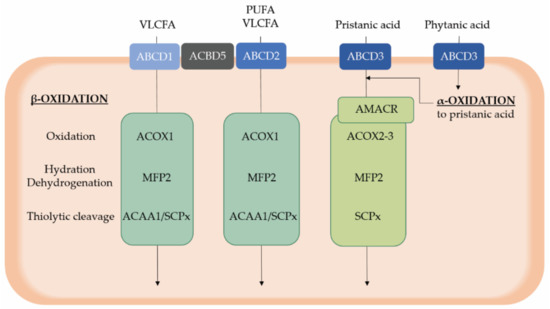
Figure 2.
Overview of peroxisomal α- and β-oxidation of substrates relevant for retinal homeostasis. Phytanic acid is catabolized via peroxisomal α-oxidation, resulting in the formation of the 2-methyl branched chain fatty acid pristanic acid that subsequently undergoes peroxisomal β-oxidation similar to other substrates, including VLCFAs and PUFAs. After activation to a CoA ester, substrates are translocated in peroxisomes via three ABCD transporters, which exert a differential, although not absolute, substrate specificity. Peroxisomal β-oxidation occurs in four steps whereby the second and third step are catalyzed by a multifunctional protein. Of note, pristanic acid first needs to be converted into the S-configuration by AMACR. Furthermore, ACBD5 is assumed to present VLCFAs to the ABCD transporters, besides its role in the tethering of peroxisomes to the endoplasmic reticulum. ABCD, ATP binding cassette subfamily D; ACAA1, acetyl-CoA acyltransferase 1; ACBD5, Acyl-CoA binding domain containing protein 5; ACOX, acyl-CoA oxidase; D/THCA, di- and trihydroxycholestanoic acid; MFP, multifunctional protein; PUFAs, polyunsaturated fatty acids; SCPx, sterol carrier protein x; VLCFAs, very long chain fatty acids.
Although the retinal deterioration is halted by restricted dietary intake of phytanic acid, which is compelling evidence that the increased levels are the disease-causing factor, the underlying mechanisms for the impaired photoreceptor survival are still elusive. Historically, two hypotheses were postulated, i.e., the antimetabolite and the molecular distortion hypothesis [49,88]. The first one claims that phytanic acid hampers the regeneration of 11-cis-retinal, while the molecular distortion hypothesis states that phytanic acid negatively alters the membrane composition and structure [88,89]. In addition, more recently, several other possible mechanisms have been postulated, such as mitochondrial toxicity [39].
4.1.2. Insights from In Vivo and In Vitro Models
To investigate the effects of increased phytanic acid levels, several animal models have been established, but a retinal phenotype similar to the human situation was not mimicked. Firstly, retinas of rats fed a phytanic acid-rich diet did not show abnormal ERG responses nor retinal degeneration [90]. Secondly, the short-term pathological effects of phytanic acid accumulation in a mouse model for Refsum disease were investigated. Hereto, PHYH deficient mice were fed a phytol-rich diet. The mice did not develop gross eye abnormalities upon microscopic investigation, although possible retinopathy was not thoroughly investigated. Therefore, it cannot be ruled out that retinal function is affected and further research is needed [91].
The impact of phytanic acid on RPE cells was studied in vitro by Bernstein et al. [88]. Hereto, both primary human and bovine RPE cells were exposed to elevated phytanic acid levels and morphological changes were investigated via electron microscopy. The changes included RPE swelling, development of presumably lipid-filled vacuoles, ER dissolution, and loss of apical microvilli, but mitochondria were not affected. Moreover, human RPE cells were more sensitive to elevated phytanic acid levels than bovine RPE cells and effects of moderately elevated levels were reversible [88].
4.2. Peroxisomal β-Oxidation Deficiency
Peroxisomal β-oxidation consists of a four-step process (i.e., oxidation, hydration, dehydrogenation, and thiolytic cleavage) and metabolizes a broad set of substrates, serving both catabolic and anabolic purposes (Figure 2). More specifically, very long chain (polyunsaturated) fatty acids (VLC-(PU)FAs; > C22), the 2-methyl branched chain fatty acid pristanic acid, dicarboxylic fatty acids and eicosanoid inflammatory mediators exclusively depend on peroxisomal β-oxidation for their breakdown. Although it was shown that peroxisomes are also able to oxidize short, medium, and long chain fatty acids (<C20), these are preferentially metabolized in mitochondria [2,92]. In addition, the bile acid intermediates dihydroxycholestanoic acid (DHCA) and trihydroxycholestanoic acid (THCA) undergo one round of peroxisomal β-oxidation in the process to form mature bile acids in the liver [2].
Peroxisomal β-oxidation executes a dual function in long chain PUFA metabolism, i.e., synthesis and degradation [2]. This is particularly interesting with respect to the specific lipid profile of the retina, which is highly enriched in PUFAs. Firstly, peroxisomal β-oxidation is essential for the synthesis of the n-3 PUFA docosahexaenoic acid (DHA, C22:6n-3) that is the most abundant PUFA in the POS. Hereto, the essential PUFA α-linolenic acid (C18:3n-3) undergoes several sequential elongation and desaturation reactions in the ER, leading to the formation of tetracosahexaenoic acid (C24:6n-3), which is subsequently oxidized to produce DHA (i.e., retroconversion to DHA). This pathway, also known as the “Sprecher shunt”, involves one cycle of peroxisomal β-oxidation (Figure 3) [93,94]. This DHA synthesis pathway was discovered in the liver, but it was shown that both RPE cells and photoreceptors are able to execute these reactions, albeit to a lesser extent [95,96,97,98]. Of note, the DHA-related PUFA in the n-6 series, i.e., C22:5n-6, undergoes similar reactions, starting from linoleic acid (C18:2n-6). Secondly, for their degradation, the long chain PUFAs also require β-oxidation. However, the contribution of peroxisomal versus mitochondrial β-oxidation was not fully elucidated and this may be species and cell type dependent [2]
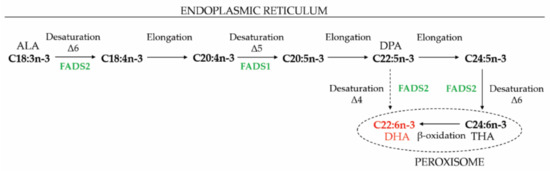
Figure 3.
De novo biosynthesis of DHA. ALA is elongated and desaturated to THA in the ER and subsequently retroconverted to DHA via one cycle of peroxisomal β-oxidation (also known as the “Sprecher shunt”). However, whereas it was previously thought that DPA cannot be directly converted to DHA, FADS2 activity on DPA was recently shown [99], but the contribution to DHA synthesis in different cell types has not been resolved. ALA, α-linolenic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; FADS, fatty acid desaturase; THA, tetrahexaenoic acid.
To date, defects in seven proteins involved in the different steps of peroxisomal β-oxidation have been identified and include (i) the ABCD1 transporter, (ii) multifunctional protein 2 (MFP2), (iii) acyl-CoA oxidase 1 (ACOX1), (iv) α-methylacyl-CoA racemase (AMACR), (v) acyl-CoA oxidase 2 (ACOX2), (vi) the ABCD3 transporter and (vii) sterol carrier protein x (SCPx), in decreasing order of occurrence [39]. Recently, Ferdinandusse et al. proposed to classify acyl-CoA binding domain containing protein 5 (ACBD5) deficiency in this subgroup, although ACBD5 does not participate in the peroxisomal β-oxidation cycle [81].
4.2.1. X-Linked Adrenoleukodystrophy
X-linked adrenoleukodystrophy (X-ALD) is caused by mutations in the ABCD1 gene. This leads to the accumulation of saturated VLCFAs, due to their impaired peroxisomal translocation preceding the degradation via β-oxidation. Even though patients can be asymptomatic or present with an isolated adrenocortical insufficiency (i.e., Addison-only phenotype), the two main disease presentations are adrenomyeloneuropathy (AMN) and cerebral ALD (CALD). AMN is mainly characterized by a progressive, non-inflammatory axonal polyneuropathy in the spinal cord, whereas CALD involves inflammatory cerebral demyelination [50,100].
The neurological decline in CALD also involves a decrease in visual acuity and development of visual field defects, cortical blindness and eye motility problems (such as strabismus). These symptoms are caused by extensive brain lesions and demyelination of the visual tract, coinciding with optic disc pallor upon fundoscopic examination [50,51,52,53]. In addition, via OCT, retinal nerve fiber layer thinning and ganglion cell loss were detected that were most pronounced in the macular region. Importantly, in contrast to other peroxisomal β-oxidation disorders, no pathology was seen in the outer retina [52,54].
4.2.2. Acyl-CoA Oxidase 1 Deficiency
ACOX1 catalyses the first desaturation step of the peroxisomal β-oxidation and is assumed to only play a role in the degradation of straight chains, including saturated and polyunsaturated VLCFAs [2]. Severely affected ACOX1 deficiency patients are indistinguishable from those suffering from Zellweger syndrome. Likewise, similar (early-onset) ocular symptoms are observed, including abnormal retinal pigmentation, retinal degeneration and optic atrophy [65,66,67,68,69,70,71].
Although most patients do not survive into adolescence, two siblings in their 50s and with a milder disease course were identified. Interestingly, fundoscopic analysis uncovered pronounced ophthalmological pathology, including retinitis pigmentosa and small lens opacities, but sparing of the optic nerve in the male patient. Unfortunately, no information on the retinal involvement in his sister was provided, due to the presence of bilateral cataracts obscuring the fundi [72].
Already more than 20 years ago, Acox1 knockout mice were generated [101], but the ocular phenotype was not investigated.
4.2.3. Multifunctional Protein 2 Deficiency
MFP2 executes the second (hydration) and third (dehydrogenation) step and displays a broad substrate specificity [2]. This leads to accumulation of VLCFAs, the branched chain fatty acid pristanic acid and bile acid intermediates and shortage of DHA in MFP2 deficient patients. Like ACOX1 deficiency, the clinical presentation of severely affected patients resembles that of Zellweger syndrome, including similar ocular symptoms [76]. More recently, MFP2 deficient patients with a milder disease phenotype with juvenile onset were described. Interestingly, despite normal visual acuity, abnormal retinal pigmentation was reported [77,78,79].
To study the mechanisms underlying the disease course of MFP2 deficiency, our laboratory generated global MFP2 knockout mice (Mfp2−/− mice) [102]. Hereto, the first three exons of the Hsd17b4 gene were deleted, resulting in the loss of both the hydratase and dehydrogenase activity. These mice displayed decreased retinal function, already at the age of 3 weeks, and reduced visual acuity. Moreover, we observed a dual phenotype, in which photoreceptors and RPE cells were severely affected [23]. On one hand, MFP2 deficiency caused impaired photoreceptor development, and more specifically impaired maturation, resulting in shortened outer and inner segments with surprisingly normal ultrastructure at the age of 3 weeks. It is noteworthy that this decrease in photoreceptor length persisted and even worsened upon aging, which is in contrast to the Pex1 knock-in mice. Together with this morphological abnormality, Mfp2−/− retinas exhibited severe changes in gene expression in almost all retinal cells, but especially in rods and cones (e.g., downregulation of genes involved in phototransduction) and in retinal glia cells (i.e., increase in inflammation related processes). The latter coincides with microglial activation and migration towards the RPE, shown via IHC for ionized calcium binding adaptor molecule 1 (Iba1). On the other hand, we also observed a degenerative component in the retinal phenotype of the Mfp2−/− mice. At the age of 9 weeks, we observed a progressive loss of photoreceptors, which was already initiated at the age of 3 weeks as shown by a marked increase in TUNEL positive photoreceptor nuclei. Moreover, RPE cells seemed to invade the POS layer. Furthermore, we performed in-depth lipid analyses on the retinas of Mfp2−/− mice, as a first approach to elucidate the mechanisms underlying the retinal pathology in peroxisomal β-oxidation deficiency. In Section 6, these results will be further discussed and matched to information from the patients. Since photoreceptors and RPE are strongly interdependent, the cause-consequence relationship of the degeneration of the outer retina still needs to be elucidated. Furthermore, in view of the abundance of Mfp2 transcripts in the inner retina, the impaired b-waves and altered transcripts of inner retinal cells (amacrine, bipolar and ganglion cells), this also deserves further research.
Unfortunately, it remains elusive to what extent the retinal pathology of the Mfp2−/− mice recapitulates that of the patients. To our knowledge, no histopathological descriptions of the retinas of MFP2 deficient patients were reported. There are, however, several similarities with the histopathological changes observed in the abovementioned Zellweger syndrome patients. Indeed, photoreceptor loss and infiltration of inflammatory cells in the retina of PEX patients was described and the RPE atrophy and protrusion of RPE cells into the POS layer of Mfp2−/− mice could correspond with the hyper-reflexive spots under the RPE upon OCT.
4.2.4. Deficient Oxidation of Branched Chain Fatty Acids and Bile Acid Synthesis
The branched chain fatty acid pristanic acid and the bile acid intermediates DHCA and THCA are imported into peroxisomes by the ABCD3 transporter [24]. Prior to their metabolism via β-oxidation by ACOX2 (pristanic acid and bile acids) or ACOX3 (pristanic acid) and subsequently by MFP2 and SCPx, the substrates are converted into their S-configuration by α-methylacyl-CoA racemase (AMACR) (Figure 2) [2]. Although these proteins are involved in the metabolism of the same substrates, the metabolic abnormalities and clinical presentation differ profoundly.
Firstly, AMACR deficiency is biochemically characterized by the accumulation of pristanic acid, DHCA and THCA. Clinically, one patient has been described with severe early-onset liver dysfunction [103], while a dozen other patients present with a late-onset neuropathy, with symptoms resembling Refsum disease, and no liver dysfunction. Importantly, the majority presents with pigmentary retinopathy, including retinitis pigmentosa, and impaired visual acuity. Additional ocular symptoms in some patients are optic atrophy, cataract and visual field defects [56,57,58,59,60,61,62,63,64]. The retina of the AMACR knockout mouse has not been investigated [104].
Given the similarity of the retinal pathology of AMACR deficiency with Refsum disease, the metabolic cause is likely the accumulation of the branched chain fatty acid pristanic acid. In this respect, it is quite surprising that the only described SCPx deficient patient does not show ocular symptoms. Yet, he had elevated pristanic acid levels and displayed profound neurological symptoms [80].
Furthermore, the few ABCD3 and ACOX2 deficient patients are characterized by relatively normal pristanic acid but elevated DHCA and THCA levels. Their clinical symptoms originate from primary liver dysfunction, and similar to the SCPx patient, no ophthalmological abnormalities were reported [55,73,74,75]. However, most of these patients were described at an early age, and it cannot be excluded that retinopathy develops later.
4.2.5. Acyl-CoA Binding Domain Containing Protein 5 Deficiency
ACBD5 is a quite recently described tail-anchored peroxisomal membrane protein that was assigned with multiple functions. Its main function is being part of a tethering complex that establishes contacts between peroxisomes and the endoplasmic reticulum (ER) [105,106,107]. The name refers to an acyl-CoA binding domain and it was postulated to present VLCFAs to the ABCD1 transporter [81,108]. ACBD5 deficiency was first described in three siblings, by using exome sequencing on a large cohort of retinopathy patients. Besides the pronounced retinal dystrophy (cone-rod dystrophy), the patients presented with severe white matter disease and spastic paraparesis [82]. Subsequently, Ferdinandusse et al. described another ACBD5 deficient patient with similar clinical symptoms [81]. Analogous to X-ALD patients, the only metabolic abnormality thus far identified in plasma was the increased levels of saturated VLCFAs, which was the reason why Ferdinandusse et al. proposed to categorize ACBD5 deficiency in the group of the peroxisomal β-oxidation deficiencies [81,108]. More recently, an additional ACBD5 deficient patient was identified, in which ophthalmological problems were the first symptoms, followed by mental and motor deterioration [83]. The retinal pathology involved cone-rod dystrophy, diagnosed by ERG analysis. In addition, fundoscopy revealed optic nerve pallor, attenuation of blood vessels and diffuse granularity of the RPE, symptoms also observed in ZSD, and ACOX1 and MFP2 deficiency.
Recently, the initial characterization of the retinal pathology of an ACBD5 deficient mouse model was described, consisting of loss of photoreceptors and microglial and astrocyte activation at the age of 1 year, but a more thorough investigation is warranted [109].
5. Ether Phospholipid Deficiency
5.1. Rhizomelic Chondrodysplasia Punctata
To date, five types of the disease rhizomelic chondrodysplasia punctata (RCDP) are identified, with mutations in five different genes. Type 1 and 5 are considered as peroxisomal biogenesis disorders leading to impaired import of PTS2 proteins due to mutations in PEX7 and in exon 9 of PEX5, respectively. Type 2, 3, and 4 belong to the single enzyme deficiencies, due to mutations in the GNPAT, AGPS, and FAR1 genes [110]. These mutations lead to a selective deficiency in the synthesis of ether phospholipids (also known as plasmanyl phospholipids) that represent a glycerophospholipid subclass in which the fatty acid at the sn-1 position is replaced by a long chain fatty alcohol, establishing the characteristic ether bond. Moreover, plasmalogens (also known as plasmenyl phospholipids) are the most abundant ether lipid subspecies, containing a vinyl-ether bond. Biosynthesis of these glycerophospholipid species requires the cooperation of peroxisomes and the ER. The first two steps are executed in the peroxisome by glycerone-phosphate O-acyltransferase (GNPAT) and AGPS. In addition, at the cytosolic side of the peroxisomal membrane, the enzyme fatty acyl-CoA reductase 1 (FAR1) produces the fatty alcohol via the reduction of the corresponding fatty acyl-CoA [84,111].
Besides characteristic bone malformations, impaired growth and a range of neurological symptoms, congenital cataract is a cardinal symptom in RCDP patients. Although plasmalogens are also important constituents of the retina, it seems to be spared [84,85].
5.2. Insights from the Different Mouse Models
Several mouse models with deficient ether phospholipid biosynthesis (Pex7 knockout [112] and hypomorphic [113] mouse, Gnpat knockout mouse [114], blind sterile 2 mouse with a spontaneous mutation in Agps [115] and two different Agps knockout mice [116]) were reported. All mouse models develop bilateral cataract, even those with residual ether phospholipid synthesis. Although lens histology is described in detail in most models, only in the hypomorphic Pex7 mouse and Gnpat knockout mouse the retinal structure was mentioned. The retina of the hypomorphic Pex7 mouse did not show any gross morphologic alterations [113]. In contrast, retinal abnormalities were reported in the Gnpat knockout mouse. Hereby, RPE abnormalities, like vacuolation, hypo- and hyperplasia, pigmentation abnormalities, and accumulation of photoreceptor degradation products were observed in addition to Bruch’s membrane thickening and optic nerve hypoplasia. The Gnpat knockout mouse also displayed retinal vasculature abnormalities, in which a persistent hyaloid artery was reported. During development, this artery, extending from the optic disc, provides nutrients to the lens and should eventually completely regress as the retinal vasculature matures [114]. Later, it was elucidated that in this mouse model, the ether phospholipid deficiency interferes with the different stages of retinal vascularization, resulting in a disorganized and dysfunctional network in the mature retina [117]. As there are, to our knowledge, no reports of retinal abnormalities in RCDP patients, it is unclear how to translate the observations from the Gnpat knockout mice.
6. Candidate Metabolites Causing Retinopathy
Combining the data from the different patient groups, it emerges that retinopathy is a frequent pathology. It appears that ether lipid deficiency does not have a detrimental impact on the retina, but the metabolic origin of the retinopathy in peroxisomal α- and β-oxidation deficient patients seems to be diverse. Recently, we performed in-depth lipid analyses on retinas of Mfp2−/− mice, as a first approach to elucidate the mechanisms underlying the retinal pathology in peroxisomal β-oxidation deficiency [23]. This was the first time that in a mouse model mimicking peroxisomal dysfunction the retinal lipid composition was associated with the retinal pathology. Given the broad substrate specificity of MFP2, it is instrumental to discuss the biochemical alterations and to put them into context of the retinal pathology in patients with deficient peroxisomal α- and β-oxidation. Hereby, several candidate metabolites deserve a more thorough discussion, including saturated VLCFAs, branched chain fatty acids, DHA and VLC-PUFAs.
6.1. Saturated Very Long Chain Fatty Acids
A first metabolic disturbance worth considering is the vast accumulation of saturated VLCFAs in Mfp2−/− retinas. We found for example a severe increase in the disease marker 1-hexacosanoyl-2-hydroxy-sn-glycero-3-phosphocholine (Lyso-PC 26:0) [118]. However, their involvement in the observed retinal anomalies is questionable because the outer retina in ABCD1 deficient patients, characterized by the accumulation of VLCFAs as sole metabolic abnormality, is always spared [52,54].
6.2. Branched Chain Fatty Acids
MFP2 is also involved in the metabolism of the branched chain fatty acid pristanic acid that is formed by the α-oxidation of phytanic acid [2]. It is quite clear that elevated levels of branched chain fatty acids are detrimental for the human retina (cf. Refsum disease and AMACR deficiency), but the situation for the rodent retina is less evident. Rats fed a phytol-rich diet did not develop retinopathy, while the retina of mice with impaired branched chain fatty acid metabolism remains understudied [90,91,104].
Phytanic and pristanic acid in the plasma and brain of Mfp2−/− mice fed a standard rodent chow were slightly elevated compared to wild type mice, but the levels in plasma remain two orders of magnitude below the levels in Refsum disease or ZSD patients [119]. Although we did not measure branched chain fatty acid levels in the Mfp2−/− retinas, we hypothesize that their contribution to the retinal degeneration will be limited, but feeding a phytol rich diet could underscore this assumption.
6.3. Polyunsaturated Fatty Acids
As already mentioned, the fatty acid composition of POS phospholipids diverges from other membranes, being highly enriched in PUFAs. The most abundant PUFA is docosahexaenoic acid (DHA, C22:6n-3), an n-3 PUFA that amounts up to approximately 50% of the POS phospholipid fatty acid side chains [120,121,122]. Notably, in some phospholipid species DHA occurs in both the sn-1 and sn-2 position [123]. Even more exceptional is the relative enrichment in phospholipids containing very long chain polyunsaturated fatty acids (VLC-PUFAs, >C30) in the sn-1 and DHA in the sn-2 position [124,125,126]. It is noteworthy that cones contain significantly less DHA and VLC-PUFAs than rods (approximately 2-fold). It is, therefore, postulated that cones and cone signalling might have other lipid requirements [127].
6.3.1. Docosahexaenoic Acid
The two main retinal DHA sources are the diet (e.g., fatty fish) and de novo biosynthesis starting from α-linolenic acid (C18:3n-3) [128]. The latter occurs primarily in liver and involves one cycle of peroxisomal β-oxidation (Figure 3) [94]. Already, in the 1970s, it was shown that DHA deficiency has a negative impact on retinal function. More specifically, DHA deficient rats displayed reduced ERG responses that could be restored by DHA replenishment [129,130]. The observations of decreased visual function were confirmed in other species, including mouse [131], guinea pig [132,133], rhesus monkeys [134,135,136] and humans (neonates) [137,138,139]. Moreover, supplementing preterm infants with fish oil, generally rich in DHA and eicosapentaenoic acid (EPA, C20:5n-3), improved the development of visual function [140].
In view of the presumed role of peroxisomal β-oxidation in PUFA homeostasis and the importance of DHA for retinal function, it was hypothesized that reduced retinal DHA levels lie at the basis of the retinopathy in ZSD and MFP2 deficient patients [141]. Reduced DHA levels are indeed frequently observed in these patients [76,141]. Moreover, in one Zellweger syndrome patient retinal lipids were analysed and a shortage of DHA was found [141]. However, it should be noted that not all MFP2 deficient patients that present with retinopathy display reduced plasma DHA levels, obscuring the possible link between DHA deficiency and retinopathy [76].
In this respect, DHA supplementation to prevent deterioration of visual acuity seemed a plausible treatment option, but two different clinical trials produced contradictory results. More specifically, an open trial showed promising stabilization of the deteriorating retinal function in mildly affected patients [30], while a randomized, double blind and placebo-controlled clinical trial detected no effect of DHA treatment on retinal function [142]. Possibly, the retinal deterioration cannot be halted when it is already ongoing and DHA supplementation should, therefore, start as soon as possible.
6.3.2. Very Long Chain Polyunsaturated Fatty Acids
Synthesis of VLC-PUFAs with more than 26 carbons is mediated by the enzyme elongation of very long chain fatty acids-4 (ELOVL4) in the ER. This enzyme is only found in tissues that contain VLCFAs and VLC-PUFAs with >26 carbons (retina, lens, brain, testis and skin), which implies that the latter are locally produced. In the retina, ELOVL4 is mainly found in the photoreceptor inner segments [126,143]. Although both n-3 and n-6 fatty acids can be used as elongation substrates, it was shown that EPA is preferentially elongated over arachidonic acid (AA, C20:4n-6) and DHA (EPA > AA > DHA) [144,145]. However, this observation has to be put into context. The DHA pool is much larger compared to the EPA and AA pool (±10-fold), and, although it was speculated that DHA is mainly esterified, there is still a considerable amount that is elongated into VLC-PUFAs. In addition, if EPA is indeed the preferred substrate for elongation in the inner segments, it has to be considered that its level in the circulation is very low (Das Y., Vaz F. and Baes M., unpublished observations). In this respect, it can be speculated that the more abundant DHA first undergoes one cycle of peroxisomal β-oxidation and subsequently a desaturation to produce EPA that is further elongated. This could possibly be an additional role of peroxisomes in the photoreceptors, besides local DHA synthesis. After elongation, VLC-PUFAs are esterified to form phospholipids that are mostly integrated into the disc membranes, although it was shown that they are also localised in the synapses [124,146].
The importance of VLC-PUFAs is highlighted by the discovery of mutations in the ELOVL4 gene, causing autosomal dominant Stargardt 3 Macular Dystrophy (STGD3). Most mutations lead to the synthesis of a truncated and mislocalized protein [143]. In addition to macular photoreceptor degeneration, STGD3 is typically characterized with optic nerve pallor and well-circumscribed macular RPE and choriocapillaris atrophy surrounded by yellow flecks upon fundoscopic examination [126]. Although, to date, the exact mechanism of photoreceptor death in STGD3 still remains elusive, three possible mechanisms are postulated: (i) VLC-PUFA reduction affects photoreceptor function and structure, (ii) the mislocalized truncated protein causes cellular stress, (iii) toxic molecules are generated by the limited function of the truncated protein [143,147]. In line with the first mechanism, STGD3 patients with ELOVL4-loss-of-function mutations have been described, which underscores the essential role of VLC-PUFAs in the retina [143].
In addition to the synthesis, it can be predicted that peroxisomal β-oxidation is required for the breakdown of VLC-PUFAs, as this is the only pathway that can handle such long carboxylate chains. The retinal VLC-PUFA status in ZSD and peroxisomal β-oxidation deficiency patients has, however, never been investigated. In the brains of Zellweger syndrome patients, an accumulation of VLC-PUFAs was reported [148,149,150]. More recently, increased levels of VLC-PUFAs were also found in fibroblasts from an ACBD5 deficient patient, but not in X-ALD fibroblasts [108]. These observations lead to the assumption that the ER and peroxisomes need to work together to set a balance between VLC-PUFA synthesis and catabolism.
6.3.3. Retinal PUFA Status of Mfp2−/− Mice
With respect to the considerations above, we assessed the PUFA status of the Mfp2−/− retinas in more detail (Figure 4) [23]. Firstly, we found an overall decrease in DHA-containing phospholipid species, with a striking virtual depletion of those containing two DHA moieties (i.e., PC(44:12)). Of note, these decreases were accompanied by a compensatory increase in phospholipids containing n-6 PUFAs (such as arachidonic acid). Secondly, it was shown that phospholipids with ≥52 carbons and 10-12 double bonds in their side chains are composed of a tetra-, penta- or hexaenoic VLC-PUFA, respectively, in the sn-1 position and DHA in the sn-2 position. These phospholipid species displayed a peculiar profile. More specifically, phospholipids containing VLC-PUFAs with ≤34 carbons in their side chains were severely reduced, while those containing >34 carbons accumulated.
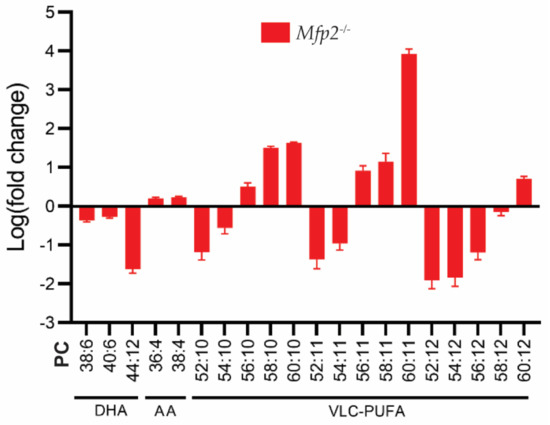
Figure 4.
PUFA profile of Mfp2−/− neural retinas. Lipidome analysis on Mfp2−/− neural retinas revealed a severe decrease in DHA- and an increase in AA-containing PC phospholipids. The PC phospholipids containing VLC-PUFAs show a peculiar profile, as phospholipids containing ≤56 carbons in their side chains are severely reduced, while those containing >56 carbons accumulate. These PC species contain a VLC-PUFA and DHA moiety in the sn-1 and sn-2 position, respectively. Data are presented as log transformation of relative values to WT values.
Keeping these observations in mind, two pertinent questions emerged: (i) Are these changes caused by a systemic or a local defect in peroxisomal β-oxidation? (ii) How do these changes in retinal lipidome relate to the observed phenotype?
6.3.4. The Origin of the Altered PUFA Profile
It is generally accepted that the majority of the retinal DHA content is delivered by the systemic circulation [128]. Interestingly, the Mfp2−/− mice suffered from a hampered retinal supply, as evidenced by a decrease in DHA-containing phospholipids in plasma [23]. This could be due to impaired DHA synthesis in the liver, but also originate from a severely impaired bile acid synthesis and steatorrhea during the lactation period, resulting in a decreased intestinal lipid uptake [102]. In addition, the Mfp2−/− retinas showed suppressed levels of Adiponectin receptor 1 (ADIPOR1), a protein involved in retinal DHA retention and traffic, suggesting hampered retinal handling [23,151]. Interesting to note is that transcripts of the transporter Major facilitator superfamily domain-containing protein 2a (MFSD2a) were not significantly reduced in Mfp2−/− mice [23]. Like ADIPOR1, MFSD2a is an essential contributor to maintain appropriate retinal DHA levels [152,153].
In contrast, it is by far less clear what the local role of peroxisomal β-oxidation in the retina encompasses. It could be involved in the synthesis of DHA in photoreceptors and RPE cells, although it is generally accepted that the yield and rate are rather limited to meet the needs for POS synthesis [95,96,97,98]. We hypothesize that there is a clear distinction in its role in the two cell types. In photoreceptors, peroxisomal β-oxidation could be involved in the retroconversion of DHA into EPA, needed for the synthesis of VLC-PUFAs as mentioned before [151]. The reduced levels of VLC-PUFAs with ≤34 carbons in the Mfp2−/− neural retina could thus be due to lower levels of DHA and/or EPA. An alternative task of peroxisomal β-oxidation could be to counter excessive PUFA elongation in the ER, in order to establish a balance between synthesis and degradation, and to maintain appropriate relative VLC-PUFA levels. The accumulation of VLC-PUFAs with >C34 carbons in the Mfp2−/− neural retina is in line with the latter hypothesis and is likely the result of an uncountered elongation [23].
In the RPE, on the other hand, the potential role of peroxisomal β-oxidation seems more straightforward as these cells are exposed to a high PUFA load following the daily phagocytosis of shed POS [8,9]. It is generally accepted that these PUFAs are mostly recycled [128]. This is underscored by the fact that n-3 deficient rats exhibited similar levels of retinal DHA compared to control rats, highlighting the avid conservation of DHA in these circumstances [121]. It is, however, plausible that some PUFAs need to be degraded, which requires peroxisomal β-oxidation for fatty acids with ≥C22. In this respect, it was shown that DHA, originating from ingested POS, can be used by RPE cells for the synthesis of ketone bodies that subsequently mainly serve as energy source for photoreceptors [154].
6.3.5. Link between the Altered PUFA Profile and Retinal Phenotype
The question remains whether the peculiar PUFA profile underlies the observed retinal abnormalities. To date, the exact function of retinal DHA and VLC-PUFAs is still under debate, but several lines of evidence demonstrate that they (directly) interact with the phototransduction process and that they have a role in photoreceptor development and survival [128,155]. In view of the latter, it is interesting that DHA and VLC-PUFAs can be converted into cytoprotective and anti-inflammatory lipid mediators in case of uncompensated retinal stress, such as neuroprotection D1 and elovanoids, respectively [156,157]. In view of the substrate shortage in the retina, it can be speculated that this leads to decreased synthesis of these mediators, possibly contributing to the retinopathy.
Moreover, valuable information can be obtained by comparing the retinal pathology of the Mfp2−/− mice with that of models with genetic ablation of proteins involved in retinal PUFA homeostasis. These proteins are involved in retinal DHA uptake and traffic (i.e., ADIPOR1 [151] and MFSD2a) [152,153] and membrane-type frizzled-related protein (MFRP) [158]) and incorporation of DHA into phospholipids (lysophosphatidic acid acyltransferase 3 (LPAAT3) [159]). The retinas of the corresponding mouse models display severe reductions of phospholipids containing DHA and VLC-PUFAs, with a compensatory increase in those containing AA. Overall, frequently observed defects include decreased retinal function, POS length reduction (i.e., impaired photoreceptor maturation) and progressive photoreceptor loss (i.e., impaired photoreceptor survival). However, there is large variability between these models. Even two knockout models of the same gene (Mfsd2a) display a diverging phenotype despite comparable changes in PUFA levels [152,153]. This variability makes it challenging to clearly determine the link between the observed deregulated PUFA profile and retinal pathologies. Still, considering the similarity with the retinal pathology of Mfp2−/− mice, it is highly likely that the alterations in the retinal PUFA levels play a crucial role.
7. Conclusions and Future Prospects
Both data from patients and mouse models indicate that proper peroxisomal function is essential to maintain retinal homeostasis, but the underlying mechanisms still remain largely unresolved.
By analysing diseases in which only a single peroxisomal protein was defective, leading to specific metabolic profiles, it became clear that saturated VLCFA accumulation does not cause discernible retinal damage, the effect of ether lipid deficiency is currently unclear and the accumulation of branched chain fatty acids causes significant retinal pathology.
Furthermore, it is highly likely that a disturbed retinal PUFA homeostasis strongly contributes to the retinopathy observed in ZSD and peroxisomal β-oxidation (e.g., ACOX1 and MFP2) deficiency patients. Unfortunately, no hard evidence is available from patients, but the severe PUFA deregulation coinciding with the early-onset retinal pathology in the Mfp2−/− mice supports this statement. It requires, however, further research to unravel (i) the exact local role of peroxisomal β-oxidation for retinal PUFA homeostasis, (ii) the contribution of systemic versus local peroxisomal β-oxidation deficiency to the retinal phenotype, and (iii) which cell type drives the retinal pathology. Future investigations on cell type selective (photoreceptors and RPE) MFP2 knockout mice, including lipid analyses, will shed light on these open questions.
It is striking that the retinal pathology of the Mfp2−/− mice is more severe compared to the Pex1 knock-in and ACBD5 knockout mice. It will be instructive to perform a thorough comparison of the lipid content and the retinal morphology of these mouse models.
Although we focused in this review on the role of peroxisomes in lipid metabolism, other important functions cannot be excluded. For example, they might have an important role in the retinal antioxidant system, which is illustrated by the high expression of the antioxidant enzyme catalase in the RPE [12,13,14,15,16,17,18]. This is not surprising as these cells are constantly challenged by ROS stress due to their very active metabolism and exposure to high ambient oxygen partial pressures [8,160]. Furthermore, a possible role of peroxisomes in ciliogenesis has recently been postulated by several laboratories. Several lines of evidence were provided: (i) knockdown of PEX genes partially impaired ciliogenesis, (ii) a serine-threonine kinase, NDR2, which is essential in ciliogenesis, localizes to peroxisomes, (iii) peroxisomes mediate trafficking of cholesterol into ciliary membranes [161,162]. Interestingly, the POS are a form of primary cilia that also contain a large amount of cholesterol in the plasma membrane and in the membranes of discs at the base [163]. It could be inferred that also via these roles in ciliogenesis peroxisomes are important in the retina.
One major drawback in the ongoing retinal research in peroxisomal disorders is the lack of reports on histopathological changes and lipid content in (mild) patients, which makes it challenging to translate the findings from the available mouse models to the human situation. Nevertheless, these models are useful to elucidate the underlying mechanisms, which should drive the development of future therapies to combat these blinding diseases.
Author Contributions
Y.D., D.S. and M.B. searched the literature, wrote the manuscript and made figures. All authors have read and agreed to the published version of the manuscript.
Funding
M.B. is supported by grants from the KU Leuven (C14/18/088) and from the Research Foundation–Flanders (FWO G0A8619N).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of KU Leuven (P166/2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wanders, R.J.; Waterham, H.R. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 2006, 75, 295–332. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhoven, P.P. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J. Lipid Res. 2010, 51, 2863–2895. [Google Scholar] [CrossRef] [PubMed]
- Waterham, H.R.; Ferdinandusse, S.; Wanders, R.J. Human disorders of peroxisome metabolism and biogenesis. Biochim. Biophys. Acta 2016, 1863, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Folz, S.J.; Trobe, J.D. The peroxisome and the eye. Surv. Ophthalmol. 1991, 35, 353–368. [Google Scholar] [CrossRef]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84. [Google Scholar] [CrossRef]
- Molday, R.S.; Moritz, O.L. Photoreceptors at a glance. J. Cell Sci. 2015, 128, 4039–4045. [Google Scholar] [CrossRef]
- Baehr, W.; Hanke-Gogokhia, C.; Sharif, A.; Reed, M.; Dahl, T.; Frederick, J.M.; Ying, G. Insights into photoreceptor ciliogenesis revealed by animal models. Prog. Retin. Eye Res. 2019, 71, 26–56. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Lakkaraju, A.; Umapathy, A.; Tan, L.X.; Daniele, L.; Philp, N.J.; Boesze-Battaglia, K.; Williams, D.S. The cell biology of the retinal pigment epithelium. Prog. Retin. Eye Res. 2020, 100846. [Google Scholar] [CrossRef]
- Kanow, M.A.; Giarmarco, M.M.; Jankowski, C.S.; Tsantilas, K.; Engel, A.L.; Du, J.; Linton, J.D.; Farnsworth, C.C.; Sloat, S.R.; Rountree, A.; et al. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. Elife 2017, 6. [Google Scholar] [CrossRef]
- Islinger, M.; Cardoso, M.J.; Schrader, M. Be different—The diversity of peroxisomes in the animal kingdom. Biochim. Biophys. Acta 2010, 1803, 881–897. [Google Scholar] [CrossRef]
- Robison, W.G., Jr.; Kuwabara, T. Microperoxisomes in retinal pigment epithelium. Investig. Ophthalmol. 1975, 14, 866–872. [Google Scholar]
- Leuenberger, P.M.; Novikoff, A.B. Studies on microperoxisomes. VII. Pigment epithelial cells and other cell types in the retina of rodents. J. Cell Biol. 1975, 65, 324–334. [Google Scholar] [CrossRef]
- Beard, M.E.; Davies, T.; Holloway, M.; Holtzman, E. Peroxisomes in pigment epithelium and Muller cells of amphibian retina possess D-amino acid oxidase as well as catalase. Exp. Eye Res. 1988, 47, 795–806. [Google Scholar] [CrossRef]
- Deguchi, J.; Yamamoto, A.; Fujiki, Y.; Uyama, M.; Tsukahara, I.; Tashiro, Y. Localization of nonspecific lipid transfer protein (nsLTP = sterol carrier protein 2) and acyl-CoA oxidase in peroxisomes of pigment epithelial cells of rat retina. J. Histochem. Cytochem. 1992, 40, 403–410. [Google Scholar] [CrossRef]
- Hazlett, L.D.; Hazlett, J.C.; Ireland, M.; Bradley, R.H. Microperoxisomes in retinal epithelium and tapetum lucidum of the American opossum. Exp. Eye Res. 1978, 27, 343–348. [Google Scholar] [CrossRef]
- Atalla, L.; Fernandez, M.A.; Rao, N.A. Immunohistochemical localization of catalase in ocular tissue. Curr. Eye Res. 1987, 6, 1181–1187. [Google Scholar] [CrossRef]
- Das, Y.; Roose, N.; De Groef, L.; Fransen, M.; Moons, L.; Van Veldhoven, P.P.; Baes, M. Differential distribution of peroxisomal proteins points to specific roles of peroxisomes in the murine retina. Mol. Cell. Biochem. 2019, 456, 53–62. [Google Scholar] [CrossRef]
- Argyriou, C.; Polosa, A.; Cecyre, B.; Hsieh, M.; Di Pietro, E.; Cui, W.; Bouchard, J.F.; Lachapelle, P.; Braverman, N. A longitudinal study of retinopathy in the PEX1-Gly844Asp mouse model for mild Zellweger Spectrum Disorder. Exp. Eye Res. 2019, 186, 107713. [Google Scholar] [CrossRef]
- Zaki, M.S.; Heller, R.; Thoenes, M.; Nurnberg, G.; Stern-Schneider, G.; Nurnberg, P.; Karnati, S.; Swan, D.; Fateen, E.; Nagel-Wolfrum, K.; et al. PEX6 is Expressed in Photoreceptor Cilia and Mutated in Deafblindness with Enamel Dysplasia and Microcephaly. Hum. Mutat. 2016, 37, 170–174. [Google Scholar] [CrossRef]
- Smith, C.E.; Poulter, J.A.; Levin, A.V.; Capasso, J.E.; Price, S.; Ben-Yosef, T.; Sharony, R.; Newman, W.G.; Shore, R.C.; Brookes, S.J.; et al. Spectrum of PEX1 and PEX6 variants in Heimler syndrome. Eur. J. Hum. Genet. 2016, 24, 1565–1571. [Google Scholar] [CrossRef]
- Daniele, L.L.; Caughey, J.; Volland, S.; Sharp, R.C.; Dhingra, A.; Williams, D.S.; Philp, N.J.; Boesze-Battaglia, K. Peroxisome turnover and diurnal modulation of antioxidant activity in retinal pigment epithelia utilizes microtubule-associated protein 1 light chain 3B (LC3B). Am. J. Physiol. Cell Physiol. 2019, 317, C1194–C1204. [Google Scholar] [CrossRef]
- Das, Y.; Swinkels, D.; Kocherlakota, S.; Vinckier, S.; Vaz, F.M.; Wever, E.; van Kampen, A.H.C.; Jun, B.; Do, K.V.; Moons, L.; et al. Peroxisomal Multifunctional Protein 2 Deficiency Perturbs Lipid Homeostasis in the Retina and Causes Visual Dysfunction in Mice. Front. Cell Dev. Biol. 2021, 9, 632930. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Morita, M. ABC Transporter Subfamily D: Distinct Differences in Behavior between ABCD1-3 and ABCD4 in Subcellular Localization, Function, and Human Disease. Biomed. Res. Int. 2016, 2016, 6786245. [Google Scholar] [CrossRef]
- Lo, W.K.; Bernstein, M.H. Daily patterns of the retinal pigment epithelium. Microperoxisomes and phagosomes. Exp. Eye Res. 1981, 32, 1–10. [Google Scholar] [CrossRef]
- Argyriou, C.; D’Agostino, M.D.; Braverman, N. Peroxisome biogenesis disorders. Transl. Sci. Rare Dis. 2016, 1, 111–144. [Google Scholar] [CrossRef]
- Ebberink, M.S.; Mooijer, P.A.; Gootjes, J.; Koster, J.; Wanders, R.J.; Waterham, H.R. Genetic classification and mutational spectrum of more than 600 patients with a Zellweger syndrome spectrum disorder. Hum. Mutat. 2011, 32, 59–69. [Google Scholar] [CrossRef]
- Berendse, K.; Engelen, M.; Ferdinandusse, S.; Majoie, C.B.; Waterham, H.R.; Vaz, F.M.; Koelman, J.H.; Barth, P.G.; Wanders, R.J.; Poll-The, B.T. Zellweger spectrum disorders: Clinical manifestations in patients surviving into adulthood. J. Inherit. Metab. Dis. 2016, 39, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Courtney, R.J.; Pennesi, M.E. Interval spectral-domain optical coherence tomography and electrophysiology findings in neonatal adrenoleukodystrophy. JAMA Ophthalmol. 2013, 131, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Noguer, M.T.; Martinez, M. Visual follow-up in peroxisomal-disorder patients treated with docosahexaenoic Acid ethyl ester. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2277–2285. [Google Scholar] [CrossRef]
- Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.R.; Kriss, A.; Taylor, D.; Coffey, R.; Pembrey, M. Follow-up and diagnostic reappraisal of 75 patients with Leber’s congenital amaurosis. Am. J. Ophthalmol. 1989, 107, 624–631. [Google Scholar] [CrossRef]
- Majewski, J.; Wang, Z.; Lopez, I.; Al Humaid, S.; Ren, H.; Racine, J.; Bazinet, A.; Mitchel, G.; Braverman, N.; Koenekoop, R.K. A new ocular phenotype associated with an unexpected but known systemic disorder and mutation: Novel use of genomic diagnostics and exome sequencing. J. Med. Genet. 2011, 48, 593–596. [Google Scholar] [CrossRef]
- Michelakakis, H.M.; Zafeiriou, D.I.; Moraitou, M.S.; Gootjes, J.; Wanders, R.J. PEX1 deficiency presenting as Leber congenital amaurosis. Pediatr. Neurol. 2004, 31, 146–149. [Google Scholar] [CrossRef]
- Raas-Rothschild, A.; Wanders, R.J.; Mooijer, P.A.; Gootjes, J.; Waterham, H.R.; Gutman, A.; Suzuki, Y.; Shimozawa, N.; Kondo, N.; Eshel, G.; et al. A PEX6-defective peroxisomal biogenesis disorder with severe phenotype in an infant, versus mild phenotype resembling Usher syndrome in the affected parents. Am. J. Hum. Genet. 2002, 70, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, G.; Sanchez-Navarro, I.; Aller, E.; Jaijo, T.; Fuster-Garcia, C.; Rodriguez-Munoz, A.; Vallejo, E.; Telleria, J.J.; Vazquez, S.; Beltran, S.; et al. Exome sequencing identifies PEX6 mutations in three cases diagnosed with Retinitis Pigmentosa and hearing impairment. Mol. Vis. 2020, 26, 216–225. [Google Scholar]
- Barillari, M.R.; Karali, M.; Di Iorio, V.; Contaldo, M.; Piccolo, V.; Esposito, M.; Costa, G.; Argenziano, G.; Serpico, R.; Carotenuto, M.; et al. Mild form of Zellweger Spectrum Disorders (ZSD) due to variants in PEX1: Detailed clinical investigation in a 9-years-old female. Mol. Genet. Metab. Rep. 2020, 24, 100615. [Google Scholar] [CrossRef]
- Daich Varela, M.; Jani, P.; Zein, W.M.; D’Souza, P.; Wolfe, L.; Chisholm, J.; Zalewski, C.; Adams, D.; Warner, B.M.; Huryn, L.A.; et al. The peroxisomal disorder spectrum and Heimler syndrome: Deep phenotyping and review of the literature. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 618–630. [Google Scholar] [CrossRef]
- De Munter, S.; Verheijden, S.; Regal, L.; Baes, M. Peroxisomal Disorders: A Review on Cerebellar Pathologies. Brain Pathol. 2015, 25, 663–678. [Google Scholar] [CrossRef]
- Cohen, S.M.; Brown, F.R., 3rd; Martyn, L.; Moser, H.W.; Chen, W.; Kistenmacher, M.; Punnett, H.; de la Cruz, Z.C.; Chan, N.R.; Green, W.R. Ocular histopathologic and biochemical studies of the cerebrohepatorenal syndrome (Zellweger’s syndrome) and its relationship to neonatal adrenoleukodystrophy. Am. J. Ophthalmol. 1983, 96, 488–501. [Google Scholar] [CrossRef]
- Glasgow, B.J.; Brown, H.H.; Hannah, J.B.; Foos, R.Y. Ocular pathologic findings in neonatal adrenoleukodystrophy. Ophthalmology 1987, 94, 1054–1060. [Google Scholar] [CrossRef]
- Tao, L.W.; Wu, Z.; Guymer, R.H.; Luu, C.D. Ellipsoid zone on optical coherence tomography: A review. Clin. Exp. Ophthalmol. 2016, 44, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Ratbi, I.; Jaouad, I.C.; Elorch, H.; Al-Sheqaih, N.; Elalloussi, M.; Lyahyai, J.; Berraho, A.; Newman, W.G.; Sefiani, A. Severe early onset retinitis pigmentosa in a Moroccan patient with Heimler syndrome due to novel homozygous mutation of PEX1 gene. Eur. J. Med. Genet. 2016, 59, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.J.; Hu, F.Y.; Xu, P.; Qi, Y.H.; Li, J.K.; Zhang, Y.J.; Chen, F.; Chang, Q.; Song, F.; Shen, S.M.; et al. Expanding the clinical and genetic spectrum of Heimler syndrome. Orphanet J. Rare Dis. 2019, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Wangtiraumnuay, N.; Alnabi, W.A.; Tsukikawa, M.; Thau, A.; Capasso, J.; Sharony, R.; Inglehearn, C.F.; Levin, A.V. Ophthalmic manifestations of Heimler syndrome due to PEX6 mutations. Ophthalmic Genet. 2018, 39, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.H.; Barbazetto, I.A.; Chen, R.; Yannuzzi, L.A.; Tsang, S.H.; Spaide, R.F. Macular dystrophy in Heimler syndrome. Ophthalmic Genet. 2011, 32, 97–100. [Google Scholar] [CrossRef]
- Hiebler, S.; Masuda, T.; Hacia, J.G.; Moser, A.B.; Faust, P.L.; Liu, A.; Chowdhury, N.; Huang, N.; Lauer, A.; Bennett, J.; et al. The Pex1-G844D mouse: A model for mild human Zellweger spectrum disorder. Mol. Genet. Metab. 2014, 111, 522–532. [Google Scholar] [CrossRef]
- Jones, B.W.; Watt, C.B.; Marc, R.E. Retinal remodelling. Clin. Exp. Optom. 2005, 88, 282–291. [Google Scholar] [CrossRef]
- Ruether, K.; Baldwin, E.; Casteels, M.; Feher, M.D.; Horn, M.; Kuranoff, S.; Leroy, B.P.; Wanders, R.J.; Wierzbicki, A.S. Adult Refsum disease: A form of tapetoretinal dystrophy accessible to therapy. Surv. Ophthalmol. 2010, 55, 531–538. [Google Scholar] [CrossRef]
- Kemp, S.; Berger, J.; Aubourg, P. X-linked adrenoleukodystrophy: Clinical, metabolic, genetic and pathophysiological aspects. Biochim. Biophys. Acta 2012, 1822, 1465–1474. [Google Scholar] [CrossRef]
- Kaplan, P.W.; Kruse, B.; Tusa, R.J.; Shankroff, J.; Rignani, J.; Moser, H.W. Visual system abnormalities in adrenomyeloneuropathy. Ann. Neurol. 1995, 37, 550–552. [Google Scholar] [CrossRef]
- Ohkuma, Y.; Hayashi, T.; Yoshimine, S.; Tsuneoka, H.; Terao, Y.; Akiyama, M.; Ida, H.; Ohashi, T.; Okumura, A.; Ebihara, N.; et al. Retinal Ganglion Cell Loss in X-linked Adrenoleukodystrophy with an ABCD1 Mutation (Gly266Arg). Neuroophthalmology 2014, 38, 331–335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Traboulsi, E.I.; Maumenee, I.H. Ophthalmologic manifestations of X-linked childhood adrenoleukodystrophy. Ophthalmology 1987, 94, 47–52. [Google Scholar] [CrossRef]
- Grainger, B.T.; Papchenko, T.L.; Danesh-Meyer, H.V. Optic nerve atrophy in adrenoleukodystrophy detectable by optic coherence tomography. J. Clin. Neurosci. 2010, 17, 122–124. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Jimenez-Sanchez, G.; Koster, J.; Denis, S.; Van Roermund, C.W.; Silva-Zolezzi, I.; Moser, A.B.; Visser, W.F.; Gulluoglu, M.; Durmaz, O.; et al. A novel bile acid biosynthesis defect due to a deficiency of peroxisomal ABCD3. Hum. Mol. Genet. 2015, 24, 361–370. [Google Scholar] [CrossRef]
- Haugarvoll, K.; Johansson, S.; Tzoulis, C.; Haukanes, B.I.; Bredrup, C.; Neckelmann, G.; Boman, H.; Knappskog, P.M.; Bindoff, L.A. MRI characterisation of adult onset alpha-methylacyl-coA racemase deficiency diagnosed by exome sequencing. Orphanet J. Rare Dis. 2013, 8, 1. [Google Scholar] [CrossRef]
- Stewart, M.W.; Vavra, M.W.; Whaley, N.R. Fundus Findings in a Patient with alpha-methlyacyl-coa racemase deficiency. Retin. Cases Brief Rep. 2011, 5, 262–266. [Google Scholar] [CrossRef]
- Kapina, V.; Sedel, F.; Truffert, A.; Horvath, J.; Wanders, R.J.; Waterham, H.R.; Picard, F. Relapsing rhabdomyolysis due to peroxisomal alpha-methylacyl-coa racemase deficiency. Neurology 2010, 75, 1300–1302. [Google Scholar] [CrossRef]
- Smith, E.H.; Gavrilov, D.K.; Oglesbee, D.; Freeman, W.D.; Vavra, M.W.; Matern, D.; Tortorelli, S. An adult onset case of alpha-methyl-acyl-CoA racemase deficiency. J. Inherit. Metab. Dis. 2010, 33 (Suppl. S3), S349–S353. [Google Scholar] [CrossRef]
- Thompson, S.A.; Calvin, J.; Hogg, S.; Ferdinandusse, S.; Wanders, R.J.; Barker, R.A. Relapsing encephalopathy in a patient with alpha-methylacyl-CoA racemase deficiency. BMJ Case Rep. 2009, 2009, bcr08.2008.0814. [Google Scholar] [CrossRef]
- Clarke, C.E.; Alger, S.; Preece, M.A.; Burdon, M.A.; Chavda, S.; Denis, S.; Ferdinandusse, S.; Wanders, R.J. Tremor and deep white matter changes in alpha-methylacyl-CoA racemase deficiency. Neurology 2004, 63, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Dick, D.; Horvath, R.; Chinnery, P.F. AMACR mutations cause late-onset autosomal recessive cerebellar ataxia. Neurology 2011, 76, 1768–1770. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandusse, S.; Denis, S.; Clayton, P.T.; Graham, A.; Rees, J.E.; Allen, J.T.; McLean, B.N.; Brown, A.Y.; Vreken, P.; Waterham, H.R.; et al. Mutations in the gene encoding peroxisomal alpha-methylacyl-CoA racemase cause adult-onset sensory motor neuropathy. Nat. Genet. 2000, 24, 188–191. [Google Scholar] [CrossRef]
- McLean, B.N.; Allen, J.; Ferdinandusse, S.; Wanders, R.J. A new defect of peroxisomal function involving pristanic acid: A case report. J. Neurol. Neurosurg. Psychiatry 2002, 72, 396–399. [Google Scholar] [CrossRef]
- Carrozzo, R.; Bellini, C.; Lucioli, S.; Deodato, F.; Cassandrini, D.; Cassanello, M.; Caruso, U.; Rizzo, C.; Rizza, T.; Napolitano, M.L.; et al. Peroxisomal acyl-CoA-oxidase deficiency: Two new cases. Am. J. Med. Genet. A 2008, 146A, 1676–1681. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Denis, S.; Hogenhout, E.M.; Koster, J.; van Roermund, C.W.T.; IJlst, L.; Moser, A.B.; Wanders, R.J.; Waterham, H.R. Clinical, biochemical, and mutational spectrum of peroxisomal acyl-coenzyme A oxidase deficiency. Hum. Mutat. 2007, 28, 904–912. [Google Scholar] [CrossRef]
- Suzuki, Y.; Iai, M.; Kamei, A.; Tanabe, Y.; Chida, S.; Yamaguchi, S.; Zhang, Z.; Takemoto, Y.; Shimozawa, N.; Kondo, N. Peroxisomal acyl CoA oxidase deficiency. J. Pediatr. 2002, 140, 128–130. [Google Scholar] [CrossRef]
- Suzuki, Y.; Shimozawa, N.; Yajima, S.; Tomatsu, S.; Kondo, N.; Nakada, Y.; Akaboshi, S.; Lai, M.; Tanabe, Y.; Hashimoto, T.; et al. Novel subtype of peroxisomal acyl-CoA oxidase deficiency and bifunctional enzyme deficiency with detectable enzyme protein: Identification by means of complementation analysis. Am. J. Hum. Genet. 1994, 54, 36–43. [Google Scholar]
- Wang, R.Y.; Monuki, E.S.; Powers, J.; Schwartz, P.H.; Watkins, P.A.; Shi, Y.; Moser, A.; Shrier, D.A.; Waterham, H.R.; Nugent, D.J.; et al. Effects of hematopoietic stem cell transplantation on acyl-CoA oxidase deficiency: A sibling comparison study. J. Inherit. Metab. Dis. 2014, 37, 791–799. [Google Scholar] [CrossRef]
- Kurian, M.A.; Ryan, S.; Besley, G.T.; Wanders, R.J.; King, M.D. Straight-chain acyl-CoA oxidase deficiency presenting with dysmorphia, neurodevelopmental autistic-type regression and a selective pattern of leukodystrophy. J. Inherit. Metab. Dis. 2004, 27, 105–108. [Google Scholar] [CrossRef]
- Poll-The, B.T.; Roels, F.; Ogier, H.; Scotto, J.; Vamecq, J.; Schutgens, R.B.; Wanders, R.J.; van Roermund, C.W.; van Wijland, M.J.; Schram, A.W.; et al. A new peroxisomal disorder with enlarged peroxisomes and a specific deficiency of acyl-CoA oxidase (pseudo-neonatal adrenoleukodystrophy). Am. J. Hum. Genet. 1988, 42, 422–434. [Google Scholar]
- Ferdinandusse, S.; Barker, S.; Lachlan, K.; Duran, M.; Waterham, H.R.; Wanders, R.J.; Hammans, S. Adult peroxisomal acyl-coenzyme A oxidase deficiency with cerebellar and brainstem atrophy. J. Neurol. Neurosurg. Psychiatry 2010, 81, 310–312. [Google Scholar] [CrossRef]
- Vilarinho, S.; Sari, S.; Mazzacuva, F.; Bilguvar, K.; Esendagli-Yilmaz, G.; Jain, D.; Akyol, G.; Dalgic, B.; Gunel, M.; Clayton, P.T.; et al. ACOX2 deficiency: A disorder of bile acid synthesis with transaminase elevation, liver fibrosis, ataxia, and cognitive impairment. Proc. Natl. Acad. Sci. USA 2016, 113, 11289–11293. [Google Scholar] [CrossRef]
- Monte, M.J.; Alonso-Pena, M.; Briz, O.; Herraez, E.; Berasain, C.; Argemi, J.; Prieto, J.; Marin, J.J.G. ACOX2 deficiency: An inborn error of bile acid synthesis identified in an adolescent with persistent hypertransaminasemia. J. Hepatol. 2017, 66, 581–588. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Denis, S.; van Roermund, C.W.T.; Preece, M.A.; Koster, J.; Ebberink, M.S.; Waterham, H.R.; Wanders, R.J.A. A novel case of ACOX2 deficiency leads to recognition of a third human peroxisomal acyl-CoA oxidase. Biochim. Biophys. Acta 2018, 1864, 952–958. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Denis, S.; Mooyer, P.A.; Dekker, C.; Duran, M.; Soorani-Lunsing, R.J.; Boltshauser, E.; Macaya, A.; Gartner, J.; Majoie, C.B.; et al. Clinical and biochemical spectrum of D-bifunctional protein deficiency. Ann. Neurol. 2006, 59, 92–104. [Google Scholar] [CrossRef]
- Amor, D.J.; Marsh, A.P.; Storey, E.; Tankard, R.; Gillies, G.; Delatycki, M.B.; Pope, K.; Bromhead, C.; Leventer, R.J.; Bahlo, M.; et al. Heterozygous mutations in HSD17B4 cause juvenile peroxisomal D-bifunctional protein deficiency. Neurol. Genet. 2016, 2, e114. [Google Scholar] [CrossRef] [PubMed]
- Lines, M.A.; Jobling, R.; Brady, L.; Marshall, C.R.; Scherer, S.W.; Rodriguez, A.R.; Lee, L.; Lang, A.E.; Mestre, T.A.; Wanders, R.J.; et al. Peroxisomal D-bifunctional protein deficiency: Three adults diagnosed by whole-exome sequencing. Neurology 2014, 82, 963–968. [Google Scholar] [CrossRef] [PubMed]
- McMillan, H.J.; Worthylake, T.; Schwartzentruber, J.; Gottlieb, C.C.; Lawrence, S.E.; Mackenzie, A.; Beaulieu, C.L.; Mooyer, P.A.; Wanders, R.J.; Majewski, J.; et al. Specific combination of compound heterozygous mutations in 17beta-hydroxysteroid dehydrogenase type 4 (HSD17B4) defines a new subtype of D-bifunctional protein deficiency. Orphanet J. Rare Dis. 2012, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandusse, S.; Kostopoulos, P.; Denis, S.; Rusch, H.; Overmars, H.; Dillmann, U.; Reith, W.; Haas, D.; Wanders, R.J.; Duran, M.; et al. Mutations in the gene encoding peroxisomal sterol carrier protein X (SCPx) cause leukencephalopathy with dystonia and motor neuropathy. Am. J. Hum. Genet. 2006, 78, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandusse, S.; Falkenberg, K.D.; Koster, J.; Mooyer, P.A.; Jones, R.; van Roermund, C.W.T.; Pizzino, A.; Schrader, M.; Wanders, R.J.A.; Vanderver, A.; et al. ACBD5 deficiency causes a defect in peroxisomal very long-chain fatty acid metabolism. J. Med. Genet. 2017, 54, 330–337. [Google Scholar] [CrossRef]
- Abu-Safieh, L.; Alrashed, M.; Anazi, S.; Alkuraya, H.; Khan, A.O.; Al-Owain, M.; Al-Zahrani, J.; Al-Abdi, L.; Hashem, M.; Al-Tarimi, S.; et al. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 2013, 23, 236–247. [Google Scholar] [CrossRef]
- Bartlett, M.; Nasiri, N.; Pressman, R.; Bademci, G.; Forghani, I. First reported adult patient with retinal dystrophy and leukodystrophy caused by a novel ACBD5 variant: A case report and review of literature. Am. J. Med. Genet. A 2021, 4, 1236–1241. [Google Scholar] [CrossRef]
- Malheiro, A.R.; da Silva, T.F.; Brites, P. Plasmalogens and fatty alcohols in rhizomelic chondrodysplasia punctata and Sjogren-Larsson syndrome. J. Inherit. Metab. Dis. 2015, 38, 111–121. [Google Scholar] [CrossRef]
- Saab, S.; Mazzocco, J.; Creuzot-Garcher, C.P.; Bron, A.M.; Bretillon, L.; Acar, N. Plasmalogens in the retina: From occurrence in retinal cell membranes to potential involvement in pathophysiology of retinal diseases. Biochimie 2014, 107 Pt A, 58–65. [Google Scholar] [CrossRef]
- Braverman, N.; Chen, L.; Lin, P.; Obie, C.; Steel, G.; Douglas, P.; Chakraborty, P.K.; Clarke, J.T.; Boneh, A.; Moser, A.; et al. Mutation analysis of PEX7 in 60 probands with rhizomelic chondrodysplasia punctata and functional correlations of genotype with phenotype. Hum. Mutat. 2002, 20, 284–297. [Google Scholar] [CrossRef]
- Van den Brink, D.M.; Brites, P.; Haasjes, J.; Wierzbicki, A.S.; Mitchell, J.; Lambert-Hamill, M.; de Belleroche, J.; Jansen, G.A.; Waterham, H.R.; Wanders, R.J. Identification of PEX7 as the second gene involved in Refsum disease. Am. J. Hum. Genet. 2003, 72, 471–477. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Lloyd, M.B.; O’Day, W.T.; Bok, D. Effect of phytanic acid on cultured retinal pigment epithelium: An in vitro model for Refsum’s disease. Exp. Eye Res. 1992, 55, 869–878. [Google Scholar] [CrossRef]
- Young, S.P.; Johnson, A.W.; Muller, D.P. Effects of phytanic acid on the vitamin E status, lipid composition and physical properties of retinal cell membranes: Implications for adult Refsum disease. Clin. Sci. 2001, 101, 697–705. [Google Scholar] [CrossRef]
- Steinberg, D.; Avigan, J.; Mize, C.E.; Baxter, J.H.; Cammermeyer, J.; Fales, H.M.; Highet, P.F. Effects of dietary phytol and phytanic acid in animals. J. Lipid Res. 1966, 7, 684–691. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Zomer, A.W.; Komen, J.C.; van den Brink, C.E.; Thanos, M.; Hamers, F.P.; Wanders, R.J.; van der Saag, P.T.; Poll-The, B.T.; Brites, P. Ataxia with loss of Purkinje cells in a mouse model for Refsum disease. Proc. Natl. Acad. Sci. USA 2008, 105, 17712–17717. [Google Scholar] [CrossRef] [PubMed]
- Violante, S.; Achetib, N.; van Roermund, C.W.T.; Hagen, J.; Dodatko, T.; Vaz, F.M.; Waterham, H.R.; Chen, H.; Baes, M.; Yu, C.; et al. Peroxisomes can oxidize medium- and long-chain fatty acids through a pathway involving ABCD3 and HSD17B4. FASEB J. 2019, 33, 4355–4364. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Reinhart, M.; Sankarappa, S.; Sprecher, H. The metabolism of 7,10,13,16,19-docosapentaenoic acid to 4,7,10,13,16,19-docosahexaenoic acid in rat liver is independent of a 4-desaturase. J. Biol. Chem. 1991, 266, 19995–20000. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Denis, S.; Mooijer, P.A.; Zhang, Z.; Reddy, J.K.; Spector, A.A.; Wanders, R.J. Identification of the peroxisomal beta-oxidation enzymes involved in the biosynthesis of docosahexaenoic acid. J. Lipid Res. 2001, 42, 1987–1995. [Google Scholar] [CrossRef]
- Wang, N.; Anderson, R.E. Synthesis of docosahexaenoic acid by retina and retinal pigment epithelium. Biochemistry 1993, 32, 13703–13709. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, N.P.; Pennacchiotti, G.L.; Sprecher, H.; Aveldano, M.I. Active synthesis of C24:5, n-3 fatty acid in retina. Biochem. J. 1996, 316 Pt 3, 859–864. [Google Scholar] [CrossRef][Green Version]
- Simon, M.V.; Agnolazza, D.L.; German, O.L.; Garelli, A.; Politi, L.E.; Agbaga, M.P.; Anderson, R.E.; Rotstein, N.P. Synthesis of docosahexaenoic acid from eicosapentaenoic acid in retina neurons protects photoreceptors from oxidative stress. J. Neurochem. 2016, 136, 931–946. [Google Scholar] [CrossRef]
- Bazan, H.E.; Careaga, M.M.; Sprecher, H.; Bazan, N.G. Chain elongation and desaturation of eicosapentaenoate to docosahexaenoate and phospholipid labeling in the rat retina in vivo. Biochim. Biophys. Acta 1982, 712, 123–128. [Google Scholar] [CrossRef]
- Park, H.G.; Park, W.J.; Kothapalli, K.S.; Brenna, J.T. The fatty acid desaturase 2 (FADS2) gene product catalyzes Delta4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells. FASEB J. 2015, 29, 3911–3919. [Google Scholar] [CrossRef]
- Engelen, M.; Kemp, S.; de Visser, M.; van Geel, B.M.; Wanders, R.J.; Aubourg, P.; Poll-The, B.T. X-linked adrenoleukodystrophy (X-ALD): Clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J. Rare Dis. 2012, 7, 51. [Google Scholar] [CrossRef]
- Fan, C.Y.; Pan, J.; Chu, R.; Lee, D.; Kluckman, K.D.; Usuda, N.; Singh, I.; Yeldandi, A.V.; Rao, M.S.; Maeda, N.; et al. Hepatocellular and hepatic peroxisomal alterations in mice with a disrupted peroxisomal fatty acyl-coenzyme A oxidase gene. J. Biol. Chem. 1996, 271, 24698–24710. [Google Scholar] [CrossRef]
- Baes, M.; Huyghe, S.; Carmeliet, P.; Declercq, P.E.; Collen, D.; Mannaerts, G.P.; Van Veldhoven, P.P. Inactivation of the peroxisomal multifunctional protein-2 in mice impedes the degradation of not only 2-methyl-branched fatty acids and bile acid intermediates but also of very long chain fatty acids. J. Biol. Chem. 2000, 275, 16329–16336. [Google Scholar] [CrossRef]
- Setchell, K.D.; Heubi, J.E.; Bove, K.E.; O’Connell, N.C.; Brewsaugh, T.; Steinberg, S.J.; Moser, A.; Squires, R.H., Jr. Liver disease caused by failure to racemize trihydroxycholestanoic acid: Gene mutation and effect of bile acid therapy. Gastroenterology 2003, 124, 217–232. [Google Scholar] [CrossRef]
- Savolainen, K.; Kotti, T.J.; Schmitz, W.; Savolainen, T.I.; Sormunen, R.T.; Ilves, M.; Vainio, S.J.; Conzelmann, E.; Hiltunen, J.K. A mouse model for alpha-methylacyl-CoA racemase deficiency: Adjustment of bile acid synthesis and intolerance to dietary methyl-branched lipids. Hum. Mol. Genet. 2004, 13, 955–965. [Google Scholar] [CrossRef]
- Costello, J.L.; Castro, I.G.; Hacker, C.; Schrader, T.A.; Metz, J.; Zeuschner, D.; Azadi, A.S.; Godinho, L.F.; Costina, V.; Findeisen, P.; et al. ACBD5 and VAPB mediate membrane associations between peroxisomes and the ER. J. Cell Biol. 2017, 216, 331–342. [Google Scholar] [CrossRef]
- Hua, R.; Cheng, D.; Coyaud, E.; Freeman, S.; Di Pietro, E.; Wang, Y.; Vissa, A.; Yip, C.M.; Fairn, G.D.; Braverman, N.; et al. VAPs and ACBD5 tether peroxisomes to the ER for peroxisome maintenance and lipid homeostasis. J. Cell Biol. 2017, 216, 367–377. [Google Scholar] [CrossRef]
- Nazarko, T.Y.; Ozeki, K.; Till, A.; Ramakrishnan, G.; Lotfi, P.; Yan, M.; Subramani, S. Peroxisomal Atg37 binds Atg30 or palmitoyl-CoA to regulate phagophore formation during pexophagy. J. Cell Biol. 2014, 204, 541–557. [Google Scholar] [CrossRef]
- Yagita, Y.; Shinohara, K.; Abe, Y.; Nakagawa, K.; Al-Owain, M.; Alkuraya, F.S.; Fujiki, Y. Deficiency of a Retinal Dystrophy Protein, Acyl-CoA Binding Domain-containing 5 (ACBD5), Impairs Peroxisomal beta-Oxidation of Very-long-chain Fatty Acids. J. Biol. Chem. 2017, 292, 691–705. [Google Scholar] [CrossRef]
- Darwisch, W.; von Spangenberg, M.; Lehmann, J.; Singin, Ö.; Deubert, G.; Kühl, S.; Roos, J.; Horstmann, H.; Körber, C.; Hoppe, S.; et al. Cerebellar and hepatic alterations in ACBD5-deficient mice are associated with unexpected, distinct alterations in cellular lipid homeostasis. Commun. Biol. 2020, 3, 713. [Google Scholar] [CrossRef]
- Dorninger, F.; Forss-Petter, S.; Berger, J. From peroxisomal disorders to common neurodegenerative diseases—The role of ether phospholipids in the nervous system. FEBS Lett. 2017, 591, 2761–2788. [Google Scholar] [CrossRef]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 2012, 1822, 1442–1452. [Google Scholar] [CrossRef]
- Brites, P.; Motley, A.M.; Gressens, P.; Mooyer, P.A.; Ploegaert, I.; Everts, V.; Evrard, P.; Carmeliet, P.; Dewerchin, M.; Schoonjans, L.; et al. Impaired neuronal migration and endochondral ossification in Pex7 knockout mice: A model for rhizomelic chondrodysplasia punctata. Hum. Mol. Genet. 2003, 12, 2255–2267. [Google Scholar] [CrossRef]
- Braverman, N.; Zhang, R.; Chen, L.; Nimmo, G.; Scheper, S.; Tran, T.; Chaudhury, R.; Moser, A.; Steinberg, S. A Pex7 hypomorphic mouse model for plasmalogen deficiency affecting the lens and skeleton. Mol. Genet. Metab. 2010, 99, 408–416. [Google Scholar] [CrossRef]
- Rodemer, C.; Thai, T.P.; Brugger, B.; Kaercher, T.; Werner, H.; Nave, K.A.; Wieland, F.; Gorgas, K.; Just, W.W. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum. Mol. Genet. 2003, 12, 1881–1895. [Google Scholar] [CrossRef]
- Liegel, R.; Chang, B.; Dubielzig, R.; Sidjanin, D.J. Blind sterile 2 (bs2), a hypomorphic mutation in Agps, results in cataracts and male sterility in mice. Mol. Genet. Metab. 2011, 103, 51–59. [Google Scholar] [CrossRef]
- Liegel, R.P.; Ronchetti, A.; Sidjanin, D.J. Alkylglycerone phosphate synthase (AGPS) deficient mice: Models for rhizomelic chondrodysplasia punctate type 3 (RCDP3) malformation syndrome. Mol. Genet. Metab. Rep. 2014, 1, 299–311. [Google Scholar] [CrossRef]
- Saab, S.; Buteau, B.; Leclere, L.; Bron, A.M.; Creuzot-Garcher, C.P.; Bretillon, L.; Acar, N. Involvement of plasmalogens in post-natal retinal vascular development. PLoS ONE 2014, 9, e101076. [Google Scholar] [CrossRef]
- Jaspers, Y.R.J.; Ferdinandusse, S.; Dijkstra, I.M.E.; Barendsen, R.W.; van Lenthe, H.; Kulik, W.; Engelen, M.; Goorden, S.M.I.; Vaz, F.M.; Kemp, S. Comparison of the Diagnostic Performance of C26:0-Lysophosphatidylcholine and Very Long-Chain Fatty Acids Analysis for Peroxisomal Disorders. Front. Cell Dev. Biol. 2020, 8, 690. [Google Scholar] [CrossRef]
- Huyghe, S.; Schmalbruch, H.; Hulshagen, L.; Veldhoven, P.V.; Baes, M.; Hartmann, D. Peroxisomal multifunctional protein-2 deficiency causes motor deficits and glial lesions in the adult central nervous system. Am. J. Pathol. 2006, 168, 1321–1334. [Google Scholar] [CrossRef][Green Version]
- Fliesler, S.J.; Anderson, R.E. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983, 22, 79–131. [Google Scholar] [CrossRef]
- Anderson, R.E.; Maude, M.B. Lipids of ocular tissues. 8. The effects of essential fatty acid deficiency on the phospholipids of the photoreceptor membranes of rat retina. Arch. Biochem. Biophys. 1972, 151, 270–276. [Google Scholar] [CrossRef]
- Tinoco, J. Dietary requirements and functions of alpha-linolenic acid in animals. Prog. Lipid Res. 1982, 21, 1–45. [Google Scholar] [CrossRef]
- Anderson, D.M.; Ablonczy, Z.; Koutalos, Y.; Spraggins, J.; Crouch, R.K.; Caprioli, R.M.; Schey, K.L. High resolution MALDI imaging mass spectrometry of retinal tissue lipids. J. Am. Soc. Mass Spectrom. 2014, 25, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Aveldano, M.I. A novel group of very long chain polyenoic fatty acids in dipolyunsaturated phosphatidylcholines from vertebrate retina. J. Biol. Chem. 1987, 262, 1172–1179. [Google Scholar] [CrossRef]
- Aveldano, M.I.; Sprecher, H. Very long chain (C24 to C36) polyenoic fatty acids of the n-3 and n-6 series in dipolyunsaturated phosphatidylcholines from bovine retina. J. Biol. Chem. 1987, 262, 1180–1186. [Google Scholar] [CrossRef]
- Agbaga, M.P.; Mandal, M.N.; Anderson, R.E. Retinal very long-chain PUFAs: New insights from studies on ELOVL4 protein. J. Lipid Res. 2010, 51, 1624–1642. [Google Scholar] [CrossRef]
- Agbaga, M.P.; Merriman, D.K.; Brush, R.S.; Lydic, T.A.; Conley, S.M.; Naash, M.I.; Jackson, S.; Woods, A.S.; Reid, G.E.; Busik, J.V.; et al. Differential composition of DHA and very-long-chain PUFAs in rod and cone photoreceptors. J. Lipid Res. 2018, 59, 1586–1596. [Google Scholar] [CrossRef]
- Bazan, N.G. Overview of how N32 and N34 elovanoids sustain sight by protecting retinal pigment epithelial cells and photoreceptors. J. Lipid Res. 2021, 100058. [Google Scholar] [CrossRef]
- Benolken, R.M.; Anderson, R.E.; Wheeler, T.G. Membrane fatty acids associated with the electrical response in visual excitation. Science 1973, 182, 1253–1254. [Google Scholar] [CrossRef]
- Wheeler, T.G.; Benolken, R.M.; Anderson, R.E. Visual membranes: Specificity of fatty acid precursors for the electrical response to illumination. Science 1975, 188, 1312–1314. [Google Scholar] [CrossRef]
- Senapati, S.; Gragg, M.; Samuels, I.S.; Parmar, V.M.; Maeda, A.; Park, P.S. Effect of dietary docosahexaenoic acid on rhodopsin content and packing in photoreceptor cell membranes. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1403–1413. [Google Scholar] [CrossRef]
- Weisinger, H.S.; Vingrys, A.J.; Sinclair, A.J. Effect of dietary n-3 deficiency on the electroretinogram in the guinea pig. Ann. Nutr. Metab. 1996, 40, 91–98. [Google Scholar] [CrossRef]
- Weisinger, H.S.; Vingrys, A.J.; Bui, B.V.; Sinclair, A.J. Effects of dietary n-3 fatty acid deficiency and repletion in the guinea pig retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 327–338. [Google Scholar]
- Neuringer, M.; Connor, W.E.; Van Petten, C.; Barstad, L. Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J. Clin. Investig. 1984, 73, 272–276. [Google Scholar] [CrossRef]
- Neuringer, M.; Connor, W.E.; Lin, D.S.; Barstad, L.; Luck, S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc. Natl. Acad. Sci. USA 1986, 83, 4021–4025. [Google Scholar] [CrossRef]
- Jeffrey, B.G.; Mitchell, D.C.; Gibson, R.A.; Neuringer, M. n-3 fatty acid deficiency alters recovery of the rod photoresponse in rhesus monkeys. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2806–2814. [Google Scholar]
- Uauy, R.D.; Birch, D.G.; Birch, E.E.; Tyson, J.E.; Hoffman, D.R. Effect of dietary omega-3 fatty acids on retinal function of very-low-birth-weight neonates. Pediatr. Res. 1990, 28, 485–492. [Google Scholar] [CrossRef]
- Birch, E.E.; Birch, D.G.; Hoffman, D.R.; Uauy, R. Dietary essential fatty acid supply and visual acuity development. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3242–3253. [Google Scholar]
- Birch, D.G.; Birch, E.E.; Hoffman, D.R.; Uauy, R.D. Retinal development in very-low-birth-weight infants fed diets differing in omega-3 fatty acids. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2365–2376. [Google Scholar]
- O’Connor, D.L.; Hall, R.; Adamkin, D.; Auestad, N.; Castillo, M.; Connor, W.E.; Connor, S.L.; Fitzgerald, K.; Groh-Wargo, S.; Hartmann, E.E.; et al. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: A prospective, randomized controlled trial. Pediatrics 2001, 108, 359–371. [Google Scholar] [CrossRef]
- Martinez, M. Abnormal profiles of polyunsaturated fatty acids in the brain, liver, kidney and retina of patients with peroxisomal disorders. Brain Res. 1992, 583, 171–182. [Google Scholar] [CrossRef]
- Paker, A.M.; Sunness, J.S.; Brereton, N.H.; Speedie, L.J.; Albanna, L.; Dharmaraj, S.; Moser, A.B.; Jones, R.O.; Raymond, G.V. Docosahexaenoic acid therapy in peroxisomal diseases: Results of a double-blind, randomized trial. Neurology 2010, 75, 826–830. [Google Scholar] [CrossRef]
- Deak, F.; Anderson, R.E.; Fessler, J.L.; Sherry, D.M. Novel Cellular Functions of Very Long Chain-Fatty Acids: Insight from ELOVL4 Mutations. Front. Cell. Neurosci. 2019, 13, 428. [Google Scholar] [CrossRef]
- Suh, M.; Clandinin, M.T. 20:5n-3 but not 22:6n-3 is a preferred substrate for synthesis of n-3 very-long- chain fatty acids (C24-C36) in retina. Curr. Eye Res. 2005, 30, 959–968. [Google Scholar] [CrossRef]
- Yu, M.; Benham, A.; Logan, S.; Brush, R.S.; Mandal, M.N.; Anderson, R.E.; Agbaga, M.P. ELOVL4 protein preferentially elongates 20:5n3 to very long chain PUFAs over 20:4n6 and 22:6n3. J. Lipid Res. 2012, 53, 494–504. [Google Scholar] [CrossRef]
- Bennett, L.D.; Hopiavuori, B.R.; Brush, R.S.; Chan, M.; Van Hook, M.J.; Thoreson, W.B.; Anderson, R.E. Examination of VLC-PUFA-deficient photoreceptor terminals. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4063–4072. [Google Scholar] [CrossRef]
- Logan, S.; Anderson, R.E. Dominant Stargardt Macular Dystrophy (STGD3) and ELOVL4. Adv. Exp. Med. Biol. 2014, 801, 447–453. [Google Scholar] [CrossRef]
- Poulos, A.; Sharp, P.; Johnson, D.; Easton, C. The occurrence of polyenoic very long chain fatty acids with greater than 32 carbon atoms in molecular species of phosphatidylcholine in normal and peroxisome-deficient (Zellweger’s syndrome) brain. Biochem. J. 1988, 253, 645–650. [Google Scholar] [CrossRef]
- Poulos, A.; Sharp, P.; Singh, H.; Johnson, D.; Fellenberg, A.; Pollard, A. Detection of a homologous series of C26-C38 polyenoic fatty acids in the brain of patients without peroxisomes (Zellweger’s syndrome). Biochem. J. 1986, 235, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.; Poulos, A.; Fellenberg, A.; Johnson, D. Structure and lipid distribution of polyenoic very-long-chain fatty acids in the brain of peroxisome-deficient patients (Zellweger syndrome). Biochem. J. 1987, 248, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.S.; Calandria, J.M.; Gordon, W.C.; Jun, B.; Zhou, Y.; Gelfman, C.M.; Li, S.; Jin, M.; Knott, E.J.; Chang, B.; et al. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat. Commun. 2015, 6, 6228. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.H.; Chan, J.P.; Cazenave-Gassiot, A.; Poh, R.W.; Foo, J.C.; Galam, D.L.; Ghosh, S.; Nguyen, L.N.; Barathi, V.A.; Yeo, S.W.; et al. Mfsd2a Is a Transporter for the Essential omega-3 Fatty Acid Docosahexaenoic Acid (DHA) in Eye and Is Important for Photoreceptor Cell Development. J. Biol. Chem. 2016, 291, 10501–10514. [Google Scholar] [CrossRef] [PubMed]
- Lobanova, E.S.; Schuhmann, K.; Finkelstein, S.; Lewis, T.R.; Cady, M.A.; Hao, Y.; Keuthan, C.; Ash, J.D.; Burns, M.E.; Shevchenko, A.; et al. Disrupted Blood-Retina Lysophosphatidylcholine Transport Impairs Photoreceptor Health but Not Visual Signal Transduction. J. Neurosci. 2019, 39, 9689–9701. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Reveles, J.; Dhingra, A.; Alexander, D.; Bragin, A.; Philp, N.J.; Boesze-Battaglia, K. Phagocytosis-dependent ketogenesis in retinal pigment epithelium. J. Biol. Chem. 2017, 292, 8038–8047. [Google Scholar] [CrossRef]
- German, O.L.; Agnolazza, D.L.; Politi, L.E.; Rotstein, N.P. Light, lipids and photoreceptor survival: Live or let die? Photochem. Photobiol. Sci. 2015, 14, 1737–1753. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA 2004, 101, 8491–8496. [Google Scholar] [CrossRef]
- Jun, B.; Mukherjee, P.K.; Asatryan, A.; Kautzmann, M.A.; Heap, J.; Gordon, W.C.; Bhattacharjee, S.; Yang, R.; Petasis, N.A.; Bazan, N.G. Elovanoids are novel cell-specific lipid mediators necessary for neuroprotective signaling for photoreceptor cell integrity. Sci. Rep. 2017, 7, 5279. [Google Scholar] [CrossRef]
- Kautzmann, M.I.; Gordon, W.C.; Jun, B.; Do, K.V.; Matherne, B.J.; Fang, Z.; Bazan, N.G. Membrane-type frizzled-related protein regulates lipidome and transcription for photoreceptor function. FASEB J. 2020, 34, 912–929. [Google Scholar] [CrossRef]
- Shindou, H.; Koso, H.; Sasaki, J.; Nakanishi, H.; Sagara, H.; Nakagawa, K.M.; Takahashi, Y.; Hishikawa, D.; Iizuka-Hishikawa, Y.; Tokumasu, F.; et al. Docosahexaenoic acid preserves visual function by maintaining correct disc morphology in retinal photoreceptor cells. J. Biol. Chem. 2017, 292, 12054–12064. [Google Scholar] [CrossRef]
- Handa, J.T. How does the macula protect itself from oxidative stress? Mol. Asp. Med. 2012, 33, 418–435. [Google Scholar] [CrossRef]
- Abe, S.; Nagai, T.; Masukawa, M.; Okumoto, K.; Homma, Y.; Fujiki, Y.; Mizuno, K. Localization of Protein Kinase NDR2 to Peroxisomes and Its Role in Ciliogenesis. J. Biol. Chem. 2017, 292, 4089–4098. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hosoba, K.; Itabashi, T.; Iwane, A.H.; Akutsu, S.N.; Ochiai, H.; Saito, Y.; Yamamoto, T.; Matsuura, S. Insufficiency of ciliary cholesterol in hereditary Zellweger syndrome. EMBO J. 2020, 39, e103499. [Google Scholar] [CrossRef]
- Albert, A.; Alexander, D.; Boesze-Battaglia, K. Cholesterol in the rod outer segment: A complex role in a “simple” system. Chem. Phys. Lipids 2016, 199, 94–105. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).