The Role of Selected Wavelengths of Light in the Activity of Photosystem II in Gloeobacter violaceus
Abstract
1. Introduction
2. Results
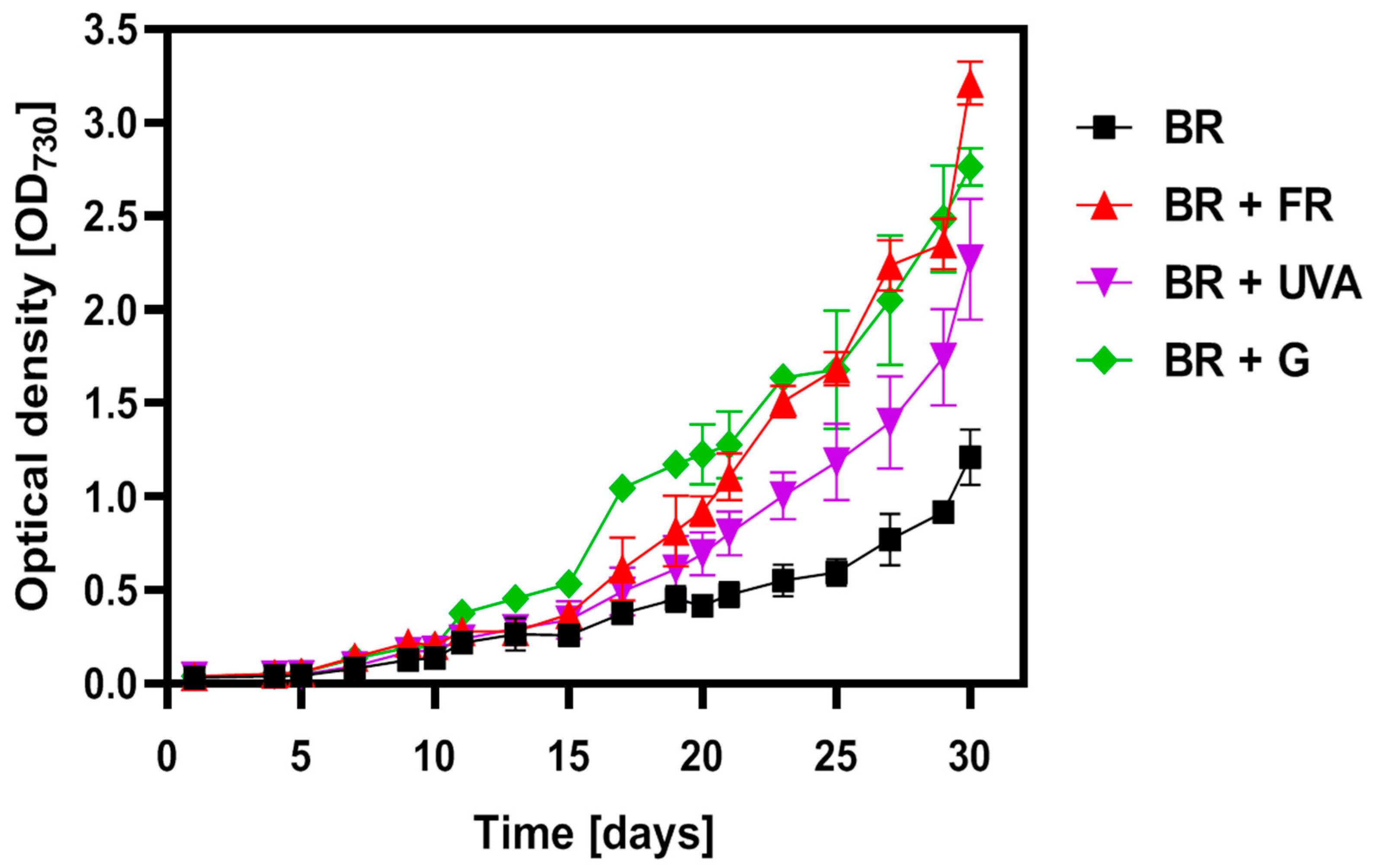
2.1. Growth of the Gloeobacter violaceus Culture Depends on LED Conditions
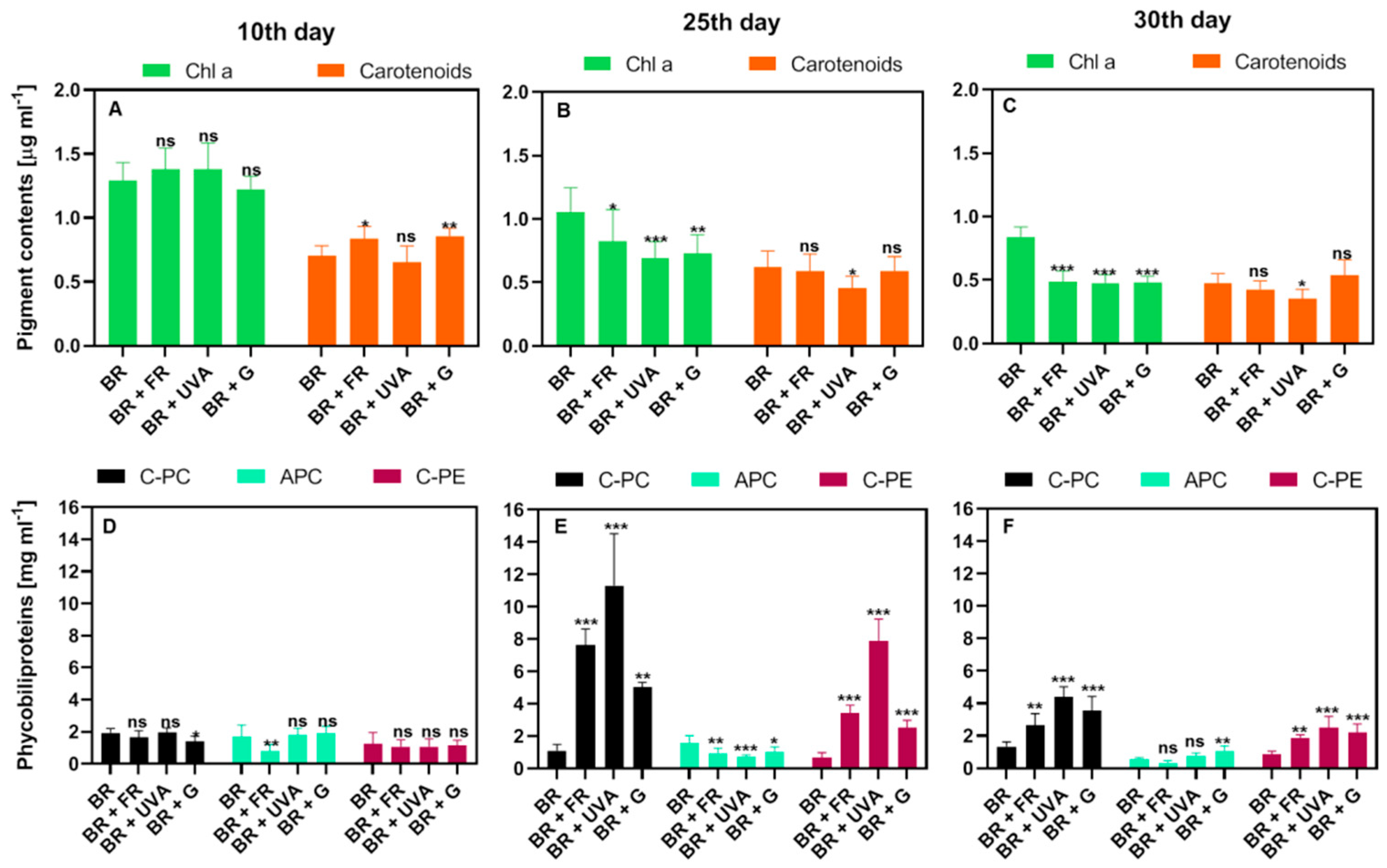
2.2. Light Conditions Modify Photosynthetic Pigments Content in Gloeobacter violaceus
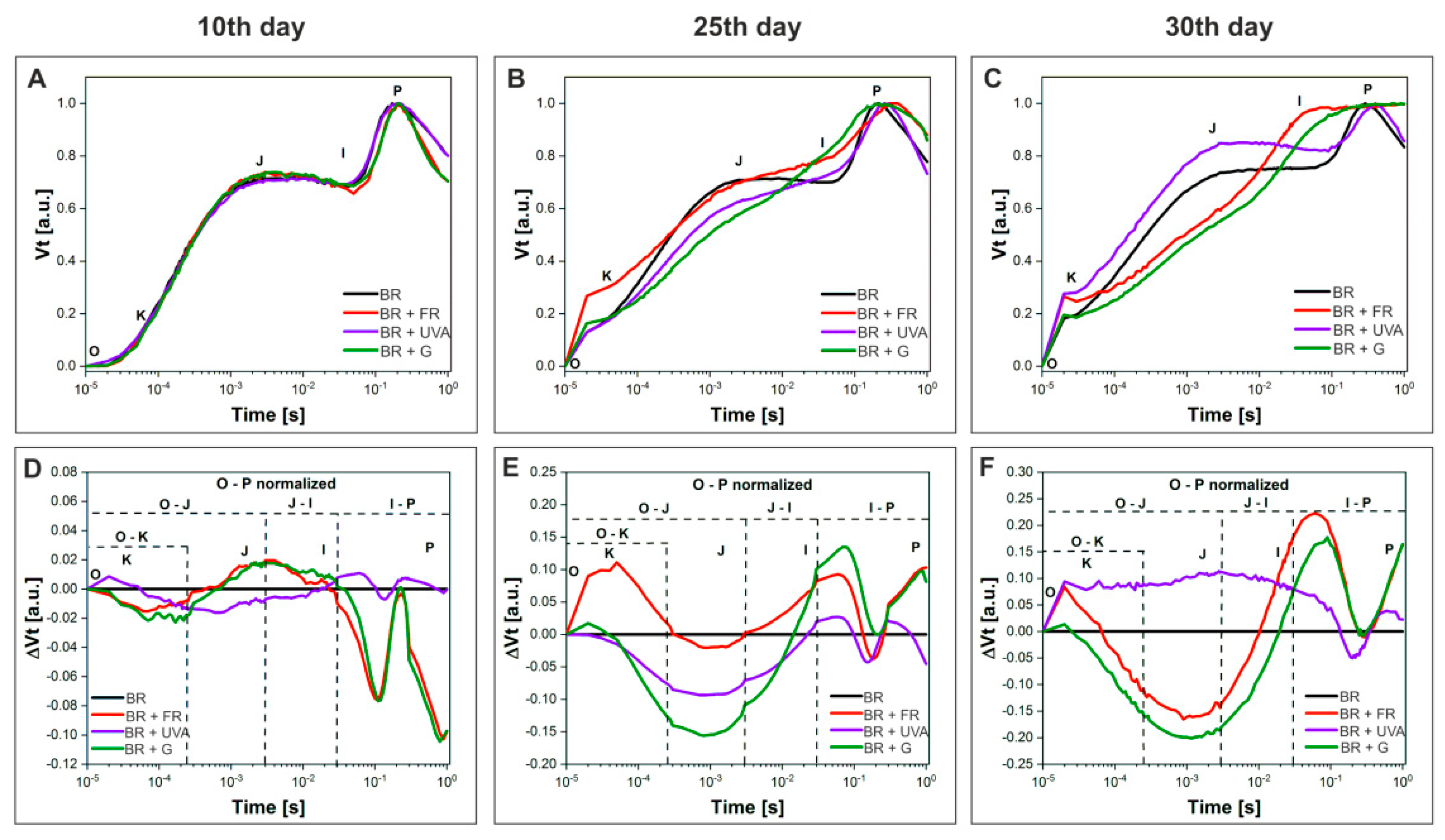
2.3. PSII Activity in Gloeobacter violaceus Is Affected by the Spectral Region of Light
2.3.1. OJIP Test
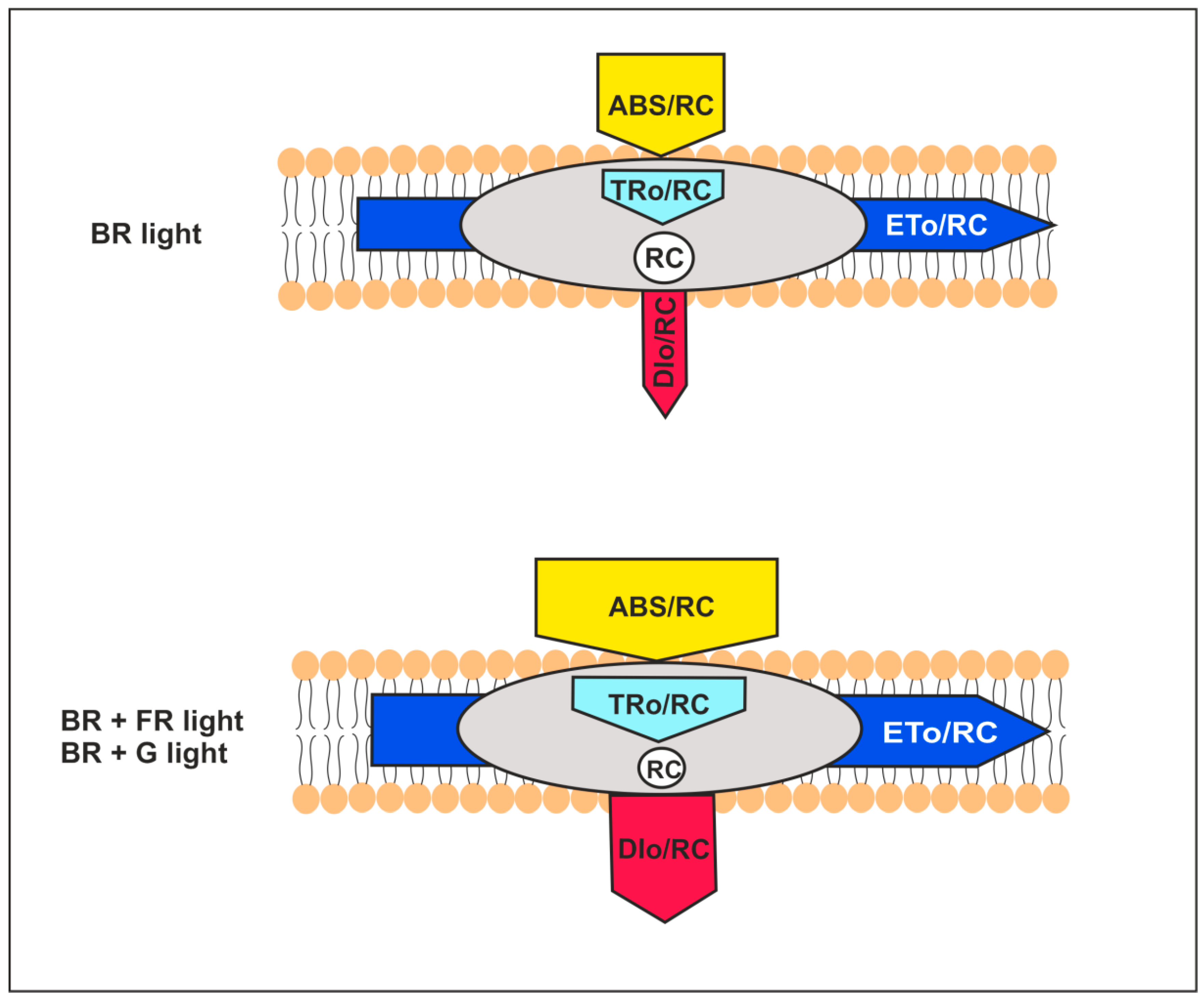
2.3.2. The Energy Flux Pipeline Model
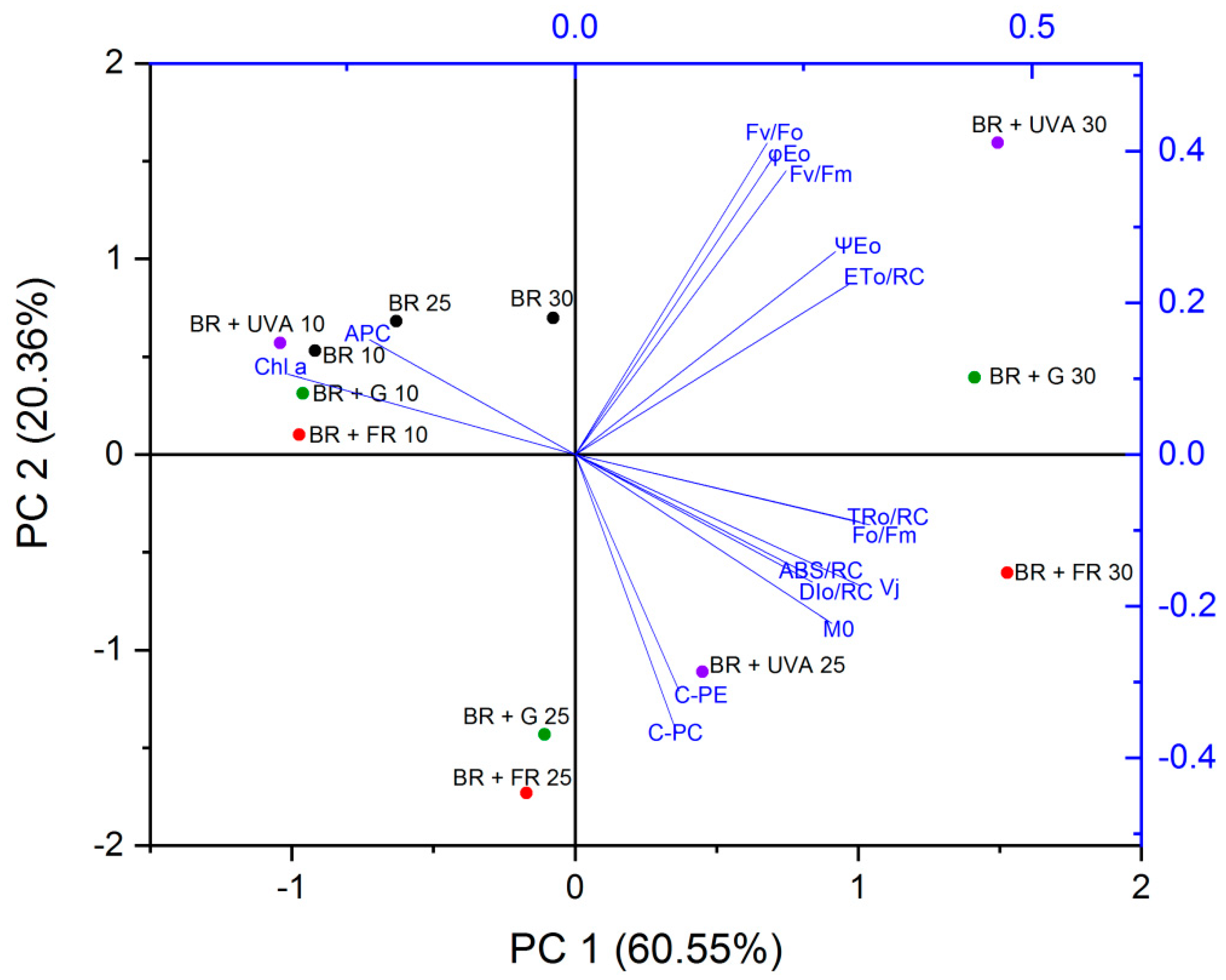
2.3.3. Principal Component Analysis (PCA)
3. Discussion
3.1. The Spectral Composition of Light Affects the Growth of Gloeobacter violaceus
3.2. Light Wavelengths Modify Pigments Content in Gloeobacter violaceus
3.3. The Activity of PSII in Gloeobacter violaceus Depends on the Spectral Region of Light
4. Materials and Methods
4.1. Cyanobacteria
4.2. Growth Conditions
4.3. Light Sources
4.4. Determination of Biomass Growth
4.5. Size of Gloeobacter violaceus Cells
4.6. Content of Photosynthetic Pigments in the Gloeobacter violaceus Culture
4.6.1. Chlorophyll and Carotenoids
4.6.2. Phycobiliproteins
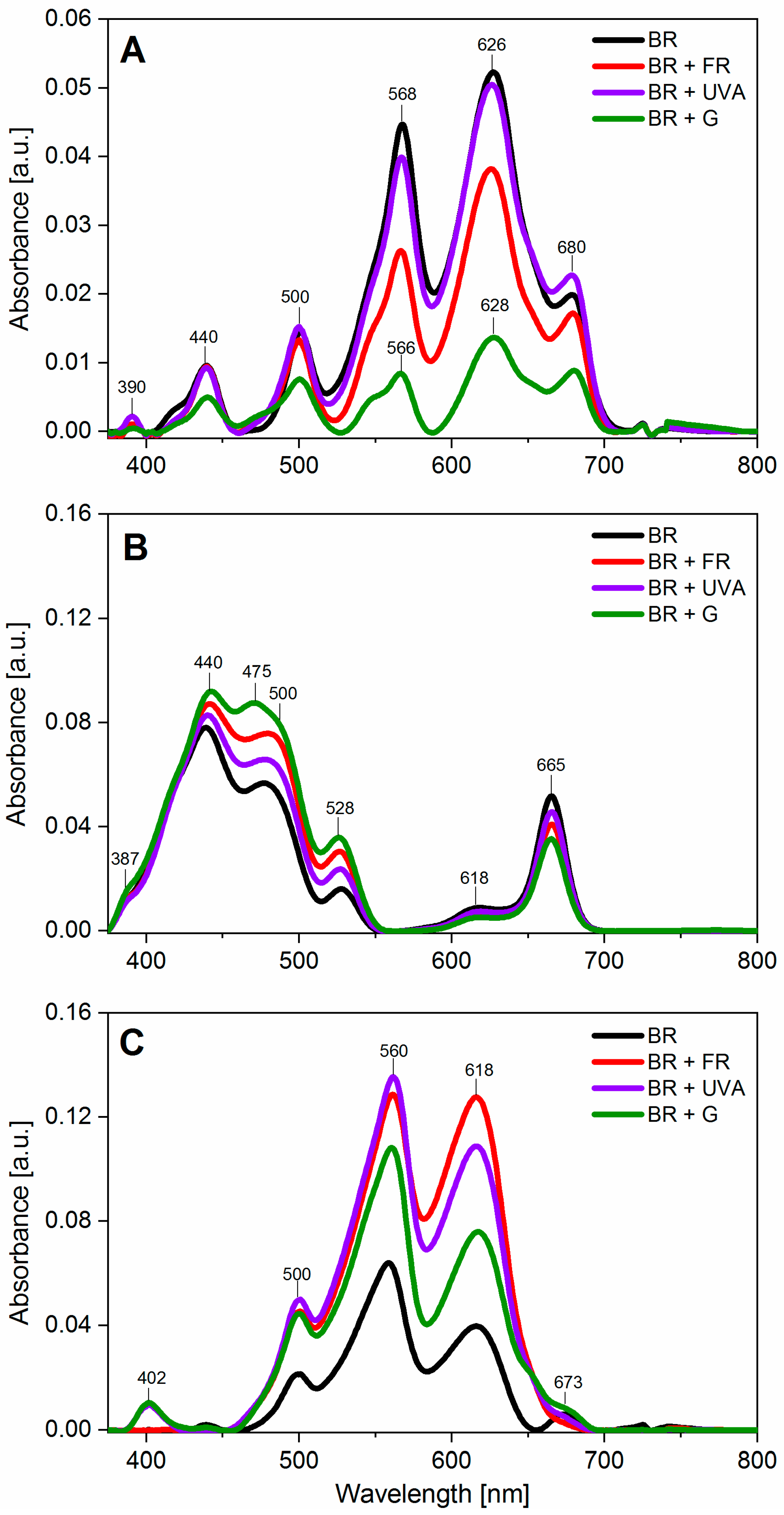
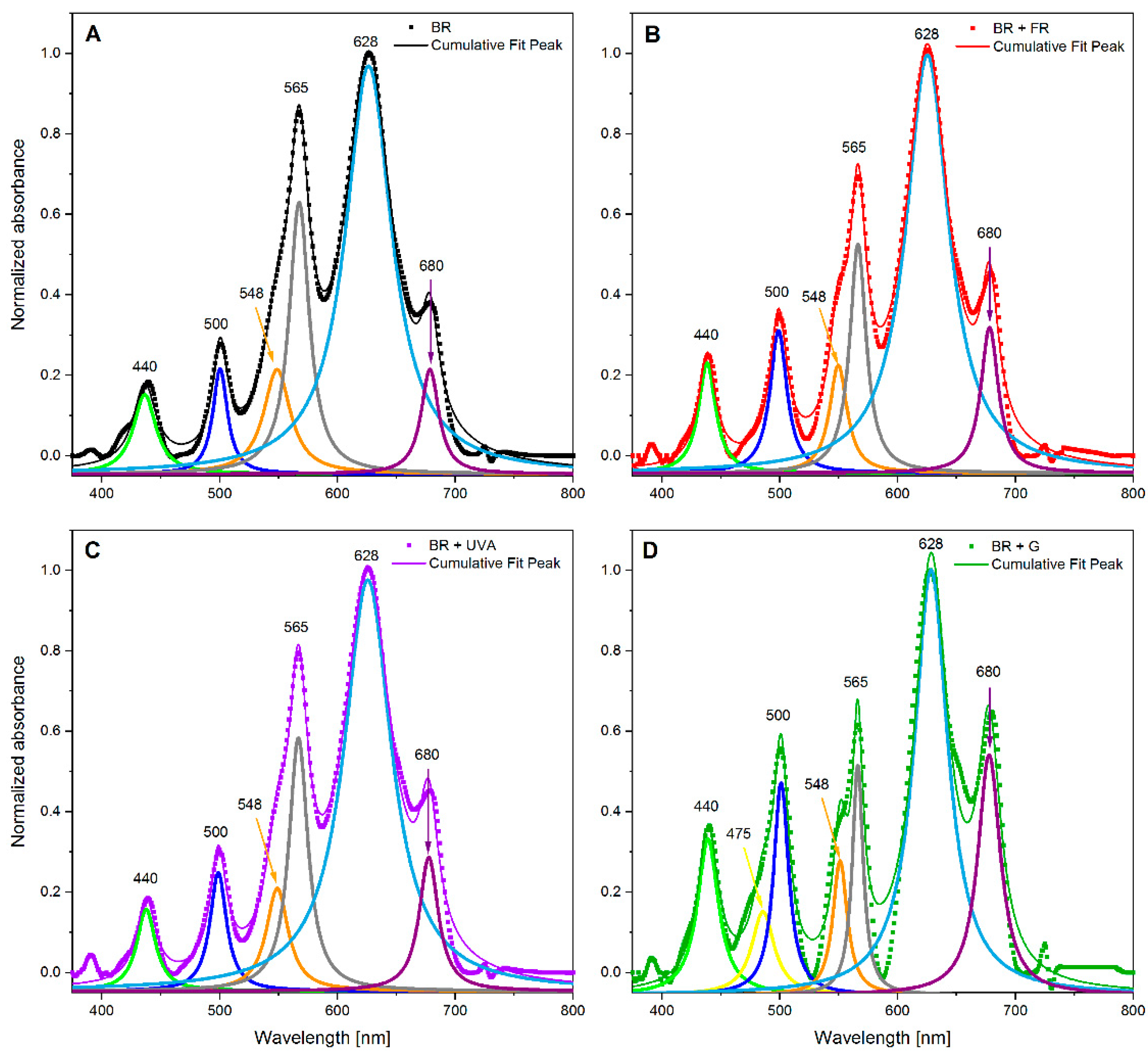
4.7. Absorption Spectroscopy
4.8. Photosystem II Activity
Principal Component Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | allophycocyanin |
| B-PE | B-phycoerythrin |
| C-PC | phycocyanin |
| C-PE | phycoerythrin |
| car | carotenoids |
| chl | chlorophyll |
| chl a | chlorophyll a |
| chl b | chlorophyll b |
| GAF | cGMP-specific phosphodiesterases/cyanobacterial adenylate cyclases/FhlA domain |
| GR | gloeorhodopsin |
| PAS | Per/ARNT/Sim domain |
| PHY | phytochrome-specific domain |
| PUB | phycourobilin |
| RC | reaction center |
| R-PE | R-phycoerythrin |
References
- Krogmann, D.W.; Pérez-Gómez, B.; Gutiérrez-Cirlos, E.B.; Chagolla-López, A.; González De La Vara, L.; Gómez-Lojero, C. The presence of multidomain linkers determines the bundle-shape structure of the phycobilisome of the cyanobacterium Gloeobacter violaceus PCC 7421. Photosynth. Res. 2007, 93, 27–43. [Google Scholar] [CrossRef]
- Tamary, E.; Kiss, V.; Nevo, R.; Adam, Z.; Bernát, G.; Rexroth, S.; Rögner, M.; Reich, Z. Structural and functional alterations of cyanobacterial phycobilisomes induced by high-light stress. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 319–327. [Google Scholar] [CrossRef]
- Zlenko, D.V.; Krasilnikov, P.M.; Stadnichuk, I.N. Structural modeling of the phycobilisome core and its association with the photosystems. Photosynth. Res. 2016, 130, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Zlenko, D.V.; Galochkina, T.V.; Krasilnikov, P.M.; Stadnichuk, I.N. Coupled rows of PBS cores and PSII dimers in cyanobacteria: Symmetry and structure. Photosynth. Res. 2017, 133, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Montejano, G.; Becerra-Absalón, I.; Gold-Morgan, M.; Osorio-Santos, K. Gloeobacter violaceus: Primitive reproductive scheme and its significance. Plant Syst. Evol. 2018, 304, 1221–1229. [Google Scholar] [CrossRef]
- Mareš, J.; Strunecký, O.; Bučinská, L.; Wiedermannová, J. Evolutionary patterns of thylakoid architecture in cyanobacteria. Front. Microbiol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A. Govindjee Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Campbell, D.; Hurry, V.; Clarke, A.K.; Gustafsson, P.; Öquist, G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol. Mol. Biol. Rev. 1998, 62, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Kaňa, R.; Prášil, O.; Komárek, O.; Papageorgiou, G.C. Govindjee Spectral characteristic of fluorescence induction in a model cyanobacterium, Synechococcus sp. (PCC 7942). Biochim. Biophys. Acta Bioenerg. 2009, 1787, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Grossman, A.R.; Bhaya, D.; He, Q. Tracking the light environment by cyanobacteria and the dynamic naure of light harvesting. J. Biol. Chem. 2001, 276, 11449–11452. [Google Scholar] [CrossRef]
- Yokono, M.; Akimoto, S.; Koyama, K.; Tsuchiya, T.; Mimuro, M. Energy transfer processes in Gloeobacter violaceus PCC 7421 that possesses phycobilisomes with a unique morphology. Biochim. Biophys. Acta Bioenerg. 2008, 1777, 55–65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horton, P.; Wentworth, M.; Ruban, A. Control of the light harvesting function of chloroplast membranes: The LHCII-aggregation model for non-photochemical quenching. FEBS Lett. 2005, 579, 4201–4206. [Google Scholar] [CrossRef] [PubMed]
- Stoitchkova, K.; Zsiros, O.; Jávorfi, T.; Páli, T.; Andreeva, A.; Gombos, Z.; Garab, G. Heat- and light-induced reorganizations in the phycobilisome antenna of Synechocystis sp. PCC 6803. Thermo-optic effect. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Büchel, C. Evolution and function of light harvesting proteins. J. Plant Physiol. 2015, 172, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, D.M. Chromatic adaptation and the evolution of light color sensing in cyanobacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 9029–9030. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, N.C.; Lagarias, J.C. Phytochrome diversification in cyanobacteria and eukaryotic algae. Curr. Opin. Plant Biol. 2018, 37, 87–93. [Google Scholar] [CrossRef]
- Sicora, C.I.; Appleton, S.E.; Brown, C.M.; Chung, J.; Chandler, J.; Cockshutt, A.M.; Vass, I.; Campbell, D. Cyanobacterial psbA families in Anabaena and Synechocystis encode trace, constitutive and UVB-induced D1 isoforms. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sicora, C.I.; Brown, C.M.; Cheregi, O.; Vass, I.; Campbell, D. The psbA gene family responds differentially to light and UVB stress in Gloeobacter violaceus PCC 7421, a deeply divergent cyanobacterium. Biochim. Biophys. Acta Bioenerg. 2008, 1777, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Ma, Z. Photosynthesis and growth of Arthrospira (Spirulina) platensis (Cyanophyta) in response to solar UV radiation, with special reference to its minor variant. Environ. Exp. Bot. 2008, 63, 123–129. [Google Scholar] [CrossRef]
- Das, P.; Lei, W.; Aziz, S.S.; Obbard, J.P. Enhanced algae growth in both phototrophic and mixotrophic culture under blue light. Bioresour. Technol. 2011, 102, 3883–3887. [Google Scholar] [CrossRef] [PubMed]
- Satoh, S.; Ikeuchi, M.; Mimuro, M.; Tanaka, A. Chlorophyll b expressed in cyanobacteria functions as a light-harvesting antenna in photosystem I through flexibility of the proteins. J. Biol. Chem. 2001, 276, 4293–4297. [Google Scholar] [CrossRef]
- Xia, Z.; Meijerink, A. Ce3+-Doped garnet phosphors: Composition modification, luminescence properties and applications. Chem. Soc. Rev. 2017, 46, 275–299. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhuang, Y.; Lv, Y.; Ruan, K.; Xie, R.J. A high-performance non-rare-earth deep-red-emitting Ca14−xSrxZn6Al10O35:Mn4+ phosphor for high-power plant growth LEDs. J. Alloys Compd. 2019, 781, 702–709. [Google Scholar] [CrossRef]
- Chen, H.B.; Wu, J.Y.; Wang, C.F.; Fu, C.C.; Shieh, C.J.; Chen, C.I.; Wang, C.Y.; Liu, Y.C. Modeling on chlorophyll a and phycocyanin production by Spirulina platensis under various light-emitting diodes. Biochem. Eng. J. 2010, 53, 52–56. [Google Scholar] [CrossRef]
- Meng, F.; Cui, H.; Wang, Y.; Li, X. Responses of a new isolated cyanobacterium aponinum strain to temperature, pH, CO2 and light quality. J. Appl. Phycol. 2018, 30, 1525–1532. [Google Scholar] [CrossRef]
- Zhou, Z.; Xia, M.; Zhong, Y.; Gai, S.; Huang, S.; Tian, Y.; Lu, X.; Zhou, N. Dy3+Mn4+ co-doped Ca14Ga10−mAlmZn6O35 far-red emitting phosphors with high brightness and improved luminescence and energy transfer properties for plant growth LED lights. J. Mater. Chem. C 2017, 5, 8201–8210. [Google Scholar] [CrossRef]
- Bernát, G.; Schreiber, U.; Sendtko, E.; Stadnichuk, I.N.; Rexroth, S.; Rögner, M.; Koenig, F. Unique properties vs. Common themes: The atypical cyanobacterium Gloeobacter violaceus PCC 7421 is capable of state transitions and blue-light-induced fluorescence quenching. Plant Cell Physiol. 2012, 53, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, B.; De Baere, R.; Wilmotte, A.; De Wachter, R. Phylogenetic relationships of nonaxenic filamentous cyanobacterial strains based on 16S rRNA sequence analysis. J. Mol. Evol. 1996, 42, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kaneko, T.; Sato, S.; Mimuro, M.; Miyashita, H.; Tsuchiya, T.; Sasamoto, S.; Watanabe, A.; Kawashima, K.; Kishida, Y.; et al. Complete genome structure of Gloeobacter violaceus PCC 7421, a cyanobacterium that lacks thylakoids. DNA Res. 2003, 10, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Mareš, J.; Hrouzek, P.; Kaňa, R.; Ventura, S.; Strunecký, O.; Komárek, J. The primitive thylakoid-less cyanobacterium Gloeobacter is a common rock-dwelling organism. PLoS ONE 2013, 8, e66323. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Waterbury, J.; Cohen-Bazire, G. A cyanobacterium which lacks thylakoids. Arch. Microbiol. 1974, 100, 419–436. [Google Scholar] [CrossRef]
- Geitler, L. Neue Blaualgen aus Lunz. Arch. Protistenkd. 1928, 60, 440–448. [Google Scholar]
- Mareš, J.; Komárek, J.; Compère, P.; Oren, A. Validation of the generic name Gloeobacter Rippka et al. 1974, Cyanophyceae. Cryptogam. Algol. 2013, 34, 255–262. [Google Scholar] [CrossRef]
- Herdman, M.; Castenholz, R.W.; Rippka, R. Form-Genus VII. Gloeobacter Rippka, Waterbury and Cohen-Bazire 1974. In Bergey’s Manual of Systematic Bacteriology; Boone, D.R., Castenholz, R.W., Eds.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Adir, N. Elucidation of the molecular structures of components of the phycobilisome: Reconstructing a giant. Photosynth. Res. 2005, 85, 15–32. [Google Scholar] [CrossRef]
- Guglielmi, G.; Cohen-Bazire, G.; Bryant, D.A. The structure of Gloeobacter violaceus and its phycobilisomes. Arch. Microbiol 1981, 129, 181–189. [Google Scholar] [CrossRef]
- Sutter, M.; Wilson, A.; Leverenz, R.L.; Lopez-Igual, R.; Thurotte, A.; Salmeen, A.E.; Kirilovsky, D.; Kerfeld, C.A. Crystal structure of the FRP and identification of the active site for modulation of OCP-mediated photoprotection in cyanobacteria. Proc. Natl. Acad. Sci. USA 2013, 110, 10022–10027. [Google Scholar] [CrossRef]
- Herrmann, A.J.; Gehringer, M.M. An investigation into the effects of increasing salinity on photosynthesis in freshwater unicellular cyanobacteria during the late Archaean. Geobiology 2019, 17, 343–359. [Google Scholar] [CrossRef]
- Koenig, F.; Schmidt, M. Gloeobacter violaceus—Investigation of an unusual photosynthetic apparatus. Absence of the long wavelength emission of photosystem I in 77 K fluorescence spectra. Physiol. Plant 1995, 94, 621–628. [Google Scholar] [CrossRef]
- Ganapathy, S.; Venselaar, H.; Chen, Q.; De Groot, H.J.M.; Hellingwerf, K.J.; De Grip, W.J. Retinal-based proton pumping in the near infrared. J. Am. Chem. Soc. 2017, 139, 2338–2344. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Choi, A.R.; Shi, L.; Brown, L.S.; Jung, K.H. Cyanobacterial light-driven proton pump, Gloeobacter rhodopsin: Complementarity between rhodopsin-based energy production and photosynthesis. PLoS ONE 2014, 9, e110643. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.C. Govindjee Photosystem II fluorescence: Slow changes—Scaling from the past. J. Photochem. Photobiol. B Biol. 2011, 104, 258–270. [Google Scholar] [CrossRef]
- Stirbet, A. Govindjee on the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A. Govindjee Chlorophyll a fluorescence induction: A personal perspective of the thermal phase, the J–I–P rise. Photosynth. Res. 2012, 113, 15–61. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2000; pp. 443–480. ISBN 0748408215. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Zushi, K.; Kajiwara, S.; Matsuzoe, N. Chlorophyll a fluorescence OJIP transient as a tool to characterize and evaluate response to heat and chilling stress in tomato leaf and fruit. Sci. Hortic. 2012, 148, 39–46. [Google Scholar] [CrossRef]
- Perales-Vela, H.V.; García, R.V.; Gómez- Juárez, E.A.; Salcedo-Álvarez, M.O.; Cañizares-Villanueva, R.O. Streptomycin affects the growth and photochemical activity of the alga Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2016, 132, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Guisse, B.; Srivastava, A.; Strasser, R.J. The polyphasic rise of the chlorophyll a fluorescence (O-K-J-I-P) in heat stressed leaves. Arch. Sci. 1995, 48, 147–160. [Google Scholar]
- Krüger, G.H.J.; Tsimilli-Michael, M.; Strasser, R.J. Light stress provokes plastic and elastic modifications in structure and function of photosystem II in camellia leaves. Physiol. Plant 1997, 101, 265–277. [Google Scholar] [CrossRef]
- Glazer, A.N.; Chan, C.; Williams, R.C.; Yeh, S.W.; Clark, J.H. Kinetics of energy flow in the phycobilisome core. Science 1985, 230, 1051–1053. [Google Scholar] [CrossRef]
- Kehoe, D.M.; Grossman, A.R. Similarity of a chromatic adaptation sensor to phytochrome and ethylene receptors. Science 1996, 273, 1409–1412. [Google Scholar] [CrossRef]
- Grossman, A.R.; Kehoe, D.M. Phosphorelay control of phycobilisome biogenesis during complementary chromatic adaptation. Photosynth. Res. 1997, 53, 95–108. [Google Scholar] [CrossRef]
- Yamanaka, G.; Glazer, A.N. Dynamic aspects of phycobilisome structure. Arch. Microbiol. 1980, 124, 39–47. [Google Scholar] [CrossRef]
- Collier, J.L.; Herbert, S.K.; Fork, D.C.; Grossman, A.R. Changes in the cyanobacterial photosynthetic apparatus during acclimation to macronutrient deprivation. Photosynth. Res. 1994, 42, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Golubic, S.; Campbell, S.E. Analogous microbial forms in recent subaerial habi- tats and in precambrian cherts: Gloeothece coerulea Geitler and Eosynechococcus moorei Hofmann. Precambrian Res. 1979, 8, 201–217. [Google Scholar] [CrossRef]
- Dayani, S.; Heydarizadeh, P.; Sabzalian, M.R. Efficiency of light-emitting diodes for future photosynthesis. In Handbook of Photosynthesis; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2016; pp. 761–783. [Google Scholar]
- Stanier, R.Y.; Deruelles, J.; Rippka, R.; Herdman, M.; Waterbury, J.B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Montgomery, B.L. Light-dependent governance of cell shape dimensions in cyanobacteria. Front. Microbiol. 2015, 6, 514. [Google Scholar] [CrossRef] [PubMed]
- Bordowitz, J.R.; Montgomery, B.L. Photoregulation of cellular morphology during complementary chromatic adaptation requires sensor-kinase-class protein RcaE in Fremyella diplosiphon. J. Bacteriol. 2008, 190, 4069–4074. [Google Scholar] [CrossRef]
- Kula, M.; Kalaji, H.M.; Skoczowski, A. Culture density influence on the photosynthetic efficiency of microalgae growing under different spectral compositions of light. J. Photochem. Photobiol. B Biol. 2017, 167, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Szwarc, D.; Zieliński, M. Effect of lighting on the intensification of phycocyanin production in a culture of Arthrospira platensis. MDPI Proc. 2018, 2, 1305. [Google Scholar] [CrossRef]
- Stevens, S.E.; Balkwill, D.L.; Paone, D.A.M. The effects of nitrogen limitation on the ultrastructure of the cyanobacterium Agmenellum quadruplicatum. Arch. Microbiol. 1981, 130, 204–212. [Google Scholar] [CrossRef]
- Bhaya, D.; Grossman, A.R.; Steunou, A.S.; Khuri, N.; Cohan, F.M.; Hamamura, N.; Melendrez, M.C.; Bateson, M.M.; Ward, D.M.; Heidelberg, J.F. Population level functional diversity in a microbial community revealed by comparative genomic and metagenomic analyses. ISME J. 2007, 1, 703–713. [Google Scholar] [CrossRef]
- Bald, D.; Kruip, J.; Rögner, M. Supramolecular architecture of cyanobacterial thylakoid membranes: How is the phycobilisome connected with the photosystems? Photosynth. Res. 1996, 49, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.M.; Tulk, S.E.; Jeans, J.A.; Campbell, D.A.; Bibby, T.S.; Cockshutt, A.M. Photophysiological and Photosynthetic Complex Changes during Iron Starvation in Synechocystis sp. PCC 6803 and Synechococcus elongatus PCC 7942. PLoS ONE 2013, 8, e59861. [Google Scholar] [CrossRef]
- Liu, H.; Blankenship, R.E. On the interface of light-harvesting antenna complexes and reaction centers in oxygenic photosynthesis. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 148079. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.L.; Conde-Alvarez, R.; Gomez, I. Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynth. Res. 2003, 75, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Yu, G.; Wu, Z.; Peng, X.; Li, R. Responses of Synechocystis sp. PCC 6803 (cyanobacterium) photosystem II to pyrene stress. J. Environ. Sci. 2010, 22, 1091–1095. [Google Scholar] [CrossRef]
- Tóth, T.; Zsiros, O.; Kis, M.; Garab, G.; Kovács, L. Cadmium exerts its toxic effects on photosynthesis via a cascade mechanism in the cyanobacterium, Synechocystis PCC 6803. Plant Cell Environ. 2012, 35, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Schuurmans, R.M.; Van Alphen, P.; Schuurmans, J.M.; Matthijs, H.C.P.; Hellingwerf, K.J. Comparison of the photosynthetic yield of cyanobacteria and green algae: Different methods give different answers. PLoS ONE 2015, 10, e0139061. [Google Scholar] [CrossRef] [PubMed]
- Touloupakis, E.; Cicchi, B.; Torzillo, G. A bioenergetic assessment of photosynthetic growth of Synechocystis sp. PCC 6803 in continuous cultures. Biotechnol. Biofuels 2015, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Touloupakis, E.; Cicchi, B.; Benavides, A.M.S.; Torzillo, G. Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.). Appl. Microbiol. Biotechnol. 2016, 100, 1333–1341. [Google Scholar] [CrossRef]
- Wu, L.; Lei, Y.; Lan, S.; Hu, C. Photosynthetic recovery and acclimation to excess light intensity in the rehydrated lichen soil crusts. PLoS ONE 2017, 12, e0172537. [Google Scholar] [CrossRef]
- Misumi, M.; Katoh, H.; Tomo, T.; Sonoike, K. Relationship between photochemical quenching and non-photochemical quenching in six species of cyanobacteria reveals species difference in redox state and species commonality in energy dissipation. Plant Cell Physiol. 2016, 57, 1510–1517. [Google Scholar] [CrossRef]
- Akimoto, S.; Yokono, M.; Hamada, F.; Teshigahara, A.; Aikawa, S.; Kondo, A. Adaptation of light-harvesting systems of Arthrospira platensis to light conditions, probed by time-resolved fluorescence spectroscopy. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Öquist, G. Predicting light acclimation in cyanobacteria from nonphotochemical quenching of photosystem II fluorescence, which reflects state transitions in these organisms. Plant Physiol. 1996, 111, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.J.; Stöckel, J.; Srivastava, A.; Strasser, R.J. Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environ. Pollut. 2001, 115, 49–64. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; Ritchie, R.J.; Raven, J.A. Living off the Sun: Chlorophylls, bacteriochlorophylls and rhodopsins. Photosynthetica 2018, 56, 11–43. [Google Scholar] [CrossRef]
- Gómez-Consarnau, L.; González, J.M.; Coll-Lladó, M.; Gourdon, P.; Pascher, T.; Neutze, R.; Pedrós-Alió, C.; Pinhassi, J. Light stimulates growth of proteorhodopsin-containing marine Flavobacteria. Nature 2007, 445, 210–213. [Google Scholar] [CrossRef]
- Gómez-Consarnau, L.; Akram, N.; Lindell, K.; Pedersen, A.; Neutze, R.; Milton, D.L.; González, J.M.; Pinhassi, J. Proteorhodopsin phototrophy promotes survival of marine bacteria during starvation. PLoS Biol. 2010, 8, e1000358. [Google Scholar] [CrossRef]
- Kimura, H.; Young, C.R.; Martinez, A.; DeLong, E.F. Light-induced transcriptional responses associated with proteorhodopsin-enhanced growth in a marine flavobacterium. ISME J. 2011, 5, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Steindler, L.; Schwalbach, M.S.; Smith, D.P.; Chan, F.; Giovannoni, S.J. Energy starved Candidatus pelagibacter ubique substitutes light-mediated ATP production for endogenous carbon respiration. PLoS ONE 2011, 6, e19725. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; O’Shaughnessy, T.J.; Soto, C.M.; Rahbar, A.M.; Robertson, K.L.; Lebedev, N.; Vora, G.J. Function and regulation of Vibrio campbellii proteorhodopsin: Acquired phototrophy in a classical organoheterotroph. PLoS ONE 2012, 7, e38749. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.; Palovaara, J.; Forsberg, J.; Lindh, M.V.; Milton, D.L.; Luo, H.; González, J.M.; Pinhassi, J. Regulation of proteorhodopsin gene expression by nutrient limitation in the marine bacterium Vibrio sp. AND4. Environ. Microbiol. 2013, 15, 1400–1415. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Powell, S.M.; Wilson, R.; Bowman, J.P. Light-stimulated growth of proteorhodopsin-bearing sea-ice psychrophile Psychroflexus torquis is salinity dependent. ISME J. 2013, 7, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Palovaara, J.; Akram, N.; Baltar, F.; Bunse, C.; Forsberg, J.; Pedros-Alio, C.; Gonzalez, J.M.; Pinhassi, J. Stimulation of growth by proteorhodopsin phototrophy involves regulation of central metabolic pathways in marine planktonic bacteria. Proc. Natl. Acad. Sci. USA 2014, E3650–E3658. [Google Scholar] [CrossRef]
- Vogt, A.; Wietek, J.; Hegemann, P. Gloeobacter rhodopsin, limitation of proton pumping at high electrochemical load. Biophys. J. 2013, 105, 2055–2063. [Google Scholar] [CrossRef]
- Rippka, R.; Herdman, M. Pasteur culture collection of cyanobacterial strains in axenic culture. In Catalogue of Strains; Institut Pasteur: Paris, France, 1992; p. 103. ISBN 9783440126349. [Google Scholar]
- Thiel, V.; Tank, M.; Bryant, D.A. Diversity of chlorophototrophic bacteria revealed in the omics era. Annu. Rev. Plant Biol. 2018, 69, 21–49. [Google Scholar] [CrossRef]
- Leu, K.L.; Hsu, B.D. A programmed cell disintegration of Chlorella after heat stress. Plant Sci. 2005, 168, 145–152. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual; Argentine, J., Irwin, N., Janssen, K.A., Curtis, S., Zierler, M., McInerny, N., Brown, D., Schaefer, S., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Bennett, A.; Bogobad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Force, L.; Critchley, C.; Van Rensen, J.J.S. New fluorescence parameters for monitoring photosynthesis in plants. Photosynth. Res. 2003, 78, 17–33. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Goltsev, V.N.; Zuk-Golaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence, Understanding Crop Performance; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781498764490. [Google Scholar]
- Bąba, W.; Kalaji, H.M.; Kompała-Baba, A.; Goltsev, V. Acclimatization of photosynthetic apparatus of tor grass (Brachypodium pinnatum) during expansion. PLoS ONE 2016, 11, e0156201. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska, A.H.; Pawluśkiewicz, B.; Paunov, M.; Alexandrov, V.; Goltsev, V.; Kalaji, M.H. Prompt chlorophyll a fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in Perennial ryegrass. J. Photochem. Photobiol. B Biol. 2016, 157, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2016, 132, 13–66. [Google Scholar] [CrossRef]

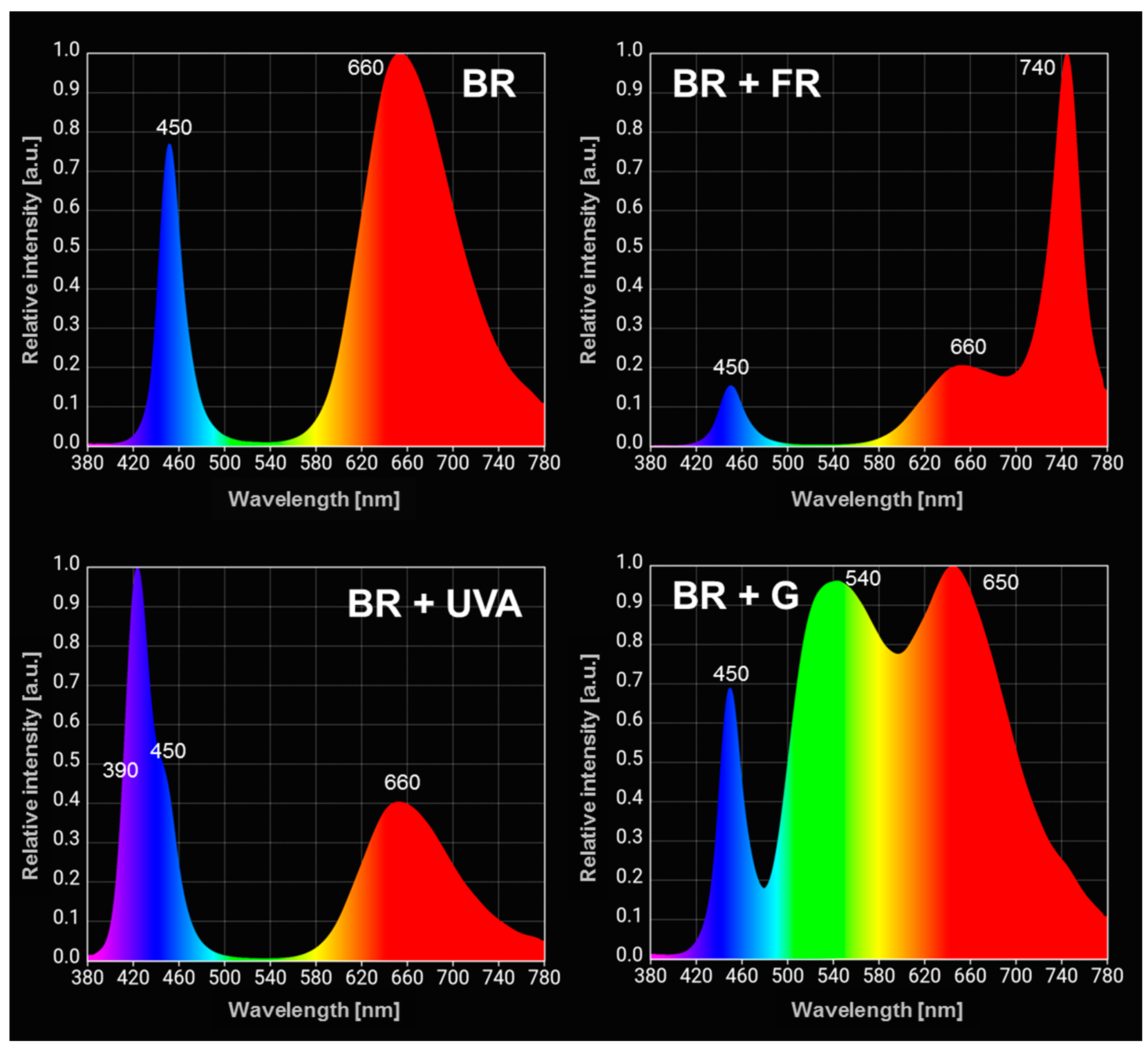
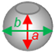 | The Spectral Composition of Light | |||
|---|---|---|---|---|
| BR (Control) | BR + FR | BR + UVA | BR + G | |
| Minor axis (a) (µm) | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.2 |
| Major axis (b) (µm) | 1.3 ± 0.2 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.5 ± 0.3 |
| Ratio a:b | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.6 ± 0.1 * |
| The model of the cell shape |  |  |  |  |
| Component (Photosynthetic Pigments) | Peak Maximum Wavelength (nm) | BR | BR + FR | BR + UVA | BR + G |
|---|---|---|---|---|---|
| Peak Area (a.u.) | |||||
| chl a | 440 | 7 | 8 | 6 | 13 |
| car | 475 | - | - | - | 8 |
| R-PE, car, PUB | 500 | 6 | 10 | 8 | 13 |
| C-PE, GR, B-PE | 548 | 11 | 8 | 9 | 8 |
| C-PE, R-PE | 565 | 22 | 15 | 19 | 10 |
| C-PC | 628 | 77 | 73 | 78 | 58 |
| chl a, APC | 680 | 7 | 10 | 10 | 22 |
| Energy Flow Rates | |
|---|---|
| Fo/Fm | quantum yield baseline, it is used as an indicator of the physiological state (state change index); |
| Fv/Fm | a value that is related to the maximum quantum yield of PSII; |
| Fv/Fo | a value that is proportional to the activity of the water-splitting complex on the donor side of the PSII; |
| Vj | relative variable fluorescence at phase J of the fluorescence induction curve; |
| M0 | the approximated initial slope of the fluorescent transient, which is related to the rate of closure of reaction centers; |
| Specific Energy Fluxes | |
| ABS/RC | the antenna size of active PSII per reaction center (RC) and light absorption energy flux (for PSII antenna chlorophylls); |
| DIo/RC | total energy dissipated per reaction center (RC) as heat, fluorescence, and energy transfer to PSI; |
| TRo/RC | trapping flux leading to QA reduction per reaction center (RC); |
| ETo/RC | the electron flux transferred per active reaction center (RC); |
| Quantum Yields | |
| ψEo (Ψo) | probability (at time 0) that a trapped exciton moves an electron into the electron transport chain beyond QA−; |
| φEo | the quantum efficiency of electron transfer from QA− to plastoquinone. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kula-Maximenko, M.; Zieliński, K.J.; Ślesak, I. The Role of Selected Wavelengths of Light in the Activity of Photosystem II in Gloeobacter violaceus. Int. J. Mol. Sci. 2021, 22, 4021. https://doi.org/10.3390/ijms22084021
Kula-Maximenko M, Zieliński KJ, Ślesak I. The Role of Selected Wavelengths of Light in the Activity of Photosystem II in Gloeobacter violaceus. International Journal of Molecular Sciences. 2021; 22(8):4021. https://doi.org/10.3390/ijms22084021
Chicago/Turabian StyleKula-Maximenko, Monika, Kamil Jan Zieliński, and Ireneusz Ślesak. 2021. "The Role of Selected Wavelengths of Light in the Activity of Photosystem II in Gloeobacter violaceus" International Journal of Molecular Sciences 22, no. 8: 4021. https://doi.org/10.3390/ijms22084021
APA StyleKula-Maximenko, M., Zieliński, K. J., & Ślesak, I. (2021). The Role of Selected Wavelengths of Light in the Activity of Photosystem II in Gloeobacter violaceus. International Journal of Molecular Sciences, 22(8), 4021. https://doi.org/10.3390/ijms22084021






