Abstract
Our understanding of the function and development of the lymphatic system is expanding rapidly due to the identification of specific molecular markers and the availability of novel genetic approaches. In connection, it has been demonstrated that mechanical forces contribute to the endothelial cell fate commitment and play a critical role in influencing lymphatic endothelial cell shape and alignment by promoting sprouting, development, maturation of the lymphatic network, and coordinating lymphatic valve morphogenesis and the stabilization of lymphatic valves. However, the mechanosignaling and mechanotransduction pathways involved in these processes are poorly understood. Here, we provide an overview of the impact of mechanical forces on lymphatics and summarize the current understanding of the molecular mechanisms involved in the mechanosensation and mechanotransduction by lymphatic endothelial cells. We also discuss how these mechanosensitive pathways affect endothelial cell fate and regulate lymphatic development and function. A better understanding of these mechanisms may provide a deeper insight into the pathophysiology of various diseases associated with impaired lymphatic function, such as lymphedema and may eventually lead to the discovery of novel therapeutic targets for these conditions.
1. Introduction
Two vascular networks are present in mammals: the blood and the lymphatic systems. The blood vasculature is a circulatory vessel system that is essential for the transportation of respiratory gases, nutrients, and signaling molecules. Lymphatic vessels form a blind-ended, linear vessel system that is distinguished from the blood vasculature in its structure, function, and development, as reviewed previously by many investigators [1,2,3,4,5,6,7,8,9,10,11,12].
Both vessel systems are comprised of endothelial cells (EC), which are surrounded by smooth muscle cells, pericytes, and basal membrane in some vascular beds. ECs are differentiated to blood endothelial cells (BEC) in the blood vessel network and lymphatic endothelial cells (LEC) in the lymphatic vasculature. Initial lymphatics are comprised of loosely connected lymphatic endothelial cells forming button-like junctions [13,14], which allow the lymphatic capillaries to take up and transport fluid, macromolecules, and cells from the interstitial compartment. Lymph is transported from the initial lymphatic capillaries through the pre-collecting lymphatics to the collecting lymphatic vessels [4,5,7,10,12,15,16,17,18,19,20]. Pre-collecting lymphatics display sporadic intraluminal valves and a discontinuous vascular smooth muscle cell layer and basal lamina [21]. Collecting lymphatic vessels have intraluminal valves and are covered by a continuous vascular smooth muscle cell layer and basal lamina in most tissues. In contrast to the initial lymphatics, LECs of the collecting lymphatic vessels are interconnected with continuous cell junctions (zipper-like junctions), resulting in a reduced uptake of fluid, molecules, and cells from the interstitium [22]. Pulsative contractions of the vascular smooth muscle cells and the activity of surrounding skeletal muscles are the main driving factors of lymph flow, while intraluminal valves of pre-collecting and collecting lymphatics prevent backflow [23,24,25,26,27]. Lymph is propelled from the collecting lymphatic vessels into the subclavian veins through the thoracic duct or the right lymphatic trunk [1,2,3,4,5,6,7,10,12,15,16,17,18,19,20,28,29].
In association with the well-known roles of the lymphatic system in maintaining body fluid balance, cell uptake and trafficking, and dietary lipid absorption, lymphatic function contributes to the orchestration of inflammation and immune responses, tumor cell dissemination, and metastasis formation, while lymphatic malfunction has a central role in lymphedema formation [1,2,3,4,5,6,7,15,16,18,20,30,31,32,33,34,35]. Importantly, recent studies revealed the importance of organ-specific lymphatic function in a great variety of conditions, including cardiovascular diseases, obesity, or diseases affecting the central nervous system [20,36,37]. These recent findings emphasize a need for better understanding of how flow-generated mechanical forces contribute to the development and function of the lymphatic system.
2. Importance of Flow-Induced Mechanical Forces in Lymphatics
Experimental data demonstrated that ECs sense flow magnitude, direction, amplitude, and frequency of pulsatile flow [38,39,40,41,42,43,44]. A pulsatile, high-pressure flow is characteristic of the arterial system, while venous flow has low-pressure laminar nature, and low-pressure oscillatory flow is observed in lymphatic vessels. Lymphatic flow velocity and shear stress (the force per area acting on the vessel wall) rates [45,46,47] are below the values measured in blood vessels [48,49,50,51,52]. Although many studies focus on the effects of fluid shear stress and flow characteristics on blood vessels and BECs [48,53,54,55,56,57,58,59], the molecular interactions involved in shear stress mechanosensation and mechanotransduction are still poorly characterized in these structures. Our understanding on the molecular mechanisms involved in flow-induced signaling in LECs is even more limited [60].
2.1. Flow-Induced Cellular Changes in ECs
Previous in vitro experiments demonstrated that LECs and BECs react differently to the same mechanical conditions [61,62]. Under 4 dynes/cm2 planar shear, both LECs and BECs elongate parallel to the flow direction, while under interstitial flow with 10 μm/s average velocity, LECs form large vacuoles and exhibit long dendritic extensions in contrast to BECs that form branching, lumenized structures [61]. Prior in vitro experiments reported that acute shear stress induces a Ca2+-dependent reorganization of the cytoskeleton in BECs [63,64,65,66,67,68]. Dynamic reorganization of focal adhesion sites was observed in BECs upon acute shear stress that also supports flow-dependent cytoskeleton reorganization and cell alignment [69,70]. Another in vitro study demonstrated that although under 4 dynes/cm2 laminar shear stress, LECs become elongated and aligned with flow, LECs are more cuboidal under 4 dynes/cm2, ¼ Hz oscillatory shear stress (OSS), and their alignment is less dependent on flow direction [71]. The authors also revealed that flow-induced cytoskeleton reorganization and cell alignment of LECs are mediated by transcription factors forkhead box protein C2 (FOXC2) and prospero homeobox protein 1 (PROX1) [71]. In a recent in vivo study, FAT tumor suppressor homolog 4 (FAT4), encoded by the GATA binding protein 2 (GATA2) target gene Fat4, was identified as a key player in shear stress-dependent polarization of LECs [72].
An in vitro study revealed that slow interstitial flow with an average velocity of 4.2 μm/s synergizes with molecular factors promoting blood and lymphatic vessels sprouting by enhancing the availability of matrix-bound growth factors to the cells [73]. Similarly, in a recent study, it was demonstrated in a 3D in vitro model that interstitial flow with 1 μm/s average velocity augments the effects of pro-lymphangiogenic molecular factors and determines the direction of lymphatic sprouting [74].
According to these results, flow characteristics affect LEC shape, alignment, and sprouting, and these findings propose that fluid pressure and flow-generated mechanical forces may play a critical role in the development and function of lymphatic vessels.
2.2. Mechanical Forces in the Developmental Program of the Lymphatic Vasculature
2.2.1. Early Steps of Lymphatic Development
The development of the lymphatic system begins between the 6th and 7th week of the 40-week-long pregnancy in humans [5] and approximately on the 9th embryonic day (E9.0) of the 21-day-long pregnancy in mice, when the first PROX1–lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) double positive lymphatic progenitor cells bud from the cardinal vein and form the jugular lymph sac [75,76,77,78,79,80,81,82,83,84,85]. In mice, vascular endothelial growth factor receptor 3 (VEGFR-3) expression becomes restricted to the LECs from E10.5 [86,87,88]. In parallel, these cells start to express podoplanin (PDPN (other common aliases: T1α, gp38, E11 antigen)) [89,90]. A recent study revealed the role of GATA2 in VEGFR3 upregulation of LECs, which is critical for the directed VEGF-C-dependent migration of LECs during the early lymphatic development [91]. Immature pre-lymphatic vessels that are comprised of LECs carrying the molecular markers LYVE-1, PROX1, VEGFR-3, and PDPN [84,86,88,89,90] enmesh the mouse embryos [17].
Although LECs are predominantly derived from the venous endothelium [75,77,78,79,82,83,85,92,93,94,95,96], experimental data suggest multiple possible origins of LECs. Although the concept of the non-venous origin of lymphatics was first introduced by Huntington and McClure [97], it was neglected until recently. Almost a century later, mesenchymal lymphangioblasts were suggested to contribute to the development of avian and amphibian lymphatics in addition to venous ECs [98,99,100,101,102]. A subpopulation of LECs co-expressing mesenchymal-specific (cluster of differentiation 45 (CD45)) and LEC-specific (LYVE-1, PROX-1) markers was also observed in mouse embryos [103,104]. Moreover, a hemogenic-derived subpopulation of LECs was identified in the lymphatic vessels of the mesentery [105], dermis [106], and the heart [107]. Interestingly, recent findings suggest the contribution of second heart field progenitors to the development of cardiac lymphatics [108,109]. Organ-specific characteristics of lymphatic development and the origin of LECs were summarized in multiple reviews [9,10,11,37,110,111].
Interstitial fluid volume affects elongation and proliferation of LECs, presumably due to a mechanical force-induced activation of VEGFR-3 mediated by ß1 integrin [112], and regulated by integrin-linked kinase (ILK) signaling [113]. Other studies reported the role of interstitial flow in lymphatic vessel regeneration [114,115,116]. These findings suggest the possible role of interstitial fluid pressure-related mechanical forces in lymphatic expansion. Nevertheless, this proposed role of mechanical forces in the proliferation and migration of LECs is yet to be investigated.
2.2.2. Common Steps of the Maturation of the Lymphatics
Further development and maturation of the lymphatic vessels take place in an organ-specific time and manner. During these processes, the immature pre-lymphatic plexuses connect to each other, additional lymphatic vessels are formed, and immature pre-lymphatic vessels undergo a structural remodeling that eventually leads to the formation of the initial lymphatic capillaries and collecting lymphatic vessels. Notably, mechanisms that regulate the maturation of lymphatic vessels are still poorly characterized. Most collecting lymphatics acquire a smooth muscle coverage, and lymphatic valves are formed in their lumen [17,117,118,119]. Numerous molecules and pathways, such as ephrin B2 [117] and B4 [120], angiopoietin 2 [121], integrin α9 [24], transcription factor nuclear factor of activated T cells 1 (NFATc1) [25,71], connexin 37 and 43 [71,122], bone morphogenetic protein (BMP-9) [123], mechanically induced Wnt/ß-catenin signaling [124], RAS p21 protein activator 1 (RASA1) [125], VEGFR-3 signaling [126,127] FAT4 [72,128], platelet endothelial cell adhesion molecule (PECAM) [129], vascular endothelial cadherin (VE-cadherin) [130], neuropilins, semaphorins, and plexins, [131,132] have been revealed to contribute to the development of the intraluminal lymphatic vessel valves. Recent data suggest that yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) signaling might be critical for the maturation and maintenance of lymphatic valves [133,134]. Transcription factors GATA2 [135], FOXC2 [25,136], and PROX1 [71] were identified as key molecular factors of the lymphatic valve development, and their expression is also critical for the maintenance of these structures [137,138,139]. GATA2 and FOXC2 are also essential for the establishment of a proper vascular smooth muscle coverage of the collecting lymphatic vessels [136,138]. In primary LECs isolated from the dermis of E16.5 embryonic mice, GATA2 has been demonstrated to regulate the expression of numerous genes encoding proteins involved in lymphatic valve formation, including FOXC2, PROX1, NFATc1, PECAM, angiopoietin 2, or integrin α9 [135].
2.2.3. Separation of the Blood and Lymphatic Systems
As mentioned above, the blood vasculature and the lymphatic vessel systems are connected in the jugular region where the thoracic duct and the right lymphatic trunk enter the subclavian veins. Two parallel mechanisms are responsible for the prevention of backflow of blood into the lymphatic system. Lymphovenous valves (LVV) develop between the jugular lymph sac and the cardinal vein at E11.5–E13.5 in mice (Figure 1A,B) [140,141]. Flow-generated mechanical forces and molecular factors that are involved in lymphatic valve formation, such as FOXC2, GATA2, PROX1, integrin α9, connexin 37, and Wnt/ß-catenin signaling, were shown to contribute to LVV morphogenesis [124,138,140,141,142]. The role of YAP/TAZ signaling in the development and maintenance of LVVs is also suggested by a recent study [134].
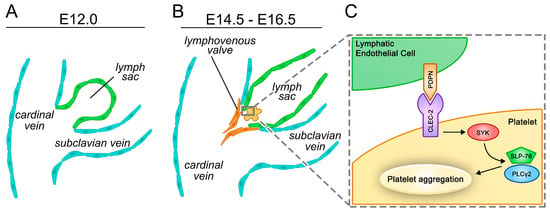
Figure 1.
Cellular and molecular mechanisms involved in the separation of the blood and lymphatic vessel systems in mice. (A,B) Overview of the separation of the cardinal vein and the lymph sac with the formation of the lymphovenous valve in parallel with the platelet-dependent activation of the C-type lectin-like receptor 2 (CLEC-2)/spleen tyrosine kinase (SYK)/SH2-domain-containing leukocyte protein of 76 kDa (SLP-76)/phospholipase C gamma 2 (PLCγ2) pathway. (C) The CLEC-2/SYK/SLP-76/PLCγ2 pathway in platelets recognizing podoplanin (PDPN) expressed on the surface of lymphatic endothelial cells (LECs) is critical for the proper separation of the two circulatory systems.
Besides LVV formation, another mechanism is required to ensure the separation of the blood and lymphatic systems. C-type lectin-like receptor 2 (CLEC-2), which is involved in the spleen tyrosine kinase (SYK)-dependent platelet activation [143,144], is a receptor for PDPN expressed on the surface of LECs [144,145,146], and PDPN activates platelets by the CLEC-2/SYK/SH2-domain-containing leukocyte protein of 76 kDa (SLP-76)/phospholipase C gamma 2 (PLCγ2) pathway [147]. This platelet-mediated molecular pathway is essential for the appropriate separation of the blood and lymphatic circulations (Figure 1C) [89,144,147,148,149,150,151,152,153,154,155,156,157,158,159].
2.2.4. Lymph Flow Promotes Organ-Specific Maturation of the Lymphatic Vasculature
Lymph flow is reportedly reduced in mice with impaired PDPN/CLEC-2/PLCγ2 signaling [160,161,162] due to an improper separation of the blood and lymphatic vessel systems [156]. These data propose that these genetic mouse models provide ideal tools to study the roles of lymphatic flow in vivo. Importantly, while the formation of immature mesenteric pre-lymphatics is intact in mice with impaired lymph flow due to a lack of CLEC-2, organ-specific maturation of the mesenteric lymphatic vessels is impaired in these embryos [160], and the phenotype is also present in PLCγ2-deficient embryos [162]. In addition, the postnatal developmental program of the meningeal lymphatic vasculature is also affected in PLCγ2-deficient mice [162]. These in vivo findings underline the importance of mechanical forces in the organ-specific developmental program of the lymphatic vessels.
Taken together, flow-induced mechanical forces regulate LEC shape and alignment, promote lymphatic sprouting and development, and contribute to the maturation of the lymphatic vessel systems. These findings suggest that LECs bear mechanisms responsible for mechanosensation and mechanotransduction that make them able to sense the surrounding mechanical forces and translate these factors to molecular levels, leading to regulation of gene expression, cell proliferation, survival, migration, and identity, in addition to cytoskeleton remodeling.
2.3. Molecular Mechanisms Involved in the Mechanosensation and Mechanotransduction of LECs
2.3.1. Shear Stress-Dependent Molecular Pathways in Lymphatic Valve Morphogenesis
Intraluminal valves develop after the increase in lymphatic flow, and it has been demonstrated that OSS (either 4 dynes/cm2, ¼ Hz or an average 0.67 dynes/cm2 with a maximum of 3.25 dynes/cm2 and a minimum of −1.25 dynes/cm2, 1 Hz) upregulates the expression of transcription factors FOXC2, GATA2 [71,138,160], and their downstream molecular factors, such as connexin 37, integrin α9, ephrin B2, neuropilin 1, and Krüppel-like factor 2 (KLF-2) [71,138,160], presumably in a VE-cadherin/Wnt/ß-catenin dependent manner [124,130,163]. In these in vitro experiments, PROX1 levels were not altered by OSS [71,160]. Nevertheless, other in vitro data suggest that GATA2 promotes PROX1 transcription [135,138]. Importantly, lymphatic valves are formed mainly at lymphatic branches [24,25,71], where the laminar nature of the flow is interrupted (Figure 2) [53,164,165]. Taken together, these findings further promote the hypothesis that the lymph flow dynamics and local shear stress characteristics play an important role in the development of the intraluminal lymphatic valves. Besides the molecular pathways mentioned before, the role of syndecan 4 was identified in the sensation of flow direction [166] and lymphatic valve formation [129]. However, the mechanism of its flow-dependent signaling that promotes lymphatic development is still poorly understood.
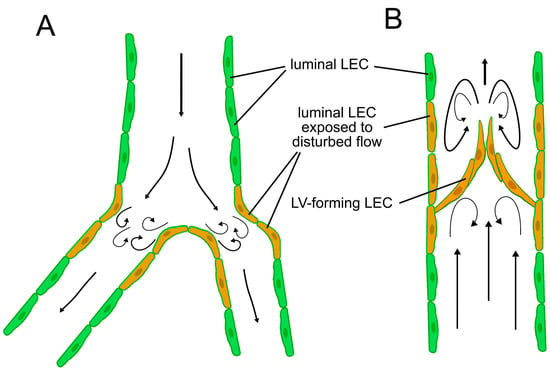
Figure 2.
Flow patterns in the lymphatic system. (A) LECs in branches are exposed to disturbed flow patterns with higher shear stress even before the establishment of intraluminal valves. (B) Flow becomes disturbed at the lymphatic valves of mature collecting lymphatic vessels. Valve-forming LECs and luminal LECs surrounding lymphatic valves are also exposed to disturbed flow, while LECs at other sites than branches, valves, and curvatures are exposed to a more laminar flow.
2.3.2. Mechanical Forces as Possible Regulators of Spatial Changes in Gene Expression during the Maturation of Lymphatic Vessels
Expression of PROX1, FOXC2, and VEGFR-3 remains high in the lymphatic valve cells but is reduced in the mature lymphatic vessel walls after the formation of lymphatic valves [25], presumably due to changes in flow patterns downstream of lymphatic valves after their development (Figure 2B). The role of steady shear flow-induced epsin signaling in the temporal and spatial regulation of VEGFR-3 abundance in LECs was demonstrated [126], and the mechanism may contribute to the mechanical force-dependent site-specific changes in gene expression in LECs.
Recent studies suggest the possible role of YAP/TAZ signaling in flow-dependent regulation of gene transcription in LECs. Transcription factor TAZ is expressed in lymphatic valve forming LECs [133,134,137], and in vitro data suggest that 4 dynes/cm2, ¼ Hz OSS enhances the nuclear translocation of YAP and TAZ, which is necessary for their function in regulating gene transcription [137]. Some investigators in their in vitro and in vivo experiments found that VEGF-C signaling is also capable of inducing the YAP/TAZ pathway that in turn enhances the expression of PROX1 [134]. However, other researchers reported that YAP/TAZ signaling is negatively regulated by the VEGF-C–VEGFR-3 pathway and represses PROX1 expression [133]. In this study, it was also observed that LEC-specific deletion of YAP and TAZ inhibits lymphatic branch formation and proper lymphatic development. Moreover, enrichment of PROX1 in LV forming LECs and downregulation of PROX1 in luminal LECs was also repressed in this model [133]. Importantly, both studies support that YAP/TAZ signaling promotes the PROX1–VEGFR-3 pro-lymphangiogenic positive feedback loop. Another study using in vitro and in vivo approaches demonstrated that FOXC2 inhibits the downstream signaling of the YAP/TAZ pathway, thereby suppressing its pro-proliferative effects [137], which may serve as an offset against the PROX1–VEGFR-3 positive feedback loop. FOXC2-dependent repression of shear stress-induced YAP/TAZ signaling may be a potential mechanism answering why PROX1 expression levels were unaltered under OSS in in vitro experiments [71,160], although GATA2 promotes the transcription of PROX1 and FOXC2 according to other in vitro experimental results [135,138]. These findings propose that YAP/TAZ signaling influenced by both local shear stress characteristics and molecular factors may play a central role in orchestrating the spatial gene expression patterns in lymphatic valve-forming and luminal LECs. Nevertheless, roles of the YAP/TAZ pathway and the underlying mechanisms of its signaling are still barely understood in LECs.
2.3.3. Ion Channels in Shear Stress-Dependent Intracellular Signaling Mechanisms
Activation of these shear stress-induced transcription factors requires mechanosensory mechanisms that regulate gene expression and cellular functions. Recent studies revealed the role of piezo-type mechanosensitive ion channel component 1 (PIEZO1)-mediated mechanotransduction in the development and maintenance of the lymphatic valves [167,168]. EC-specific deletion of PIEZO1 in mice results in the absence of lymphatic valves, chylous pleural effusion, and postnatal mortality due to impaired lymphatic function [167]. Post-developmental LEC-specific deletion of PIEZO1 leads to a significant degeneration of both lymphatic valves and lymphatic vessels in the skin and mesentery [168]. In humans, absence or loss of function mutations of the PIEZO1 gene causes hereditary lymphoedema as result of defects in the lymphatic vessel developmental program [169,170]. PIEZO1 is a pore-forming subunit of mechanically activated cation channels [171,172,173] with a rapid inactivation rate, suggesting its role as a sensor of transient stress [174]. PIEZO1 presumably contributes to shear stress-mediated development of lymphatic vessels by increasing intracellular Ca2+ levels in LECs, similarly to BECs, in which it has been demonstrated that acute shear stress elicits a rise in intracellular Ca2+ levels from intracellular stores and activation of Ca2+ channels [175,176] and PIEZO1 is essential for shear-stress dependent vascular development [177,178,179].
Importantly, PIEZO1 is not the only mechanosensitive ion channel that has been proposed to affect the maturation of the lymphatic vessels. Calcium release-activated calcium modulator 1 (ORAI1), a pore subunit of the calcium release-activated calcium (CRAC) channel, is activated upon shear stress and mediates Ca2+-influx in LECs [180]. Laminar flow induces an ORAI1-dependent upregulation of KLF-2 and KLF-4 in LECs that promote VEGF-C expression, among other molecular factors contributing to the cell cycle progression [181]. VEGF-C signaling is reportedly regulated by sphingosine-1-phosphate receptor 1 (S1PR1) in quiescent LECs [182]. In addition to their role during the development of lymphatic vessels, CRAC channels are also proposed to mediate Ca2+-dependent regulation of lymphatic barrier function [183].
Intracellular Ca2+, acting together with KLF-2 and PROX1, is suggested to play a central role in shear stress-induced lymphatic sprouting and development [180,181]. However, in vitro data suggest that OSS (an average 0.67 dynes/cm2 with a maximum of 3.25 dynes/cm2 and a minimum of −1.25 dynes/cm2, 1 Hz) induces the expression of KLF-2 but not PROX1 [160], which further supports the role of KLF-2 in lymphatic shear stress-induced signal transduction.
2.3.4. The PECAM–VE-Cadherin–VEGFR-2/3 Complex as a Potential Mechanoreceptor Complex in Lymphatic Endothelial Cells
PECAM, VE-cadherin, VEGFR-2, and VEGFR-3 form a mechanosensory complex in ECs [184,185], which is proposed to promote cell proliferation by activating the phosphatidylinositol-3-kinase (PI3K)/Akt pathway and cytoskeleton remodeling in a flow-dependent manner [185,186,187,188,189].
Pan-endothelial marker PECAM is assumed to play an upstream transmitting role in this complex [185]. However, the mechanisms that evoke a flow-dependent activation of PECAM are still poorly understood [190]. It has been revealed previously that PECAM-deficiency results in an impairment of flow response of blood vessels and inhibits arteriogenesis and vascular remodeling in mice [187,191,192]. Moreover, PECAM was also demonstrated to play a role in lymphatic mechanosensation and valve formation during the embryonic maturation of lymphatic vessels, as PECAM-null mouse embryos display lymphatic remodeling defects [129].
VE-cadherin functions as an adaptor by binding to VEGFR-2 and VEGFR-3 [184,185,189], and its distribution within the cell membrane depends on the spatial flow characteristics [193]. Importantly, LEC-specific conditional deletion of VE-cadherin results in lymphatic valve defects in mice [130].
VEGFR-2 is known for playing various important roles in the cardiovascular system [194]. VEGFR-2-related ligand-independent activation of PI3K/Akt and Erk pathways in response to shear stress promotes cell proliferation, contributes to cytoskeletal remodeling, and impairs junctional remodeling and stabilization in BECs [185,195,196,197,198,199,200,201]. In addition, VEGFR-2 has been shown to be expressed on LECs [202] and is suggested to support VEGFR-3 signaling in the lymphatic developmental program and adult lymphangiogenesis [202,203,204].
As discussed above, flow-induced mechanical forces promote the sprouting and development of lymphatic vessels and play an important role in the stabilization of mature lymphatics. Flow-induced mechanical forces also contribute to similar processes in the blood vasculature [205,206,207,208]. Importantly, ECs with different cell fate commitments react differently to the same mechanical conditions [61,62]. Flow characteristics vary greatly among different vessel types [45,46,47,48,49,50,51,52], and changes in the nature of flow are sufficient to re-program the identity of differentiated ECs [159,180,209]. Therefore, a mechanism that determines the appropriate shear stress sensitivity of the ECs is essential for the proper organization of the vascular systems. It has been demonstrated that the shear sensitivity of the ECs is largely dependent on the VEGFR-3 expression of the cells [44]. Overexpression of VEGFR-3 in BECs significantly increased their sensitivity to shear stress in vitro, which suggests that ECs with a higher VEGFR-3 expression are more sensitive to shear stress due to a lower shear stress set point. This is in line with the phenomenon that VEGFR-3 expression is restricted to lymphatic vessels [86,87,88] exposed to lower flow velocity and shear stress levels compared to those in arteries or veins [45,46,47,48,49,50,51,52].
Interestingly, in addition to its well-known roles in lymphatic vessel development and lymphangiogenesis [4,5,7,15,16,17], VEGFR-3 expression was demonstrated in the endothelium of the aorta [44,184], Schlemm’s canal [210,211,212], ascending vasa recta [213], and the spiral arteries [214] in mice. Importantly, the role of VEGF-C – VEGFR-3 signaling in the development of Schlemm’s canal and the remodeling of the spiral arteries was demonstrated [211,212,214]. VEGFR-3 was previously considered to be primarily expressed on LECs [86,87,88], promoting lymphatic commitment of the ECs [82,215,216].
In vitro results suggest that the expression of VEGFR-3 on BECs is dependent on fluid shear stress characteristics [217]. Importantly, in a mature lymphatic network, VEGFR-3 expression also differs among LECs exposed to different local flow characteristics [25,141]. These data suggest that VEGFR-3 expression in ECs is regulated, at least in part by shear stress-induced mechanisms, and VEGFR-3 expression levels may affect the shear stress sensitivity of the PECAM–VE-cadherin–VEGFR-2/3 mechanosensory complex by a still uncovered mechanism. Moreover, VEGFR-3 signaling may have a still poorly characterized function in regulating endothelial cell plasticity, which may be in relation to its presumed role in determining shear stress sensitivity of endothelial cells.
Although the PECAM–VE-cadherin–VEGFR-2/3 mechanoreceptor complex has been investigated in BECs, the results indicating the involvement of PECAM [129] and VE-cadherin [130] in lymphatic valve formation and lymphatic remodeling, in combination with the well-known functions of VEGFR-3 in lymphatic development, suggest that these molecular factors may also contribute to these mechanical force-dependent processes in LECs.
3. Closing Remarks
Taken together, the presented findings support that the flow-induced mechanical force dynamics play critical roles in the determination and maintenance of EC fate; regulate LECs shape and alignment and maturation of the lymphatic network; promote lymphatic sprouting and development; and orchestrate the morphogenesis and maintenance of the lymphatic valves and the lymphovenous valve.
Mutations affecting genes that contribute to the maturation of lymphatic vessels often result in diseases associated with lymphatic malfunction in clinical studies, such as GATA2 (primary lymphedema with myelodysplasia progressing to acute myeloid leukemia) [135,218,219], FOXC2 (lymphedema distichiasis) [220,221,222], FAT4 (Hennekam syndrome with no mutations in collagen and calcium binding EGF domains 1 gene (CCBE1) [223], ITGA9 encoding integrin α9 (fetal severe chylothorax) [224], PIEZO1 (autosomal recessive form of generalized lymphatic dysplasia) [170], FLT4 encoding VEGFR-3 (Milroy’s disease) [225,226,227], and EPHB4 encoding ephrin B4 (lymphatic-related hydrops fetalis) [120]. As most of these molecular factors are proposed to be regulated at least in part by flow-induced mechanical forces, these data emphasize the possible clinical relevance of lymph flow-induced molecular pathways. Despite the recent important advancements in understanding the mechanisms of mechanosensation and mechanotransduction in LECs (Figure 3), the molecular background of these processes is still poorly understood.
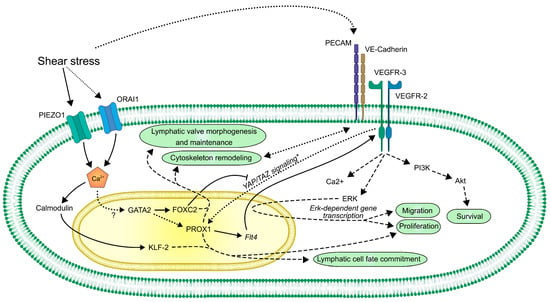
Figure 3.
A schematic overview of the currently known mechanosensory and mechanotransduction molecular pathways in LECs. Lines represent direct connections between molecules, such as regulation of transcription, complex formation, direct activation, or inactivation. Dashed lines represent an indirect connection. Dotted lines represent assumed or still unclear connections.
A better understanding of molecular pathways and interactions that are involved in the mechanical force-related signaling of LECs is needed. Uncovering the molecular background of flow-dependent mechanisms in lymphatic vessels may lead to identification of biomarkers improving the diagnosis of lymphatic diseases or may provide novel therapeutic targets for pathological conditions related to a dysfunction of lymphatics, such as lymphedema.
Author Contributions
Writing—review and editing, L.B. and Z.J.; supervision, Z.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research, Development and Innovation Office (NVKP_16-2016-1-0039 to Z.J.), the European Union and the Hungarian Government (VEKOP-2.3.2-16-2016-00002), and the Higher Education Institutional Excellence Program of the Ministry for Innovation and Technology in Hungary, within the framework of the Molecular Biology thematic program of the Semmelweis University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Gábor Kovács for careful and critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Swartz, M.A. The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 2001, 50, 3–20. [Google Scholar] [CrossRef]
- Oliver, G.; Detmar, M. The rediscovery of the lymphatic system: Old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002, 16, 773–783. [Google Scholar] [CrossRef]
- Hong, Y.K.; Shin, J.W.; Detmar, M. Development of the lymphatic vascular system: A mystery unravels. Dev. Dyn. 2004, 231, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G. Lymphatic vasculature development. Nat. Rev. Immunol 2004, 4, 35–45. [Google Scholar] [CrossRef]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef]
- Oliver, G.; Alitalo, K. The lymphatic vasculature: Recent progress and paradigms. Annu. Rev. Cell Dev. Biol. 2005, 21, 457–483. [Google Scholar] [CrossRef] [PubMed]
- Maby-El Hajjami, H.; Petrova, T.V. Developmental and pathological lymphangiogenesis: From models to human disease. Histochem Cell Biol. 2008, 130, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Bautch, V.L.; Caron, K.M. Blood and lymphatic vessel formation. Cold Spring Harb. Perspect Biol. 2015, 7, a008268. [Google Scholar] [CrossRef]
- Escobedo, N.; Oliver, G. Lymphangiogenesis: Origin, Specification, and Cell Fate Determination. Annu. Rev. Cell Dev. Biol. 2016, 32, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Ulvmar, M.H.; Makinen, T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc Res. 2016, 111, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Oliver, G. Lymphatic Endothelial Cell Plasticity in Development and Disease. Physiology (Bethesda) 2017, 32, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Makinen, T. Vascular heterogeneity and specialization in development and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Leak, L.V.; Burke, J.F. Fine structure of the lymphatic capillary and the adjoining connective tissue area. Am. J. Anat 1966, 118, 785–809. [Google Scholar] [CrossRef]
- Leak, L.V.; Burke, J.F. Ultrastructural studies on the lymphatic anchoring filaments. J. Cell Biol. 1968, 36, 129–149. [Google Scholar] [CrossRef]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef]
- Zheng, W.; Aspelund, A.; Alitalo, K. Lymphangiogenic factors, mechanisms, and applications. J. Clin. Investig. 2014, 124, 878–887. [Google Scholar] [CrossRef]
- Koltowska, K.; Betterman, K.L.; Harvey, N.L.; Hogan, B.M. Getting out and about: The emergence and morphogenesis of the vertebrate lymphatic vasculature. Development 2013, 140, 1857–1870. [Google Scholar] [CrossRef]
- Schulte-Merker, S.; Sabine, A.; Petrova, T.V. Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. 2011, 193, 607–618. [Google Scholar] [CrossRef]
- Schmid-Schonbein, G.W. Microlymphatics and lymph flow. Physiol Rev. 1990, 70, 987–1028. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21(st) Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- Sacchi, G.; Weber, E.; Agliano, M.; Raffaelli, N.; Comparini, L. The structure of superficial lymphatics in the human thigh: Precollectors. Anat. Rec. 1997, 247, 53–62. [Google Scholar] [CrossRef]
- Baluk, P.; Fuxe, J.; Hashizume, H.; Romano, T.; Lashnits, E.; Butz, S.; Vestweber, D.; Corada, M.; Molendini, C.; Dejana, E.; et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007, 204, 2349–2362. [Google Scholar] [CrossRef]
- Dougherty, P.J.; Davis, M.J.; Zawieja, D.C.; Muthuchamy, M. Calcium sensitivity and cooperativity of permeabilized rat mesenteric lymphatics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1524–R1532. [Google Scholar] [CrossRef] [PubMed]
- Bazigou, E.; Xie, S.; Chen, C.; Weston, A.; Miura, N.; Sorokin, L.; Adams, R.; Muro, A.F.; Sheppard, D.; Makinen, T. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev. Cell 2009, 17, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Norrmen, C.; Ivanov, K.I.; Cheng, J.; Zangger, N.; Delorenzi, M.; Jaquet, M.; Miura, N.; Puolakkainen, P.; Horsley, V.; Hu, J.; et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J. Cell Biol. 2009, 185, 439–457. [Google Scholar] [CrossRef]
- Davis, M.J.; Rahbar, E.; Gashev, A.A.; Zawieja, D.C.; Moore, J.E., Jr. Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H48–H60. [Google Scholar] [CrossRef] [PubMed]
- Muthuchamy, M.; Zawieja, D. Molecular regulation of lymphatic contractility. Ann. N. Y. Acad. Sci. 2008, 1131, 89–99. [Google Scholar] [CrossRef]
- Casley-Smith, J.R. The fine structure and functioning of tissue channels and lymphatics. Lymphology 1980, 13, 177–183. [Google Scholar]
- Breslin, J.W.; Yang, Y.; Scallan, J.P.; Sweat, R.S.; Adderley, S.P.; Murfee, W.L. Lymphatic Vessel Network Structure and Physiology. Compr. Physiol. 2018, 9, 207–299. [Google Scholar] [CrossRef]
- Randolph, G.J.; Ivanov, S.; Zinselmeyer, B.H.; Scallan, J.P. The Lymphatic System: Integral Roles in Immunity. Annu. Rev. Immunol. 2017, 35, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.A.; Hubbell, J.A.; Reddy, S.T. Lymphatic drainage function and its immunological implications: From dendritic cell homing to vaccine design. Semin. Immunol. 2008, 20, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Oliver, G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010, 24, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Harris, N.R.; Caron, K.M. Lymphatic Vasculature: An Emerging Therapeutic Target and Drug Delivery Route. Annu. Rev. Med. 2021, 72, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Loyola, A.; Petrova, T.V. Development and aging of the lymphatic vascular system. Adv. Drug Deliv. Rev. 2021, 169, 63–78. [Google Scholar] [CrossRef]
- Sleeman, J.; Schmid, A.; Thiele, W. Tumor lymphatics. Semin. Cancer Biol. 2009, 19, 285–297. [Google Scholar] [CrossRef]
- Wong, B.W.; Zecchin, A.; Garcia-Caballero, M.; Carmeliet, P. Emerging Concepts in Organ-Specific Lymphatic Vessels and Metabolic Regulation of Lymphatic Development. Dev. Cell 2018, 45, 289–301. [Google Scholar] [CrossRef]
- Petrova, T.V.; Koh, G.Y. Organ-specific lymphatic vasculature: From development to pathophysiology. J. Exp. Med. 2018, 215, 35–49. [Google Scholar] [CrossRef]
- Noris, M.; Morigi, M.; Donadelli, R.; Aiello, S.; Foppolo, M.; Todeschini, M.; Orisio, S.; Remuzzi, G.; Remuzzi, A. Nitric-Oxide Synthesis by Cultured Endothelial-Cells Is Modulated by Flow Conditions. Circ. Res. 1995, 76, 536–543. [Google Scholar] [CrossRef]
- Blackman, B.R.; Garcia-Cardena, G.; Gimbrone, M.A. A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J. Biomech Eng.-T Asme. 2002, 124, 397–407. [Google Scholar] [CrossRef]
- Yee, A.; Bosworth, K.A.; Conway, D.E.; Eskin, S.G.; McIntire, L.V. Gene expression of endothelial cells under pulsatile non-reversing vs. steady shear stress; comparison of nitric oxide production. Ann. Biomed. Eng. 2008, 36, 571–579. [Google Scholar] [CrossRef]
- Wang, C.; Baker, B.M.; Chen, C.S.; Schwartz, M.A. Endothelial Cell Sensing of Flow Direction. Arter. Throm Vas. 2013, 33, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Feaver, R.E.; Gelfand, B.D.; Blackman, B.R. Human haemodynamic frequency harmonics regulate the inflammatory phenotype of vascular endothelial cells. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Uzarski, J.S.; Scott, E.W.; McFetridge, P.S. Adaptation of endothelial cells to physiologically-modeled, variable shear stress. PLoS ONE 2013, 8, e57004. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, N.; Nicoli, S.; Coon, B.G.; Ross, T.D.; Van den Dries, K.; Han, J.; Lauridsen, H.M.; Mejean, C.O.; Eichmann, A.; Thomas, J.L.; et al. Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. eLife 2015, 4. [Google Scholar] [CrossRef]
- Dixon, J.B.; Greiner, S.T.; Gashev, A.A.; Cote, G.L.; Moore, J.E.; Zawieja, D.C. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 2006, 13, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; Zawieja, D.C.; Gashev, A.A.; Cote, G.L. Measuring microlymphatic flow using fast video microscopy. J. Biomed. Opt. 2005, 10, 064016. [Google Scholar] [CrossRef]
- Calnan, J.S.; Pflug, J.J.; Reis, N.D.; Taylor, L.M. Lymphatic pressures and the flow of lymph. Br. J. Plast. Surg. 1970, 23, 305–317. [Google Scholar] [CrossRef]
- Ballermann, B.J.; Dardik, A.; Eng, E.; Liu, A. Shear stress and the endothelium. Kidney Int. Suppl. 1998, 67, S100–S108. [Google Scholar] [CrossRef]
- Oyre, S.; Pedersen, E.M.; Ringgaard, S.; Boesiger, P.; Paaske, W.P. In vivo wall shear stress measured by magnetic resonance velocity mapping in the normal human abdominal aorta. Eur. J. Vasc. Endovasc. Surg. 1997, 13, 263–271. [Google Scholar] [CrossRef]
- Sun, D.; Huang, A.; Yan, E.H.; Wu, Z.; Yan, C.; Kaminski, P.M.; Oury, T.D.; Wolin, M.S.; Kaley, G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2249–H2256. [Google Scholar] [CrossRef]
- Crouch, A.C.; Cao, A.A.; Scheven, U.M.; Greve, J.M. In Vivo MRI Assessment of Blood Flow in Arteries and Veins from Head-to-Toe Across Age and Sex in C57BL/6 Mice. Ann. Biomed. Eng. 2020, 48, 329–341. [Google Scholar] [CrossRef]
- Santamaria, R.; Gonzalez-Alvarez, M.; Delgado, R.; Esteban, S.; Arroyo, A.G. Remodeling of the Microvasculature: May the Blood Flow Be With You. Front. Physiol. 2020, 11, 586852. [Google Scholar] [CrossRef]
- Hahn, C.; Schwartz, M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Y.S.; Chien, S. Shear stress-initiated signaling and its regulation of endothelial function. Arter. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef]
- Baeyens, N.; Schwartz, M.A. Biomechanics of vascular mechanosensation and remodeling. Mol. Biol. Cell 2016, 27, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, N. Fluid shear stress sensing in vascular homeostasis and remodeling: Towards the development of innovative pharmacological approaches to treat vascular dysfunction. Biochem. Pharm. 2018, 158, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Campinho, P.; Vilfan, A.; Vermot, J. Blood Flow Forces in Shaping the Vascular System: A Focus on Endothelial Cell Behavior. Front. Physiol. 2020, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, A.L.; Vignes, H.; Vermot, J.; Chow, R. Mechanotransduction in cardiovascular morphogenesis and tissue engineering. Curr. Opin. Genet. Dev. 2019, 57, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, N.; Bandyopadhyay, C.; Coon, B.G.; Yun, S.; Schwartz, M.A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Investig. 2016, 126, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Sabine, A.; Saygili Demir, C.; Petrova, T.V. Endothelial Cell Responses to Biomechanical Forces in Lymphatic Vessels. Antioxid Redox Signal 2016, 25, 451–465. [Google Scholar] [CrossRef]
- Ng, C.P.; Helm, C.L.; Swartz, M.A. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc. Res. 2004, 68, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Helm, C.L.; Zisch, A.; Swartz, M.A. Engineered blood and lymphatic capillaries in 3-D VEGF-fibrin-collagen matrices with interstitial flow. Biotechnol. Bioeng. 2007, 96, 167–176. [Google Scholar] [CrossRef]
- Wong, A.J.; Pollard, T.D.; Herman, I.M. Actin filament stress fibers in vascular endothelial cells in vivo. Science 1983, 219, 867–869. [Google Scholar] [CrossRef]
- Franke, R.P.; Grafe, M.; Schnittler, H.; Seiffge, D.; Mittermayer, C.; Drenckhahn, D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature 1984, 307, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Wechezak, A.R.; Viggers, R.F.; Sauvage, L.R. Fibronectin and F-actin redistribution in cultured endothelial cells exposed to shear stress. Lab. Investig. 1985, 53, 639–647. [Google Scholar]
- Wechezak, A.R.; Wight, T.N.; Viggers, R.F.; Sauvage, L.R. Endothelial adherence under shear stress is dependent upon microfilament reorganization. J. Cell Physiol. 1989, 139, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Kurihara, H.; Maemura, K.; Yoshizumi, M.; Nagai, R.; Yazaki, Y. Role of Ca2+ and protein kinase C in shear stress-induced actin depolymerization and endothelin 1 gene expression. Circ. Res. 1994, 75, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.M.; Izumo, S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J. Cell Sci. 1996, 109, 713–726. [Google Scholar] [PubMed]
- Davies, P.F.; Robotewskyj, A.; Griem, M.L. Quantitative studies of endothelial cell adhesion. Directional remodeling of focal adhesion sites in response to flow forces. J. Clin. Investig. 1994, 93, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Girard, P.R.; Nerem, R.M. Shear stress modulates endothelial cell morphology and F-actin organization through the regulation of focal adhesion-associated proteins. J. Cell Physiol. 1995, 163, 179–193. [Google Scholar] [CrossRef]
- Sabine, A.; Agalarov, Y.; Maby-El Hajjami, H.; Jaquet, M.; Hagerling, R.; Pollmann, C.; Bebber, D.; Pfenniger, A.; Miura, N.; Dormond, O.; et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev. Cell 2012, 22, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Betterman, K.L.; Sutton, D.L.; Secker, G.A.; Kazenwadel, J.; Oszmiana, A.; Lim, L.; Miura, N.; Sorokin, L.; Hogan, B.M.; Kahn, M.L.; et al. Atypical cadherin FAT4 orchestrates lymphatic endothelial cell polarity in response to flow. J. Clin. Investig. 2020, 130, 3315–3328. [Google Scholar] [CrossRef] [PubMed]
- Helm, C.L.; Fleury, M.E.; Zisch, A.H.; Boschetti, F.; Swartz, M.A. Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc. Natl. Acad. Sci. USA 2005, 102, 15779–15784. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chung, M.; Jeon, N.L. Three-dimensional biomimetic model to reconstitute sprouting lymphangiogenesis in vitro. Biomaterials 2016, 78, 115–128. [Google Scholar] [CrossRef]
- Sabin, F.R. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat. 1902, 1, 367–389. [Google Scholar] [CrossRef]
- Kampmeier, O.F. Evolution and Comparative Morphology of the Lymphatic System; Thomas: Springfield, IL, USA, 1969; p. 620. [Google Scholar]
- van der Putte, S.C. The early development of the lymphatic system in mouse embryos. Acta Morphol. Neerl Scand. 1975, 13, 245–286. [Google Scholar]
- Wigle, J.T.; Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 1999, 98, 769–778. [Google Scholar] [CrossRef]
- Wigle, J.T.; Harvey, N.; Detmar, M.; Lagutina, I.; Grosveld, G.; Gunn, M.D.; Jackson, D.G.; Oliver, G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002, 21, 1505–1513. [Google Scholar] [CrossRef]
- Petrova, T.V.; Makinen, T.; Makela, T.P.; Saarela, J.; Virtanen, I.; Ferrell, R.E.; Finegold, D.N.; Kerjaschki, D.; Yla-Herttuala, S.; Alitalo, K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002, 21, 4593–4599. [Google Scholar] [CrossRef]
- Hong, Y.K.; Harvey, N.; Noh, Y.H.; Schacht, V.; Hirakawa, S.; Detmar, M.; Oliver, G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 2002, 225, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.S.; Dillard, M.E.; Lagutin, O.V.; Lin, F.J.; Tsai, S.; Tsai, M.J.; Samokhvalov, I.M.; Oliver, G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007, 21, 2422–2432. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Garcia-Verdugo, J.M.; Soriano-Navarro, M.; Srinivasan, R.S.; Scallan, J.P.; Singh, M.K.; Epstein, J.A.; Oliver, G. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 2012, 120, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.; Short, K.; Secker, G.A.; Combes, A.; Schwarz, Q.; Davidson, T.L.; Smyth, I.; Hong, Y.K.; Harvey, N.L.; Koopman, P. Segmental territories along the cardinal veins generate lymph sacs via a ballooning mechanism during embryonic lymphangiogenesis in mice. Dev. Biol. 2012, 364, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Hagerling, R.; Pollmann, C.; Andreas, M.; Schmidt, C.; Nurmi, H.; Adams, R.H.; Alitalo, K.; Andresen, V.; Schulte-Merker, S.; Kiefer, F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 2013, 32, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Dumont, D.J.; Jussila, L.; Taipale, J.; Lymboussaki, A.; Mustonen, T.; Pajusola, K.; Breitman, M.; Alitalo, K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 1998, 282, 946–949. [Google Scholar] [CrossRef]
- Hamada, K.; Oike, Y.; Takakura, N.; Ito, Y.; Jussila, L.; Dumont, D.J.; Alitalo, K.; Suda, T. VEGF-C signaling pathways through VEGFR-2 and VEGFR-3 in vasculoangiogenesis and hematopoiesis. Blood 2000, 96, 3793–3800. [Google Scholar] [CrossRef]
- Kaipainen, A.; Korhonen, J.; Mustonen, T.; van Hinsbergh, V.W.; Fang, G.H.; Dumont, D.; Breitman, M.; Alitalo, K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA 1995, 92, 3566–3570. [Google Scholar] [CrossRef]
- Schacht, V.; Ramirez, M.I.; Hong, Y.K.; Hirakawa, S.; Feng, D.; Harvey, N.; Williams, M.; Dvorak, A.M.; Dvorak, H.F.; Oliver, G.; et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003, 22, 3546–3556. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Greenberg, J.M.; Akeson, A.L. NFATc1 regulates lymphatic endothelial development. Mech. Dev. 2009, 126, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.; Taddei, A.; Dierkes, C.; Martinez-Corral, I.; Fielden, M.; Ortsater, H.; Kazenwadel, J.; Calado, D.P.; Ostergaard, P.; Salminen, M.; et al. Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program. Nat. Commun. 2018, 9, 1511. [Google Scholar] [CrossRef]
- Lewis, F.T. The development of the lymphatic system in rabbits. Am. J. Anat. 1905, 5, 95–111. [Google Scholar] [CrossRef]
- Sabin, F.R. The lymphatic system in human embryos, with a consideration of the morphology of the system as a whole. Am. J. Anat. 1909, 9, 43–91. [Google Scholar] [CrossRef]
- van der Putte, S.C.; van Limborgh, J. The embryonic development of the main lymphatics in man. Acta Morphol. Neerl. Scand. 1980, 18, 323–335. [Google Scholar] [PubMed]
- Yaniv, K.; Isogai, S.; Castranova, D.; Dye, L.; Hitomi, J.; Weinstein, B.M. Live imaging of lymphatic development in the zebrafish. Nat. Med. 2006, 12, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, S.; Hong, Y.K.; Harvey, N.; Schacht, V.; Matsuda, K.; Libermann, T.; Detmar, M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am. J. Pathol. 2003, 162, 575–586. [Google Scholar] [CrossRef]
- Huntington, G.S.; McClure, C.F.W. The anatomy and development of the jugular lymph sacs in the domestic cat (Felis domestica). Am. J. Anat. 1910, 10, 177–312. [Google Scholar] [CrossRef]
- Wilting, J.; Papoutsi, M.; Othman-Hassan, K.; Rodriguez-Niedenfuhr, M.; Prols, F.; Tomarev, S.I.; Eichmann, A. Development of the avian lymphatic system. Microsc. Res. Tech. 2001, 55, 81–91. [Google Scholar] [CrossRef]
- Wilting, J.; Tomarev, S.I.; Christ, B.; Schweigerer, L. Lymphangioblasts in embryonic lymphangiogenesis. Lymphat Res. Biol. 2003, 1, 33–40. [Google Scholar] [CrossRef]
- Wilting, J.; Aref, Y.; Huang, R.; Tomarev, S.I.; Schweigerer, L.; Christ, B.; Valasek, P.; Papoutsi, M. Dual origin of avian lymphatics. Dev. Biol. 2006, 292, 165–173. [Google Scholar] [CrossRef]
- Ny, A.; Koch, M.; Schneider, M.; Neven, E.; Tong, R.T.; Maity, S.; Fischer, C.; Plaisance, S.; Lambrechts, D.; Heligon, C.; et al. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat. Med. 2005, 11, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Othman-Hassan, K.; Christ, B.; Wilting, J. Lymphangioblasts in the avian wing bud. Dev. Dyn. 1999, 216, 311–319. [Google Scholar] [CrossRef]
- Buttler, K.; Kreysing, A.; von Kaisenberg, C.S.; Schweigerer, L.; Gale, N.; Papoutsi, M.; Wilting, J. Mesenchymal cells with leukocyte and lymphendothelial characteristics in murine embryos. Dev. Dyn. 2006, 235, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Buttler, K.; Ezaki, T.; Wilting, J. Proliferating mesodermal cells in murine embryos exhibiting macrophage and lymphendothelial characteristics. BMC Dev. Biol. 2008, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Stanczuk, L.; Martinez-Corral, I.; Ulvmar, M.H.; Zhang, Y.; Lavina, B.; Fruttiger, M.; Adams, R.H.; Saur, D.; Betsholtz, C.; Ortega, S.; et al. cKit Lineage Hemogenic Endothelium-Derived Cells Contribute to Mesenteric Lymphatic Vessels. Cell Rep. 2015, 10, 1708–1721. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Corral, I.; Ulvmar, M.H.; Stanczuk, L.; Tatin, F.; Kizhatil, K.; John, S.W.; Alitalo, K.; Ortega, S.; Makinen, T. Nonvenous origin of dermal lymphatic vasculature. Circ. Res. 2015, 116, 1649–1654. [Google Scholar] [CrossRef]
- Klotz, L.; Norman, S.; Vieira, J.M.; Masters, M.; Rohling, M.; Dube, K.N.; Bollini, S.; Matsuzaki, F.; Carr, C.A.; Riley, P.R. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 2015, 522, 62–67. [Google Scholar] [CrossRef]
- Maruyama, K.; Miyagawa-Tomita, S.; Mizukami, K.; Matsuzaki, F.; Kurihara, H. Isl1-expressing non-venous cell lineage contributes to cardiac lymphatic vessel development. Dev. Biol. 2019, 452, 134–143. [Google Scholar] [CrossRef]
- Lioux, G.; Liu, X.; Temino, S.; Oxendine, M.; Ayala, E.; Ortega, S.; Kelly, R.G.; Oliver, G.; Torres, M. A Second Heart Field-Derived Vasculogenic Niche Contributes to Cardiac Lymphatics. Dev. Cell 2020, 52, 350–363. [Google Scholar] [CrossRef]
- Semo, J.; Nicenboim, J.; Yaniv, K. Development of the lymphatic system: New questions and paradigms. Development 2016, 143, 924–935. [Google Scholar] [CrossRef]
- Gancz, D.; Perlmoter, G.; Yaniv, K. Formation and Growth of Cardiac Lymphatics during Embryonic Development, Heart Regeneration, and Disease. Cold Spring Harb. Perspect. Biol. 2020, 12. [Google Scholar] [CrossRef]
- Planas-Paz, L.; Strilic, B.; Goedecke, A.; Breier, G.; Fassler, R.; Lammert, E. Mechanoinduction of lymph vessel expansion. EMBO J. 2012, 31, 788–804. [Google Scholar] [CrossRef]
- Urner, S.; Planas-Paz, L.; Hilger, L.S.; Henning, C.; Branopolski, A.; Kelly-Goss, M.; Stanczuk, L.; Pitter, B.; Montanez, E.; Peirce, S.M.; et al. Identification of ILK as a critical regulator of VEGFR3 signalling and lymphatic vascular growth. EMBO J. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.; Conley, K.A.; Raehl, A.; Bondy, D.M.; Pytowski, B.; Swartz, M.A.; Rutkowski, J.M.; Jaroch, D.B.; Ongstad, E.L. Regulation of lymphatic capillary regeneration by interstitial flow in skin. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2176–H2183. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.M.; Boardman, K.C.; Swartz, M.A. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1402–H1410. [Google Scholar] [CrossRef]
- Boardman, K.C.; Swartz, M.A. Interstitial flow as a guide for lymphangiogenesis. Circ. Res. 2003, 92, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Makinen, T.; Adams, R.H.; Bailey, J.; Lu, Q.; Ziemiecki, A.; Alitalo, K.; Klein, R.; Wilkinson, G.A. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005, 19, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, Y.; Mae, M.A.; Zhang, Y.; Ortsater, H.; Betsholtz, C.; Makinen, T.; Jakobsson, L. Smooth muscle cell recruitment to lymphatic vessels requires PDGFB and impacts vessel size but not identity. Development 2017, 144, 3590–3601. [Google Scholar] [CrossRef]
- Szotak-Ajtay, K.; Szoke, D.; Kovacs, G.; Andreka, J.; Brenner, G.B.; Giricz, Z.; Penninger, J.; Kahn, M.L.; Jakus, Z. Reduced Prenatal Pulmonary Lymphatic Function Is Observed in Clp1 (K/K) Embryos With Impaired Motor Functions Including Fetal Breathing Movements in Preparation of the Developing Lung for Inflation at Birth. Front Bioeng. Biotechnol. 2020, 8, 136. [Google Scholar] [CrossRef]
- Martin-Almedina, S.; Martinez-Corral, I.; Holdhus, R.; Vicente, A.; Fotiou, E.; Lin, S.; Petersen, K.; Simpson, M.A.; Hoischen, A.; Gilissen, C.; et al. EPHB4 kinase-inactivating mutations cause autosomal dominant lymphatic-related hydrops fetalis. J. Clin. Investig. 2016, 126, 3080–3088. [Google Scholar] [CrossRef]
- Dellinger, M.; Hunter, R.; Bernas, M.; Gale, N.; Yancopoulos, G.; Erickson, R.; Witte, M. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev. Biol. 2008, 319, 309–320. [Google Scholar] [CrossRef]
- Kanady, J.D.; Dellinger, M.T.; Munger, S.J.; Witte, M.H.; Simon, A.M. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev. Biol. 2011, 354, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Levet, S.; Ciais, D.; Merdzhanova, G.; Mallet, C.; Zimmers, T.A.; Lee, S.J.; Navarro, F.P.; Texier, I.; Feige, J.J.; Bailly, S.; et al. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood 2013, 122, 598–607. [Google Scholar] [CrossRef]
- Cha, B.; Geng, X.; Mahamud, M.R.; Fu, J.; Mukherjee, A.; Kim, Y.; Jho, E.H.; Kim, T.H.; Kahn, M.L.; Xia, L.; et al. Mechanotransduction activates canonical Wnt/beta-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves. Genes Dev. 2016, 30, 1454–1469. [Google Scholar] [CrossRef] [PubMed]
- Lapinski, P.E.; Lubeck, B.A.; Chen, D.; Doosti, A.; Zawieja, S.D.; Davis, M.J.; King, P.D. RASA1 regulates the function of lymphatic vessel valves in mice. J. Clin. Investig. 2017, 127, 2569–2585. [Google Scholar] [CrossRef]
- Liu, X.; Pasula, S.; Song, H.; Tessneer, K.L.; Dong, Y.; Hahn, S.; Yago, T.; Brophy, M.L.; Chang, B.; Cai, X.; et al. Temporal and spatial regulation of epsin abundance and VEGFR3 signaling are required for lymphatic valve formation and function. Sci. Signal 2014, 7, ra97. [Google Scholar] [CrossRef]
- Nurmi, H.; Saharinen, P.; Zarkada, G.; Zheng, W.; Robciuc, M.R.; Alitalo, K. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol. Med. 2015, 7, 1418–1425. [Google Scholar] [CrossRef]
- Pujol, F.; Hodgson, T.; Martinez-Corral, I.; Prats, A.C.; Devenport, D.; Takeichi, M.; Genot, E.; Makinen, T.; Francis-West, P.; Garmy-Susini, B.; et al. Dachsous1-Fat4 Signaling Controls Endothelial Cell Polarization During Lymphatic Valve Morphogenesis-Brief Report. Arter. Thromb. Vasc. Biol. 2017, 37, 1732–1735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Baeyens, N.; Corti, F.; Tanaka, K.; Fang, J.S.; Zhang, J.; Jin, Y.; Coon, B.; Hirschi, K.K.; Schwartz, M.A.; et al. Syndecan 4 controls lymphatic vasculature remodeling during mouse embryonic development. Development 2016, 143, 4441–4451. [Google Scholar] [CrossRef]
- Yang, Y.; Cha, B.; Motawe, Z.Y.; Srinivasan, R.S.; Scallan, J.P. VE-Cadherin Is Required for Lymphatic Valve Formation and Maintenance. Cell Rep. 2019, 28, 2397–2412. [Google Scholar] [CrossRef] [PubMed]
- Bouvree, K.; Brunet, I.; Del Toro, R.; Gordon, E.; Prahst, C.; Cristofaro, B.; Mathivet, T.; Xu, Y.; Soueid, J.; Fortuna, V.; et al. Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation. Circ. Res. 2012, 111, 437–445. [Google Scholar] [CrossRef]
- Jurisic, G.; Maby-El Hajjami, H.; Karaman, S.; Ochsenbein, A.M.; Alitalo, A.; Siddiqui, S.S.; Ochoa Pereira, C.; Petrova, T.V.; Detmar, M. An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ Res. 2012, 111, 426–436. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Ahn, J.H.; Hong, Y.K.; Makinen, T.; Lim, D.S.; Koh, G.Y. YAP and TAZ Negatively Regulate Prox1 During Developmental and Pathologic Lymphangiogenesis. Circ. Res. 2019, 124, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.; Ho, Y.C.; Geng, X.; Mahamud, M.R.; Chen, L.; Kim, Y.; Choi, D.; Kim, T.H.; Randolph, G.J.; Cao, X.; et al. YAP and TAZ maintain PROX1 expression in the developing lymphatic and lymphovenous valves in response to VEGF-C signaling. Development 2020, 147. [Google Scholar] [CrossRef] [PubMed]
- Kazenwadel, J.; Secker, G.A.; Liu, Y.J.; Rosenfeld, J.A.; Wildin, R.S.; Cuellar-Rodriguez, J.; Hsu, A.P.; Dyack, S.; Fernandez, C.V.; Chong, C.E.; et al. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood 2012, 119, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Petrova, T.V.; Karpanen, T.; Norrmen, C.; Mellor, R.; Tamakoshi, T.; Finegold, D.; Ferrell, R.; Kerjaschki, D.; Mortimer, P.; Yla-Herttuala, S.; et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat. Med. 2004, 10, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Sabine, A.; Bovay, E.; Demir, C.S.; Kimura, W.; Jaquet, M.; Agalarov, Y.; Zangger, N.; Scallan, J.P.; Graber, W.; Gulpinar, E.; et al. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J. Clin. Investig. 2015, 125, 3861–3877. [Google Scholar] [CrossRef]
- Kazenwadel, J.; Betterman, K.L.; Chong, C.E.; Stokes, P.H.; Lee, Y.K.; Secker, G.A.; Agalarov, Y.; Demir, C.S.; Lawrence, D.M.; Sutton, D.L.; et al. GATA2 is required for lymphatic vessel valve development and maintenance. J. Clin. Investig. 2015, 125, 2979–2994. [Google Scholar] [CrossRef]
- Johnson, N.C.; Dillard, M.E.; Baluk, P.; McDonald, D.M.; Harvey, N.L.; Frase, S.L.; Oliver, G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008, 22, 3282–3291. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.S.; Oliver, G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev. 2011, 25, 2187–2197. [Google Scholar] [CrossRef]
- Geng, X.; Cha, B.; Mahamud, M.R.; Lim, K.C.; Silasi-Mansat, R.; Uddin, M.K.M.; Miura, N.; Xia, L.; Simon, A.M.; Engel, J.D.; et al. Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development. Dev. Biol. 2016, 409, 218–233. [Google Scholar] [CrossRef]
- Mahamud, M.R.; Geng, X.; Ho, Y.C.; Cha, B.; Kim, Y.; Ma, J.; Chen, L.; Myers, G.; Camper, S.; Mustacich, D.; et al. GATA2 controls lymphatic endothelial cell junctional integrity and lymphovenous valve morphogenesis through miR-126. Development 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Inoue, K.; Fuller, G.L.; Garcia, A.; Eble, J.A.; Pohlmann, S.; Inoue, O.; Gartner, T.K.; Hughan, S.C.; Pearce, A.C.; Laing, G.D.; et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood 2006, 107, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, C.C.; Schmaier, A.A.; Mericko, P.; Hess, P.R.; Zou, Z.; Chen, M.; Chen, C.Y.; Xu, B.; Lu, M.M.; Zhou, D.; et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 2010, 116, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Inoue, K.; Kato, Y.; Inoue, O.; Kaneko, M.K.; Mishima, K.; Yatomi, Y.; Yamazaki, Y.; Narimatsu, H.; Ozaki, Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J. Biol. Chem. 2007, 282, 25993–26001. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Suzuki-Inoue, K.; Inoue, O. Novel interactions in platelet biology: CLEC-2/podoplanin and laminin/GPVI. J. Thromb. Haemost. 2009, 7, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Uhrin, P.; Zaujec, J.; Breuss, J.M.; Olcaydu, D.; Chrenek, P.; Stockinger, H.; Fuertbauer, E.; Moser, M.; Haiko, P.; Fassler, R.; et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood 2010, 115, 3997–4005. [Google Scholar] [CrossRef]
- Turner, M.; Mee, P.J.; Costello, P.S.; Williams, O.; Price, A.A.; Duddy, L.P.; Furlong, M.T.; Geahlen, R.L.; Tybulewicz, V.L. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature 1995, 378, 298–302. [Google Scholar] [CrossRef]
- Cheng, A.M.; Rowley, B.; Pao, W.; Hayday, A.; Bolen, J.B.; Pawson, T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature 1995, 378, 303–306. [Google Scholar] [CrossRef]
- Wang, D.; Feng, J.; Wen, R.; Marine, J.C.; Sangster, M.Y.; Parganas, E.; Hoffmeyer, A.; Jackson, C.W.; Cleveland, J.L.; Murray, P.J.; et al. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity 2000, 13, 25–35. [Google Scholar] [CrossRef]
- Abtahian, F.; Guerriero, A.; Sebzda, E.; Lu, M.M.; Zhou, R.; Mocsai, A.; Myers, E.E.; Huang, B.; Jackson, D.G.; Ferrari, V.A.; et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science 2003, 299, 247–251. [Google Scholar] [CrossRef]
- Sebzda, E.; Hibbard, C.; Sweeney, S.; Abtahian, F.; Bezman, N.; Clemens, G.; Maltzman, J.S.; Cheng, L.; Liu, F.; Turner, M.; et al. Syk and Slp-76 mutant mice reveal a cell-autonomous hematopoietic cell contribution to vascular development. Dev. Cell 2006, 11, 349–361. [Google Scholar] [CrossRef]
- Fu, J.; Gerhardt, H.; McDaniel, J.M.; Xia, B.; Liu, X.; Ivanciu, L.; Ny, A.; Hermans, K.; Silasi-Mansat, R.; McGee, S.; et al. Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J. Clin. Investig. 2008, 118, 3725–3737. [Google Scholar] [CrossRef]
- Ichise, H.; Ichise, T.; Ohtani, O.; Yoshida, N. Phospholipase Cgamma2 is necessary for separation of blood and lymphatic vasculature in mice. Development 2009, 136, 191–195. [Google Scholar] [CrossRef]
- Suzuki-Inoue, K.; Inoue, O.; Ding, G.; Nishimura, S.; Hokamura, K.; Eto, K.; Kashiwagi, H.; Tomiyama, Y.; Yatomi, Y.; Umemura, K.; et al. Essential in vivo roles of the C-type lectin receptor CLEC-2: Embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J. Biol. Chem. 2010, 285, 24494–24507. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.R.; Rawnsley, D.R.; Jakus, Z.; Yang, Y.; Sweet, D.T.; Fu, J.; Herzog, B.; Lu, M.; Nieswandt, B.; Oliver, G.; et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J. Clin. Investig. 2014, 124, 273–284. [Google Scholar] [CrossRef]
- Bianchi, R.; Russo, E.; Bachmann, S.B.; Proulx, S.T.; Sesartic, M.; Smaadahl, N.; Watson, S.P.; Buckley, C.D.; Halin, C.; Detmar, M. Postnatal Deletion of Podoplanin in Lymphatic Endothelium Results in Blood Filling of the Lymphatic System and Impairs Dendritic Cell Migration to Lymph Nodes. Arter. Thromb. Vasc. Biol. 2017, 37, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.D.; Kahn, M.L.; Sweet, D.T. Lymphovenous hemostasis and the role of platelets in regulating lymphatic flow and lymphatic vessel maturation. Blood 2016, 128, 1169–1173. [Google Scholar] [CrossRef]
- Chen, C.Y.; Bertozzi, C.; Zou, Z.; Yuan, L.; Lee, J.S.; Lu, M.; Stachelek, S.J.; Srinivasan, S.; Guo, L.; Vicente, A.; et al. Blood flow reprograms lymphatic vessels to blood vessels. J. Clin. Investig. 2012, 122, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.T.; Jimenez, J.M.; Chang, J.; Hess, P.R.; Mericko-Ishizuka, P.; Fu, J.; Xia, L.; Davies, P.F.; Kahn, M.L. Lymph flow regulates collecting lymphatic vessel maturation in vivo. J. Clin. Investig. 2015, 125, 2995–3007. [Google Scholar] [CrossRef]
- Reed, H.O.; Wang, L.; Sonett, J.; Chen, M.; Yang, J.; Li, L.; Aradi, P.; Jakus, Z.; D’Armiento, J.; Hancock, W.W.; et al. Lymphatic impairment leads to pulmonary tertiary lymphoid organ formation and alveolar damage. J. Clin. Investig. 2019, 129, 2514–2526. [Google Scholar] [CrossRef]
- Balint, L.; Ocskay, Z.; Deak, B.A.; Aradi, P.; Jakus, Z. Lymph Flow Induces the Postnatal Formation of Mature and Functional Meningeal Lymphatic Vessels. Front. Immunol. 2019, 10, 3043. [Google Scholar] [CrossRef]
- Cha, B.; Geng, X.; Mahamud, M.R.; Zhang, J.Y.; Chen, L.; Kim, W.; Jho, E.H.; Kim, Y.; Choi, D.; Dixon, J.B.; et al. Complementary Wnt Sources Regulate Lymphatic Vascular Development via PROX1-Dependent Wnt/beta-Catenin Signaling. Cell Rep. 2018, 25, 571–584. [Google Scholar] [CrossRef]
- Karino, T.; Goldsmith, H.L. Particle flow behavior in models of branching vessels. II. Effects of branching angle and diameter ratio on flow patterns. Biorheology 1985, 22, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Karino, T.; Goldsmith, H.L.; Motomiya, M.; Mabuchi, S.; Sohara, Y. Flow patterns in vessels of simple and complex geometries. Ann. N. Y. Acad. Sci. 1987, 516, 422–441. [Google Scholar] [CrossRef]
- Baeyens, N.; Mulligan-Kehoe, M.J.; Corti, F.; Simon, D.D.; Ross, T.D.; Rhodes, J.M.; Wang, T.Z.; Mejean, C.O.; Simons, M.; Humphrey, J.; et al. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 17308–17313. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, K.; Lukacs, V.; Sweet, D.T.; Goddard, L.M.; Kanie, A.; Whitwam, T.; Ranade, S.S.; Fujimori, T.; Kahn, M.L.; Patapoutian, A. Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc. Natl. Acad. Sci. USA 2018, 115, 12817–12822. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Park, E.; Jung, E.; Cha, B.; Lee, S.; Yu, J.; Kim, P.M.; Lee, S.; Hong, Y.J.; Koh, C.J.; et al. Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, V.; Mathur, J.; Mao, R.; Bayrak-Toydemir, P.; Procter, M.; Cahalan, S.M.; Kim, H.J.; Bandell, M.; Longo, N.; Day, R.W.; et al. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat. Commun. 2015, 6, 8329. [Google Scholar] [CrossRef] [PubMed]
- Fotiou, E.; Martin-Almedina, S.; Simpson, M.A.; Lin, S.; Gordon, K.; Brice, G.; Atton, G.; Jeffery, I.; Rees, D.C.; Mignot, C.; et al. Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non-immune hydrops fetalis. Nat. Commun. 2015, 6, 8085. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef]
- Ge, J.; Li, W.; Zhao, Q.; Li, N.; Chen, M.; Zhi, P.; Li, R.; Gao, N.; Xiao, B.; Yang, M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 2015, 527, 64–69. [Google Scholar] [CrossRef]
- Gottlieb, P.A.; Bae, C.; Sachs, F. Gating the mechanical channel Piezo1: A comparison between whole-cell and patch recording. Channels (Austin) 2012, 6, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Geiger, R.V.; Berk, B.C.; Alexander, R.W.; Nerem, R.M. Flow-induced calcium transients in single endothelial cells: Spatial and temporal analysis. Am. J. Physiol. 1992, 262, C1411–C1417. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G.; Droogmans, G.; Nilius, B. Shear stress induced membrane currents and calcium transients in human vascular endothelial cells. Pflugers Arch. 1992, 421, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 integration of vascular architecture with physiological force. Nature 2014, 515, 279–282. [Google Scholar] [CrossRef]
- Ranade, S.S.; Qiu, Z.; Woo, S.H.; Hur, S.S.; Murthy, S.E.; Cahalan, S.M.; Xu, J.; Mathur, J.; Bandell, M.; Coste, B.; et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10347–10352. [Google Scholar] [CrossRef]
- Hyman, A.J.; Tumova, S.; Beech, D.J. Piezo1 Channels in Vascular Development and the Sensing of Shear Stress. Curr. Top Membr. 2017, 79, 37–57. [Google Scholar] [CrossRef]
- Choi, D.; Park, E.; Jung, E.; Seong, Y.J.; Yoo, J.; Lee, E.; Hong, M.; Lee, S.; Ishida, H.; Burford, J.; et al. Laminar flow downregulates Notch activity to promote lymphatic sprouting. J. Clin. Investig. 2017, 127, 1225–1240. [Google Scholar] [CrossRef]
- Choi, D.; Park, E.; Jung, E.; Seong, Y.J.; Hong, M.; Lee, S.; Burford, J.; Gyarmati, G.; Peti-Peterdi, J.; Srikanth, S.; et al. ORAI1 Activates Proliferation of Lymphatic Endothelial Cells in Response to Laminar Flow Through Kruppel-Like Factors 2 and 4. Circ. Res. 2017, 120, 1426–1439. [Google Scholar] [CrossRef]
- Geng, X.; Yanagida, K.; Akwii, R.G.; Choi, D.; Chen, L.; Ho, Y.; Cha, B.; Mahamud, M.R.; Berman de Ruiz, K.; Ichise, H.; et al. S1PR1 regulates the quiescence of lymphatic vessels by inhibiting laminar shear stress-dependent VEGF-C signaling. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Si, H.; Wang, J.; Meininger, C.J.; Peng, X.; Zawieja, D.C.; Zhang, S.L. Ca(2+) release-activated Ca(2+) channels are responsible for histamine-induced Ca(2+) entry, permeability increase, and interleukin synthesis in lymphatic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1283–H1295. [Google Scholar] [CrossRef]
- Coon, B.G.; Baeyens, N.; Han, J.; Budatha, M.; Ross, T.D.; Fang, J.S.; Yun, S.; Thomas, J.L.; Schwartz, M.A. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J. Cell Biol. 2015, 208, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; DeLisser, H.; Schwartz, M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.; Fisslthaler, B.; Dixit, M.; Busse, R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J. Cell Sci. 2005, 118, 4103–4111. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tzima, E. PECAM-1 is necessary for flow-induced vascular remodeling. Arter. Thromb. Vasc. Biol. 2009, 29, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Guilluy, C.; Welch, C.; O’Brien, E.T.; Hahn, K.; Superfine, R.; Burridge, K.; Tzima, E. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr. Biol. 2012, 22, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.E.; Breckenridge, M.T.; Hinde, E.; Gratton, E.; Chen, C.S.; Schwartz, M.A. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol. 2013, 23, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.E.; Schwartz, M.A. Mechanotransduction of shear stress occurs through changes in VE-cadherin and PECAM-1 tension: Implications for cell migration. Cell Adh. Migr. 2015, 9, 335–339. [Google Scholar] [CrossRef]
- Chen, Z.; Rubin, J.; Tzima, E. Role of PECAM-1 in arteriogenesis and specification of preexisting collaterals. Circ. Res. 2010, 107, 1355–1363. [Google Scholar] [CrossRef]
- Bagi, Z.; Frangos, J.A.; Yeh, J.C.; White, C.R.; Kaley, G.; Koller, A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arter. Thromb. Vasc. Biol. 2005, 25, 1590–1595. [Google Scholar] [CrossRef]
- Miao, H.; Hu, Y.L.; Shiu, Y.T.; Yuan, S.; Zhao, Y.; Kaunas, R.; Wang, Y.; Jin, G.; Usami, S.; Chien, S. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: In vivo and in vitro investigations. J. Vasc. Res. 2005, 42, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Fulton, D.; Gratton, J.P.; McCabe, T.J.; Fontana, J.; Fujio, Y.; Walsh, K.; Franke, T.F.; Papapetropoulos, A.; Sessa, W.C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 1999, 399, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C.; Hwang, J.; Sykes, M.; Michell, B.J.; Kemp, B.E.; Lum, H.; Jo, H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1819–H1828. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.G.; Ueba, H.; Tanimoto, T.; Lungu, A.O.; Frame, M.D.; Berk, B.C. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ. Res. 2003, 93, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Kadohama, T.; Nishimura, K.; Hoshino, Y.; Sasajima, T.; Sumpio, B.E. Effects of different types of fluid shear stress on endothelial cell proliferation and survival. J. Cell Physiol. 2007, 212, 244–251. [Google Scholar] [CrossRef]
- Deng, Y.; Larrivee, B.; Zhuang, Z.W.; Atri, D.; Moraes, F.; Prahst, C.; Eichmann, A.; Simons, M. Endothelial RAF1/ERK activation regulates arterial morphogenesis. Blood 2013, 121, 3988–3996. [Google Scholar] [CrossRef]
- Angulo-Urarte, A.; Casado, P.; Castillo, S.D.; Kobialka, P.; Kotini, M.P.; Figueiredo, A.M.; Castel, P.; Rajeeve, V.; Mila-Guasch, M.; Millan, J.; et al. Endothelial cell rearrangements during vascular patterning require PI3-kinase-mediated inhibition of actomyosin contractility. Nat. Commun. 2018, 9, 4826. [Google Scholar] [CrossRef]
- Saaristo, A.; Veikkola, T.; Tammela, T.; Enholm, B.; Karkkainen, M.J.; Pajusola, K.; Bueler, H.; Yla-Herttuala, S.; Alitalo, K. Lymphangiogenic gene therapy with minimal blood vascular side effects. J. Exp. Med. 2002, 196, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, M.T.; Meadows, S.M.; Wynne, K.; Cleaver, O.; Brekken, R.A. Vascular endothelial growth factor receptor-2 promotes the development of the lymphatic vasculature. PLoS ONE 2013, 8, e74686. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.; Rutkowski, J.M.; Shields, J.D.; Pasquier, M.C.; Cui, Y.; Schmokel, H.G.; Willey, S.; Hicklin, D.J.; Pytowski, B.; Swartz, M.A. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J. 2007, 21, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Gebala, V.; Collins, R.; Geudens, I.; Phng, L.K.; Gerhardt, H. Blood flow drives lumen formation by inverse membrane blebbing during angiogenesis in vivo. Nat. Cell Biol. 2016, 18, 443–450. [Google Scholar] [CrossRef]
- Lucitti, J.L.; Jones, E.A.; Huang, C.; Chen, J.; Fraser, S.E.; Dickinson, M.E. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 2007, 134, 3317–3326. [Google Scholar] [CrossRef]
- Franco, C.A.; Jones, M.L.; Bernabeu, M.O.; Geudens, I.; Mathivet, T.; Rosa, A.; Lopes, F.M.; Lima, A.P.; Ragab, A.; Collins, R.T.; et al. Dynamic endothelial cell rearrangements drive developmental vessel regression. PLoS Biol. 2015, 13, e1002125. [Google Scholar] [CrossRef]
- Mattsson, E.J.; Kohler, T.R.; Vergel, S.M.; Clowes, A.W. Increased blood flow induces regression of intimal hyperplasia. Arter. Thromb Vasc. Biol. 1997, 17, 2245–2249. [Google Scholar] [CrossRef]
- le Noble, F.; Moyon, D.; Pardanaud, L.; Yuan, L.; Djonov, V.; Matthijsen, R.; Breant, C.; Fleury, V.; Eichmann, A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development 2004, 131, 361–375. [Google Scholar] [CrossRef]
- Kizhatil, K.; Ryan, M.; Marchant, J.K.; Henrich, S.; John, S.W. Schlemm’s canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol. 2014, 12, e1001912. [Google Scholar] [CrossRef]
- Aspelund, A.; Tammela, T.; Antila, S.; Nurmi, H.; Leppanen, V.M.; Zarkada, G.; Stanczuk, L.; Francois, M.; Makinen, T.; Saharinen, P.; et al. The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J. Clin. Investig. 2014, 124, 3975–3986. [Google Scholar] [CrossRef]
- Park, D.Y.; Lee, J.; Park, I.; Choi, D.; Lee, S.; Song, S.; Hwang, Y.; Hong, K.Y.; Nakaoka, Y.; Makinen, T.; et al. Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. J. Clin. Investig. 2014, 124, 3960–3974. [Google Scholar] [CrossRef]
- Kenig-Kozlovsky, Y.; Scott, R.P.; Onay, T.; Carota, I.A.; Thomson, B.R.; Gil, H.J.; Ramirez, V.; Yamaguchi, S.; Tanna, C.E.; Heinen, S.; et al. Ascending Vasa Recta Are Angiopoietin/Tie2-Dependent Lymphatic-Like Vessels. J. Am. Soc. Nephrol. 2018, 29, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.B.; Balint, L.; Lim, L.; Ma, W.; Davis, R.B.; Benyo, Z.; Soares, M.J.; Oliver, G.; Kahn, M.L.; Jakus, Z.; et al. Lymphatic mimicry in maternal endothelial cells promotes placental spiral artery remodeling. J. Clin. Investig. 2019. [Google Scholar] [CrossRef] [PubMed]
- You, L.R.; Lin, F.J.; Lee, C.T.; DeMayo, F.J.; Tsai, M.J.; Tsai, S.Y. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 2005, 435, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Yoshimatsu, Y.; Morishita, Y.; Miyazono, K.; Watabe, T. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes Cells 2009, 14, 425–434. [Google Scholar] [CrossRef]
- Park, Y.G.; Choi, J.; Jung, H.K.; Song, I.K.; Shin, Y.; Park, S.Y.; Seol, J.W. Fluid shear stress regulates vascular remodeling via VEGFR-3 activation, although independently of its ligand, VEGF-C, in the uterus during pregnancy. Int. J. Mol. Med. 2017, 40, 1210–1216. [Google Scholar] [CrossRef][Green Version]
- Hahn, C.N.; Chong, C.E.; Carmichael, C.L.; Wilkins, E.J.; Brautigan, P.J.; Li, X.C.; Babic, M.; Lin, M.; Carmagnac, A.; Lee, Y.K.; et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 2011, 43, 1012–1017. [Google Scholar] [CrossRef]
- Ostergaard, P.; Simpson, M.A.; Connell, F.C.; Steward, C.G.; Brice, G.; Woollard, W.J.; Dafou, D.; Kilo, T.; Smithson, S.; Lunt, P.; et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat. Genet. 2011, 43, 929–931. [Google Scholar] [CrossRef]
- Brice, G.; Mansour, S.; Bell, R.; Collin, J.R.; Child, A.H.; Brady, A.F.; Sarfarazi, M.; Burnand, K.G.; Jeffery, S.; Mortimer, P.; et al. Analysis of the phenotypic abnormalities in lymphoedema-distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. J. Med. Genet. 2002, 39, 478–483. [Google Scholar] [CrossRef]
- Fang, J.; Dagenais, S.L.; Erickson, R.P.; Arlt, M.F.; Glynn, M.W.; Gorski, J.L.; Seaver, L.H.; Glover, T.W. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am. J. Hum. Genet. 2000, 67, 1382–1388. [Google Scholar] [CrossRef]
- van Steensel, M.A.; Damstra, R.J.; Heitink, M.V.; Bladergroen, R.S.; Veraart, J.; Steijlen, P.M.; van Geel, M. Novel missense mutations in the FOXC2 gene alter transcriptional activity. Hum. Mutat. 2009, 30, E1002–E1009. [Google Scholar] [CrossRef] [PubMed]
- Alders, M.; Al-Gazali, L.; Cordeiro, I.; Dallapiccola, B.; Garavelli, L.; Tuysuz, B.; Salehi, F.; Haagmans, M.A.; Mook, O.R.; Majoie, C.B.; et al. Hennekam syndrome can be caused by FAT4 mutations and be allelic to Van Maldergem syndrome. Hum. Genet. 2014, 133, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.C.; Liu, C.S.; Chang, S.P.; Yeh, K.T.; Ke, Y.Y.; Chen, T.H.; Wang, B.B.; Kuo, S.J.; Shih, J.C.; Chen, M. A recurrent ITGA9 missense mutation in human fetuses with severe chylothorax: Possible correlation with poor response to fetal therapy. Prenat. Diagn. 2008, 28, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Irrthum, A.; Karkkainen, M.J.; Devriendt, K.; Alitalo, K.; Vikkula, M. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am. J. Hum. Genet. 2000, 67, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, R.E.; Levinson, K.L.; Esman, J.H.; Kimak, M.A.; Lawrence, E.C.; Barmada, M.M.; Finegold, D.N. Hereditary lymphedema: Evidence for linkage and genetic heterogeneity. Hum. Mol. Genet. 1998, 7, 2073–2078. [Google Scholar] [CrossRef]
- Butler, M.G.; Dagenais, S.L.; Rockson, S.G.; Glover, T.W. A novel VEGFR3 mutation causes Milroy disease. Am. J. Med. Genet A 2007, 143A, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).