Molecular Imaging of Apoptosis: The Case of Caspase-3 Radiotracers
Abstract
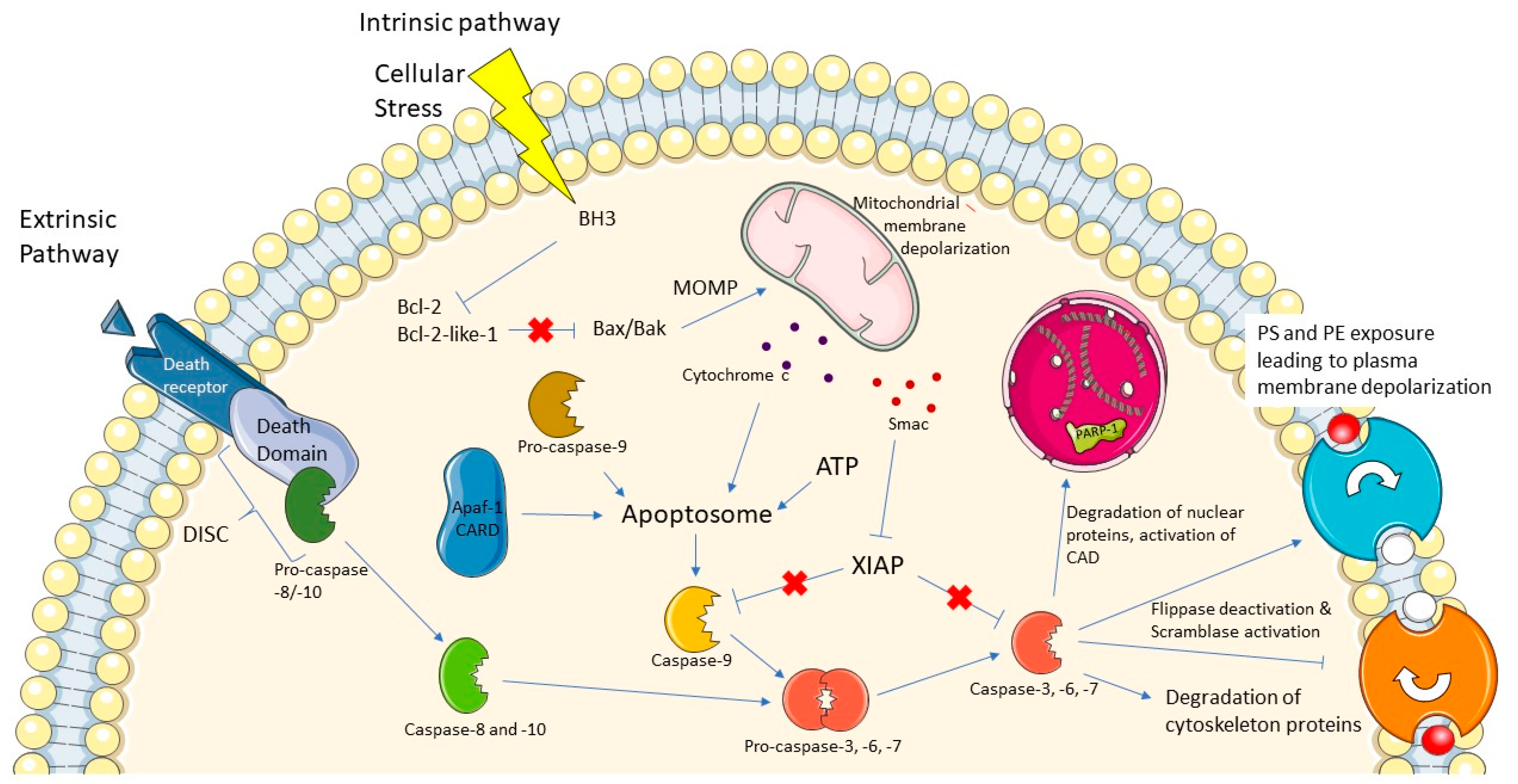
1. Introduction
2. Challenges of Caspase-3 Probes
3. Small Molecule Probes
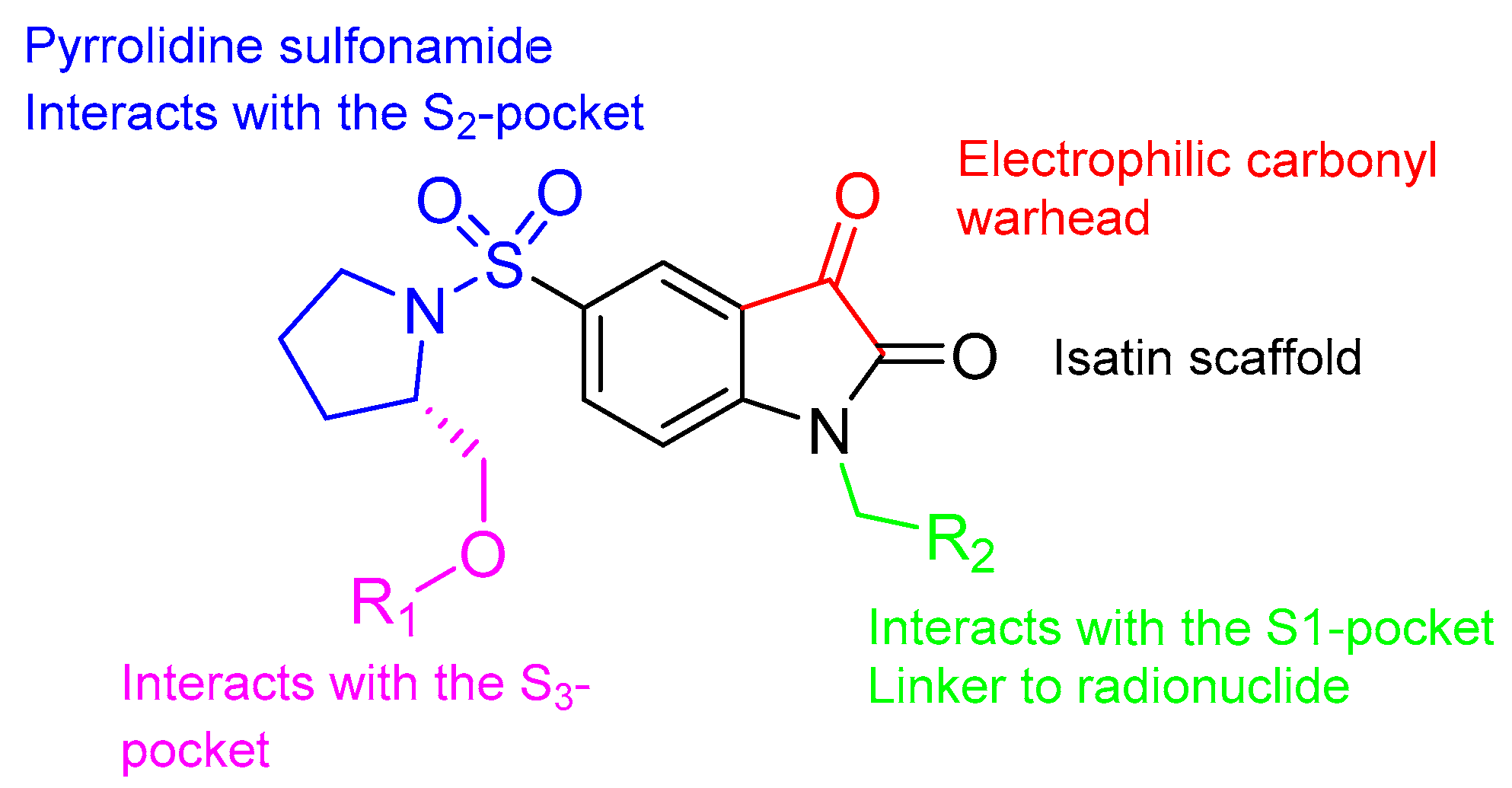
3.1. Isatin Sulfonamide Family
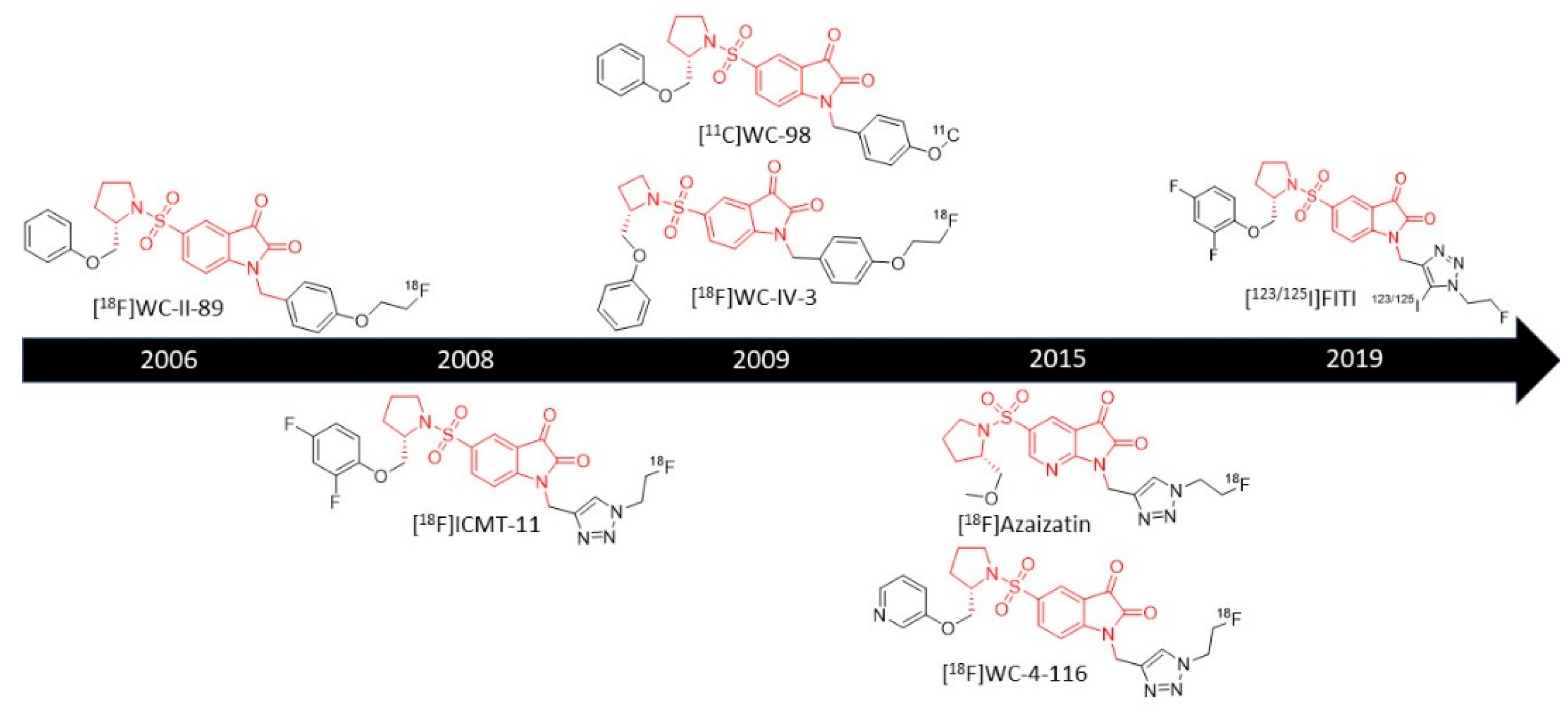
3.1.1. 18F-Labeled Isatin Sulfonamides
[18F]WC-II-89
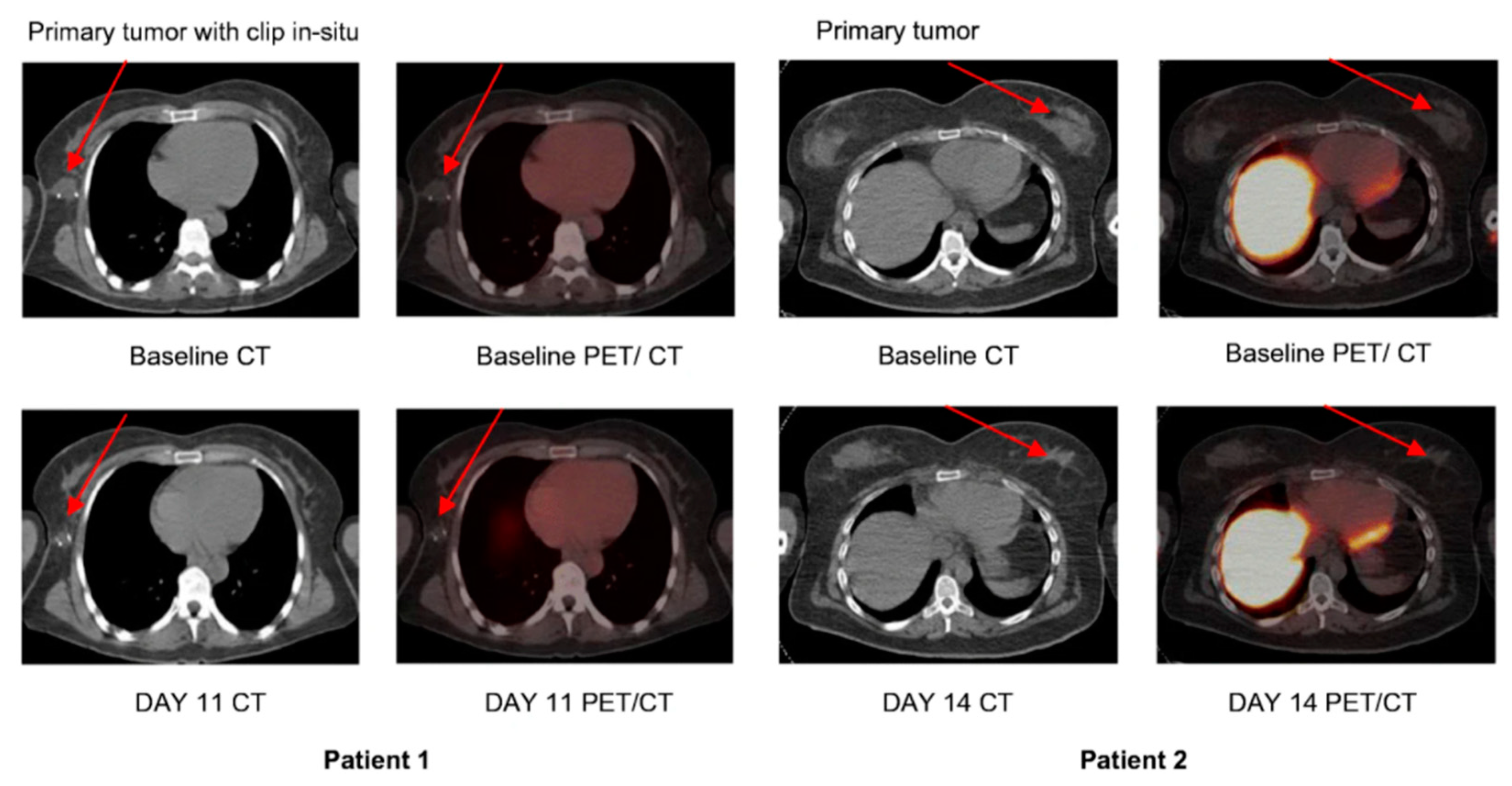
[18F]ICMT-11
[11C]WC-98
[18F]WC-IV-3
[18F]WC-4-116
[18F]Azaisatin
[123/125I]FITI
3.2. Other Small-Molecule Radiotracers
[18F]pyrimidoindolone
4. Peptide-Based Caspase-3 Probes
4.1. Substrate-Based Probes
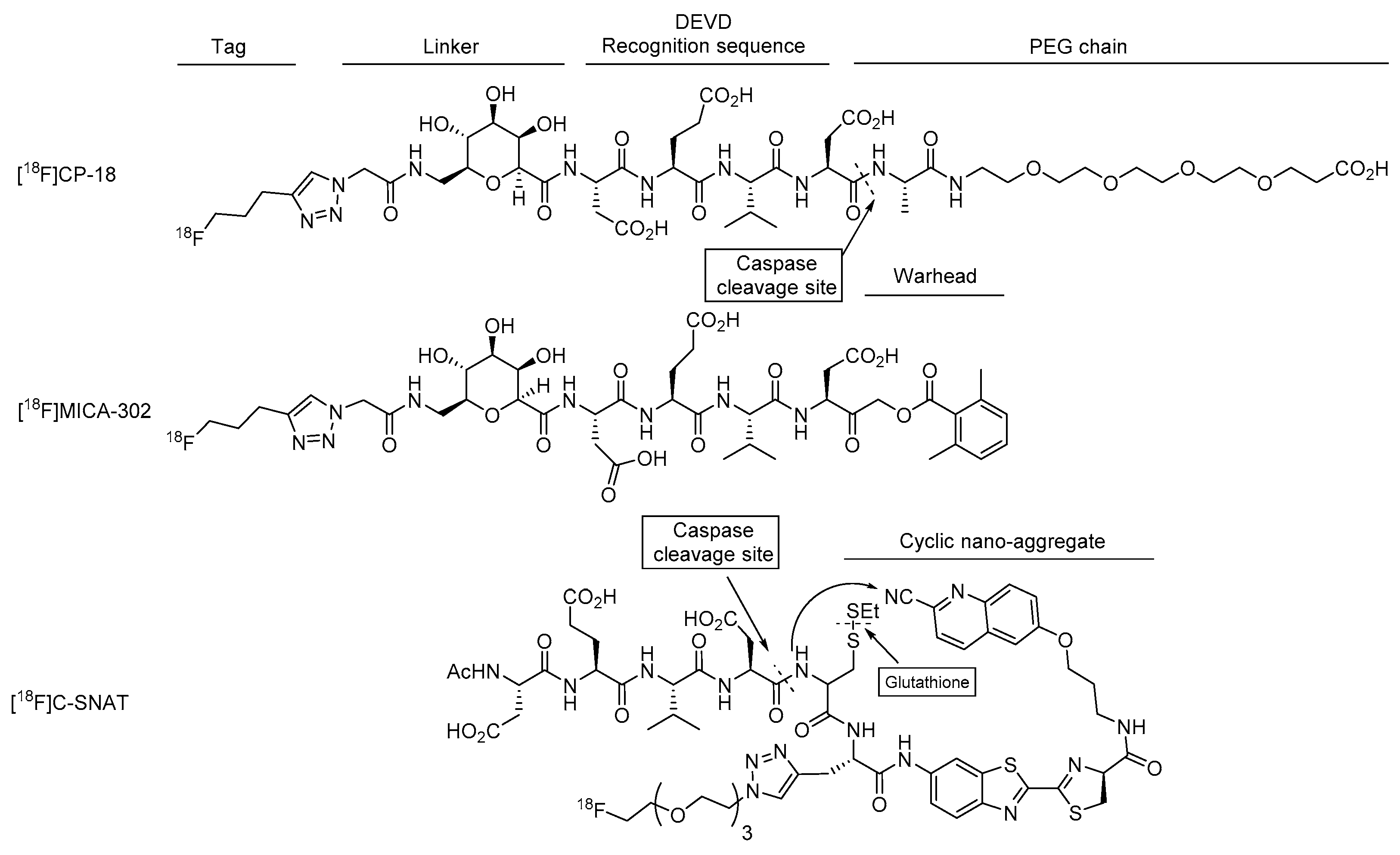
4.1.1. [18F]CP-18
4.1.2. [18F]TBD
4.1.3. [68Ga]Ga-TC3-OGDOTA
4.2. Activity-Based Probes
4.2.1. [18F]FB-VAD-FMK
4.2.2. [18F]MICA-302
4.3. Nanoaggregate Probes
4.3.1. [18F]C-SNAT
4.3.2. DEVD-Cys(StBu)-PPG(CBT)-AmBF3
4.3.3. TCO-C-SNAT4
5. Conclusions and Further Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Rybczynska, A.A.; Boersma, H.H.; De Jong, S.; Gietema, J.A.; Noordzij, W.; Dierckx, R.A.J.O.; Elsinga, P.H.; Van Waarde, A. Avenues to molecular imaging of dying cells: Focus on cancer. Med. Res. Rev. 2018, 38, 1713–1768. [Google Scholar] [CrossRef]
- Creagh, E.M. Caspase crosstalk: Integration of apoptotic and innate immune signalling pathways. Trends Immunol. 2014, 35, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- McComb, S.; Chan, P.K.; Guinot, A.; Hartmannsdottir, H.; Jenni, S.; Dobay, M.P.; Bourquin, J.-P.; Bornhauser, B.C. Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Sci. Adv. 2019, 5, eaau9433. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Teringova, E.; Tousek, P. Apoptosis in ischemic heart disease. J. Transl. Med. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Rahmanian, N.; Hosseinimehr, S.J.; Khalaj, A. The paradox role of caspase cascade in ionizing radiation therapy. J. Biomed. Sci. 2016, 23, 1–13. [Google Scholar] [CrossRef]
- Schmitt, E.; Sané, A.-T.; Bertrand, R. Activation and role of caspases in chemotherapy-induced apoptosis. Drug Resist. Updat. 1999, 2, 21–29. [Google Scholar] [CrossRef]
- Liang, Y.; Yan, C.; Schor, N.F. Apoptosis in the absence of caspase 3. Oncogene 2001, 20, 6570–6578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nat. Cell Biol. 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Liu, X.-L.; Zhao, R. Induction of Pyroptosis and Its Implications in Cancer Management. Front. Oncol. 2019, 9, 971. [Google Scholar] [CrossRef]
- Vickers, C.J.; González-Páez, G.E.; Wolan, D.W. Selective Detection of Caspase-3 versus Caspase-7 Using Activity-Based Probes with Key Unnatural Amino Acids. ACS Chem. Biol. 2013, 8, 1558–1566. [Google Scholar] [CrossRef]
- Elvas, F.; Berghe, T.V.; Adriaenssens, Y.; Vandenabeele, P.; Augustyns, K.; Staelens, S.; Stroobants, S.; Van Der Veken, P.; Wyffels, L. Caspase-3 probes for PET imaging of apoptotic tumor response to anticancer therapy. Org. Biomol. Chem. 2019, 17, 4801–4824. [Google Scholar] [CrossRef]
- Solania, A.; González-Páez, G.E.; Wolan, D.W. Selective and Rapid Cell-Permeable Inhibitor of Human Caspase-3. ACS Chem. Biol. 2019, 14, 2463–2470. [Google Scholar] [CrossRef]
- Poręba, M.; Stróżyk, A.; Salvesen, G.S.; Drąg, M. Caspase Substrates and Inhibitors. Cold Spring Harb. Perspect. Biol. 2013, 5, a008680. [Google Scholar] [CrossRef] [PubMed]
- Edgington, L.E.; Verdoes, M.; Bogyo, M. Functional imaging of proteases: Recent advances in the design and application of substrate-based and activity-based probes. Curr. Opin. Chem. Biol. 2011, 15, 798–805. [Google Scholar] [CrossRef]
- Patel, S.; Homaei, A.; El-Seedi, H.R.; Akhtar, N. Cathepsins: Proteases that are vital for survival but can also be fatal. Biomed. Pharmacother. 2018, 105, 526–532. [Google Scholar] [CrossRef]
- Dall, E.; Brandstetter, H. Structure and function of legumain in health and disease. Biochimie 2016, 122, 126–150. [Google Scholar] [CrossRef]
- Elliott, M.R.; Ravichandran, K.S. The Dynamics of Apoptotic Cell Clearance. Dev. Cell 2016, 38, 147–160. [Google Scholar] [CrossRef]
- Crestoni, M.E. Radiopharmaceuticals for Diagnosis and Therapy. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier BV: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Limpachayaporn, P.; Schäfers, M.; Schober, O.; Kopka, K.; Haufe, G. Synthesis of new fluorinated, 2-substituted 5-pyrrolidinylsulfonyl isatin derivatives as caspase-3 and caspase-7 inhibitors: Nonradioactive counterparts of putative PET-compatible apoptosis imaging agents. Bioorg. Med. Chem. 2013, 21, 2025–2036. [Google Scholar] [CrossRef]
- Limpachayaporn, P.; Schäfers, M.; Haufe, G. Isatin sulfonamides: Potent caspases-3 and -7 inhibitors, and promising PET and SPECT radiotracers for apoptosis imaging. Futur. Med. Chem. 2015, 7, 1173–1196. [Google Scholar] [CrossRef]
- García-Argüello, S.F.; Lopez-Lorenzo, B.; Cornelissen, B.; Smith, G. Development of [(18)F]ICMT-11 for Imaging Caspase-3/7 Activity during Therapy-Induced Apoptosis. Cancers 2020, 12, 2191. [Google Scholar] [CrossRef]
- Firoozpour, L.; Gao, L.; Moghimi, S.; Pasalar, P.; Davoodi, J.; Wang, M.-W.; Rezaei, Z.; Dadgar, A.; Yahyavi, H.; Amanlou, M.; et al. Efficient synthesis, biological evaluation, and docking study of isatin based derivatives as caspase inhib-itors. J. Enzym. Inhib. Med. Chem. 2020, 35, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chu, W.; Rothfuss, J.; Zeng, C.; Xu, J.; Jones, L.; Welch, M.J.; Mach, R.H. Synthesis, radiolabeling, and in vivo evaluation of an 18F-labeled isatin analog for imaging caspase-3 activa-tion in apoptosis. Bioorg. Med. Chem. Lett. 2006, 16, 5041–5046. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Zhou, D.; Chu, W.; Herrbrich, P.E.; Jones, L.A.; Rothfuss, J.M.; Engle, J.T.; Geraci, M.; Welch, M.J.; Mach, R.H. Comparison of radiolabeled isatin analogs for imaging apoptosis with positron emission tomography. Nucl. Med. Biol. 2009, 36, 651–658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vassileva, V.; Stribbling, S.M.; Barnes, C.; Carroll, L.; Braga, M.; Abrahams, J.; Heinzmann, K.; Haegeman, C.; Macfarlane, M.; Simpson, K.L.; et al. Evaluation of apoptosis imaging biomarkers in a genetic model of cell death. EJNMMI Res. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Smith, G.; Glaser, M.; Perumal, M.; Nguyen, Q.D.; Shan, B.; Årstad, E.; Aboagye, E.O. Design, synthesis, and biological characterization of a caspase 3/7 selective isatin labeled with 2-[18F]fluoroethylazide. J. Med. Chem. 2008, 51, 8057–8067. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.-D.; Smith, G.; Glaser, M.; Perumal, M.; Arstad, E.; Aboagye, E.O. Positron emission tomography imaging of drug-induced tumor apoptosis with a caspase-3/7 specific [18F]-labeled isatin sulfonamide. Proc. Natl. Acad. Sci. USA 2009, 106, 16375–16380. [Google Scholar] [CrossRef]
- Fortt, R.; Smith, G.; Awais, R.O.; Luthra, S.K.; Aboagye, E.O. Automated GMP Synthesis of [18F]ICMT-11 for In Vivo Imaging of Caspase-3 Activity. Nucl. Med. Biol. 2012, 39, 1000–1005. [Google Scholar] [CrossRef]
- Challapalli, A.; Kenny, L.M.; Hallett, W.A.; Kozlowski, K.; Tomasi, G.; Gudi, M.; Al-Nahhas, A.; Coombes, R.C.; Aboagye, E.O. 18F-ICMT-11, a Caspase-3-Specific PET Tracer for Apoptosis: Biodistribution and Radiation Dosimetry. J. Nucl. Med. 2013, 54, 1551–1556. [Google Scholar] [CrossRef]
- Dubash, S.R.; Merchant, S.; Heinzmann, K.; Mauri, F.; Lavdas, I.; Inglese, M.; Kozlowski, K.; Rama, N.; Masrour, N.; Steel, J.F.; et al. Clinical translation of [(18)F]ICMT-11 for measuring chemotherapy-induced caspase 3/7 activation in breast and lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2285–2299. [Google Scholar] [CrossRef]
- Kapty, J.; Murray, D.; Mercer, J. Radiotracers for Noninvasive Molecular Imaging of Tumor Cell Death. Cancer Biother. Radiopharm. 2010, 25, 615–628. [Google Scholar] [CrossRef]
- Chen, D.L.; Engle, J.T.; Griffin, E.A.; Miller, J.P.; Chu, W.; Zhou, D.; Mach, R.H. Imaging Caspase-3 Activation as a Marker of Apoptosis-Targeted Treatment Response in Cancer. Mol. Imaging Biol. 2015, 17, 384–393. [Google Scholar] [CrossRef]
- Chu, W.; Rothfuss, J.; Zhou, D.; Mach, R.H. Synthesis and evaluation of isatin analogs as caspase-3 inhibitors: Introduction of a hydrophilic group in-creases potency in a whole cell assay. Bioorg. Med. Chem. Lett. 2011, 21, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Thukkani, A.K.; Shoghi, K.I.; Zhou, D.; Xu, J.; Chu, W.; Novak, E.; Chen, D.L.; Gropler, R.J.; Mach, R.H. PET imaging of in vivo caspase-3/7 activity following myocardial ischemia-reperfusion injury with the radiolabeled isatin sulfonamide analogue [(18)F]WC-4-116. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 110. [Google Scholar]
- Waldmann, C.M.; Hermann, S.; Faust, A.; Riemann, B.; Schober, O.; Schäfers, M.; Haufe, G.; Kopka, K. Novel fluorine-18 labeled 5-(1-pyrrolidinylsulfonyl)-7-azaisatin derivatives as potential PET tracers for in vivo imaging of activated caspases in apoptosis. Bioorg. Med. Chem. 2015, 23, 5734–5739. [Google Scholar] [CrossRef] [PubMed]
- Glaser, M.; Rajkumar, V.; Diocou, S.; Gendron, T.; Yan, R.; Sin, P.K.B.; Sander, K.; Carroll, L.; Pedley, R.B.; Aboagye, E.O.; et al. One-Pot Radiosynthesis and Biological Evaluation of a Caspase-3 Selective 5-[123,125I]iodo-1,2,3-triazole derived Isatin SPECT Tracer. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, G. Nuclear Medicine in Oncology: Molecular Imaging and Target Therapy; Springer: Singapore, 2019. [Google Scholar]
- Reshef, A.; Shirvan, A.; Akselrod-Ballin, A.; Wall, A.; Ziv, I. Small-Molecule Biomarkers for Clinical PET Imaging of Apoptosis. J. Nucl. Med. 2010, 51, 837–840. [Google Scholar] [CrossRef]
- Udemba, A.; Smith, G.; Nguyen, Q.D.; Kaliszczak, M.; Carroll, L.; Fortt, R.; Fuchter, M.J.; Aboagye, E.O. Design, synthesis and initial characterisation of a radiolabelled [(18)F]pyrimidoindolone probe for detect-ing activated caspase-3/7. Org. Biomol. Chem. 2015, 13, 5418–5423. [Google Scholar] [CrossRef]
- Vickers, C.J.; Gonzalez-Paez, G.E.; Wolan, D.W. Selective detection and inhibition of active caspase-3 in cells with opti-mized peptides. J. Am. Chem. Soc. 2013, 135, 12869–12876. [Google Scholar] [CrossRef]
- Zeng, W.; Miao, W. Development of small molecular probes for the molecular imaging of apoptosis. Anti-Cancer Agents Med. Chem. 2009, 9, 986–995. [Google Scholar] [CrossRef]
- Blum, G.; Weimer, R.M.; Edgington, L.E.; Adams, W.; Bogyo, M. Comparative Assessment of Substrates and Activity Based Probes as Tools for Non-Invasive Optical Imaging of Cysteine Protease Activity. PLoS ONE 2009, 4, e6374. [Google Scholar] [CrossRef]
- Ofori, L.O.; Withana, N.P.; Prestwood, T.R.; Verdoes, M.; Brady, J.J.; Winslow, M.M.; Sorger, J.; Bogyo, M. Design of Protease Activated Optical Contrast Agents That Exploit a Latent Lysosomotropic Effect for Use in Fluorescence-Guided Surgery. ACS Chem. Biol. 2015, 10, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Palner, M.; Shen, B.; Jeon, J.; Lin, J.; Chin, F.T.; Rao, J. Preclinical Kinetic Analysis of the Caspase-3/7 PET Tracer 18F-C-SNAT: Quantifying the Changes in Blood Flow and Tumor Retention After Chemotherapy. J. Nucl. Med. 2015, 56, 1415–1421. [Google Scholar] [CrossRef]
- Bauer, C.; Bauder-Wuest, U.; Mier, W.; Haberkorn, U.; Eisenhut, M. 131I-labeled peptides as caspase substrates for apoptosis imaging. J. Nucl. Med. 2005, 46, 1066–1074. [Google Scholar]
- Su, H.; Chen, G.; Gangadharmath, U.; Gomez, L.F.; Liang, Q.; Mu, F.; Mocharla, V.P.; Szardenings, A.K.; Walsh, J.C.; Xia, C.-F.; et al. Evaluation of [18F]-CP18 as a PET Imaging Tracer for Apoptosis. Mol. Imaging Biol. 2013, 15, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Rapic, S.; Vangestel, C.; Elvas, F.; Verhaeghe, J.; Van den Wyngaert, T.; Pauwels, P.; Staelens, S.; Stroobants, S. Evaluation of [(18)F]CP18 as a Substrate-Based Apoptosis Imaging Agent for the Assessment of Early Treat-ment Response in Oncology. Mol. Imaging Biol. 2017, 19, 560–569. [Google Scholar] [CrossRef] [PubMed]
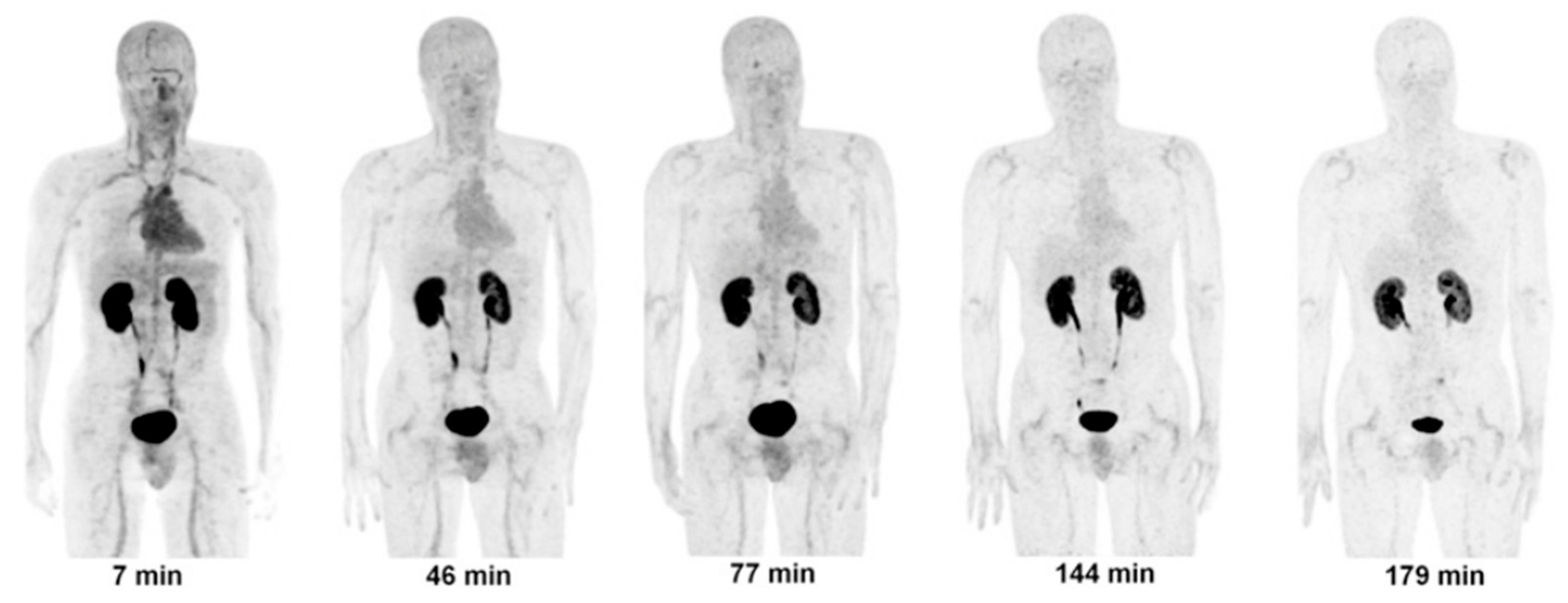
- Doss, M.; Kolb, H.C.; Walsh, J.C.; Mocharla, V.; Fan, H.; Chaudhary, A.; Zhu, Z.; Alpaugh, R.K.; Lango, M.N.; Yu, J.Q. Biodistribution and Radiation Dosimetry of 18F-CP-18, a Potential Apoptosis Imaging Agent, as Determined from PET/CT Scans in Healthy Volunteers. J. Nucl. Med. 2013, 54, 2087–2092. [Google Scholar] [CrossRef]
- Xia, C.-F.; Chen, G.; Gangadharmath, U.; Gomez, L.F.; Liang, Q.; Mu, F.; Mocharla, V.P.; Su, H.; Szardenings, A.K.; Walsh, J.C.; et al. In Vitro and In Vivo Evaluation of the Caspase-3 Substrate-Based Radiotracer [18F]-CP18 for PET Imaging of Apoptosis in Tumors. Mol. Imaging Biol. 2013, 15, 748–757. [Google Scholar] [CrossRef]
- Su, H.; Gorodny, N.; Gomez, L.F.; Gangadharmath, U.; Mu, F.; Chen, G.; Walsh, J.C.; Szardenings, K.; Kolb, H.C.; Tamarappoo, B. Noninvasive Molecular Imaging of Apoptosis in a Mouse Model of Anthracycline-Induced Cardiotoxicity. Circ. Cardiovasc. Imaging 2015, 8, e001952. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.; Dauer, Z.; Pandit-Taskar, N.; Schoder, H.; Dauer, L.T. Radiation dosimetry of 18F-FDG PET/CT: Incorporating exam-specific parameters in dose estimates. BMC Med. Imaging 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Engel, B.J.; Gammon, S.T.; Chaudhari, R.; Lu, Z.; Pisaneschi, F.; Yang, H.; Ornelas, A.; Yan, V.; Kelderhouse, L.; Najjar, A.M.; et al. Caspase-3 Substrates for Noninvasive Pharmacodynamic Imaging of Apoptosis by PET/CT. Bioconjugate Chem. 2018, 29, 3180–3195. [Google Scholar] [CrossRef]
- Méthot, N.; Vaillancourt, J.P.; Huang, J.; Colucci, J.; Han, Y.; Ménard, S.; Zamboni, R.; Toulmond, S.; Nicholson, D.W.; Roy, S. A Caspase Active Site Probe Reveals High Fractional Inhibition Needed to Block DNA Fragmentation. J. Biol. Chem. 2004, 279, 27905–27914. [Google Scholar] [CrossRef] [PubMed]
- Ostapchenko, V.G.; Snir, J.; Suchy, M.; Fan, J.; Cobb, M.R.; Chronik, B.A.; Kovacs, M.; Prado, V.F.; Hudson, R.H.E.; Pasternak, S.H.; et al. Detection of Active Caspase-3 in Mouse Models of Stroke and Alzheimer’s Disease with a Novel Dual Positron Emission Tomography/Fluorescent Tracer [68Ga]Ga-TC3-OGDOTA. Contrast Media Mol. Imaging 2019, 2019, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hight, M.R.; Cheung, Y.-Y.; Nickels, M.L.; Dawson, E.S.; Zhao, P.; Saleh, S.; Buck, J.R.; Tang, D.; Washington, M.K.; Coffey, R.J.; et al. A Peptide-Based Positron Emission Tomography Probe for In Vivo Detection of Caspase Activity in Apoptotic Cells. Clin. Cancer Res. 2014, 20, 2126–2135. [Google Scholar] [CrossRef] [PubMed]
- Ekert, P.G.; Silke, J.; Vaux, D.L. Caspase inhibitors. Cell Death Differ. 1999, 6, 1081–1086. [Google Scholar] [CrossRef]
- Chen, Z.; Rao, J. Positron Emission Tomography Imaging of Tumor Apoptosis with a Caspase-Sensitive Nano-Aggregation Tracer [18F]C-SNAT. Methods Mol. Biol. 2018, 1790, 181–195. [Google Scholar] [CrossRef]
- Witney, T.H.; Hoehne, A.; Reeves, R.E.; Ilovich, O.; Namavari, M.; Shen, B.; Chin, F.T.; Rao, J.; Gambhir, S.S. A Systematic Comparison of 18F-C-SNAT to Established Radiotracer Imaging Agents for the Detection of Tumor Response to Treatment. Clin. Cancer Res. 2015, 21, 3896–3905. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Jeon, J.; Palner, M.; Ye, D.; Shuhendler, A.; Chin, F.T.; Rao, J. Positron emission tomography imaging of drug-induced tumor apoptosis with a caspase-triggered nanoag-gregation probe. Angew. Chem. Int. Ed. Engl. 2013, 52, 10511–10514. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, M.; Zhou, K.; Rao, J. Pre-targeted Imaging of Protease Activity through In Situ Assembly of Nanoparticles. Angew. Chem. Int. Ed. 2020, 59, 7864–7870. [Google Scholar] [CrossRef] [PubMed]
- Gammon, S.T.; Engel, B.J.; Gores, G.J.; Cressman, E.; Piwnica-Worms, D.; Millward, S.W. Mistiming Death: Modeling the Time-Domain Variability of Tumor Apoptosis and Implications for Mo-lecular Imaging of Cell Death. Mol. Imaging Biol. 2020, 22, 1310–1323. [Google Scholar] [CrossRef] [PubMed]
- Aloj, L.; Zannetti, A.; Caracó, C.; Del Vecchio, S.; Salvatore, M. Bcl-2 overexpression prevents 99mTc-MIBI uptake in breast cancer cell lines. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.C.; Torres, J.B.; Goldin, R.; Mosley, M.; Dias, G.M.; Bravo, L.C.; Kersemans, V.; Allen, P.D.; Mukherjee, S.; Smart, S.C.; et al. Early Detection in a Mouse Model of Pancreatic Cancer by Imaging DNA Damage Response Signaling. J. Nucl. Med. 2020, 61, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Elvas, F.; Vangestel, C.; Pak, K.; Vermeulen, P.; Gray, B.; Stroobants, S.; Wyffels, L. Early prediction of tumor response to treatment: Preclinical validation of 99mTc-duramycin. J. Nucl. Med. 2016, 57, 805–811. [Google Scholar] [CrossRef]

| Inhibitor | Caspase-3 Affinity IC50 (nM) | Selectivity Index | Log P | ||||
|---|---|---|---|---|---|---|---|
| −1 | −6 | −7 | −8 | −9 | |||
| WC-II-89 | 9.7 | >5000 | >361 | 2.4 | >5000 | N/A | 4.19 |
| ICMT-11 | 0.5 | >10,000 | >10,000 | 5.0 | >10,000 | N/A | 1.61 |
| WC-98 | 14.5 | >1300 | >680 | 1.5 | >1300 | N/A | 3.97 |
| WC-IV-3 | 8.6 | >2300 | 580 | 3.0 | >2300 | N/A | 3.65 |
| WC-4-116 | 4.5 | >4400 | 2989 | 0.8 | >11,000 | N/A | 0.73 (calculated) |
| Azaisatin | 21 | >2380 | >2380 | 4.6 | N/A | N/A | −1.32 |
| FITI | Ki 6.1 | N/A | N/A | N/A | >8190 | N/A | 1.6 |
| Pyrimidoindolone | 100.4 | N/A | N/A | N/A | >49.8 | N/A | N/A |
| FB-VAD-fmk | 225.0 | N/A | N/A | N/A | N/A | N/A | N/A |
| MICA-302 | 1.0 | 1000 | 20 | 13.2 | 23.6 | >1000 | −3.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beroske, L.; Van den Wyngaert, T.; Stroobants, S.; Van der Veken, P.; Elvas, F. Molecular Imaging of Apoptosis: The Case of Caspase-3 Radiotracers. Int. J. Mol. Sci. 2021, 22, 3948. https://doi.org/10.3390/ijms22083948
Beroske L, Van den Wyngaert T, Stroobants S, Van der Veken P, Elvas F. Molecular Imaging of Apoptosis: The Case of Caspase-3 Radiotracers. International Journal of Molecular Sciences. 2021; 22(8):3948. https://doi.org/10.3390/ijms22083948
Chicago/Turabian StyleBeroske, Lucas, Tim Van den Wyngaert, Sigrid Stroobants, Pieter Van der Veken, and Filipe Elvas. 2021. "Molecular Imaging of Apoptosis: The Case of Caspase-3 Radiotracers" International Journal of Molecular Sciences 22, no. 8: 3948. https://doi.org/10.3390/ijms22083948
APA StyleBeroske, L., Van den Wyngaert, T., Stroobants, S., Van der Veken, P., & Elvas, F. (2021). Molecular Imaging of Apoptosis: The Case of Caspase-3 Radiotracers. International Journal of Molecular Sciences, 22(8), 3948. https://doi.org/10.3390/ijms22083948






