Sinularin, an Anti-Cancer Agent Causing Mitochondria-Modulated Apoptosis and Cytoskeleton Disruption in Human Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
2.1. Sinularin Significantly Induced Apoptosis through Caspase-Activated DNA Fragmentation
2.2. Production Levels of Intracellular and Mitochondrial ROS (mtROS) Were Augmented, While the Mitochondrial Membrane Potential (ΔΨ) Was Reduced after Sinularin Treatments
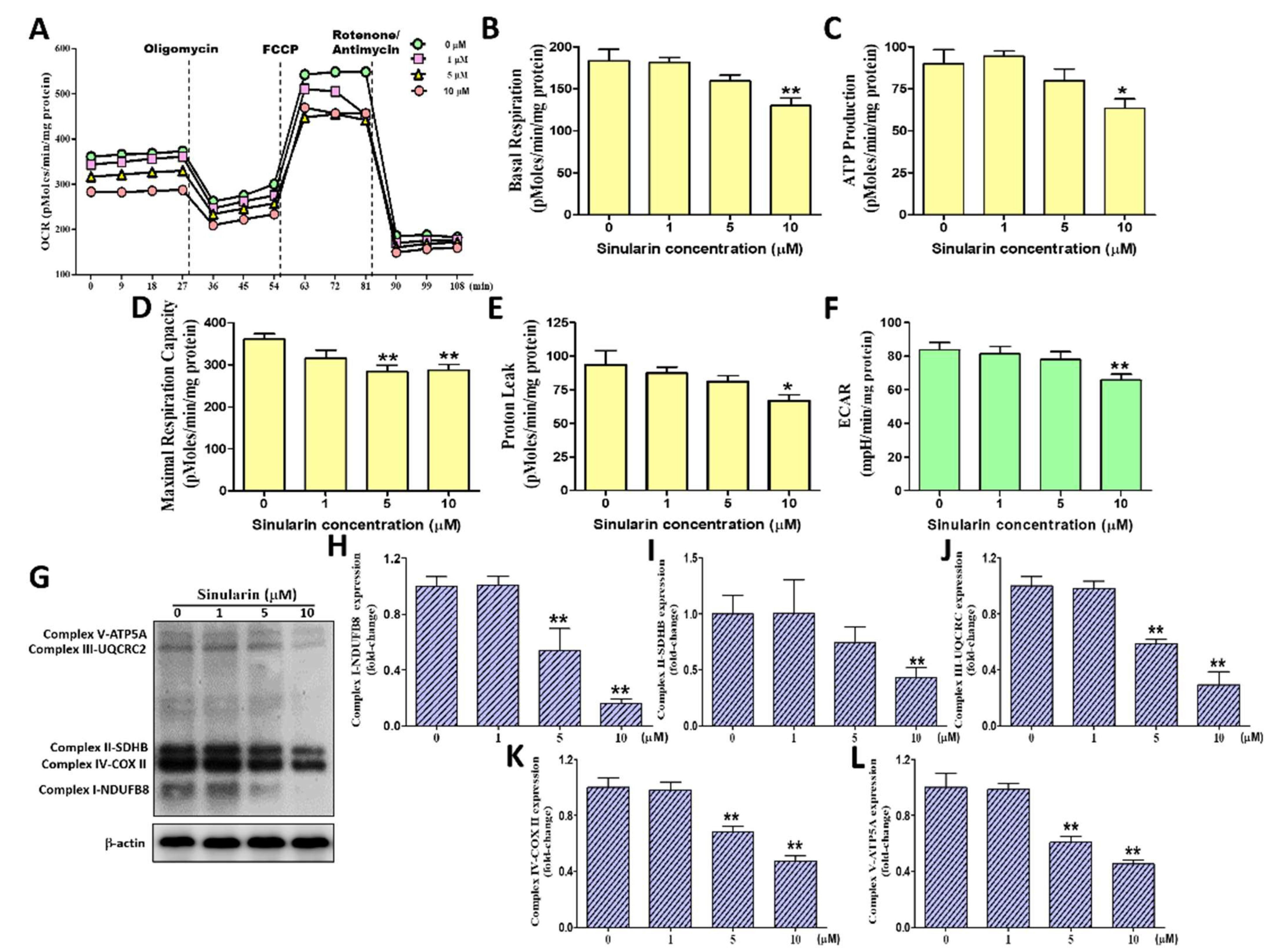
2.3. Effect of Sinularin on the Oxygen Consumption Rate, Extracellular Acidification Rate, and ETC Complex I–V Proteins of Mitochondria in SK-HEP-1 Cells
2.4. Sinularin Significantly Ablated Migration Ability and Colony Formation Potential
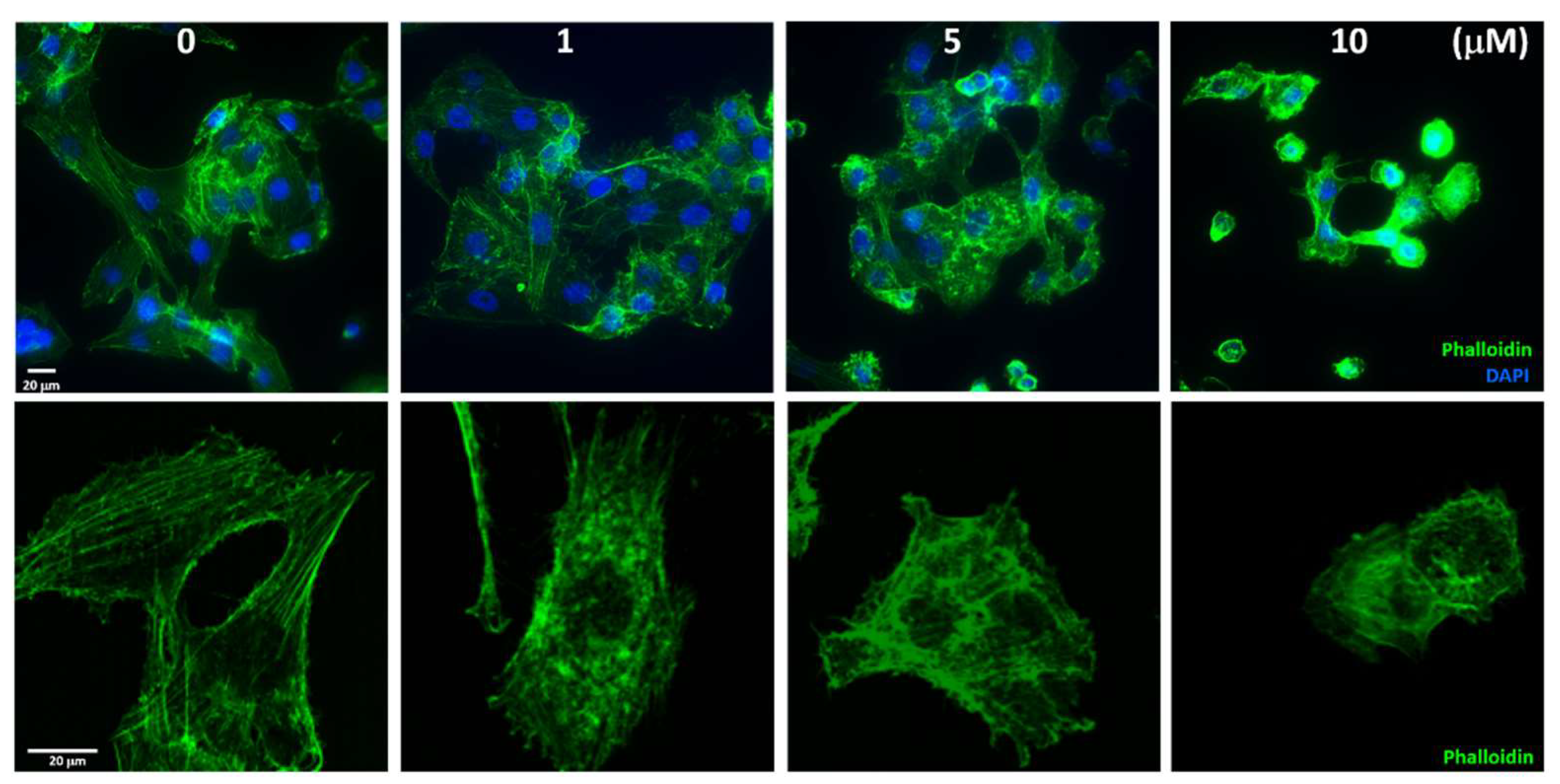
2.5. Sinularin Influenced the Distribution of Actin Filaments
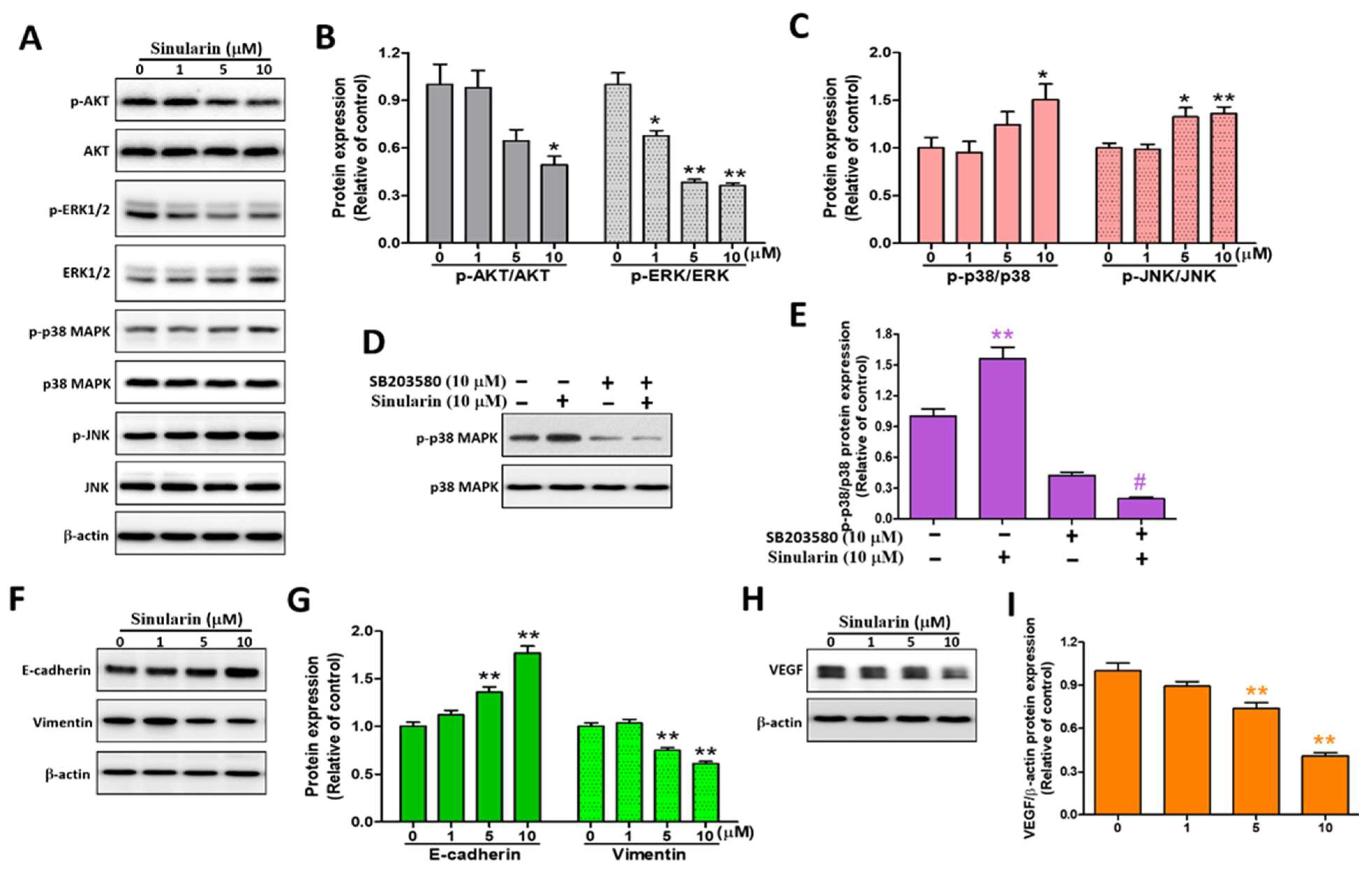
2.6. Sinularin Induces Apoptosis via MAPK, Epithelial-Mesenchymal Transition (EMT) and VEGF Signaling Pathways in SK-HEP-1 Cells
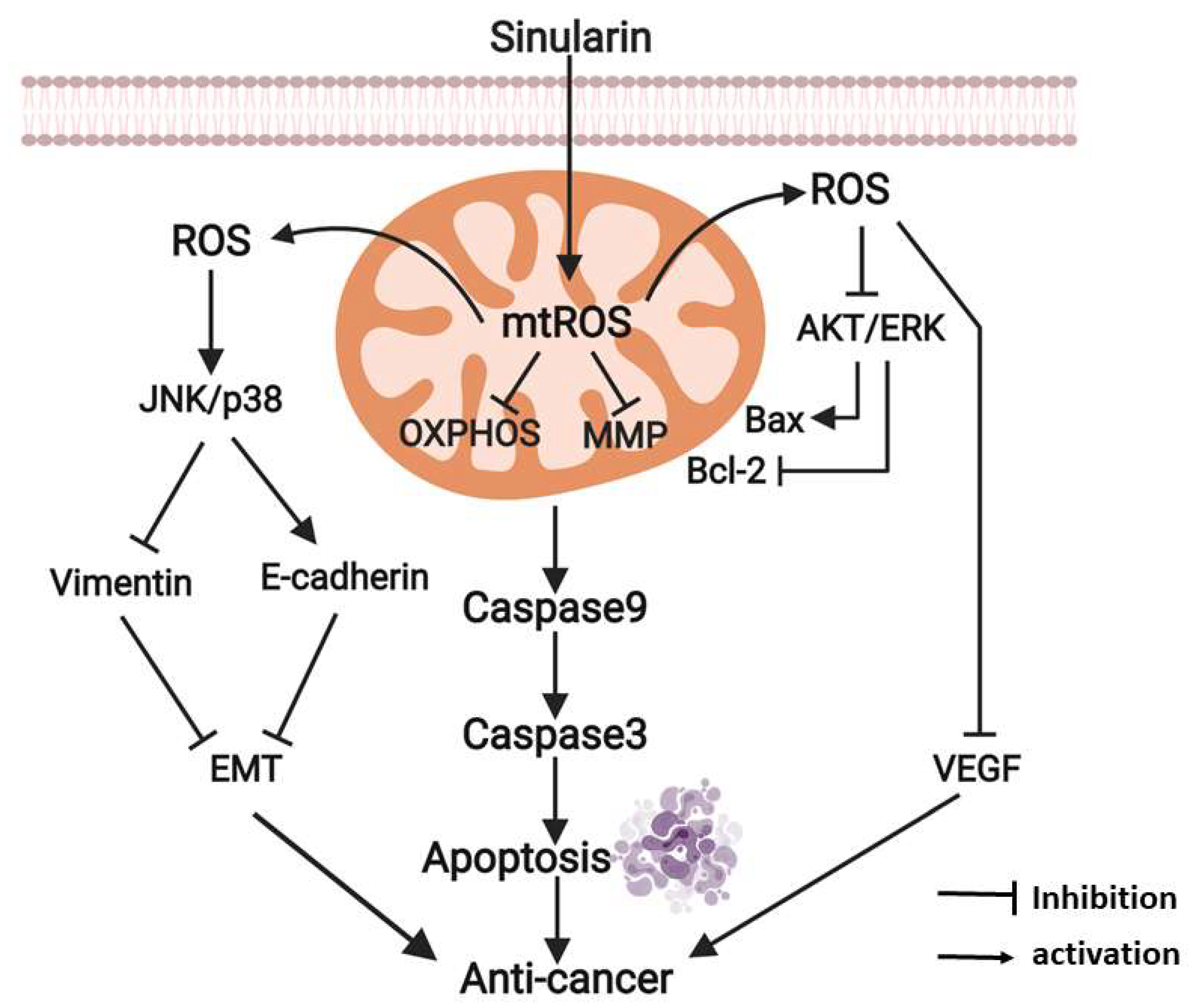
3. Discussion
4. Materials and Methods
4.1. Compound and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Annexin V-FITC/PI Staining
4.5. Terminal Transferase-Mediated dUTP Nick-End Labeling (TUNEL) Assay
4.6. Western Blot Analysis
4.7. Measurement of Cleaved Caspase-3 and VEGF by ELISA Kit
4.8. Measurement of Intracellular and Mitochondrial ROS (Reactive Oxygen Species) Levels
4.9. Measurement of Mitochondrial Membrane Potential (ΔΨ) by Flow Cytometry
4.10. Mitochondrial Function Measurements
4.11. Wound Healing Measurement (Scratch-Test Assay)
4.12. Transwell Migration Chamber Assay
4.13. Colony Formation Assay
4.14. Soft Agar Colony Formation Assay
4.15. Confocal Microscopy for Immunofluorescence Phalloidin Staining of Cells
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, Y.; Yuan, H.; Fang, Q.; Cai, N.; Suo, C.; Jin, L.; Zhang, T.; Chen, X. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J. Hepatol. 2019, 70, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Center, M.M.; Jemal, A. International trends in liver cancer incidence rates. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2362–2368. [Google Scholar] [CrossRef]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Tejeda-Maldonado, J.; García-Juárez, I.; Aguirre-Valadez, J.; González-Aguirre, A.; Vilatobá-Chapa, M.; Armengol-Alonso, A.; Escobar-Penagos, F.; Torre, A.; Sánchez-Ávila, J.F.; Carrillo-Pérez, D.L. Diagnosis and treatment of hepatocellular carcinoma: An update. World J. Hepatol. 2015, 7, 362. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Prager, G.W.; Poettler, M. Angiogenesis in cancer*: Basic mechanisms and therapeutic advances. Hamostaseologie 2012, 32, 105–114. [Google Scholar]
- Huang, C.; Jacobson, K.; Schaller, M.D. MAP kinases and cell migration. J. Cell Sci. 2004, 117, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Yang, J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef]
- Strippoli, R.; Benedicto, I.; Foronda, M.; Perez-Lozano, M.L.; Sánchez-Perales, S.; López-Cabrera, M.; Del Pozo, M.Á. p38 maintains E-cadherin expression by modulating TAK1-NF-κB during epithelial-to-mesenchymal transition. J. Cell Sci. 2010, 123, 4321–4331. [Google Scholar] [CrossRef]
- Lau, M.T.; So, W.K.; Leung, P.C.K. Fibroblast Growth Factor 2 Induces E-Cadherin Down-Regulation via PI3K/Akt/mTOR and MAPK/ERK Signaling in Ovarian Cancer Cells. PLoS ONE 2013, 8, e59083. [Google Scholar] [CrossRef]
- Wang, B.; Tan, Z.; Guan, F. Tumor-derived exosomes mediate the instability of cadherins and promote tumor progression. Int. J. Mol. Sci. 2019, 20, 3652. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.H.; Ma, Y.L.; Shih, P.C.; Chen, C.T.; Cheng, S.Y.; Pan, C.Y.; Jean, Y.H.; Chu, Y.M.; Lin, S.C.; Lai, Y.C.; et al. The antimicrobial peptide tilapia piscidin 3 induces mitochondria-modulated intrinsic apoptosis of osteosarcoma cells. Biochem. Pharmacol. 2020, 178, 114064. [Google Scholar] [CrossRef]
- Denisenko, T.V.; Gorbunova, A.S.; Zhivotovsky, B. Mitochondrial Involvement in Migration, Invasion and Metastasis. Front. Cell Dev. Biol. 2019, 7, 355. [Google Scholar] [CrossRef]
- Weinheimer, A.J.; Matson, J.A.; Hossain, M.B.; van der Helm, D. Marine anticancer agents: Sinularin and dihydrosinularin, new cembranolides from the soft coral, Sinularia flexibilis. Tetrahedron Lett. 1977, 18, 2923–2926. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Wells, R.J.; Schönholzer, P.; Coll, J.C. Cembranoid constituents from an australian collection of the soft coral sinularia flexibilis. Aust. J. Chem. 1978, 31, 1817–1824. [Google Scholar] [CrossRef]
- Buckle, P.J.; Baldo, B.A.; Taylor, K.M. The anti-inflammatory activity of marine natural products-6-n-tridecylsalicylic acid, flexibilide and dendalone 3-hydroxybutyrate. Agents Actions 1980, 10, 361–367. [Google Scholar] [CrossRef]
- Aceret, T.L.; Brown, L.; Miller, J.; Coll, J.C.; Sammarco, P.W. Cardiac end vascular responses of isolated rat tissues treated with diterpenes from Sinularia flexibilis (Coelenterata: Octocorallia). Toxicon 1996, 34, 1165–1171. [Google Scholar] [CrossRef]
- Aceret, T.L.; Coll, J.C.; Uchio, Y.; Sammarco, P.W. Antimicrobial activity of the diterpenes flexibilide and sinulariolide derived from Sinularia flexibilis Quoy and Gaimard 1833 (Coelenterata: Alcyonacea, Octocorallia). Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998, 120, 121–126. [Google Scholar] [CrossRef]
- Ma, Q.; Meng, X.Y.; Wu, K.R.; Cao, J.Z.; Yu, R.; Yan, Z.J. Sinularin exerts anti-tumor effects against human renal cancer cells relies on the generation of ROS. J. Cancer 2019, 10, 5114–5123. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Tang, J.Y.; Ou-Yang, F.; Wang, H.R.; Guan, P.Y.; Huang, C.Y.; Chen, C.Y.; Hou, M.F.; Sheu, J.H.; Chang, H.W. Sinularin selectively kills breast cancer cells showing G2/M arrest, apoptosis, and oxidative DNA damage. Molecules 2018, 23, 849. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wong, B.S.; Yea, S.H.; Lu, C.I.; Weng, S.H. Sinularin induces apoptosis through mitochondria dysfunction and inactivation of the pI3K/Akt/mTOR pathway in gastric carcinoma cells. Mar. Drugs 2016, 14, 142. [Google Scholar] [CrossRef]
- Su, T.R.; Lin, J.J.; Chiu, C.C.; Chen, J.Y.F.; Su, J.H.; Cheng, Z.J.; Hwang, W.I.; Huang, H.H.; Wu, Y.J. Proteomic investigation of anti-tumor activities exerted by sinularin against A2058 melanoma cells. Electrophoresis 2012, 33, 1139–1152. [Google Scholar] [CrossRef]
- Chang, Y.T.; Wu, C.Y.; Tang, J.Y.; Huang, C.Y.; Liaw, C.C.; Wu, S.H.; Sheu, J.H.; Chang, H.W. Sinularin induces oxidative stress-mediated G2/M arrest and apoptosis in oral cancer cells. Environ. Toxicol. 2017, 32, 2124–2132. [Google Scholar] [CrossRef]
- Chung, T.W.; Lin, S.C.; Su, J.H.; Chen, Y.K.; Lin, C.C.; Chan, H.L. Sinularin induces DNA damage, G2/M phase arrest, and apoptosis in human hepatocellular carcinoma cells. BMC Complement. Altern. Med. 2017, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.; Raes, M.; Toussaint, O.; Remacle, J. Importance of SE-glutathione peroxidase, catalase, and CU/ZN-SOD for cell survival against oxidative stress. Free Radic. Biol. Med. 1994, 17, 235–248. [Google Scholar] [CrossRef]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio-protocol 2019, 9, e3128. [Google Scholar] [CrossRef]
- Enomoto, A.; Murakami, H.; Asai, N.; Morone, N.; Watanabe, T.; Kawai, K.; Murakumo, Y.; Usukura, J.; Kaibuchi, K.; Takahashi, M. Akt/PKB regulates actin organization and cell motility via girdin/APE. Dev. Cell 2005, 9, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Hossain, R.; Hassan, S.M.H.; Salehi, B.; Martins, N.; Sharifi-Rad, J.; Amarowicz, R. Biological activities of sinularin: A literature-based review. Cell. Mol. Biol. 2020, 66, 33–36. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Ashton, T.M.; Gillies McKenna, W.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Bi, K.W.; Kuo, H.M.; Lin, T.K.; Liao, P.L.; Wang, P.W.; Chuang, J.H.; Liou, C.W. Phyllanthus urinaria induces mitochondrial dysfunction in human osteosarcoma 143B cells associated with modulation of mitochondrial fission/fusion proteins. Mitochondrion 2014, 17, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Shih, P.C.; Kuo, H.M.; Yang, S.N.; Lin, Y.Y.; Chen, W.F.; Tzou, S.J.; Liu, H.T.; Chen, N.F. TP3, an antimicrobial peptide, inhibits infiltration and motility of glioblastoma cells via modulating the tumor microenvironment. Cancer Med. 2020, 9, 3918–3931. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.; Lai, Y.C.; Kuo, T.J.; Su, J.H.; Sung, P.J.; Feng, C.W.; Lin, Y.Y.; Chen, P.C.; Tai, M.H.; Cheng, S.Y.; et al. Rhodoptilometrin, a crinoid-derived anthraquinone, induces cell regeneration by promoting wound healing and oxidative phosphorylation in human gingival fibroblast cells. Mar. Drugs 2019, 17, 138. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, C.-Y.; Shih, P.-C.; Huang, P.-W.; Lee, Y.-H.; Chen, Y.-F.; Tai, M.-H.; Liu, C.-H.; Wen, Z.-H.; Kuo, H.-M. Sinularin, an Anti-Cancer Agent Causing Mitochondria-Modulated Apoptosis and Cytoskeleton Disruption in Human Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 3946. https://doi.org/10.3390/ijms22083946
Ko C-Y, Shih P-C, Huang P-W, Lee Y-H, Chen Y-F, Tai M-H, Liu C-H, Wen Z-H, Kuo H-M. Sinularin, an Anti-Cancer Agent Causing Mitochondria-Modulated Apoptosis and Cytoskeleton Disruption in Human Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2021; 22(8):3946. https://doi.org/10.3390/ijms22083946
Chicago/Turabian StyleKo, Chou-Yuan, Po-Chang Shih, Po-Wei Huang, Yi-Hsin Lee, Yen-Fu Chen, Ming-Hong Tai, Chi-Hao Liu, Zhi-Hong Wen, and Hsiao-Mei Kuo. 2021. "Sinularin, an Anti-Cancer Agent Causing Mitochondria-Modulated Apoptosis and Cytoskeleton Disruption in Human Hepatocellular Carcinoma" International Journal of Molecular Sciences 22, no. 8: 3946. https://doi.org/10.3390/ijms22083946
APA StyleKo, C.-Y., Shih, P.-C., Huang, P.-W., Lee, Y.-H., Chen, Y.-F., Tai, M.-H., Liu, C.-H., Wen, Z.-H., & Kuo, H.-M. (2021). Sinularin, an Anti-Cancer Agent Causing Mitochondria-Modulated Apoptosis and Cytoskeleton Disruption in Human Hepatocellular Carcinoma. International Journal of Molecular Sciences, 22(8), 3946. https://doi.org/10.3390/ijms22083946





