Contribution of Invariant Natural Killer T Cells to the Clearance of Pseudomonas aeruginosa from Skin Wounds
Abstract
1. Introduction
2. Results
2.1. Reduced Elimination of P. aeruginosa and Delayed Re-Epithelialization in Jα18KO Mice
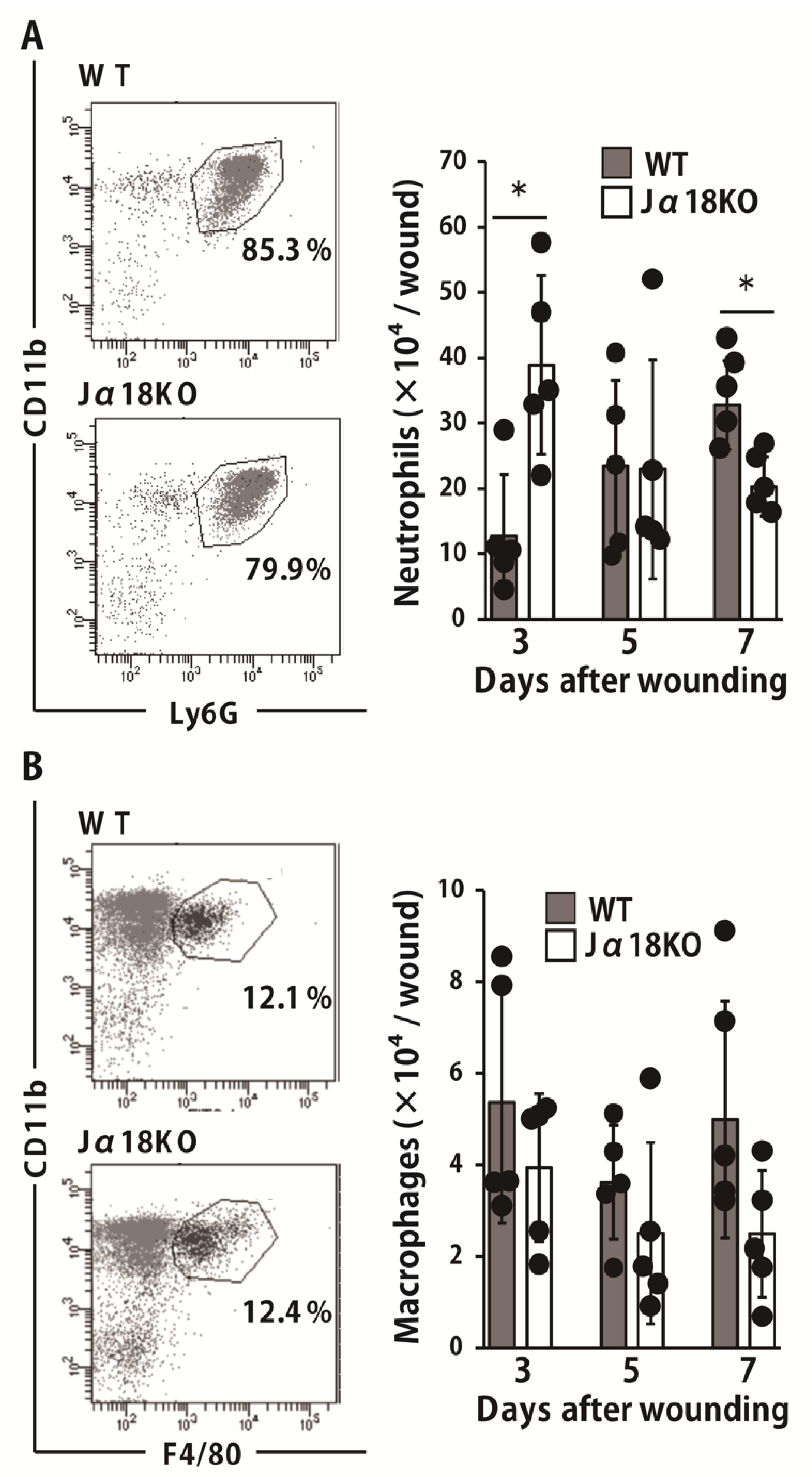
2.2. Effect of iNKT Cell Deficiency on Neutrophils and Macrophages Migration at the Wound Sites after Inoculation with P. aeruginosa
2.3. Effect of iNKT Cell Deficiency on the Production of Antimicrobial Peptides at the Wound Sites after Inoculation with P. aeruginosa
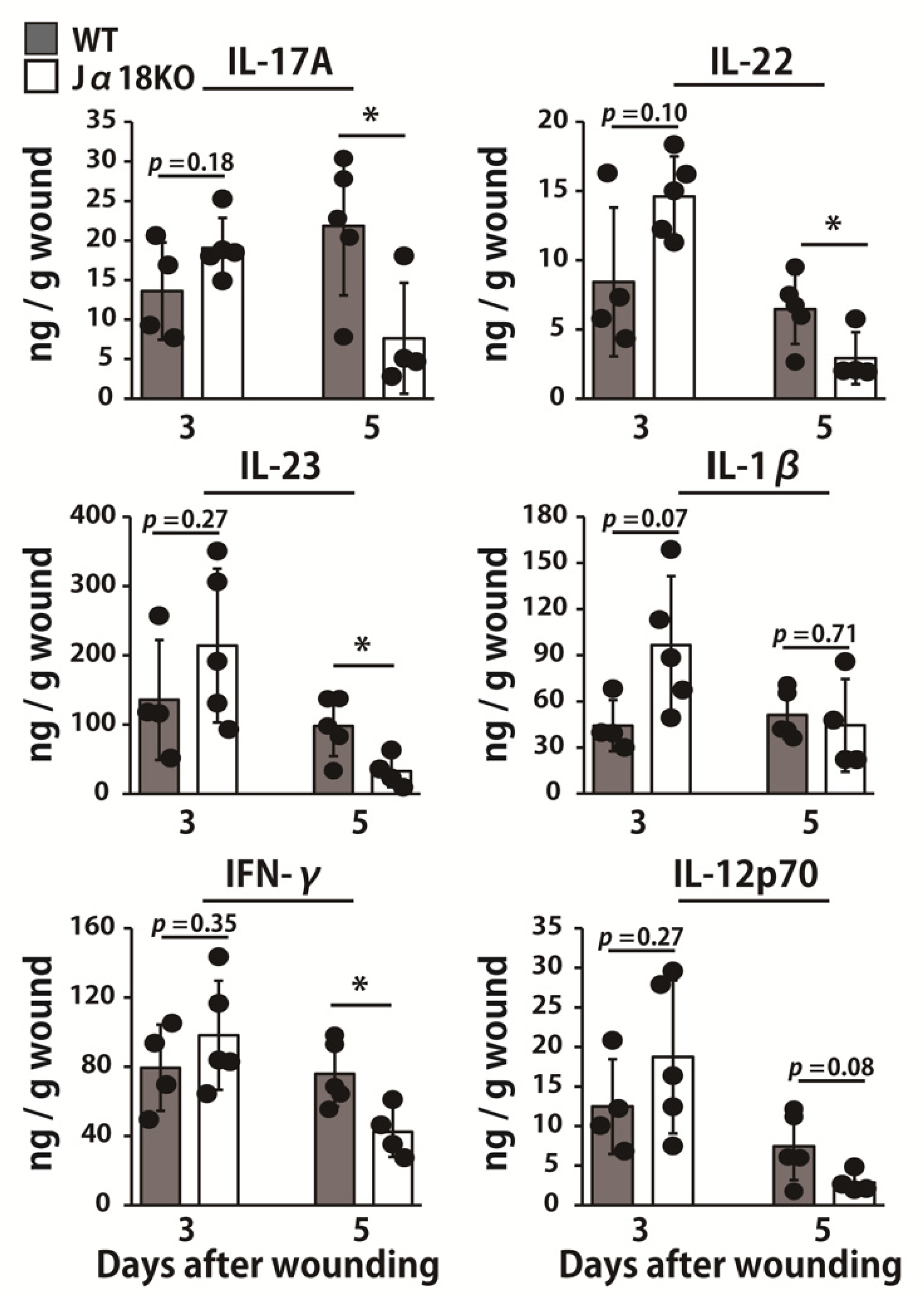
2.4. Effect of iNKT Cell Deficiency on the Production of Cytokines at the Wound Sites after Inoculation with P. aeruginosa
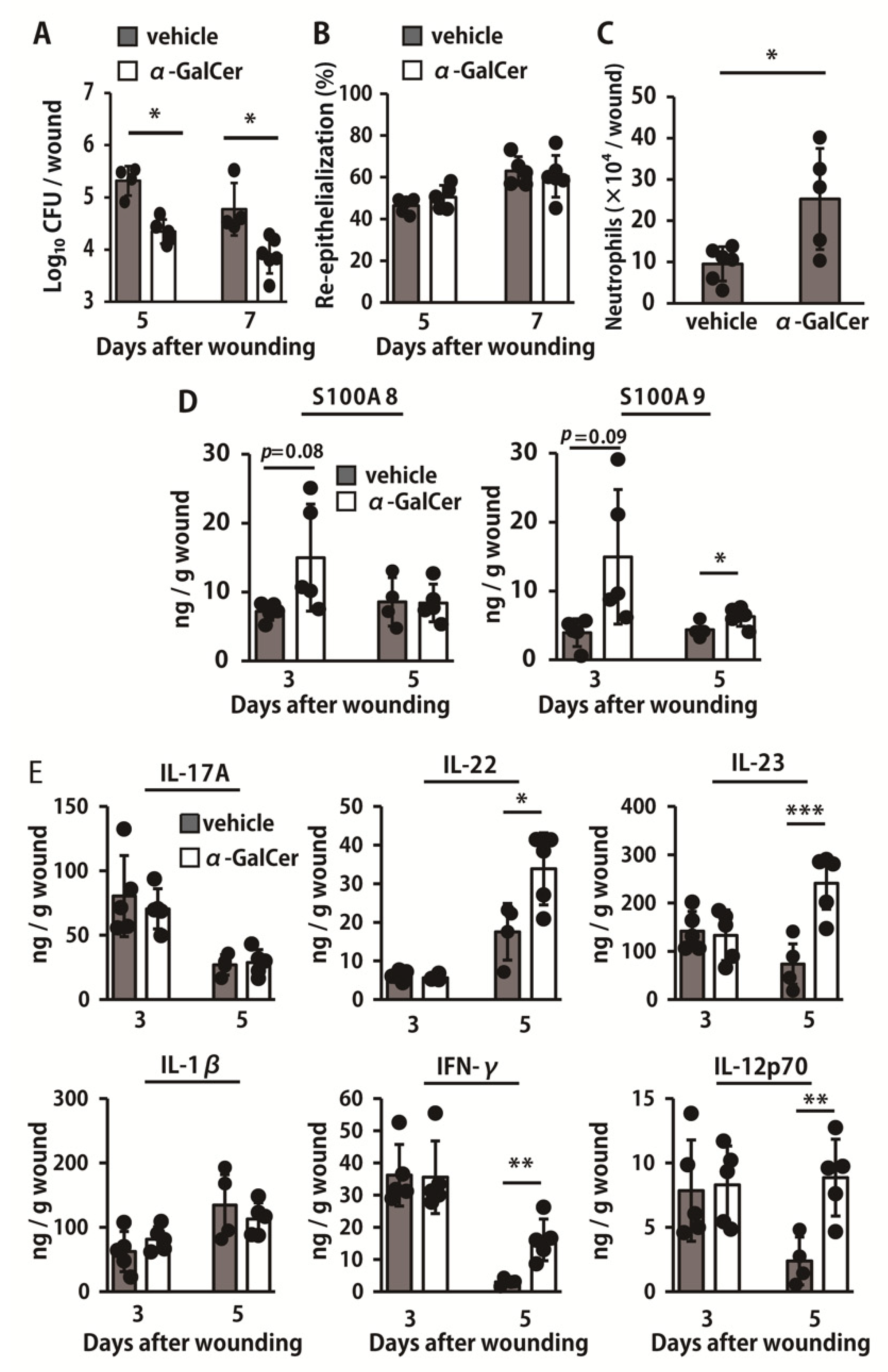
2.5. Effect of iNKT Cell Activation on the Elimination of P. aeruginosa
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Bacteria
4.3. Wound Creation and Tissue Collection
4.4. Reagents and Antibodies
4.5. Counting the Viable P. aeruginosa
4.6. Histological Analysis
4.7. RNA Extraction and Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.8. Preparation of Leukocytes in the Wounded Tissue
4.9. Flow Cytometry
4.10. Measurement of Cytokine Concentrations
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| iNKT cell | invariant natural killer T cell |
| IL | interleukin |
| IFN | interferon |
| AMP | antimicrobial peptide |
| KO | knockout |
| WT | wild type |
References
- Attinger, C.; Wolcott, R. Clinically Addressing Biofilm in Chronic Wounds. Adv. Wound Care 2012, 1, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Nusbaum, A.G.; Gil, J.; Patel, S.B.; Chen, J.; Valdes, J.; Stojadinovic, O.; Plano, L.R.; Tomic-Canic, M.; Davis, S.C. Interactions of Methicillin Resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in Polymicrobial Wound Infection. PLoS ONE 2013, 8, e56846. [Google Scholar] [CrossRef] [PubMed]
- Burmølle, M.; Thomsen, T.R.; Fazli, M.; Dige, I.; Christensen, L.; Homøe, P.; Tvede, M.; Nyvad, B.; Tolker-Nielsen, T.; Givskov, M.; et al. Biofilms in chronic infections—A matter of opportunity—Monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010, 59, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Sawa, T.; Allen, L.; Wiener-Kronish, J.; Hawgood, S. Surfactant Proteins A and D Enhance Pulmonary Clearance of Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 2006, 34, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Strieter, R.M.; Mehrad, B.; Newstead, M.W.; Zeng, X.; Standiford, T.J. CXC Chemokine Receptor CXCR2 is Essential for Protective Innate Host Response in Murine Pseudomonas aeruginosa Pneumonia. Infect. Immun. 2000, 68, 4289–4296. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Pittet, J.F.; Hong, K.; Folkesson, H.; Bagby, G.; Kobzik, L.; Frevert, C.; Watanabe, K.; Tsurufuji, S.; Wiener-Kronish, J. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am. J. Physiol. Content 1996, 270, L819–L828. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, E.E.S.; Matsumoto, T.; Exley, M.; Schleipman, R.A.; Glickman, J.; Bailey, D.T.; Corazza, N.; Colgan, S.P.; Onderdonk, A.B.; Blumberg, R.S. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat. Med. 2002, 8, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Wesselkamper, S.C.; Eppert, B.L.; Motz, G.T.; Lau, G.W.; Hassett, D.J.; Borchers, M.T. NKG2D is Critical for NK Cell Activation in Host Defense against Pseudomonas aeruginosa Respiratory Infection. J. Immunol. 2008, 181, 5481–5489. [Google Scholar] [CrossRef]
- Pène, F.; Zuber, B.; Courtine, E.; Rousseau, C.; Ouaaz, F.; Toubiana, J.; Tazi, A.; Mira, J.P.; Chiche, J.D. Dendritic Cells Modulate Lung Response to Pseudomonas aeruginosa in a Murine Model of Sepsis-Induced Immune Dysfunction. J. Immunol. 2008, 181, 8513–8520. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- MacLeod, A.S.; Hemmers, S.; Garijo, O.; Chabod, M.; Mowen, K.; Witherden, D.A.; Havran, W.L. Dendritic epidermal T cells regulate skin antimicrobial barrier function. J. Clin. Investig. 2013, 123, 4364–4374. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Li, D.; Li, C.; Muehleisen, B.; Radek, K.A.; Park, H.J.; Jiang, Z.; Li, Z.; Lei, H.; Quan, Y.; et al. The Antimicrobial Protein REG3A Regulates Keratinocyte Proliferation and Differentiation after Skin Injury. Immunity 2012, 37, 74–84. [Google Scholar] [CrossRef]
- Pichavant, M.; Sharan, R.; Le Rouzic, O.; Olivier, C.; Hennegrave, F.; Rémy, G.; Pérez-Cruz, M.; Koné, B.; Gosset, P.; Just, N.; et al. IL-22 Defect during Streptococcus pneumoniae Infection Triggers Exacerbation of Chronic Obstructive Pulmonary Disease. EBioMedicine 2015, 2, 1686–1696. [Google Scholar] [CrossRef]
- Arshad, T.; Mansur, F.; Palek, R.; Manzoor, S.; Liska, V. A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration. Front. Immunol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Takagi, N.; Kawakami, K.; Kanno, E.; Tanno, H.; Takeda, A.; Ishii, K.; Imai, Y.; Iwakura, Y.; Tachi, M. IL-17A promotes neutrophilic inflammation and disturbs acute wound healing in skin. Exp. Dermatol. 2017, 26, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Kawakami, K.; Kanno, E.; Tanno, H.; Tada, H.; Sato, N.; Masaki, A.; Yokoyama, R.; Kawamura, K.; Kitai, Y.; et al. Dectin-2–Mediated Signaling Leads to Delayed Skin Wound Healing through Enhanced Neutrophilic Inflammatory Response and Neutrophil Extracellular Trap Formation. J. Investig. Dermatol. 2019, 139, 702–711. [Google Scholar] [CrossRef]
- Tsagaratou, A. Unveiling the regulation of NKT17 cell differentiation and function. Mol. Immunol. 2019, 105, 55–61. [Google Scholar] [CrossRef]
- Crosby, C.M.; Kronenberg, M. Tissue-specific functions of invariant natural killer T cells. Nat. Rev. Immunol. 2018, 18, 559–574. [Google Scholar] [CrossRef]
- Tanno, H.; Kawakami, K.; Ritsu, M.; Kanno, E.; Suzuki, A.; Kamimatsuno, R.; Takagi, N.; Miyasaka, T.; Ishii, K.; Imai, Y.; et al. Contribution of Invariant Natural Killer T Cells to Skin Wound Healing. Am. J. Pathol. 2015, 185, 3248–3257. [Google Scholar] [CrossRef] [PubMed]
- Tanno, H.; Kawakami, K.; Kanno, E.; Suzuki, A.; Takagi, N.; Yamamoto, H.; Ishii, K.; Matuyama, R.; Tachi, M. Invariant NKT cells promote skin wound healing by preventing a prolonged neutrophilic inflammatory response. Wound Repair Regen. 2017, 25, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, N.M.S.; Bohmwald, K.; Pacheco, G.A.; Andrade, C.A.; Carreño, L.J.; Kalergis, A.M. Type I Natural Killer T Cells as Key Regulators of the Immune Response to Infectious Diseases. Clin. Microbiol. Rev. 2021, 34. [Google Scholar] [CrossRef] [PubMed]
- Dickerhof, N.; Isles, V.; Pattemore, P.; Hampton, M.B.; Kettle, A.J. Exposure of Pseudomonas aeruginosa to bactericidal hypochlorous acid during neutrophil phagocytosis is compromised in cystic fibrosis. J. Biol. Chem. 2019, 294, 13502–13514. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.Y.; Priebe, G.P.; Ray, C.; van Rooijen, N.; Pier, G.B. Inescapable Need for Neutrophils as Mediators of Cellular Innate Immunity to Acute Pseudomonas aeruginosa Pneumonia. Infect. Immun. 2009, 77, 5300–5310. [Google Scholar] [CrossRef]
- Benoit, P.; Sigounas, V.Y.; Thompson, J.L.; van Rooijen, N.; Poynter, M.E.; Wargo, M.J.; Boyson, J.E. The Role of CD1d-Restricted NKT Cells in the Clearance of Pseudomonas aeruginosa from the Lung is Dependent on the Host Genetic Background. Infect. Immun. 2015, 83, 2557–2565. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Feng, D.; Park, O.; Yin, S.; Gao, B. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: Opposite regulation by IL-4 and IFN-γ. Hepatology 2013, 58, 1474–1485. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, K.; Zhu, Q.; Wang, W.; Huang, F.; Miao, L.; Wu, X. β-catenin promotes intracellular bacterial killing via suppression of Pseudomonas aeruginosa-triggered macrophage autophagy. J. Int. Med. Res. 2017, 45, 556–569. [Google Scholar] [CrossRef]
- Leid, J.G.; Willson, C.J.; Shirtliff, M.E.; Hassett, D.J.; Parsek, M.R.; Jeffers, A.K. The Exopolysaccharide Alginate Protects Pseudomonas aeruginosa Biofilm Bacteria from IFN-γ-Mediated Macrophage Killing. J. Immunol. 2005, 175, 7512–7518. [Google Scholar] [CrossRef]
- Huang, X.; McClellan, S.A.; Barrett, R.P.; Hazlett, L.D. IL-18 Contributes to Host Resistance Against Infection with Pseudomonas aeruginosa Through Induction of IFN-γ Production. J. Immunol. 2002, 168, 5756–5763. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, D.; Wang, Y.; Liu, X.; Zhang, Y.; Qu, W.; Chen, K.; Francisco, N.M.; Feng, L.; Huang, X.; et al. Beta-Defensin 2 and 3 Promote Bacterial Clearance of Pseudomonas aeruginosa by Inhibiting Macrophage Autophagy through Downregulation of Early Growth Response Gene-1 and c-FOS. Front. Immunol. 2018, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Abtin, A.; Eckhart, L.; Gläser, R.; Gmeiner, R.; Mildner, M.; Tschachler, E. The Antimicrobial Heterodimer S100A8/S100A9 (Calprotectin) is Upregulated by Bacterial Flagellin in Human Epidermal Keratinocytes. J. Investig. Dermatol. 2010, 130, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; McClellan, S.A.; Barrett, R.P.; Zhang, Y.; Hazlett, L.D. β-Defensins 2 and 3 Together Promote Resistance to P. aeruginosa Keratitis. J. Immunol. 2009, 183, 8054–8060. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; McClellan, S.A.; Barrett, R.P.; Hazlett, L.D. β-Defensin-2 Promotes Resistance against Infection with P. aeruginosa. J. Immunol. 2009, 182, 1609–1616. [Google Scholar] [CrossRef]
- Morrison, G.M.; Davidson, D.J.; Kilanowski, F.M.; Borthwick, D.W.; Crook, K.; Maxwell, A.I.; Govan, J.R.; Dorin, J.R. Mouse beta defensin-1 is a functional homolog of human beta defensin-1. Mamm. Genome 1998, 9, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.J.; Brigl, M.; Brenner, M.B. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013, 13, 101–117. [Google Scholar] [CrossRef]
- Díaz-Basabe, A.; Strati, F.; Facciotti, F. License to Kill: When iNKT Cells are Granted the Use of Lethal Cytotoxicity. Int. J. Mol. Sci. 2020, 21, 3909. [Google Scholar] [CrossRef] [PubMed]
- Siegemund, S.; Schütze, N.; Freudenberg, M.A.; Lutz, M.B.; Straubinger, R.K.; Alber, G. Production of IL-12, IL-23 and IL-27p28 by bone marrow-derived conventional dendritic cells rather than macrophages after LPS/TLR4-dependent induction by Salmonella Enteritidis. Immunobiology 2008, 212, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Petes, C.; Wynick, C.; Guzzo, C.; Mehta, D.; Logan, S.; Banfield, B.W.; Basta, S.; Cooper, A.; Gee, K. IL-27 enhances LPS-induced IL-1β in human monocytes and murine macrophages. J. Leukoc. Biol. 2017, 102, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, N.A.; Kronenberg, M. Invariant NKT Cells Amplify the Innate Immune Response to Lipopolysaccharide. J. Immunol. 2007, 178, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Sato, K.; Yamamoto, H.; Kasamatsu, J.; Miyasaka, T.; Tanno, D.; Miyahara, A.; Kagesawa, T.; Oniyama, O.; Kawamura, K.; et al. Limited Role of Mincle in the Host Defense against Infection with Cryptococcus deneoformans. Infect. Immun. 2020, 88. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, A.; Maltha, J.; Hoff, J.V.D.; Kuijpers-Jagtman, A. Local injection of IFN-gamma reduces the number of myofibroblasts and the collagen content in palatal wounds. J. Dent. Res. 2000, 79, 1782–1788. [Google Scholar] [CrossRef]
- Keast, D.H.; Bowering, C.K.; Evans, A.W.; Mackean, G.L.; Burrows, C.; D’Souza, L. Contents. Wound Repair Regen. 2004, 12, s1–s17. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.T.; Han, Y.-P.; Yan, C.; Shaw, M.C.; Garner, W.L. TNF-α Suppresses α-Smooth Muscle Actin Expression in Human Dermal Fibroblasts: An Implication for Abnormal Wound Healing. J. Investig. Dermatol. 2007, 127, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shin, T.; Kawano, T.; Sato, H.; Kondo, E.; Toura, I.; Kaneko, Y.; Koseki, H.; Kanno, M.; Taniguchi, M. Requirement for Vα14 NKT Cells in IL-12-Mediated Rejection of Tumors. Science 1997, 278, 1623–1626. [Google Scholar] [CrossRef] [PubMed]
- Kanno, E.; Kawakami, K.; Ritsu, M.; Ishii, K.; Tanno, H.; Toriyabe, S.; Imai, Y.; Maruyama, R.; Tachi, M. Wound healing in skin promoted by inoculation with Pseudomonas aeruginosa PAO1: The critical role of tumor necrosis factor-α secreted from infiltrating neutrophils. Wound Repair Regen. 2011, 19, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Kanno, E.; Tanno, H.; Sasaki, A.; Kitai, Y.; Miura, T.; Takagi, N.; Shoji, M.; Kasamatsu, J.; Sato, K.; et al. Distinct roles for Dectin-1 and Dectin-2 in skin wound healing and neutrophilic inflammatory responses. J. Investig. Dermatol. 2020, 141, 164–176.e8. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanno, H.; Kanno, E.; Sato, S.; Asao, Y.; Shimono, M.; Kurosaka, S.; Oikawa, Y.; Ishi, S.; Shoji, M.; Sato, K.; et al. Contribution of Invariant Natural Killer T Cells to the Clearance of Pseudomonas aeruginosa from Skin Wounds. Int. J. Mol. Sci. 2021, 22, 3931. https://doi.org/10.3390/ijms22083931
Tanno H, Kanno E, Sato S, Asao Y, Shimono M, Kurosaka S, Oikawa Y, Ishi S, Shoji M, Sato K, et al. Contribution of Invariant Natural Killer T Cells to the Clearance of Pseudomonas aeruginosa from Skin Wounds. International Journal of Molecular Sciences. 2021; 22(8):3931. https://doi.org/10.3390/ijms22083931
Chicago/Turabian StyleTanno, Hiromasa, Emi Kanno, Suzuna Sato, Yu Asao, Mizuki Shimono, Shiho Kurosaka, Yukari Oikawa, Shinyo Ishi, Miki Shoji, Ko Sato, and et al. 2021. "Contribution of Invariant Natural Killer T Cells to the Clearance of Pseudomonas aeruginosa from Skin Wounds" International Journal of Molecular Sciences 22, no. 8: 3931. https://doi.org/10.3390/ijms22083931
APA StyleTanno, H., Kanno, E., Sato, S., Asao, Y., Shimono, M., Kurosaka, S., Oikawa, Y., Ishi, S., Shoji, M., Sato, K., Kasamatsu, J., Miyasaka, T., Yamamoto, H., Ishii, K., Imai, Y., Tachi, M., & Kawakami, K. (2021). Contribution of Invariant Natural Killer T Cells to the Clearance of Pseudomonas aeruginosa from Skin Wounds. International Journal of Molecular Sciences, 22(8), 3931. https://doi.org/10.3390/ijms22083931





