Evolution of Flavylium-Based Color Systems in Plants: What Physical Chemistry Can Tell Us
Abstract
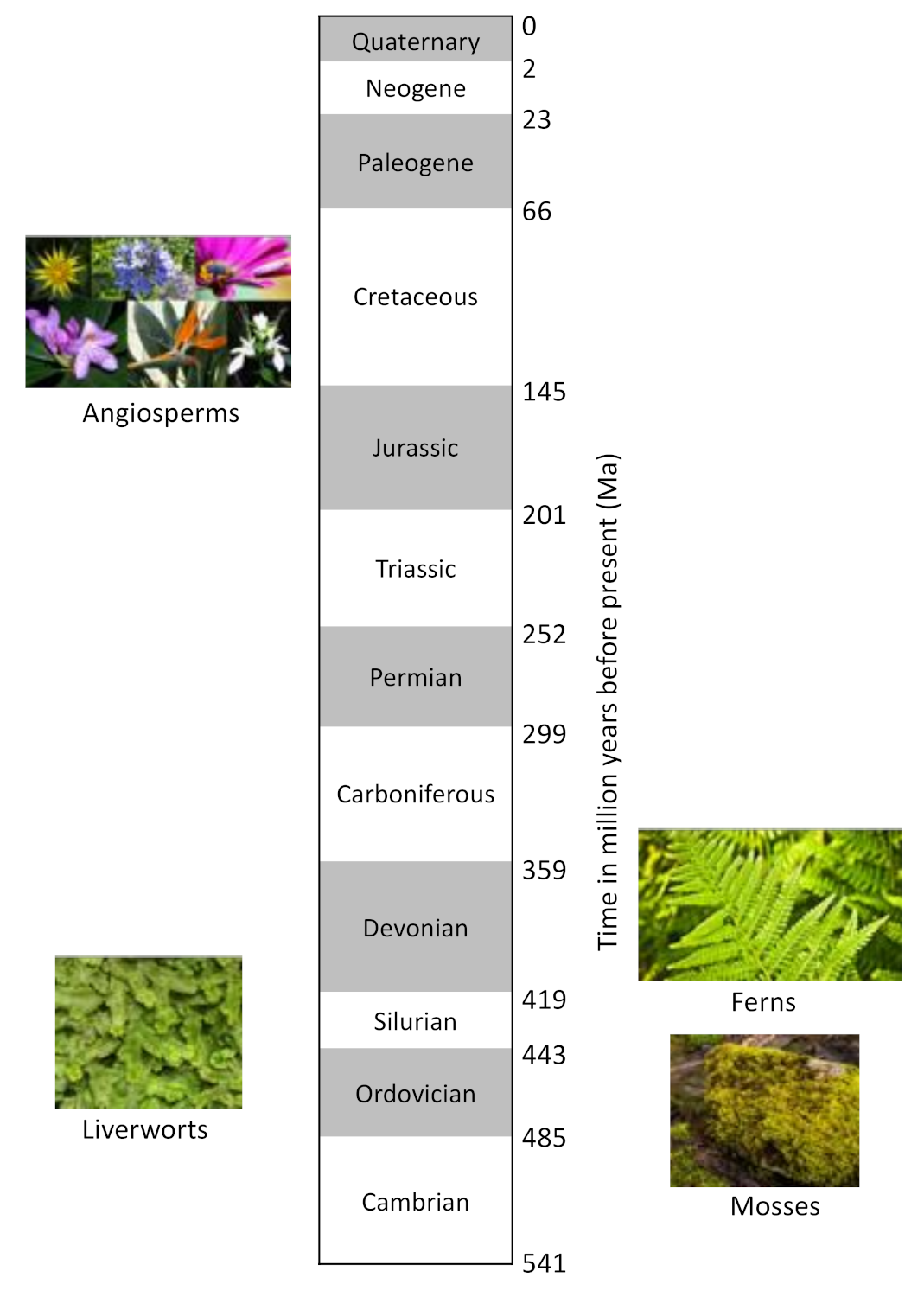
1. Introduction
2. Results and Discussion
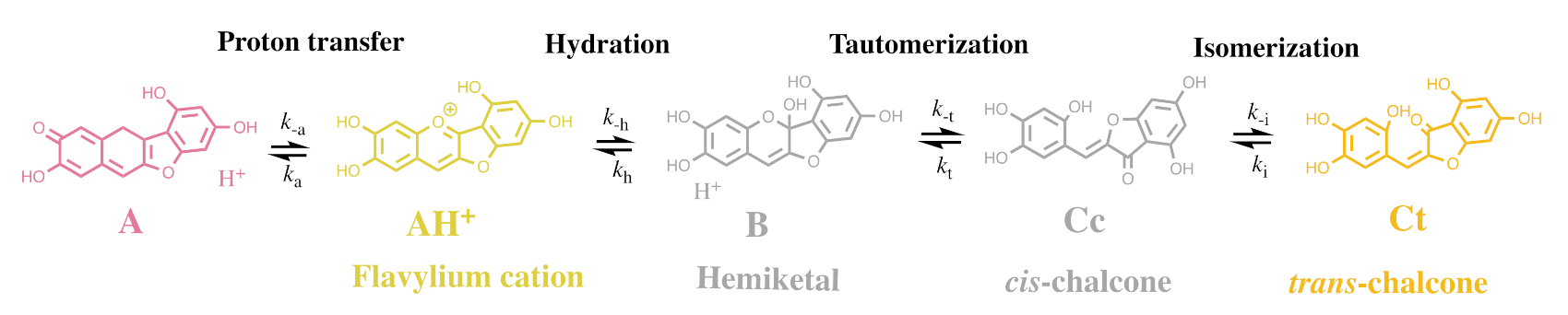
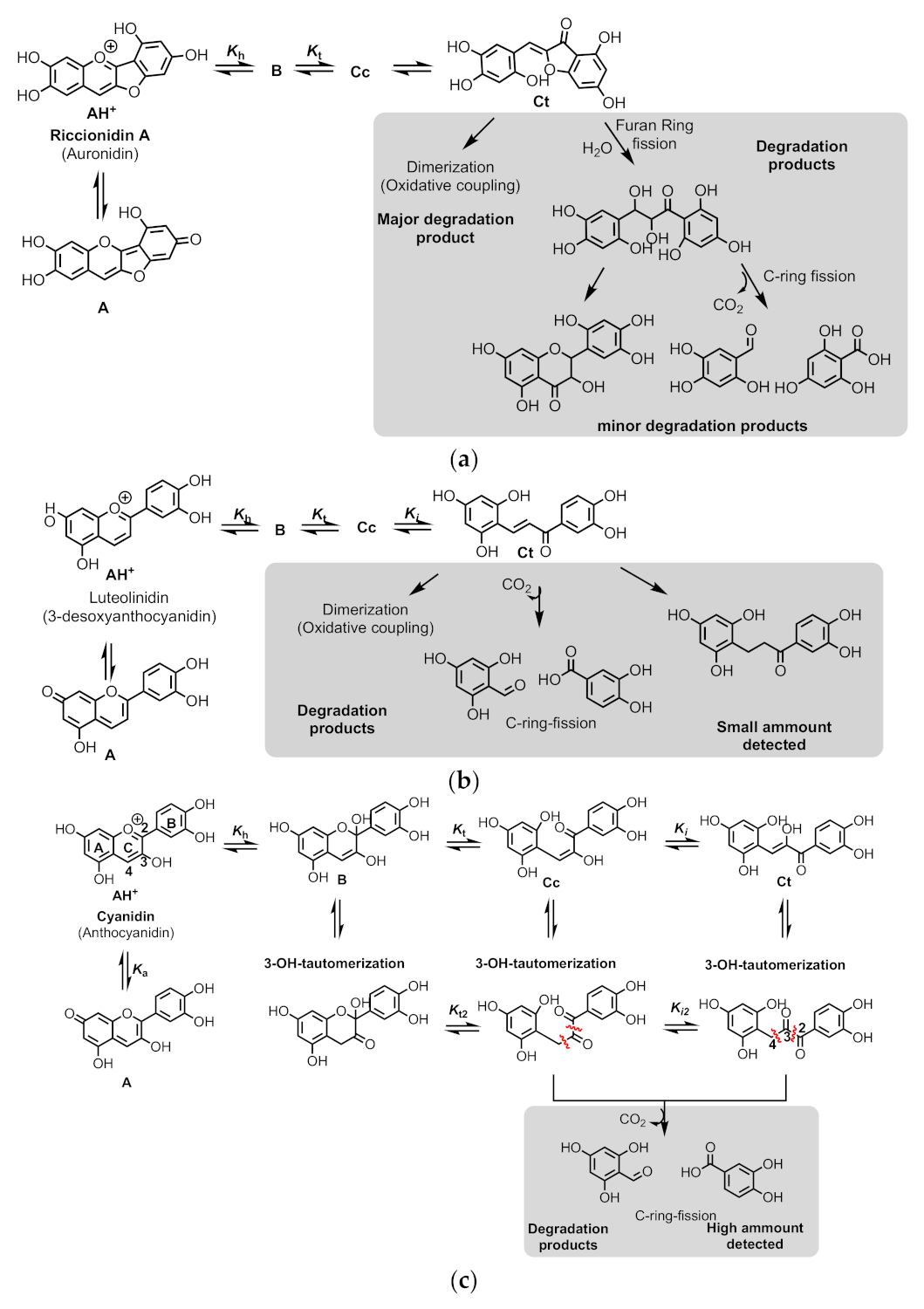
2.1. The Color System of Anthocyanins. The Case of Cyanidin-3-O-Glucoside (Kuromanin)
2.2. Anthocyanin Multistate
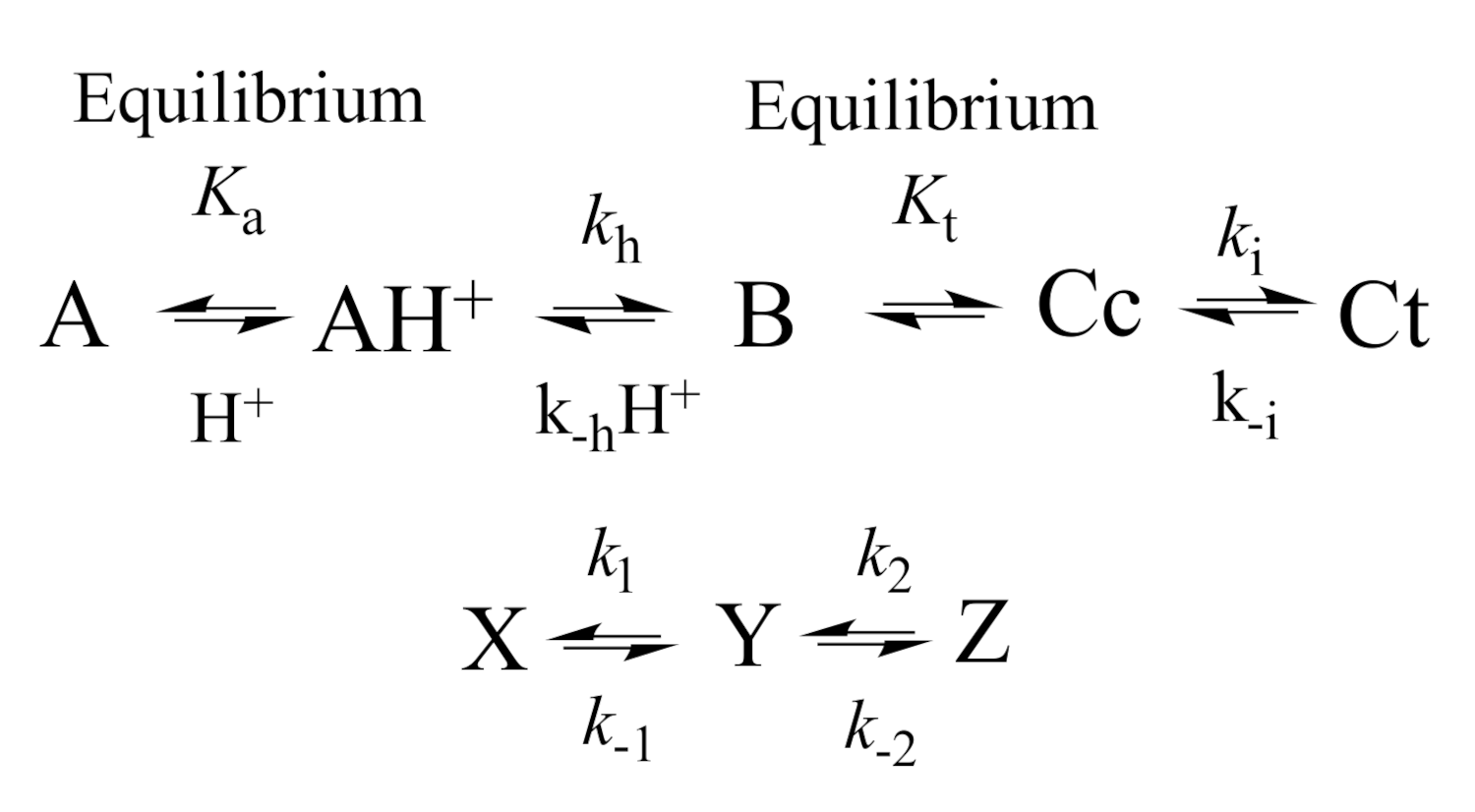
2.3. How to Calculate All Equilibrium Constants. Reverse pH Jumps: A New Paradigm
2.4. Limits of Anthocyanins to Confer Colour
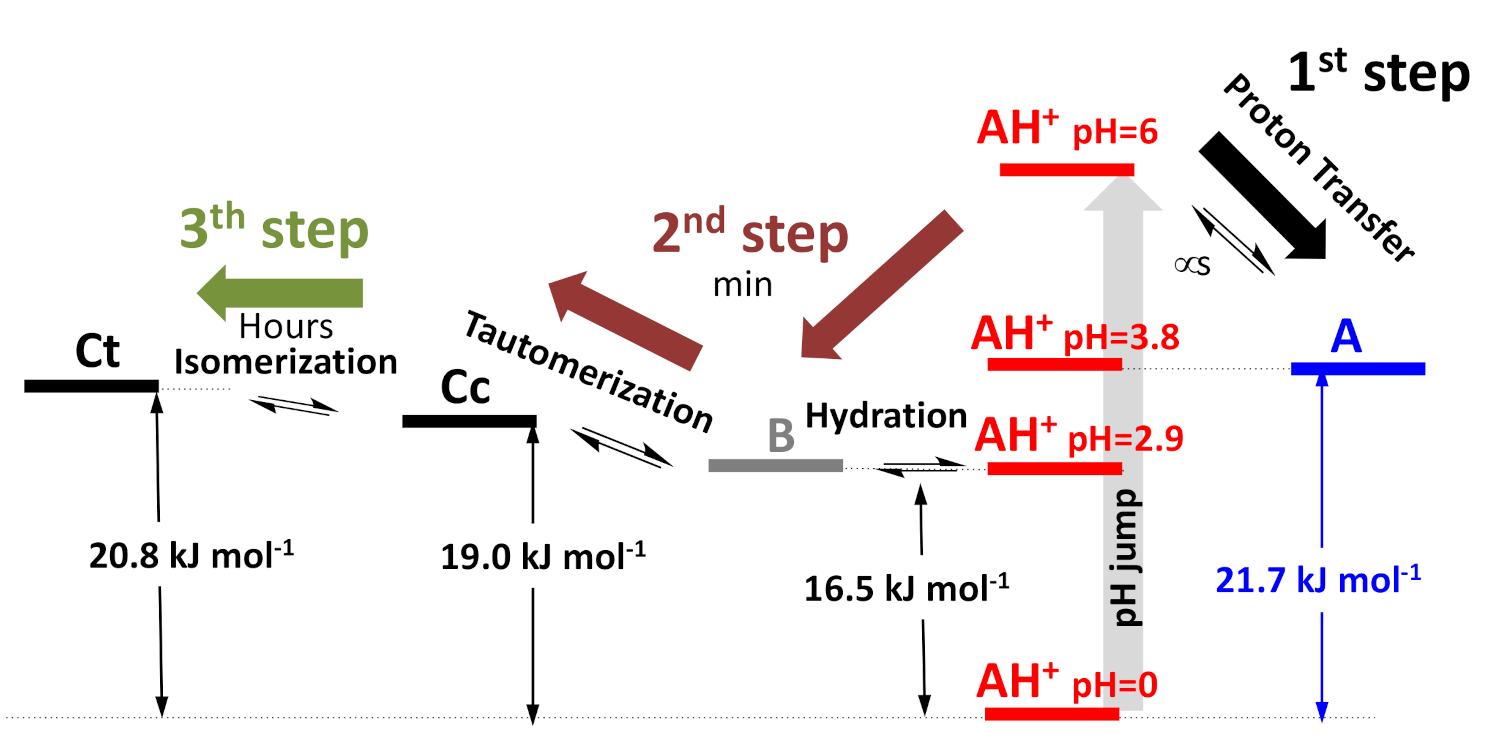
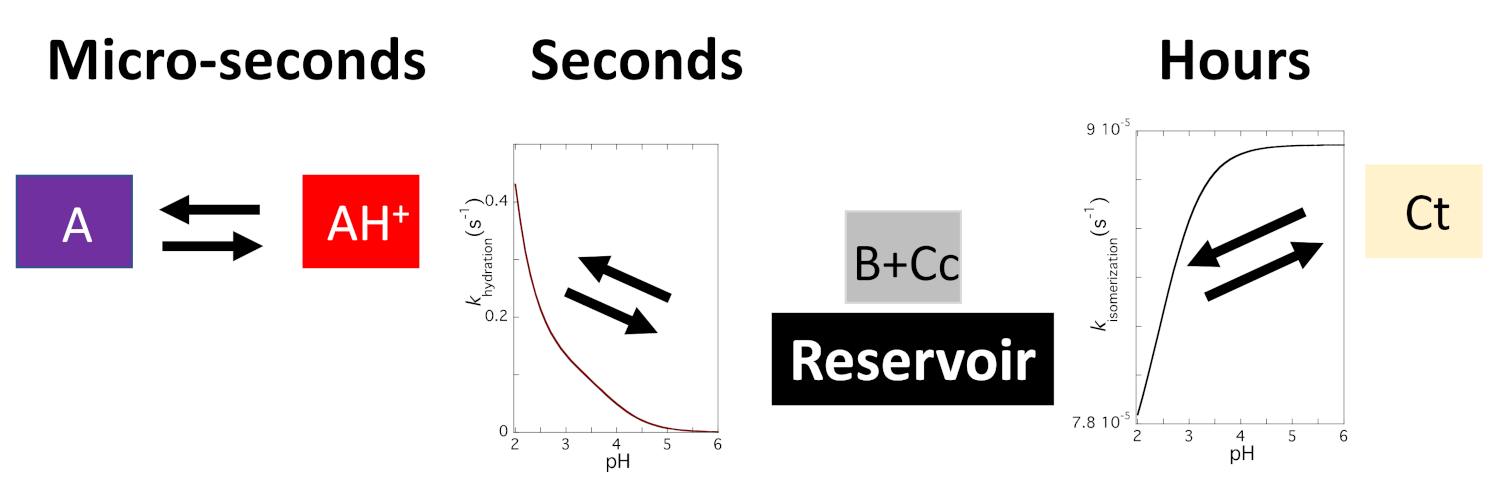
2.5. The Kinetics of the Multistate
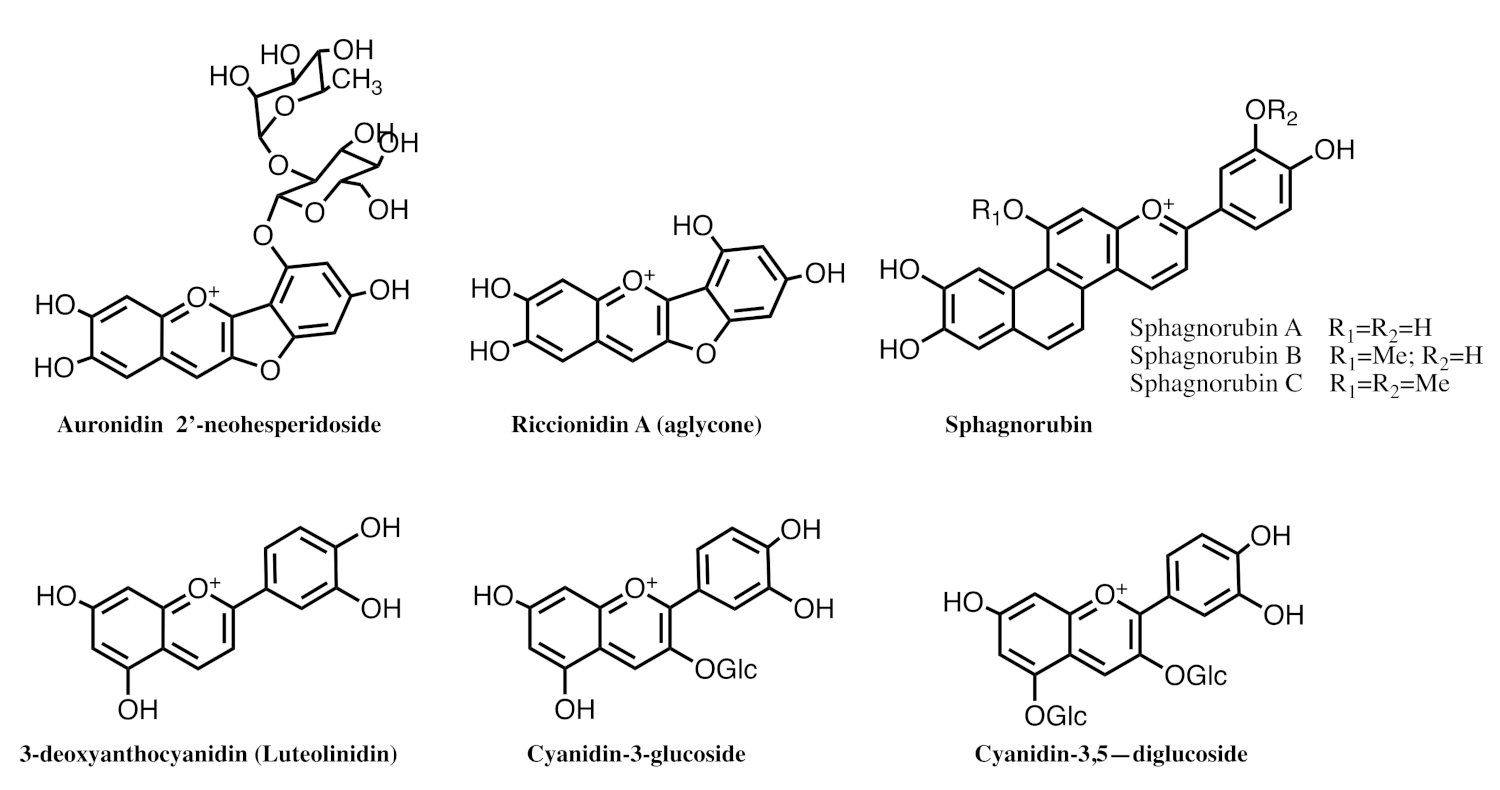
2.6. The Colour System of 3-deoxyanthocyanins
2.7. Comparison of the Colour System in Anthocyanins and 3-Deoxyanthocyanidins
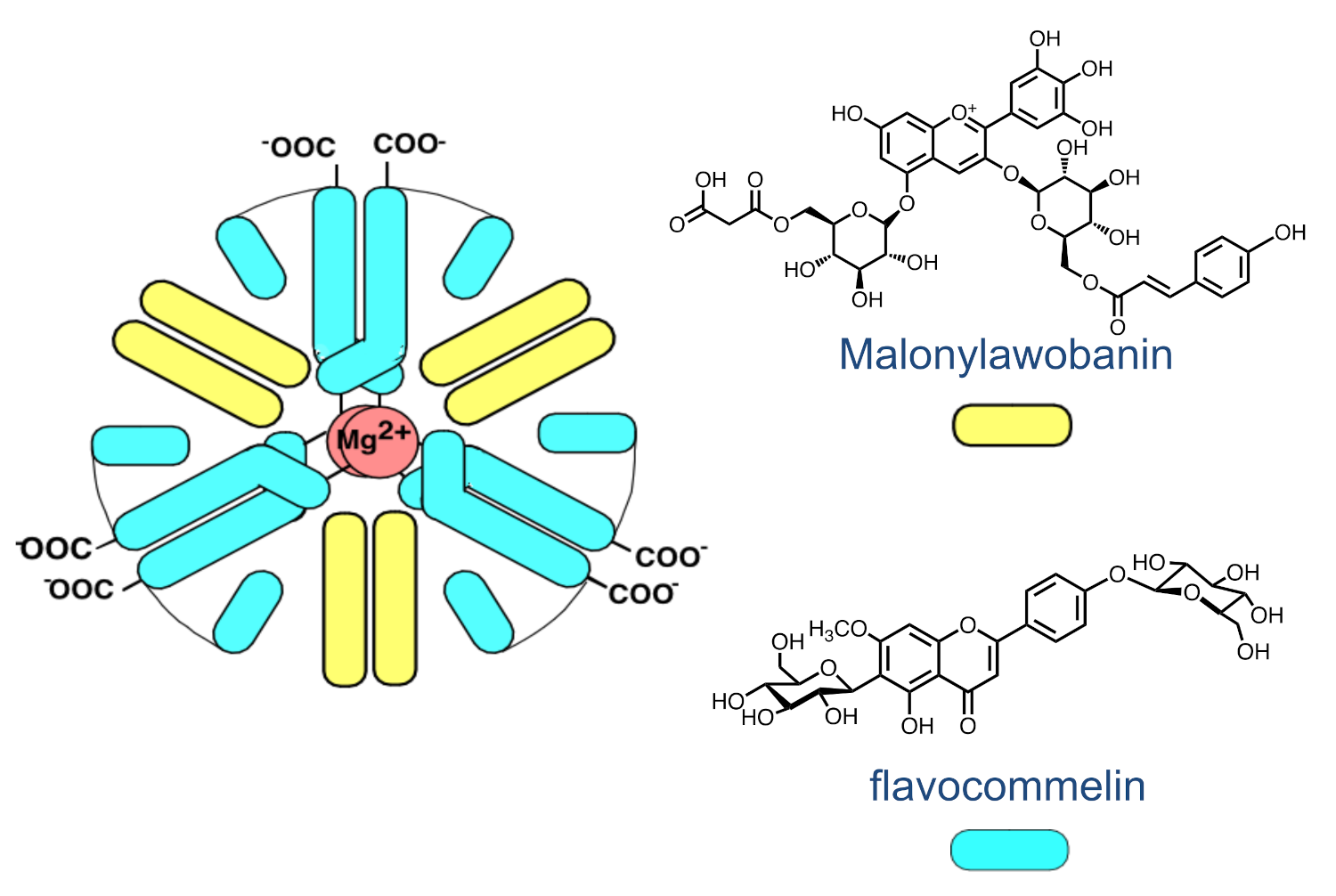
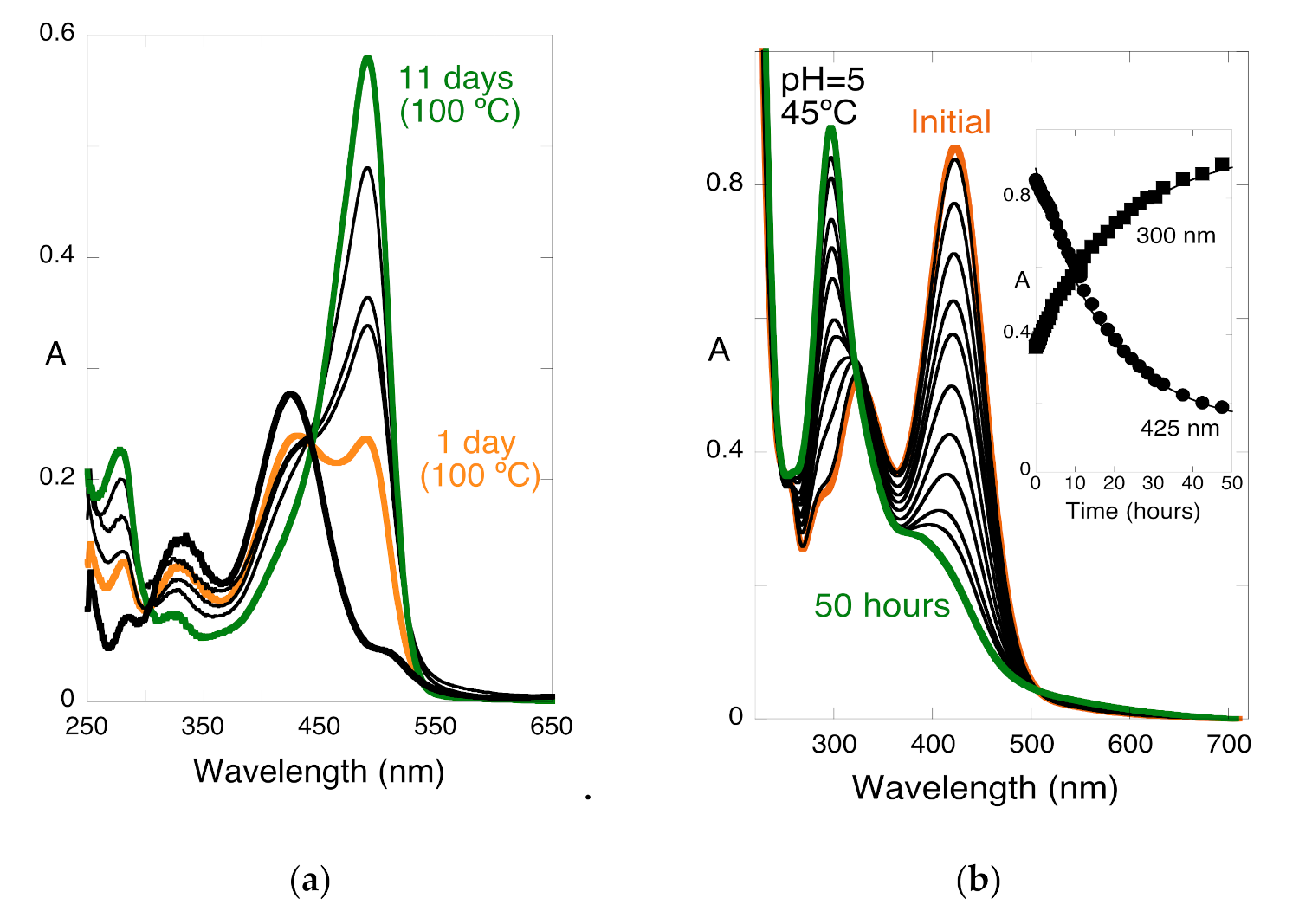
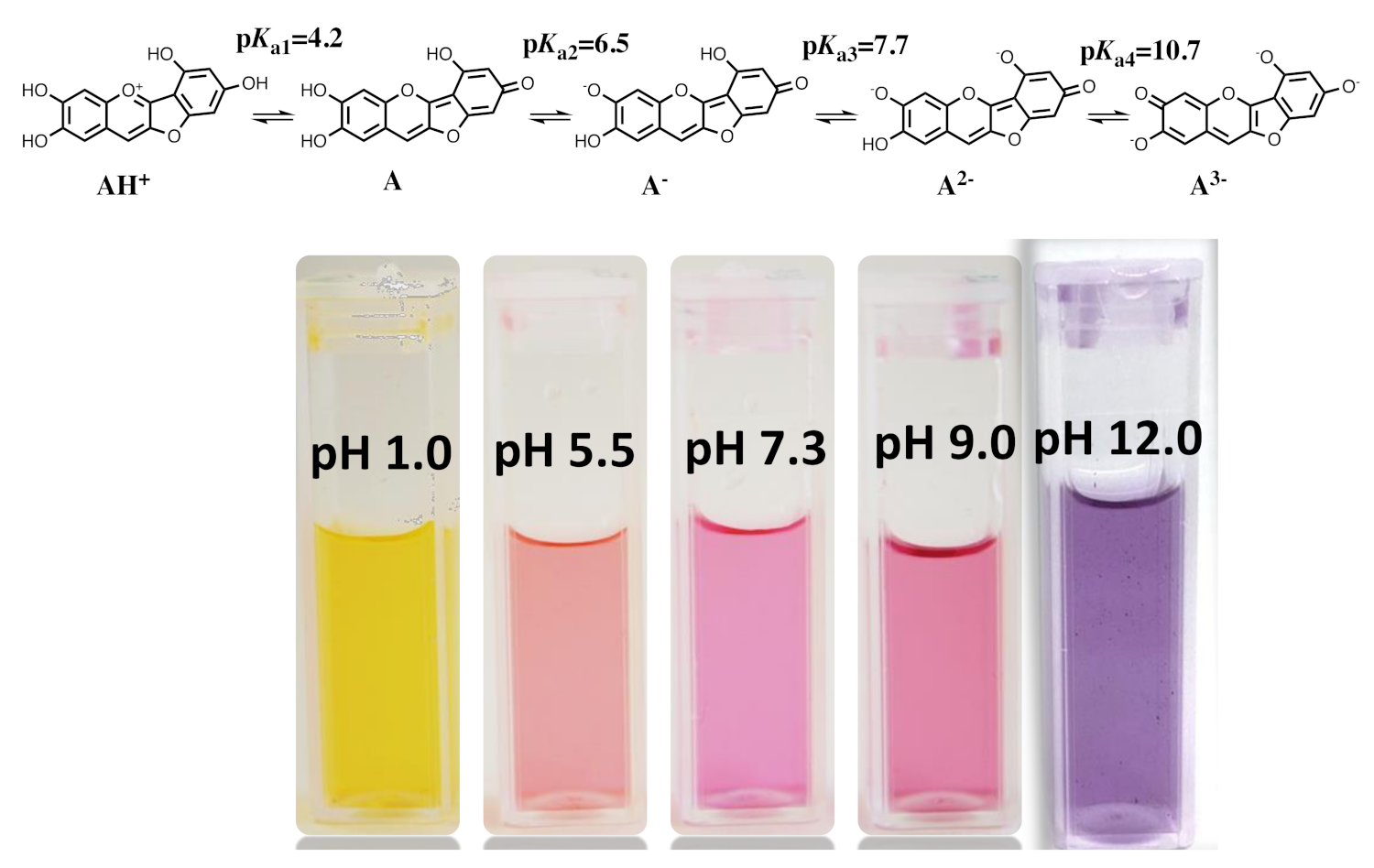
2.8. The Colour System of Auronidins
2.9. Comparison between the Auronidins and the Other Systems
3. Experimental
Thermodynamic and Kinetic Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morris, J.L.; Puttick, M.N.; Clark, J.W.; Edwards, D.; Kenrick, P.; Pressel, S.; Wellman, C.H.; Yang, Z.; Schneider, H.; Donoghue, P.C.J. The timescale of early land plant evolution. Proc. Natl. Acad. Sci. USA 2018, 115, E2274–E2283. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, J.; Hentschel, J.; Wilson, R.; Feldberg, K.; Schneider, H. Evolution of leafy liverworts (Jungermanniidae, Marchantiophyta): Estimating divergence times from chloroplast DNA sequences using penalized likelihood with integrated fossil evidence. Taxon 2007, 56, 31–44. [Google Scholar] [CrossRef]
- Cooper, E.D.; Henwood, M.J.; Brown, E.A. Are the liverworts really that old? Cretaceous origins and Cenozoic diversifications in Lepidoziaceae reflect a recurrent theme in liverwort evolution. Biol. J. Linn. Soc. 2012, 107, 425–441. [Google Scholar] [CrossRef]
- Puttick, M.N.; Morris, J.L.; Williams, T.A.; Cox, C.J.; Edwards, D.; Kenrick, P.; Pressel, S.; Wellman, C.H.; Schneider, H.; Pisani, D.; et al. The interrelationships of land plants and the nature of the ancestral embryophyte. Curr. Biol. 2018, 28, 733–745.e2. [Google Scholar] [CrossRef] [PubMed]
- Pryer, K.M.; Schuettpelz, E.; Wolf, P.G.; Schneider, H.; Smith, A.R.; Cranfill, R. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. Am. J. Bot. 2004, 91, 1582–1598. [Google Scholar] [CrossRef]
- Herendeen, P.S.; Friis, E.M.; Pedersen, K.R.; Crane, P.R. Palaeobotanical redux: Revisiting the age of the angiosperms. Nat. Plants 2017, 3, 17015. [Google Scholar] [CrossRef]
- Clifford, M.N. Anthocyanins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Vowinkel, E. Cell-wall pigments of peat mosses. 2. Structure of sphagnorubin. Chem. Ber. Recl. 1975, 108, 1166–1181. [Google Scholar] [CrossRef]
- Berland, H.; Andersen, Ø.M. Characterization of a natural, stable, reversible and colourful anthocyanidin network from sphagnum moss based mainly on the yellow trans-chalcone and red flavylium cation forms. Molecules 2021, 26, 709. [Google Scholar] [CrossRef]
- Berland, H.; Albert, N.W.; Stavland, A.; Jordheim, M.; McGhie, T.K.; Zhou, Y.; Zhang, H.; Deroles, S.C.; Schwinn, K.E.; Jordan, B.R.; et al. Auronidins are a previously unreported class of flavonoid pigments that challenges when anthocyanin biosynthesis evolved in plants. Proc. Natl. Acad. Sci. USA 2019, 116, 20232–20239. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Parola, A.J.; Pina, F. pH-dependent multistate system generated by a synthetic Furanoflavylium compound: An ancestor of the anthocyanin multistate of chemical species. ACS Omega 2019, 4, 4091–4100. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Basilio, N.; Freitas, A.A.; Maçanita, A.L.; Lima, J.C.; Parola, A.J.; Pina, F. Ground and excited state properties of furanoflavylium derivatives. Phys. Chem. Chem. Phys. 2019, 21, 21651–21662. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Mendoza, J.; Parola, A.J.; Pina, F. Chemical evolution of the colour systems generated by riccionidin A, 3-deoxyanthocyanidins and anthocyanins. Phytochemistry 2020, 174, 11. [Google Scholar] [CrossRef]
- Oliveira, J.; Azevedo, J.; Teixeira, N.; Araújo, P.; de Freitas, V.; Basílio, N.; Pina, F. On the limits of anthocyanins co-pigmentation models and respective equations. J. Agric. Food Chem. 2021, 69, 1359–1367. [Google Scholar] [CrossRef]
- Piatkowski, B.T.; Imwattana, K.; Tripp, E.A.; Weston, D.J.; Healey, A.; Schmutz, J.; Shaw, A.J. Phylogenomics reveals convergent evolution of red-violet coloration in land plants and the origins of the anthocyanin biosynthetic pathway. Mol. Phylogenet. Evol. 2020, 151, 106904. [Google Scholar] [CrossRef]
- Davies, K.M.; Jibran, R.; Zhou, Y.; Albert, N.W.; Brummell, D.A.; Jordan, B.R.; Bowman, J.L.; Schwinn, K.E. The evolution of flavonoid biosynthesis: A bryophyte perspective. Front. Plant Sci. 2020, 11, 7. [Google Scholar] [CrossRef]
- Brouillard, R.; Dubois, J.-E. Mechanism of the structural transformations of anthocyanins in acidic media. J. Am. Chem. Soc. 1977, 99, 1359–1364. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Maestri, M.; Ballardini, R.; Balzani, V. Photochromism of 4′-Methoxyflavylium Perchlorate. A “write−lock−read−unlock−erase” molecular switching system. J. Am. Chem. Soc. 1997, 119, 5556–5561. [Google Scholar] [CrossRef]
- Basílio, N.; Pina, F. Chemistry and photochemistry of anthocyanins and related compounds: A thermodynamic and kinetic approach. Molecules 2016, 21, 1502. [Google Scholar] [CrossRef]
- Brouillard, R.; Iacobucci, G.A.; Sweeny, J.G. Chemistry of anthocyanin pigments. UV-visible spectrophotometric determination of the acidity constants of apigeninidin and three related 3-deoxyflavylium salts. J. Am. Chem. Soc. 1982, 104, 7585–7590. [Google Scholar] [CrossRef]
- Brouillard, R.; Delaporte, B.; Dubois, J.E. Chemistry of anthocyanin pigments. Relaxation amplitudes in pH-jump experiments. J. Am. Chem. Soc. 1978, 100, 6202–6205. [Google Scholar] [CrossRef]
- McClelland, R.A.; Gedge, S. Hydration of the flavylium ion. J. Am. Chem. Soc. 1980, 102, 5838–5848. [Google Scholar] [CrossRef]
- Mendoza, J.; Basílio, N.; De Freitas, V.; Pina, F. New procedure to calculate all equilibrium constants in flavylium compounds: Application to the copigmentation of anthocyanins. ACS Omega 2019, 4, 12058–12070. [Google Scholar] [CrossRef] [PubMed]
- Pina, F. Anthocyanins and related compounds. Detecting the change of regime between rate control by hydration or by tautomerization. Dye. Pigment. 2014, 102, 308–314. [Google Scholar] [CrossRef]
- Felle, H.H. pH Regulation in anoxic plants. Ann. Bot. 2005, 96, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, E.; Burns, J.; Felle, H. Compartmentation and cellular conditions controlling sucrose breakdown in mature acid lime fruits. Phytochemistry 1992, 31, 4091–4095. [Google Scholar] [CrossRef]
- Yoshida, K.; Kondo, T.; Okazaki, Y.; Katou, K. Cause of blue petal colour. Nature 1995, 373, 291. [Google Scholar] [CrossRef]
- Araújo, P.; Basílio, N.; Fernandes, A.; Mateus, N.; De Freitas, V.; Piña, F.; Oliveira, J. Impact of lignosulfonates on the thermodynamic and kinetic parameters of malvidin-3-o-glucoside in aqueous solutions. J. Agric. Food Chem. 2018, 66, 6382–6387. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-Garcia, J.-C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Robinson, G.M.; Robinson, R. A survey of anthocyanins. I. Biochem. J. 1931, 25, 1687–1705. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Bechtold, L.; Schweiggert, R.M.; Carle, R. Co-pigmentation of pelargonidin derivatives in strawberry and red radish model solutions by the addition of phenolic fractions from mango peels. Food Chem. 2016, 213, 625–634. [Google Scholar] [CrossRef]
- Iwashina, T. Contribution to flower colors of flavonoids including anthocyanins: A review. Nat. Prod. Commun. 2015, 10, 529–544. [Google Scholar] [CrossRef]
- Mendoza, J.; Oliveira, J.; Araújo, P.; Basílio, N.; Teixeira, N.; Brás, N.F.; Pina, F.; Yoshida, K.; De Freitas, V. The peculiarity of malvidin 3-O-(6-O-p-coumaroyl) glucoside aggregation. Intra and intermolecular interactions. Dye. Pigment. 2020, 180, 108382. [Google Scholar] [CrossRef]
- Qian, B.-J.; Liu, J.-H.; Zhao, S.-J.; Cai, J.-X.; Jing, P. The effects of gallic/ferulic/caffeic acids on colour intensification and anthocyanin stability. Food Chem. 2017, 228, 526–532. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef]
- Maçanita, A.L.; Moreira, P.F.; Lima, J.C.; Quina, F.H.; Yihwa, C.; Vautier-Giongo, C. Proton transfer in anthocyanins and related flavylium salts. determination of ground-state rate constants with nanosecond laser flash photolysis. J. Phys. Chem. A 2002, 106, 1248–1255. [Google Scholar] [CrossRef]
- Pina, F. Chemical applications of anthocyanins and related compounds. a source of bioinspiration. J. Agric. Food Chem. 2014, 62, 6885–6897. [Google Scholar] [CrossRef]
- Hondo, T.; Yoshida, K.; Nakagawa, A.; Kawai, T.; Tamura, H.; Goto, T. Structural basis of blue-colour development in flower petals from Commelina communis. Nature 1992, 358, 515–518. [Google Scholar] [CrossRef]
- Cabrita, L.; Petrov, V.; Pina, F. On the thermal degradation of anthocyanidins: Cyanidin. RSC Adv. 2014, 4, 18939–18944. [Google Scholar] [CrossRef]
- Melo, M.J.; Moura, S.; Roque, A.; Maestri, M.; Pina, F. Photochemistry of luteolinidin—“Write-lock-read-unlock-erase” with a natural compound. J. Photochem. Photobiol. A Chem. 2000, 135, 33–39. [Google Scholar] [CrossRef]
- Chakravarty, G.; Seshadri, T.R. Study of 3,2-furanoflavylium chlorides. Indian J. Chem. 1964, 2, 319. [Google Scholar]
- Snell, K.R.S.; Kokubun, T.; Griffiths, H.; Convey, P.; Hodgson, D.A.; Newsham, K.K. Quantifying the metabolic cost to an Antarctic liverwort of responding to an abrupt increase in UVB radiation exposure. Glob. Chang. Biol. 2009, 15, 2563–2573. [Google Scholar] [CrossRef]
- Kunz, S.; Becker, H. Cell Wall pigment formation of in vitro cultures of the liverwort Ricciocarpos natans. Z. Nat. C 1995, 50, 235–240. [Google Scholar] [CrossRef][Green Version]
- Taniguchi, S.; Yazaki, K.; Yabu-Uchi, R.; Kawakami, K.-Y.; Ito, H.; Hatano, T.; Yoshida, T. Galloylglucoses and riccionidin A in Rhus javanica adventitious root cultures. Phytochemistry 2000, 53, 357–363. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Qin, Y.; Mao, Y.; Zhang, B.; Deng, Z. Effects of heat, ultrasound, and microwave processing on the stability and antioxidant activity of delphinidin and petunidin. J. Food Biochem. 2019, 43, e12818. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dykes, L.; Awika, J.M. Thermal stability of 3-deoxyanthocyanidin pigments. Food Chem. 2014, 160, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.; Gomes, R.; Parola, A.J.; Jesus, A.; Laia, C.A.; Pina, F. 2′-Hydroxyflavylium: Introducing flavanones into the flavylium network of chemical reactions. Tetrahedron 2008, 64, 714–720. [Google Scholar] [CrossRef]
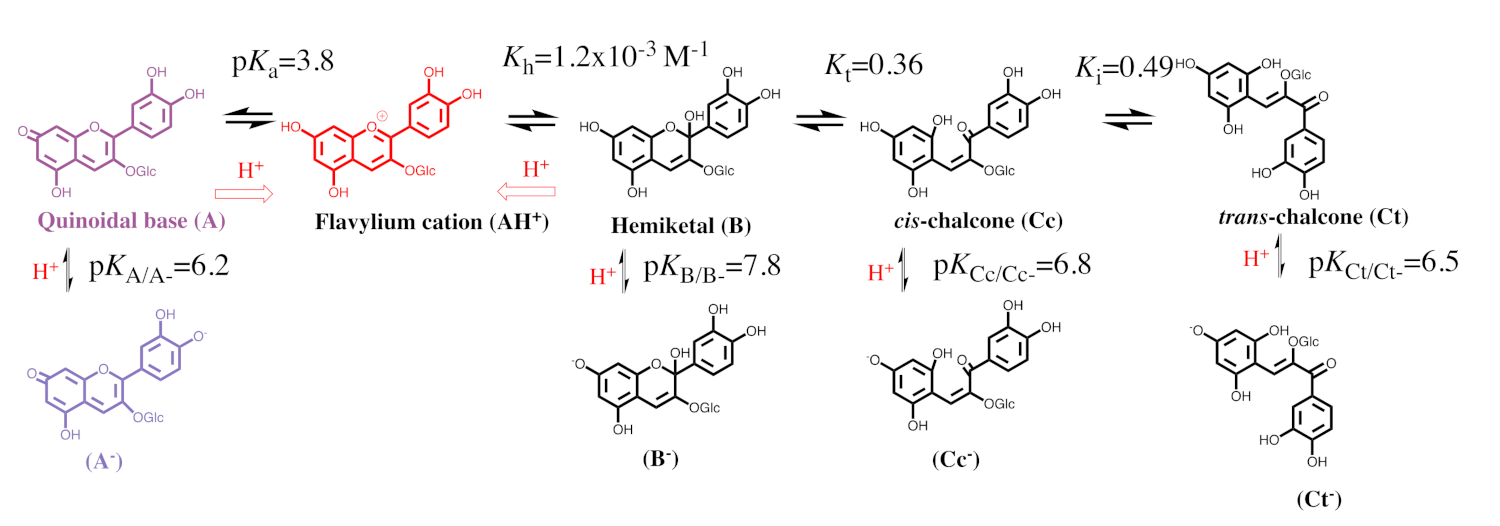

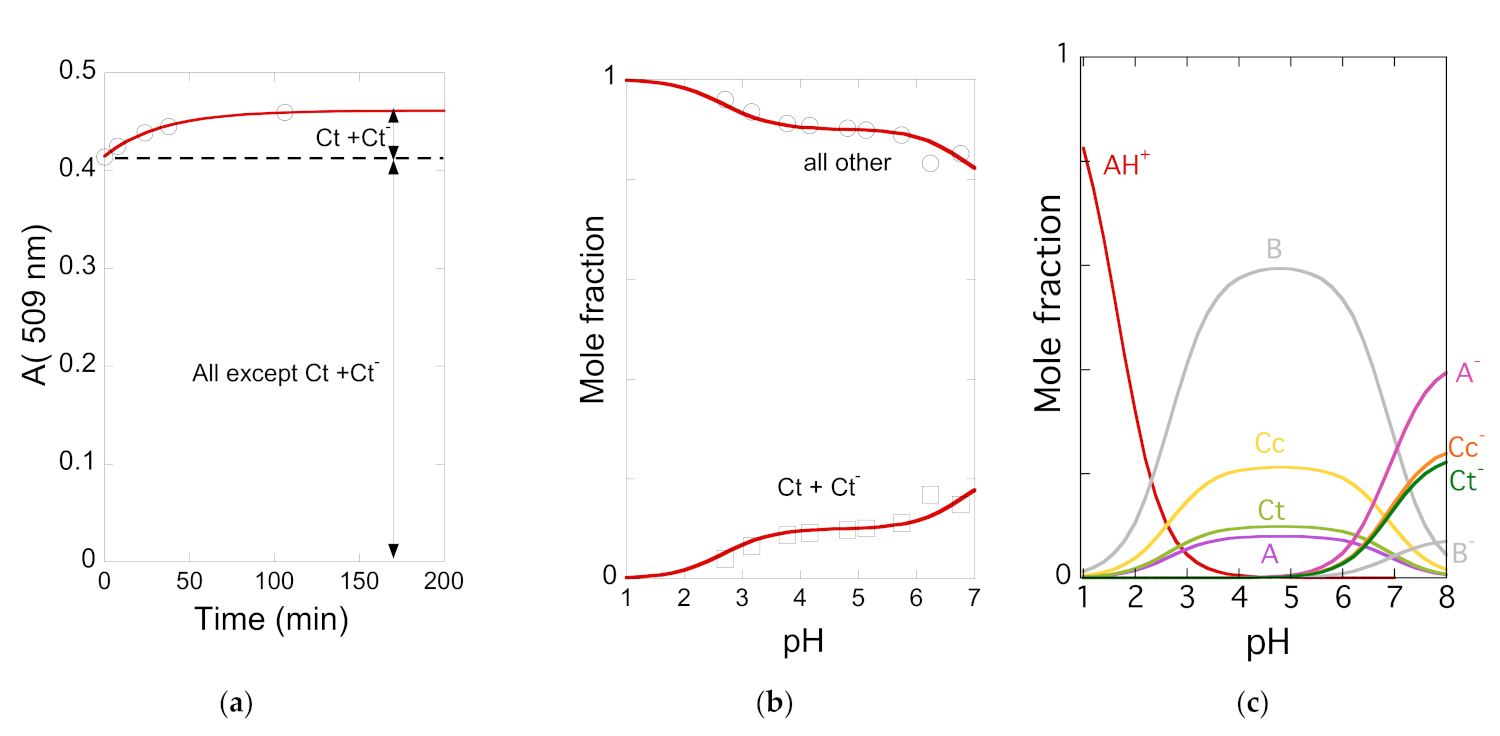
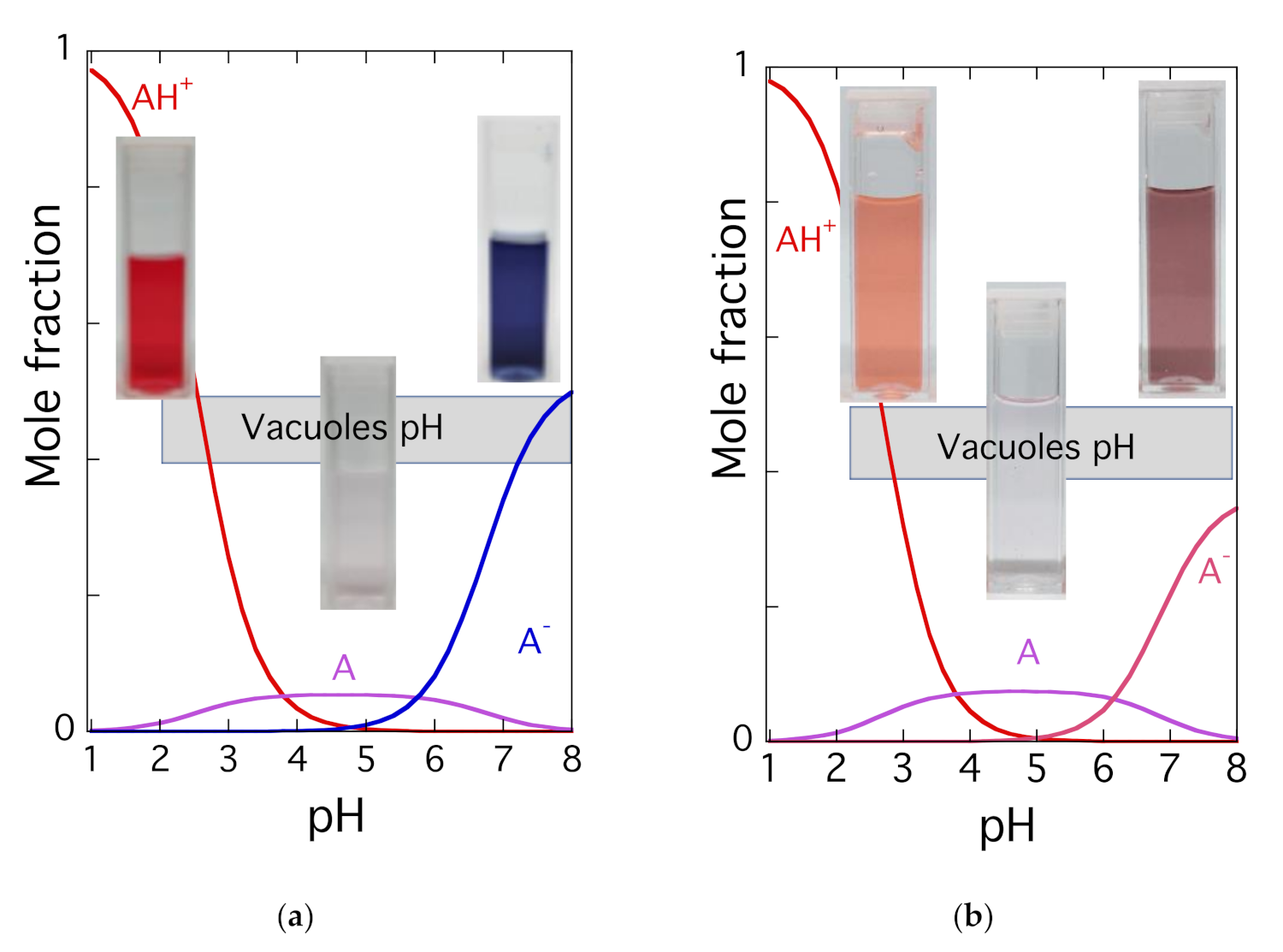
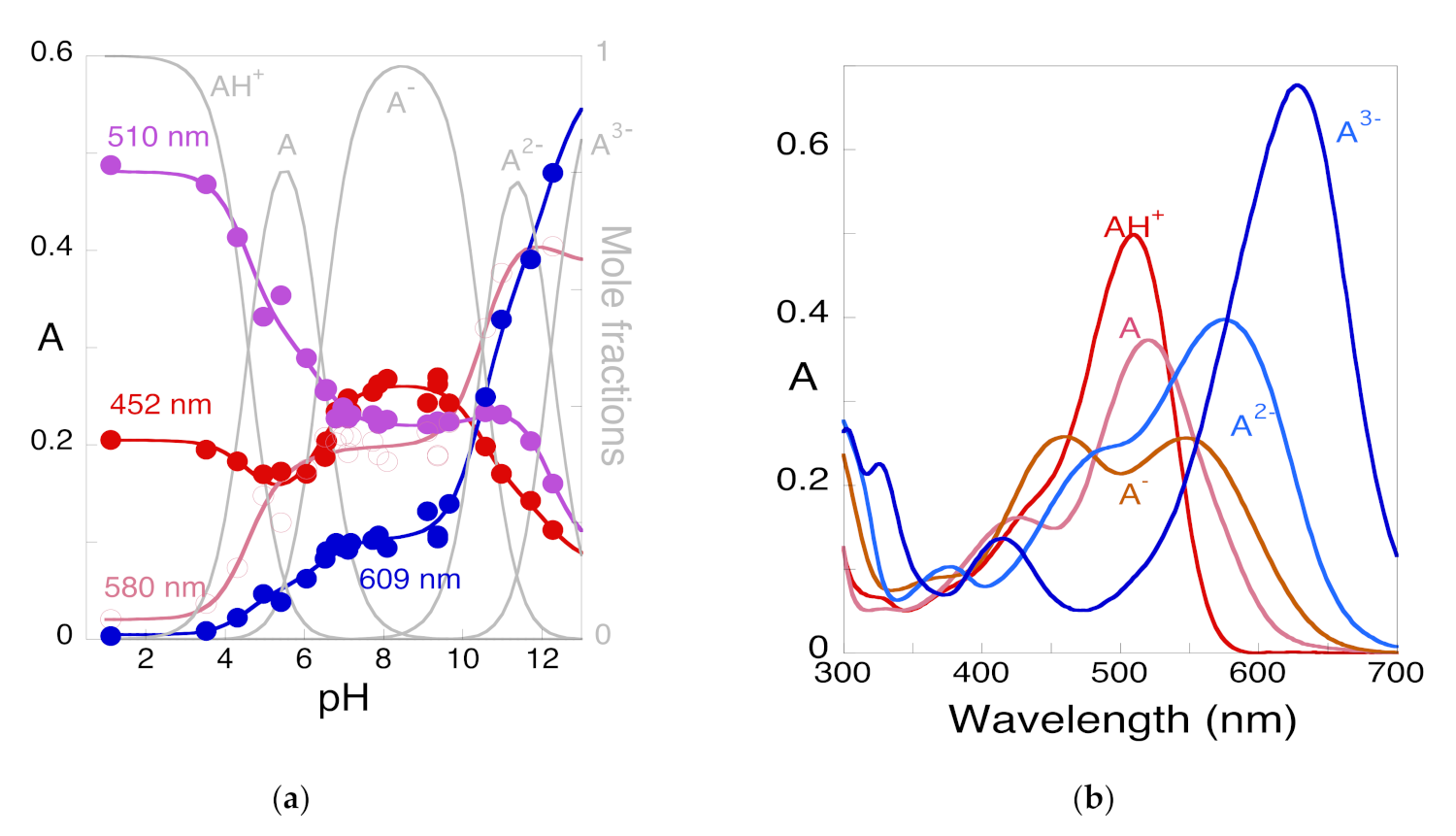
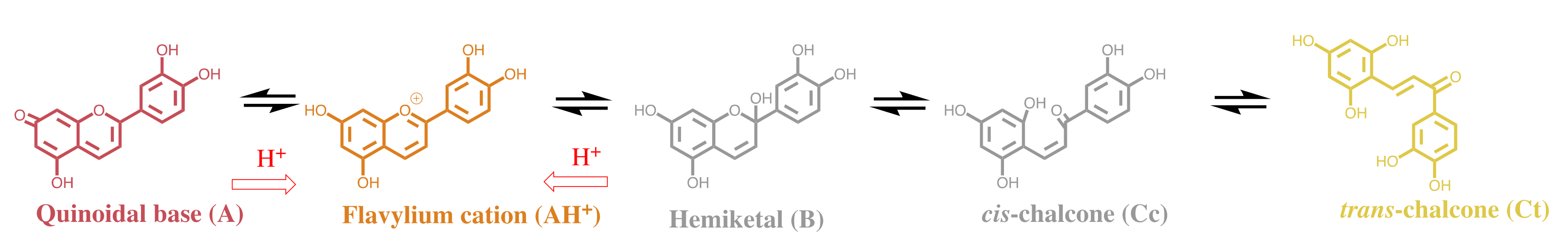



| pK′a | pK^a | pKa | Kh | Kt | Ki |
| 2.7 | 2.8 | 3.8 | 1.2 × 10−3 M−1 | 0.36 | 0.50 |
| pKA/A− | pKB/B− | pKCc/Cc− | pKCt/Ct− | ||
| 6.15 | 7.8 | 6.8 | 6.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pina, F.; Alejo-Armijo, A.; Clemente, A.; Mendoza, J.; Seco, A.; Basílio, N.; Parola, A.J. Evolution of Flavylium-Based Color Systems in Plants: What Physical Chemistry Can Tell Us. Int. J. Mol. Sci. 2021, 22, 3833. https://doi.org/10.3390/ijms22083833
Pina F, Alejo-Armijo A, Clemente A, Mendoza J, Seco A, Basílio N, Parola AJ. Evolution of Flavylium-Based Color Systems in Plants: What Physical Chemistry Can Tell Us. International Journal of Molecular Sciences. 2021; 22(8):3833. https://doi.org/10.3390/ijms22083833
Chicago/Turabian StylePina, Fernando, Alfonso Alejo-Armijo, Adelaide Clemente, Johan Mendoza, André Seco, Nuno Basílio, and António Jorge Parola. 2021. "Evolution of Flavylium-Based Color Systems in Plants: What Physical Chemistry Can Tell Us" International Journal of Molecular Sciences 22, no. 8: 3833. https://doi.org/10.3390/ijms22083833
APA StylePina, F., Alejo-Armijo, A., Clemente, A., Mendoza, J., Seco, A., Basílio, N., & Parola, A. J. (2021). Evolution of Flavylium-Based Color Systems in Plants: What Physical Chemistry Can Tell Us. International Journal of Molecular Sciences, 22(8), 3833. https://doi.org/10.3390/ijms22083833









