Differential Expression of Sphingolipid Metabolizing Enzymes in Spontaneously Hypertensive Rats: A Possible Substrate for Susceptibility to Brain and Kidney Damage
Abstract
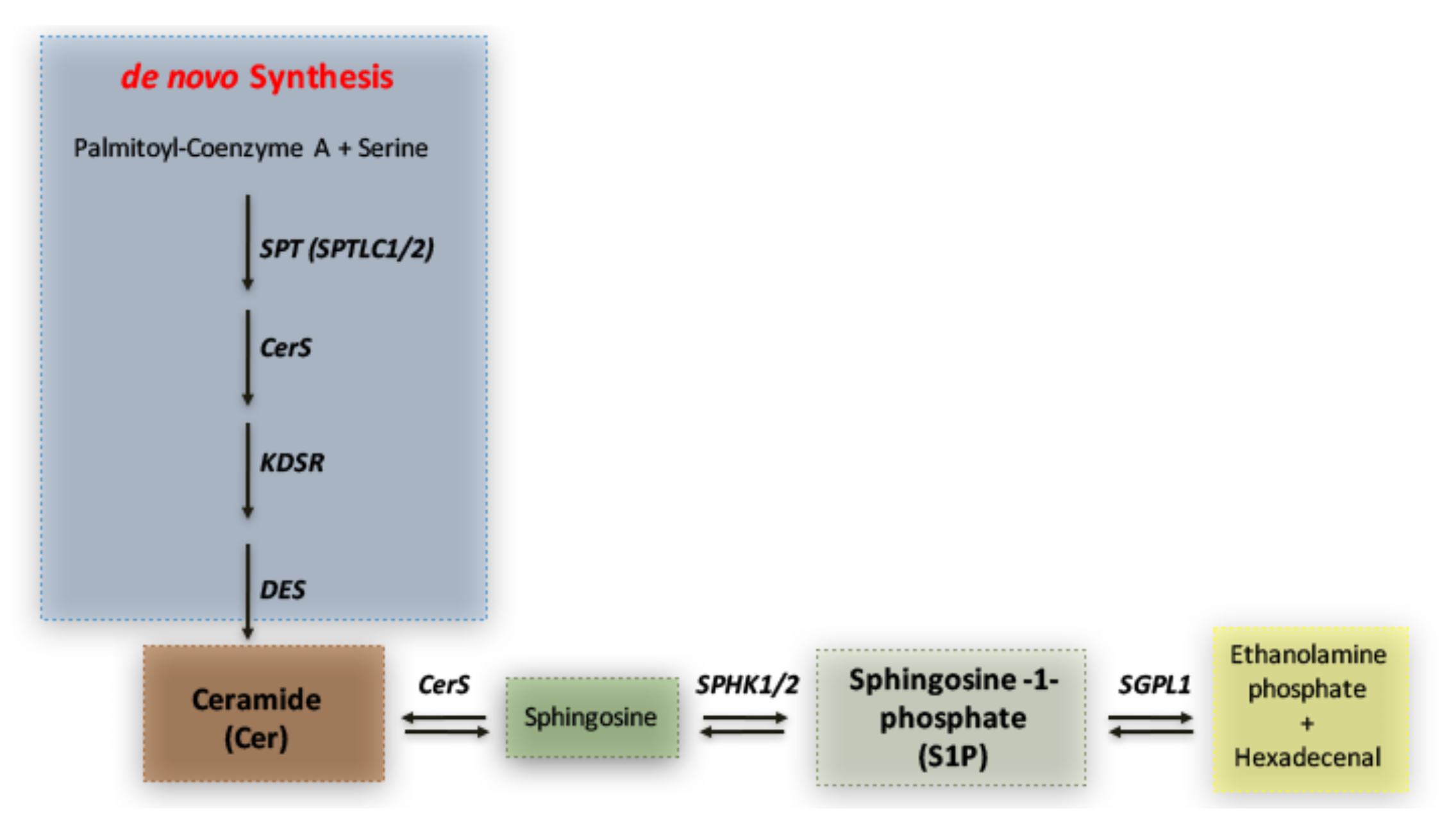
1. Introduction
2. Results
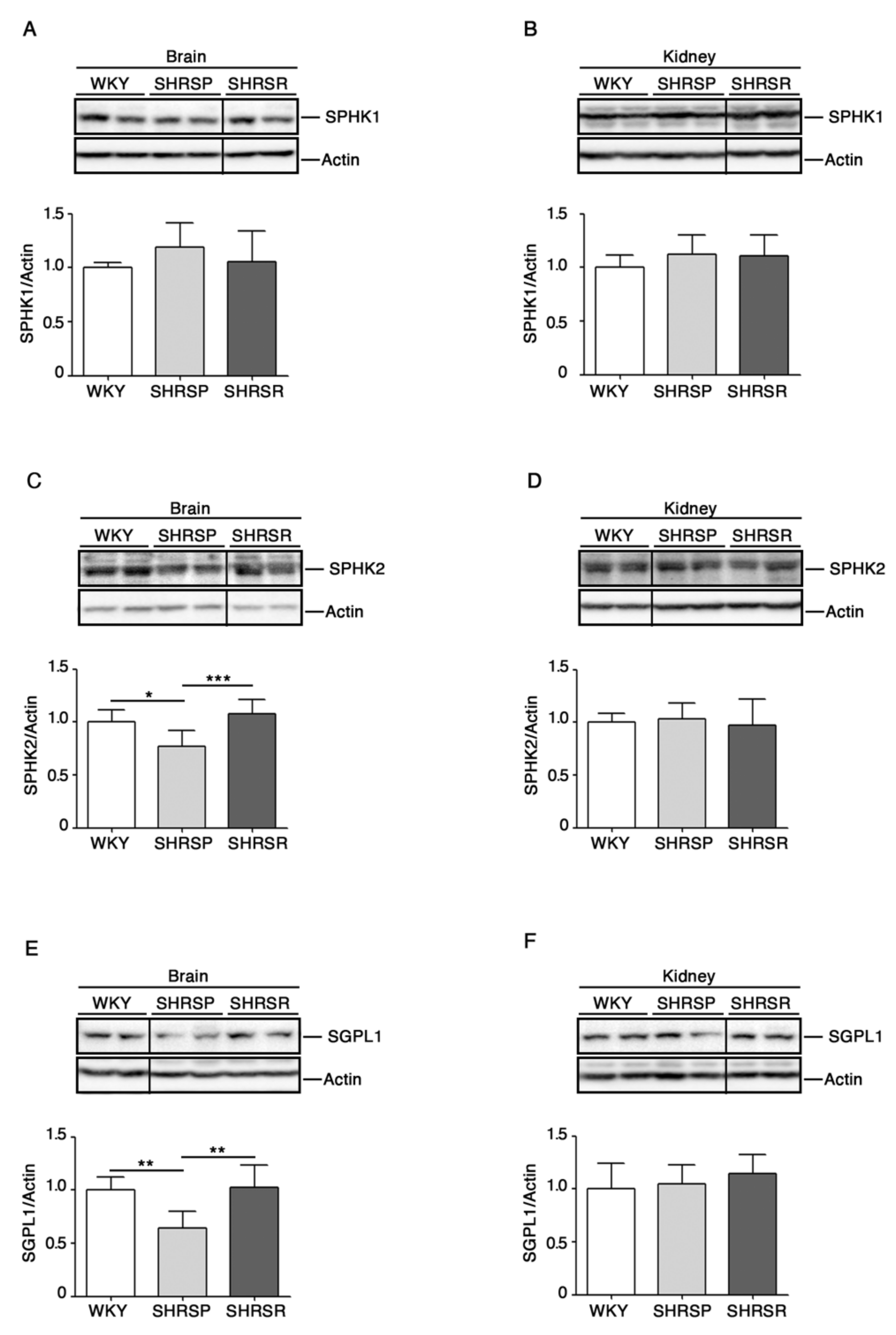
2.1. Levels of S1P-Metabolizing Enzymes Are Perturbed in SHRSP
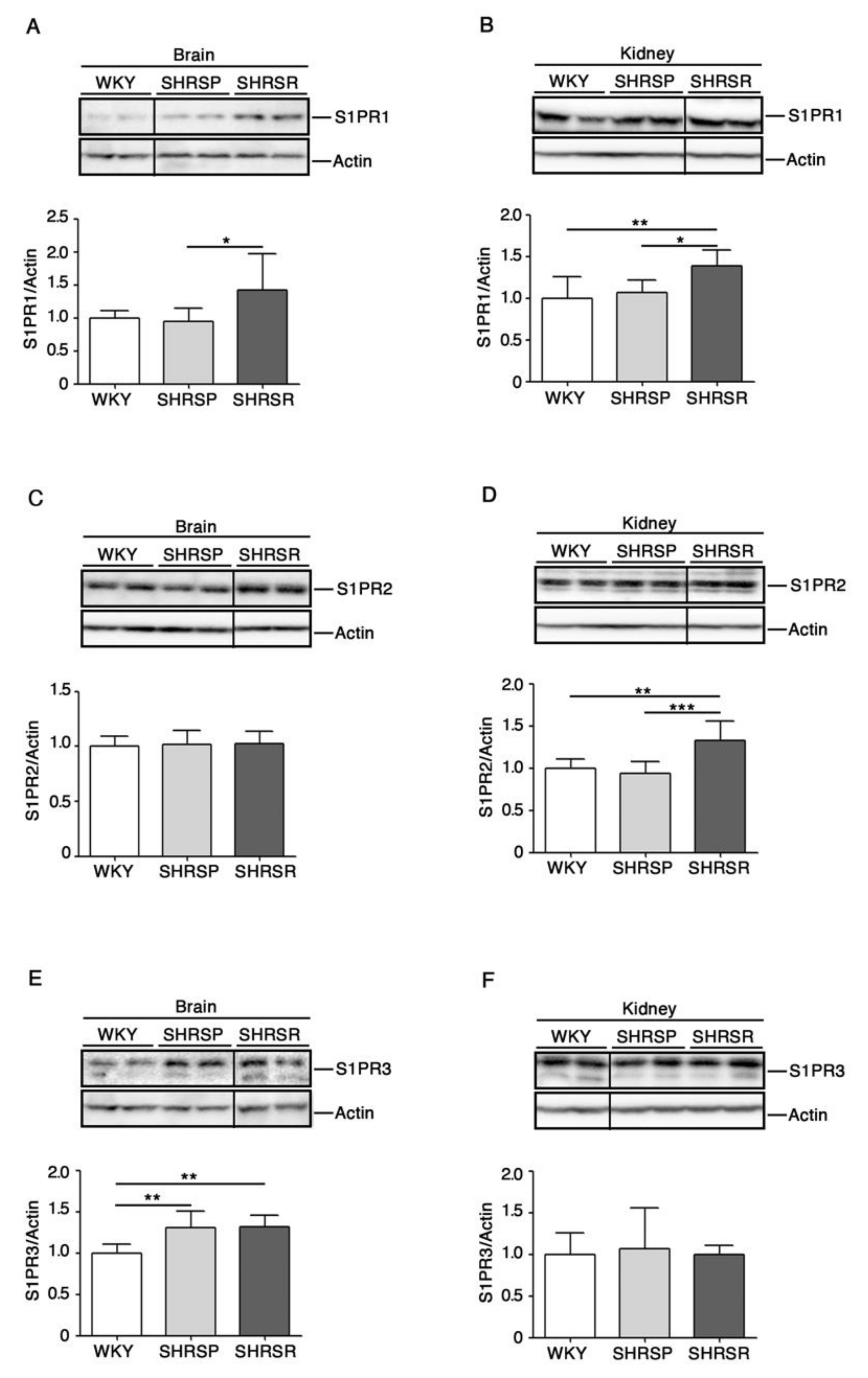
2.2. Levels of S1P Receptors Are Aberrant in Spontaneously Hypertensive Rats
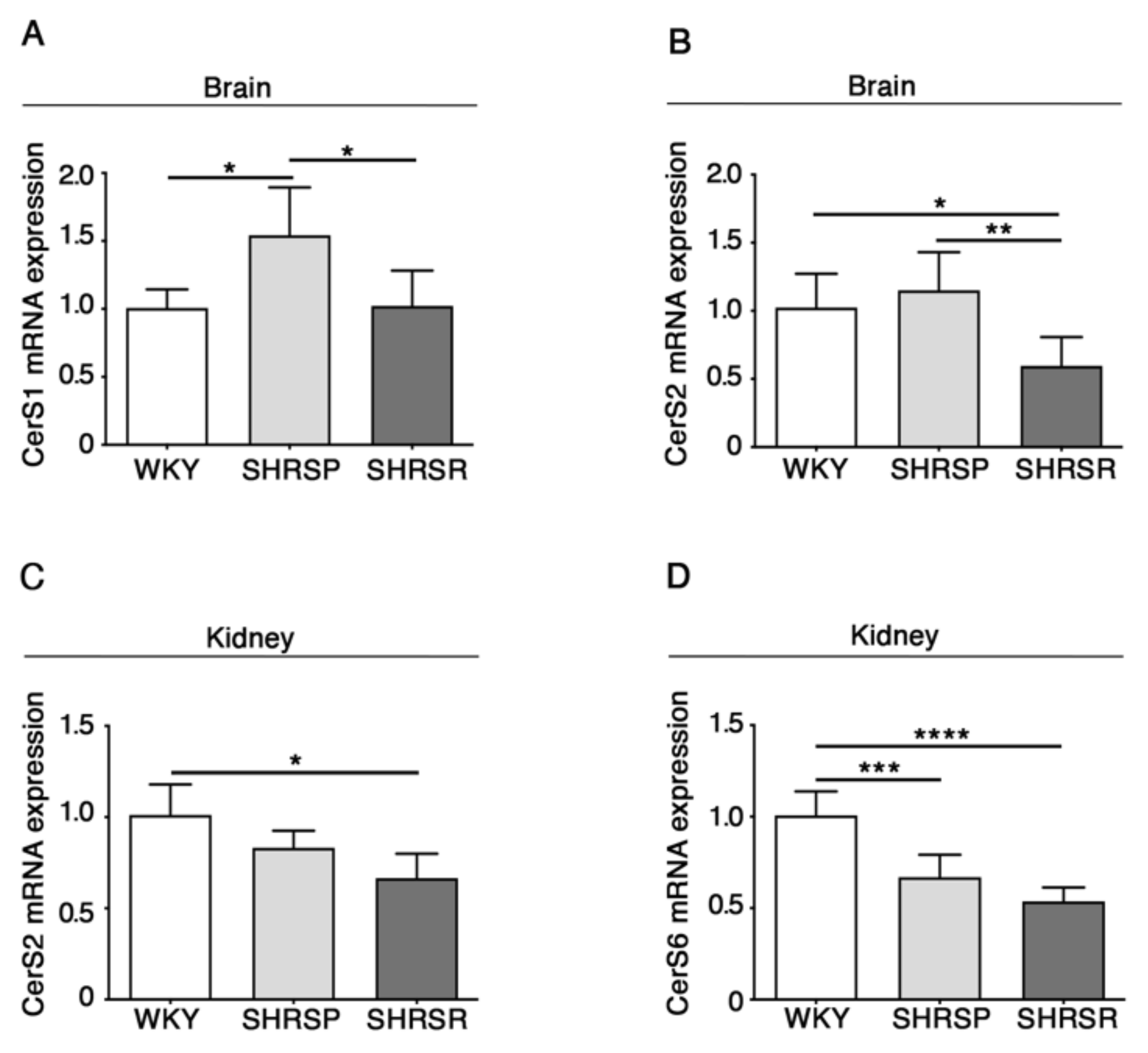
2.3. Expression of Ceramide Synthases Is Defective in Multiple Tissues from SHRSP and SHRSR Models
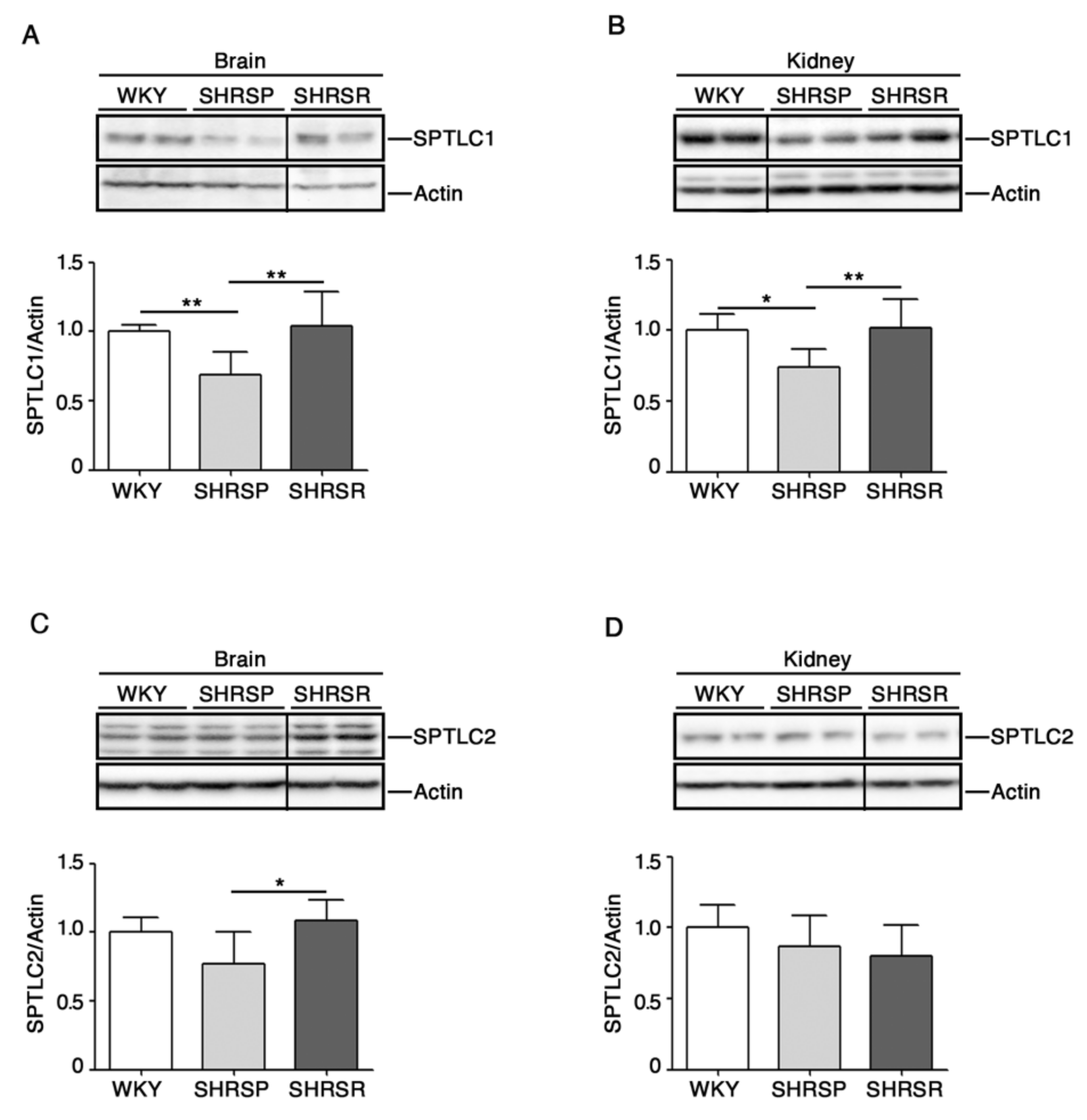
2.4. Expression of SPTLC1 Is Reduced in SHRSP Tissues
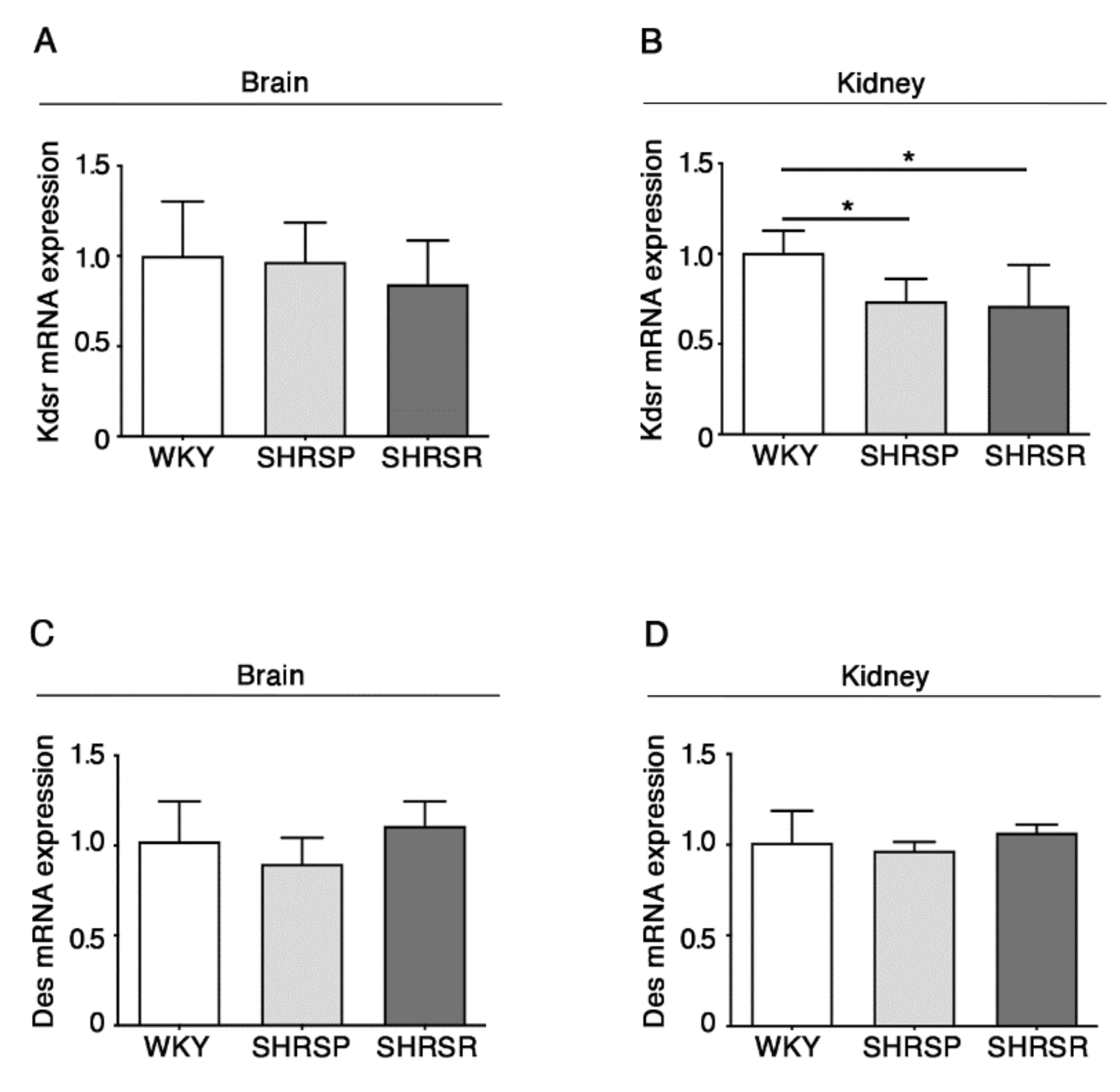
2.5. Expression of 3-Keto-Dihydrosphingosine Reductase Is Reduced in Both Spontaneously Hypertensive Rats
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.2. Immunoblottings
4.3. RNA Extraction and qPCR.
4.4. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Giliberti, R.; Volpe, M. Etiology and pathophysiology of stroke as a complex trait. Am. J. Hypertens 2000, 13, 1139–1148. [Google Scholar] [CrossRef]
- Ascherio, A.; Rimm, E.B.; Hernan, M.A.; Giovannucci, E.L.; Kawachi, I.; Stampfer, M.J.; Willett, W.C. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation 1998, 98, 1198–1204. [Google Scholar] [CrossRef]
- Yamori, Y.; Horie, R.; Tanase, H.; Fujiwara, K.; Nara, Y.; Lovenberg, W. Possible role of nutritional factors in the incidence of cerebral lesions in stroke-prone spontaneously hypertensive rats. Hypertension 1984, 6, 49–53. [Google Scholar] [CrossRef]
- Rubattu, S.; Volpe, M.; Kreutz, R.; Ganten, U.; Ganten, D.; Lindpaintner, K. Chromosomal mapping of quantitative trait loci contributing to stroke in a rat model of complex human disease. Nat. Genet. 1996, 13, 429–434. [Google Scholar] [CrossRef]
- Rubattu, S.; Stanzione, R.; Volpe, M. Mitochondrial Dysfunction Contributes to Hypertensive Target Organ Damage: Lessons from an Animal Model of Human Disease. Oxid Med. Cell Longev 2016, 2016, 1067801. [Google Scholar] [CrossRef]
- Rubattu, S.; Stanzione, R.; Gigante, B.; Volpe, M. Role of genetic factors in the etiopathogenesis of cerebrovascular accidents: From an animal model to the human disease. Cell Mol Neurobiol 2004, 24, 581–588. [Google Scholar] [CrossRef]
- Busceti, C.L.; Cotugno, M.; Bianchi, F.; Forte, M.; Stanzione, R.; Marchitti, S.; Battaglia, G.; Nicoletti, F.; Fornai, F.; Rubattu, S. Brain Overexpression of Uncoupling Protein-2 (UCP2) Delays Renal Damage and Stroke Occurrence in Stroke-Prone Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Rubattu, S.; Lee-Kirsch, M.A.; DePaolis, P.; Giliberti, R.; Gigante, B.; Lombardi, A.; Volpe, M.; Lindpaintner, K. Altered structure, regulation, and function of the gene encoding the atrial natriuretic peptide in the stroke-prone spontaneously hypertensive rat. Circ. Res. 1999, 85, 900–905. [Google Scholar] [CrossRef]
- Rubattu, S.; Stanzione, R.; Bianchi, F.; Cotugno, M.; Forte, M.; Della Ragione, F.; Fioriniello, S.; D’Esposito, M.; Marchitti, S.; Madonna, M.; et al. Reduced brain UCP2 expression mediated by microRNA-503 contributes to increased stroke susceptibility in the high-salt fed stroke-prone spontaneously hypertensive rat. Cell Death Dis. 2017, 8, e2891. [Google Scholar] [CrossRef]
- Rubattu, S.; Di Castro, S.; Schulz, H.; Geurts, A.M.; Cotugno, M.; Bianchi, F.; Maatz, H.; Hummel, O.; Falak, S.; Stanzione, R.; et al. Ndufc2 Gene Inhibition Is Associated With Mitochondrial Dysfunction and Increased Stroke Susceptibility in an Animal Model of Complex Human Disease. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Hla, T.; Venkataraman, K.; Michaud, J. The vascular S1P gradient-cellular sources and biological significance. Biochim. Biophys. Acta 2008, 1781, 477–482. [Google Scholar] [CrossRef]
- Xiong, Y.; Hla, T. S1P control of endothelial integrity. Curr. Top. Microbiol. Immunol. 2014, 378, 85–105. [Google Scholar] [CrossRef]
- Cogolludo, A.; Villamor, E.; Perez-Vizcaino, F.; Moreno, L. Ceramide and Regulation of Vascular Tone. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Wilkerson, B.A.; Argraves, K.M. The role of sphingosine-1-phosphate in endothelial barrier function. Biochim. Biophys. Acta 2014, 1841, 1403–1412. [Google Scholar] [CrossRef]
- Cantalupo, A.; Zhang, Y.; Kothiya, M.; Galvani, S.; Obinata, H.; Bucci, M.; Giordano, F.J.; Jiang, X.C.; Hla, T.; Di Lorenzo, A. Nogo-B regulates endothelial sphingolipid homeostasis to control vascular function and blood pressure. Nat. Med. 2015, 21, 1028–1037. [Google Scholar] [CrossRef]
- Sun, N.; Keep, R.F.; Hua, Y.; Xi, G. Critical Role of the Sphingolipid Pathway in Stroke: A Review of Current Utility and Potential Therapeutic Targets. Transl. Stroke Res. 2016, 7, 420–438. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Suzuki, H.; Altay, O.; Rolland, W.; Zhang, J.H. Role of the sphingosine metabolism pathway on neurons against experimental cerebral ischemia in rats. Transl. Stroke Res. 2013, 4, 524–532. [Google Scholar] [CrossRef]
- Brait, V.H.; Tarrason, G.; Gavalda, A.; Godessart, N.; Planas, A.M. Selective Sphingosine 1-Phosphate Receptor 1 Agonist Is Protective Against Ischemia/Reperfusion in Mice. Stroke 2016, 47, 3053–3056. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.; McBride, M.W.; Gaasenbeek, M.; Gilday, K.; Beattie, E.; Miller, W.H.; McClure, J.D.; Polke, J.M.; Montezano, A.; Touyz, R.M.; et al. Candidate genes that determine response to salt in the stroke-prone spontaneously hypertensive rat: Congenic analysis. Hypertension 2007, 50, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.; Gob, E.; Schuhmann, M.K.; Gobel, K.; Deppermann, C.; Thielmann, I.; Herrmann, A.M.; Lorenz, K.; Brede, M.; Stoll, G.; et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke 2013, 44, 3202–3210. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Hla, T.; Lynch, K.R.; Spiegel, S.; Moolenaar, W.H. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2010, 62, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.C.; Lee, T.H.; Chiang, C.S.; Yang, S.Y.; Kuo, C.H.; Tang, S.C. Sphingolipidomics Investigation of the Temporal Dynamics after Ischemic Brain Injury. J. Proteome Res. 2019, 18, 3470–3478. [Google Scholar] [CrossRef]
- Lee, T.H.; Cheng, C.N.; Chao, H.C.; Lee, C.H.; Kuo, C.H.; Tang, S.C.; Jeng, J.S. Plasma ceramides are associated with outcomes in acute ischemic stroke patients. J. Formos Med. Assoc. 2021. [Google Scholar] [CrossRef]
- Testai, F.D.; Hillmann, M.; Amin-Hanjani, S.; Gorshkova, I.; Berdyshev, E.; Gorelick, P.B.; Dawson, G. Changes in the cerebrospinal fluid ceramide profile after subarachnoid hemorrhage. Stroke 2012, 43, 2066–2070. [Google Scholar] [CrossRef][Green Version]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef]
- Sasset, L.; Zhang, Y.; Dunn, T.M.; Di Lorenzo, A. Sphingolipid De Novo Biosynthesis: A Rheostat of Cardiovascular Homeostasis. Trends Endocrinol. Metab. 2016, 27, 807–819. [Google Scholar] [CrossRef]
- Cantalupo, A.; Sasset, L.; Gargiulo, A.; Rubinelli, L.; Del Gaudio, I.; Benvenuto, D.; Wadsack, C.; Jiang, X.C.; Bucci, M.R.; Di Lorenzo, A. Endothelial Sphingolipid De Novo Synthesis Controls Blood Pressure by Regulating Signal Transduction and NO via Ceramide. Hypertension 2020, 75, 1279–1288. [Google Scholar] [CrossRef]
- Borodzicz, S.; Czarzasta, K.; Kuch, M.; Cudnoch-Jedrzejewska, A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015, 14, 55. [Google Scholar] [CrossRef]
- Vessey, D.A.; Li, L.; Jin, Z.Q.; Kelley, M.; Honbo, N.; Zhang, J.; Karliner, J.S. A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes responsiveness to ischemic preconditioning. Oxid. Med. Cell Longev. 2011, 2011, 961059. [Google Scholar] [CrossRef]
- Hagen, N.; Van Veldhoven, P.P.; Proia, R.L.; Park, H.; Merrill, A.H., Jr.; van Echten- Deckert, G. Subcellular origin of sphingosine 1-phosphate is essential for its toxic effect in lyase-deficient neurons. J Biol Chem. 2009, 17, 11346–11353. [Google Scholar] [CrossRef]
- Iwasawa, E.; Ishibashi, S.; Suzuki, M.; Li, F.; Ichijo, M.; Miki, K.; Yokota, T. Sphingosine-1-Phosphate Receptor 1 Activation Enhances Leptomeningeal Collateral Development and Improves Outcome after Stroke in Mice. J. Stroke Cerebrovasc. Dis. 2018, 27, 1237–1251. [Google Scholar] [CrossRef]
- Nitzsche, A.; Poittevin, M.; Benarab, A.; Bonnin, P.; Faraco, G.; Uchida, H.; Favre, J.; Garcia-Bonilla, L.; Garcia, M.C.L.; Leger, P.L.; et al. Endothelial S1P1 Signaling Counteracts Infarct Expansion in Ischemic Stroke. Circ. Res. 2021, 128, 363–382. [Google Scholar] [CrossRef]
- Li, Y.J.; Shi, S.X.; Liu, Q.; Shi, F.D.; Gonzales, R.J. Targeted role for sphingosine-1-phosphate receptor 1 in cerebrovascular integrity and inflammation during acute ischemic stroke. Neurosci. Lett. 2020, 735, 135160. [Google Scholar] [CrossRef]
- Azizkhanian, I.; Sheth, S.A.; Iavarone, A.T.; Lee, S.; Kakarla, V.; Hinman, J.D. Plasma Lipid Profiling Identifies Biomarkers of Cerebral Microvascular Disease. Front. Neurol. 2019, 10, 950. [Google Scholar] [CrossRef]
- Mielke, M.M.; Syrjanen, J.A.; Bui, H.H.; Petersen, R.C.; Knopman, D.S.; Jack, C.R.; Graff-Radford, J.; Vemuri, P. Elevated Plasma Ceramides Are Associated With Higher White Matter Hyperintensity Volume-Brief Report. Arterioscl. Throm. Vas. 2019, 39, 2431–2436. [Google Scholar] [CrossRef]
- Czubowicz, K.; Jesko, H.; Wencel, P.; Lukiw, W.J.; Strosznajder, R.P. The Role of Ceramide and Sphingosine-1-Phosphate in Alzheimer’s Disease and Other Neurodegenerative Disorders. Mol. Neurobiol. 2019, 56, 5436–5455. [Google Scholar] [CrossRef]
- Gonzalez-Cabrera, P.J.; Brown, S.; Studer, S.M.; Rosen, H. S1P signaling: New therapies and opportunities. F1000Prime Rep. 2014, 6, 109. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe, G.; Cotugno, M.; Marracino, F.; Giova, S.; Capocci, L.; Forte, M.; Stanzione, R.; Bianchi, F.; Marchitti, S.; Di Pardo, A.; et al. Differential Expression of Sphingolipid Metabolizing Enzymes in Spontaneously Hypertensive Rats: A Possible Substrate for Susceptibility to Brain and Kidney Damage. Int. J. Mol. Sci. 2021, 22, 3796. https://doi.org/10.3390/ijms22073796
Pepe G, Cotugno M, Marracino F, Giova S, Capocci L, Forte M, Stanzione R, Bianchi F, Marchitti S, Di Pardo A, et al. Differential Expression of Sphingolipid Metabolizing Enzymes in Spontaneously Hypertensive Rats: A Possible Substrate for Susceptibility to Brain and Kidney Damage. International Journal of Molecular Sciences. 2021; 22(7):3796. https://doi.org/10.3390/ijms22073796
Chicago/Turabian StylePepe, Giuseppe, Maria Cotugno, Federico Marracino, Susy Giova, Luca Capocci, Maurizio Forte, Rosita Stanzione, Franca Bianchi, Simona Marchitti, Alba Di Pardo, and et al. 2021. "Differential Expression of Sphingolipid Metabolizing Enzymes in Spontaneously Hypertensive Rats: A Possible Substrate for Susceptibility to Brain and Kidney Damage" International Journal of Molecular Sciences 22, no. 7: 3796. https://doi.org/10.3390/ijms22073796
APA StylePepe, G., Cotugno, M., Marracino, F., Giova, S., Capocci, L., Forte, M., Stanzione, R., Bianchi, F., Marchitti, S., Di Pardo, A., Sciarretta, S., Rubattu, S., & Maglione, V. (2021). Differential Expression of Sphingolipid Metabolizing Enzymes in Spontaneously Hypertensive Rats: A Possible Substrate for Susceptibility to Brain and Kidney Damage. International Journal of Molecular Sciences, 22(7), 3796. https://doi.org/10.3390/ijms22073796






