Kidney Injury Caused by Preeclamptic Pregnancy Recovers Postpartum in a Transgenic Rat Model
Abstract
1. Introduction
2. Results
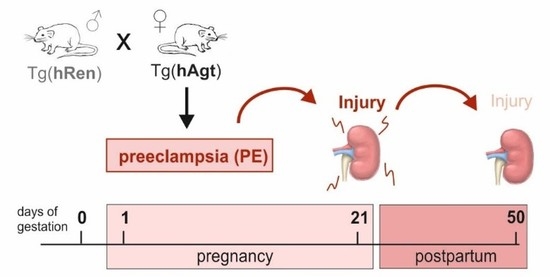
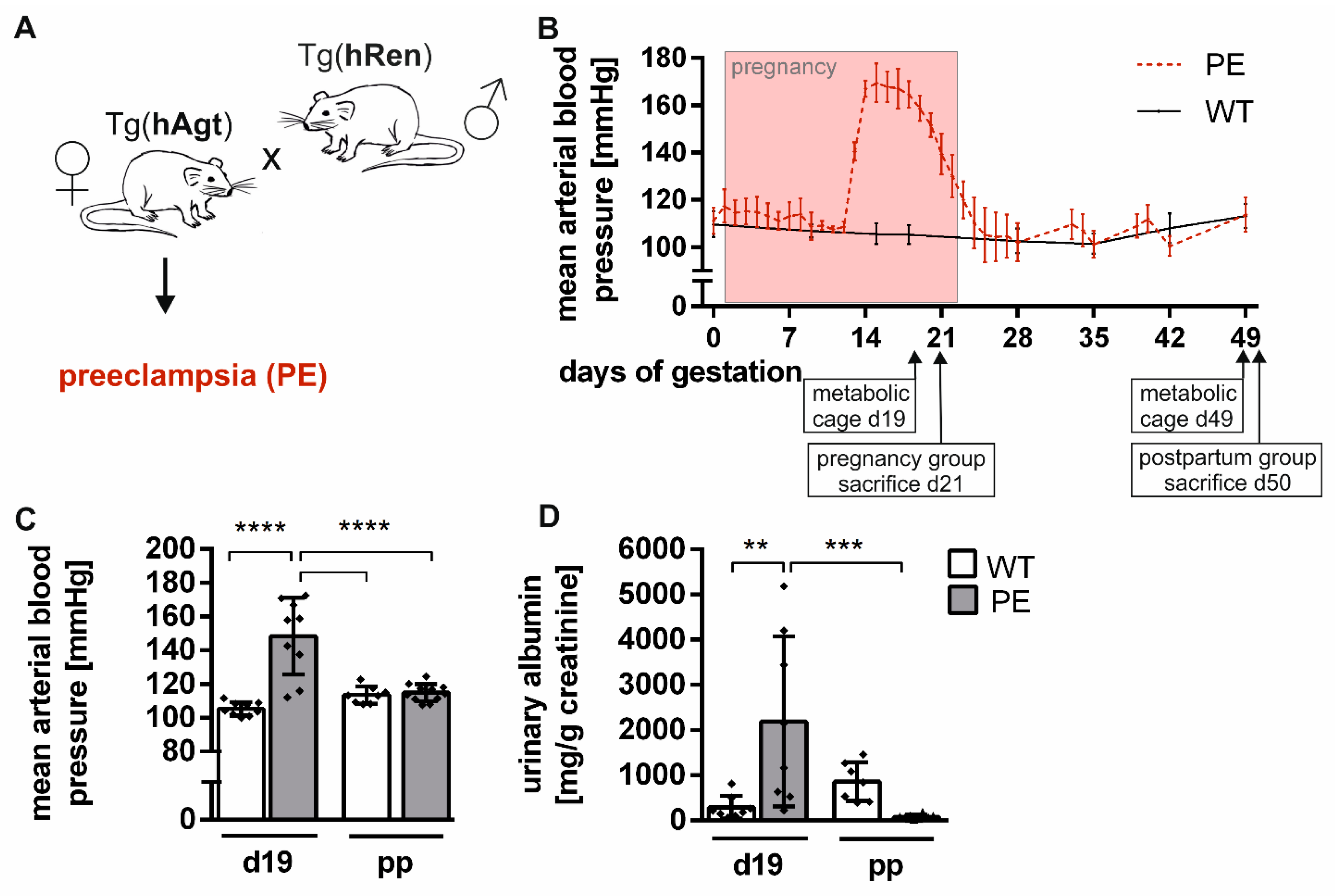
2.1. Preeclamptic Phenotype in the Transgenic Rat Model
2.2. Altered Expression of Kidney Injury Marker Genes
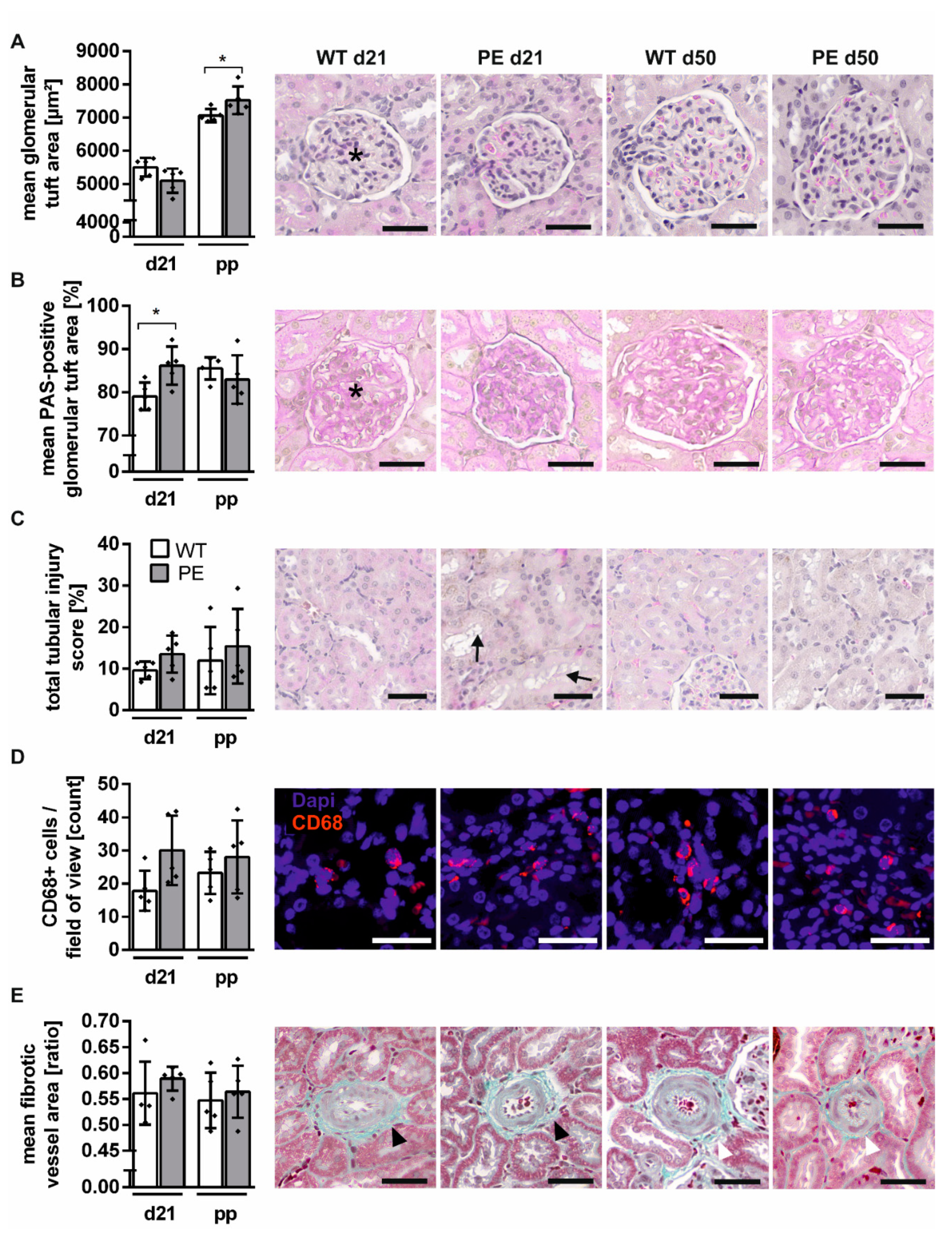
2.3. Mild Structural Renal Impairment
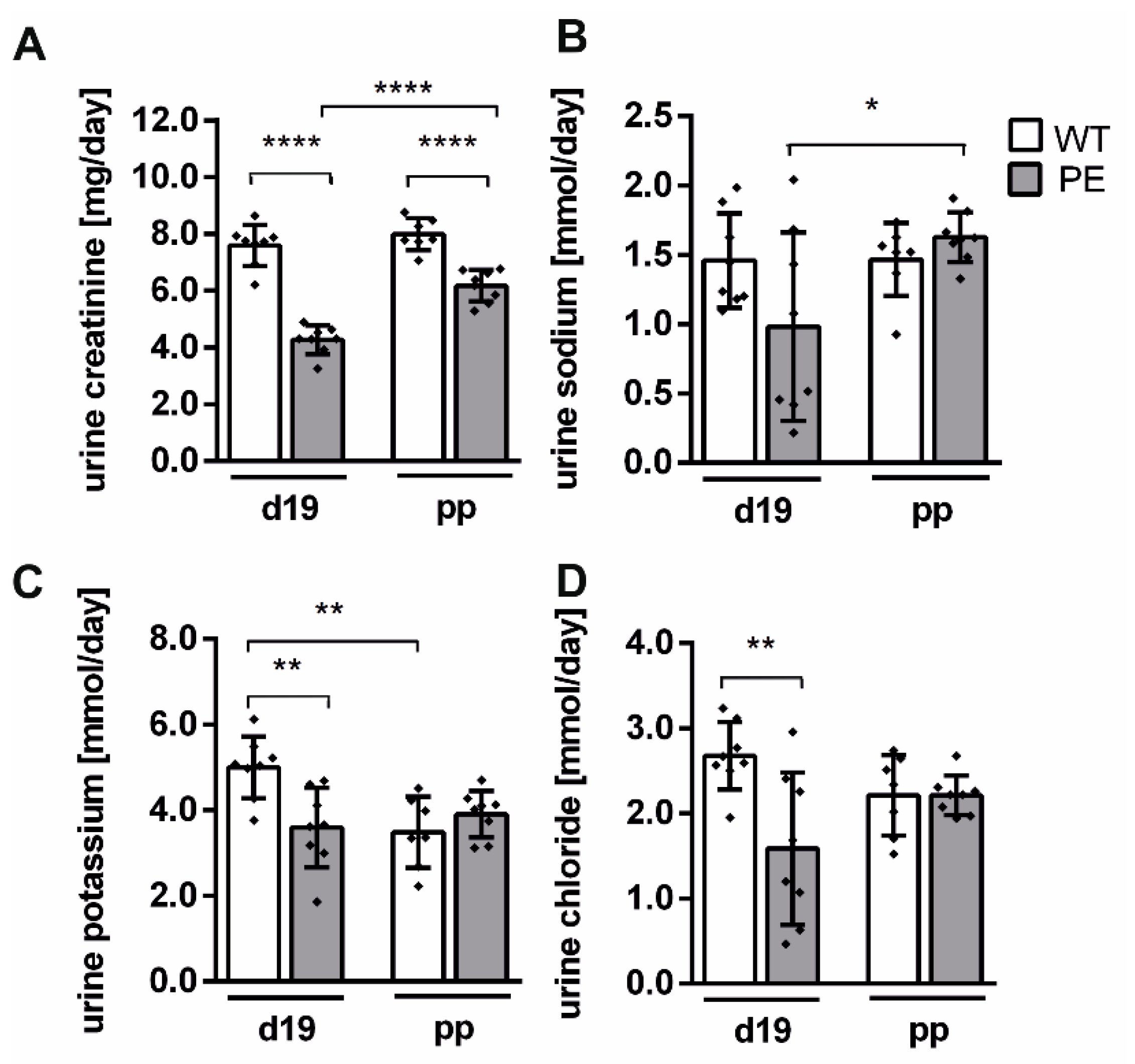
2.4. Renal Function Is Slightly Altered in PE Pregnancy
3. Discussion
4. Materials and Methods
4.1. Animal Study
4.2. Blood Pressure Measurement
4.3. Urine Measurement
4.4. Gene Expression Analysis
4.5. Immunohistochemistry
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef]
- Ghulmiyyah, L.; Sibai, B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012, 36, 56–59. [Google Scholar] [CrossRef]
- Homer, C.S.; Brown, M.A.; Mangos, G.; Davis, G.K. Non-proteinuric preeclampsia: A novel risk indicator in women with gestational hypertension. J. Hypertens. 2008, 26, 295–302. [Google Scholar] [CrossRef]
- ACOG Practice Bulletin No. 202. American College of Obstetricians and Gynecologists. Gestational hypertension and preeclampsia. Obstet. Gynecol. 2019, 133, 211–214. [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Przybyl, L.; Haase, N.; Golic, M.; Rugor, J.; Solano, M.E.; Arck, P.C.; Gauster, M.; Huppertz, B.; Emontzpohl, C.; Stoppe, C.; et al. CD74-Downregulation of Placental Macrophage-Trophoblastic Interactions in Preeclampsia. Circ. Res. 2016, 119, 55–68. [Google Scholar] [CrossRef]
- Pierik, E.; Prins, J.R.; Van Goor, H.; Dekker, G.A.; Daha, M.R.; Seelen, M.A.J.; Scherjon, S.A. Dysregulation of Complement Activation and Placental Dysfunction: A Potential Target to Treat Preeclampsia? Front. Immunol. 2019, 10, 3098. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Ayansina, D.; Black, C.; Hall, S.J.; Marks, A.; Millar, C.; Prescott, G.J.; Wilde, K.; Bhattacharya, S. Long term effects of gestational hypertension and pre-eclampsia on kidney function: Record linkage study. Pregnancy Hypertens. 2016, 6, 344–349. [Google Scholar] [CrossRef]
- McDonald, S.D.; Han, Z.; Walsh, M.W.; Gerstein, H.C.; Devereaux, P.J. Kidney Disease After Preeclampsia: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2010, 55, 1026–1039. [Google Scholar] [CrossRef]
- Vikse, B.E.; Irgens, L.M.; Leivestad, T.; Skjaerven, R.; Iversen, B.M. Preeclampsia and the Risk of End-Stage Renal Disease. N. Engl. J. Med. 2008, 359, 800–809. [Google Scholar] [CrossRef]
- Spargo, B.; McCartney, C.P.; Winemiller, R. Glomerular capillary endotheliosis in toxemia of pregnancy. Arch. Pathol. 1959, 68, 593–599. [Google Scholar]
- Sheehan, H. Renal morphology in preeclampsia. Kidney Int. 1980, 18, 241–252. [Google Scholar] [CrossRef]
- Lafayette, R.A.; Druzin, M.; Sibley, R.; Derby, G.; Malik, T.; Huie, P.; Polhemus, C.; Deen, W.M.; Myers, B.D. Nature of glomerular dysfunction in pre-eclampsia11See Editorial by Chapman, p. 1394. Kidney Int. 1998, 54, 1240–1249. [Google Scholar] [CrossRef]
- Karumanchi, S.A.; Maynard, S.E.; Stillman, I.E.; Epstein, F.H.; Sukhatme, V.P. Preeclampsia: A renal perspective. Kidney Int. 2005, 67, 2101–2113. [Google Scholar] [CrossRef]
- Strevens, H.; Wide-Swensson, D.; Hansen, A.; Horn, T.; InIngemarsson, I.; Larsen, S.; Willner, J.; Olsen, S. Glomerular endotheliosis in normal pregnancy and pre-eclampsia. Int. J. Obstet. Gynaecol. 2003, 110, 831–836. [Google Scholar] [CrossRef]
- Stillman, I.E.; Karumanchi, S.A. The Glomerular Injury of Preeclampsia: Figure 1. J. Am. Soc. Nephrol. 2007, 18, 2281–2284. [Google Scholar] [CrossRef]
- Packham, D.K.; Mathews, D.C.; Fairley, K.F.; Whitworth, J.A.; Kincaid-Smith, P.S. Morphometric analysis of pre-eclampsia in women biopsied in pregnancy and post-partum. Kidney Int. 1988, 34, 704–711. [Google Scholar] [CrossRef]
- Dai, L.; Chen, Y.; Sun, W.; Liu, S. Association Between Hypertensive Disorders During Pregnancy and the Subsequent Risk of End-Stage Renal Disease: A Population-Based Follow-Up Study. J. Obstet. Gynaecol. Can. 2018, 40, 1129–1138. [Google Scholar] [CrossRef]
- Kattah, A.G.; Scantlebury, D.C.; Agarwal, S.; Mielke, M.M.; Rocca, W.A.; Weaver, A.L.; Vaughan, L.E.; Miller, V.M.; Weissgerber, T.L.; White, W.; et al. Preeclampsia and ESRD: The Role of Shared Risk Factors. Am. J. Kidney Dis. 2017, 69, 498–505. [Google Scholar] [CrossRef]
- Granger, J.P.; Spradley, F.T.; Bakrania, B.A. The Endothelin System: A Critical Player in the Pathophysiology of Preeclampsia. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Denney, J.M.; Bird, C.E.; Gendron-Fitzpatrick, A.; Sampene, E.; Bird, I.M.; Shah, D.M. Renin-angiotensin system transgenic mouse model recapitulates pathophysiology similar to human preeclampsia with renal injury that may be mediated through VEGF. Am. J. Physiol. Physiol. 2017, 312, F445–F455. [Google Scholar] [CrossRef]
- Sugimoto, H.; Hamano, Y.; Charytan, D.; Cosgrove, D.; Kieran, M.; Sudhakar, A.; Kalluri, R. Neutralization of Circulating Vascular Endothelial Growth Factor (VEGF) by Anti-VEGF Antibodies and Soluble VEGF Receptor 1 (sFlt-1) Induces Proteinuria. J. Biol. Chem. 2003, 278, 12605–12608. [Google Scholar] [CrossRef]
- Gilbert, J.S.; Verzwyvelt, J.; Colson, D.; Arany, M.; Karumanchi, S.A.; Granger, J.P. Recombinant Vascular Endothelial Growth Factor 121 Infusion Lowers Blood Pressure and Improves Renal Function in Rats With PlacentalIschemia-Induced Hypertension. Hypertension 2010, 55, 380–385. [Google Scholar] [CrossRef]
- Li, J.; Lamarca, B.; Reckelhoff, J.F. A model of preeclampsia in rats: The reduced uterine perfusion pressure (RUPP) model. Am. J. Physiol. Circ. Physiol. 2012, 303, H1–H8. [Google Scholar] [CrossRef]
- Dechend, R.; Gratze, P.; Wallukat, G.; Shagdarsuren, E.; Plehm, R.; Bräsen, J.-H.; Fiebeler, A.; Schneider, W.; Caluwaerts, S.; Vercruysse, L.; et al. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension 2005, 45, 742–746. [Google Scholar] [CrossRef]
- Cunningham, M.W.; Vaka, V.R.; McMaster, K.; Ibrahim, T.; Cornelius, D.C.; Amaral, L.; Campbell, N.; Wallukat, G.; McDuffy, S.; Usry, N.; et al. Renal natural killer cell activation and mitochondrial oxidative stress; new mechanisms in AT1-AA mediated hypertensive pregnancy. Pregnancy Hypertens. 2019, 15, 72–77. [Google Scholar] [CrossRef]
- Lamarca, B.; Wallace, K.; Herse, F.; Wallukat, G.; Martin, J.N.; Weimer, A.; Dechend, R. Hypertension in Response to Placental Ischemia During Pregnancy. Hypertension 2011, 57, 865–871. [Google Scholar] [CrossRef]
- Vaka, V.R.; McMaster, K.M.; Cunningham, M.W.; Ibrahim, T.; Hazlewood, R.; Usry, N.; Cornelius, D.C.; Amaral, L.M.; Lamarca, B. Role of Mitochondrial Dysfunction and Reactive Oxygen Species in Mediating Hypertension in the Reduced Uterine Perfusion Pressure Rat Model of Preeclampsia. Hypertension 2018, 72, 703–711. [Google Scholar] [CrossRef]
- Paauw, N.D.; Joles, J.A.; Spradley, F.T.; Bakrania, B.; Zsengeller, Z.K.; Franx, A.; Verhaar, M.C.; Granger, J.P.; Lely, A.T. Exposure to placental ischemia impairs postpartum maternal renal and cardiac function in rats. Am. J. Physiol. Integr. Comp. Physiol. 2017, 312, R664–R670. [Google Scholar] [CrossRef] [PubMed]
- Bohlender, J.; Ganten, D.; Luft, F.C. Rats Transgenic for Human Renin and Human Angiotensinogen as a Model for Gestational Hypertension. J. Am. Soc. Nephrol. 2000, 11, 2056–2061. [Google Scholar]
- Brosnihan, K.B.; Hering, L.; Dechend, R.; Chappell, M.C.; Herse, F. Increased Angiotensin II in the Mesometrial Triangle of a Transgenic Rat Model of Preeclampsia. Hypertension 2010, 55, 562–566. [Google Scholar] [CrossRef][Green Version]
- Hering, L.; Herse, F.; Geusens, N.; Verlohren, S.; Wenzel, K.; Staff, A.C.; Brosnihan, K.B.; Huppertz, B.; Luft, F.C.; Muller, D.N.; et al. Effects of circulating and local uteroplacental angiotensin II in rat pregnancy. Hypertension 2010, 56, 311–318. [Google Scholar] [CrossRef]
- Kräker, K.; O’Driscoll, J.M.; Schütte, T.; Herse, F.; Patey, O.; Golic, M.; Geisberger, S.; Verlohren, S.; Birukov, A.; Heuser, A.; et al. Statins Reverse Postpartum Cardiovascular Dysfunction in a Rat Model of Preeclampsia. Hypertension 2020, 75, 202–210. [Google Scholar] [CrossRef]
- Jin, Y.; Shao, X.; Sun, B.; Miao, C.; Li, Z.; Shi, Y. Urinary kidney injury molecule-1 as an early diagnostic biomarker of obstructive acute kidney injury and development of a rapid detection method. Mol. Med. Rep. 2017, 15, 1229–1235. [Google Scholar] [CrossRef]
- Schmidt-Ott, K.M.; Mori, K.; Li, J.Y.; Kalandadze, A.; Cohen, D.J.; Devarajan, P.; Barasch, J. Dual Action of Neutrophil Gelatinase–Associated Lipocalin. J. Am. Soc. Nephrol. 2007, 18, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Dechend, R.; Viedt, C.; MüllerD, N.; Ugele, B.; Brandes, R.P.; Wallukat, G.; Park, J.-K.; Janke, J.; Barta, P.; Theuer, J.; et al. AT 1 Receptor Agonistic Antibodies From Preeclamptic Patients Stimulate NADPH Oxidase. Circulation 2003, 107, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, G.; Pussetto, M.; Rose, M.; Staff, A.; Blois, S.; Toblli, J. Defective trophoblast invasion underlies fetal growth restriction and preeclampsia-like symptoms in the stroke-prone spontaneously hypertensive rat. Mol. Hum. Reprod. 2017, 23, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Davisson, R.L.; Hoffmann, D.S.; Butz, G.M.; Aldape, G.; Schlager, G.; Merrill, D.C.; Sethi, S.; Weiss, R.M.; Bates, J.N. Discovery of a Spontaneous Genetic Mouse Model of Preeclampsia. Hypertension 2002, 39, 337–342. [Google Scholar] [CrossRef]
- Sandgren, J.A.; Deng, G.; Linggonegoro, D.W.; Scroggins, S.M.; Perschbacher, K.J.; Nair, A.R.; Nishimura, T.E.; Zhang, S.Y.; Agbor, L.N.; Wu, J.; et al. Arginine vasopressin infusion is sufficient to model clinical features of preeclampsia in mice. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Doridot, L.; Passet, B.; Méharts, C.; Rigourd, V.; Barbaux, S.; Ducat, A.; Mondon, F.; Vilotte, M.; Castille, J.; Breuiller-Fouché, M.; et al. Preeclampsia-Like Symptoms Induced in Mice by Fetoplacental Expression of STOX1 Are Reversed by Aspirin Treatment. Hypertension 2013, 61, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Turbeville, H.R.; Taylor, E.B.; Garrett, M.R.; Didion, S.P.; Ryan, M.J.; Sasser, J.M. Superimposed Preeclampsia Exacerbates Postpartum Renal Injury Despite Lack of Long-Term Blood Pressure Difference in the Dahl Salt-Sensitive Rat. Hypertension 2019, 73, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Cowley, A.W. Role of Pressure in Angiotensin II-Induced Renal Injury. Hypertension 2004, 43, 752–759. [Google Scholar] [CrossRef]
- Hildebrand, A.M.; Hladunewich, M.A.; Garg, A.X. Preeclampsia and the Long-term Risk of Kidney Failure. Am. J. Kidney Dis. 2017, 69, 487–488. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Haase, N.; Golic, M.; Herse, F.; Rugor, J.; Linz, D.; Solano, M.E.; Müller, M.N.; Dechend, R. Relaxin Treatment in an Ang-II-Based Transgenic Preeclamptic-Rat Model. PLoS ONE 2016, 11, e0150743. [Google Scholar] [CrossRef]
- Andersen, L.B.; Golic, M.; Przybyl, L.; Sorensen, G.L.; Jørgensen, J.S.; Fruekilde, P.; Von Versen-Höynck, F.; Herse, F.; Højskov, C.S.; Dechend, R.; et al. Vitamin D depletion does not affect key aspects of the preeclamptic phenotype in a transgenic rodent model for preeclampsia. J. Am. Soc. Hypertens. 2016, 10, 597–607.e1. [Google Scholar] [CrossRef] [PubMed]
- Vigolo, E.; Markó, L.; Hinze, C.; Müller, M.N.; Schmidt-Ullrich, R.; Schmidt-Ott, K.M. Canonical BMP signaling in tubular cells mediates recovery after acute kidney injury. Kidney Int. 2019, 95, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Rangan, G.K.; Tesch, G.H. Quantification of renal pathology by image analysis (Methods in Renal Research). Nephrology 2007, 12, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, C.D.; Döpp, E.A.; Joling, P.K.G. The heterogeneity of mononuclear phagocytes in lymphoid organs: Distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 1985, 54, 589–599. [Google Scholar] [PubMed]
- Damoiseaux, J.G.; Döpp, E.A.; Calame, W.; Chao, D.; MacPherson, G.G.; Dijkstra, C.D. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology 1994, 83, 140–147. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kedziora, S.M.; Kräker, K.; Markó, L.; Binder, J.; Sugulle, M.; Gauster, M.; Müller, D.N.; Dechend, R.; Haase, N.; Herse, F. Kidney Injury Caused by Preeclamptic Pregnancy Recovers Postpartum in a Transgenic Rat Model. Int. J. Mol. Sci. 2021, 22, 3762. https://doi.org/10.3390/ijms22073762
Kedziora SM, Kräker K, Markó L, Binder J, Sugulle M, Gauster M, Müller DN, Dechend R, Haase N, Herse F. Kidney Injury Caused by Preeclamptic Pregnancy Recovers Postpartum in a Transgenic Rat Model. International Journal of Molecular Sciences. 2021; 22(7):3762. https://doi.org/10.3390/ijms22073762
Chicago/Turabian StyleKedziora, Sarah M., Kristin Kräker, Lajos Markó, Julia Binder, Meryam Sugulle, Martin Gauster, Dominik N. Müller, Ralf Dechend, Nadine Haase, and Florian Herse. 2021. "Kidney Injury Caused by Preeclamptic Pregnancy Recovers Postpartum in a Transgenic Rat Model" International Journal of Molecular Sciences 22, no. 7: 3762. https://doi.org/10.3390/ijms22073762
APA StyleKedziora, S. M., Kräker, K., Markó, L., Binder, J., Sugulle, M., Gauster, M., Müller, D. N., Dechend, R., Haase, N., & Herse, F. (2021). Kidney Injury Caused by Preeclamptic Pregnancy Recovers Postpartum in a Transgenic Rat Model. International Journal of Molecular Sciences, 22(7), 3762. https://doi.org/10.3390/ijms22073762