Studies in a Murine Granuloma Model of Instilled Carbon Nanotubes: Relevance to Sarcoidosis
Abstract
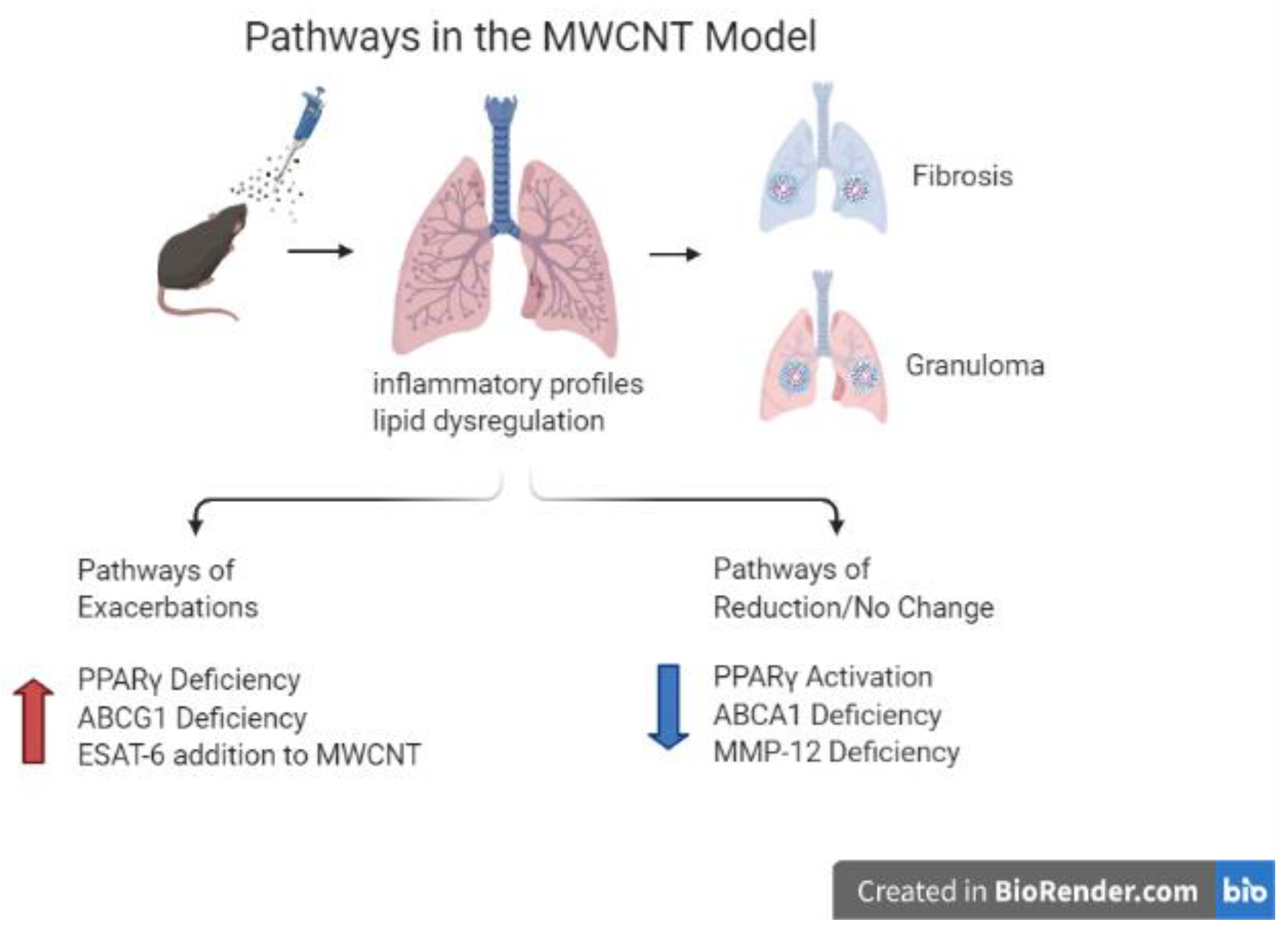
1. Background
2. Development of a Murine Multi-Wall Carbon Nanotube (MWCNT)-Induced Granuloma Model
3. Role of PPARγ in Sarcoidosis
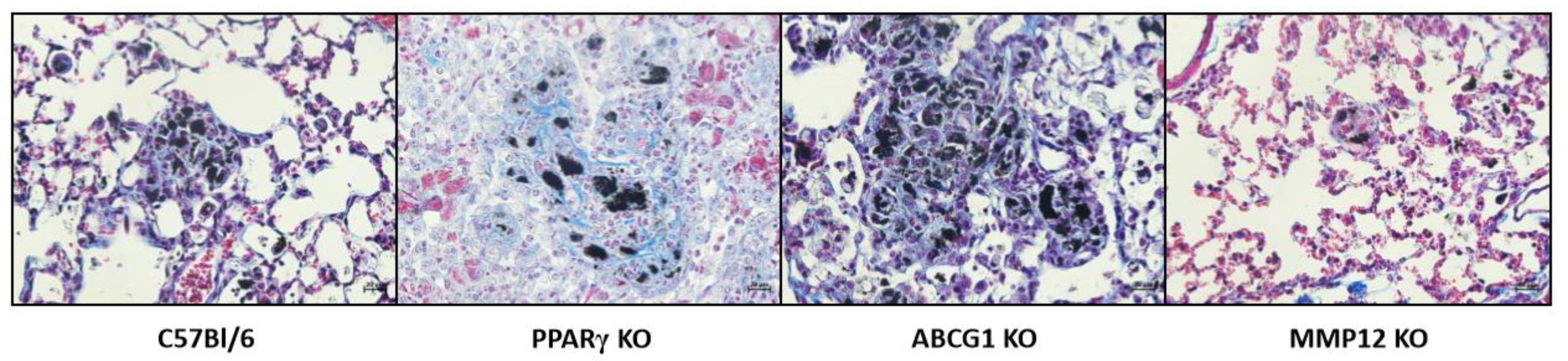
3.1. PPARγ Deficiency and Elevated Granulomatosis
3.2. PPARγ Deficiency and Elevated Fibrosis
3.3. PPARγ Deficiency and Elevated Th-17 Lymphocytes
4. ABCG1 Deficiency
4.1. ABCG1 Deficiency and the MWCNT Granuloma Model
4.2. ABCG1/ABCA1 Deficiencies and MicroRNA 33 Elevation in the MWCNT Model
5. Common Gene Pathways in Sarcoidosis and the MWCNT Model
6. MMP12 Deficiency and the MWCNT Granuloma Model
7. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arkema, E.V.; Cozier, Y.C. Epidemiology of sarcoidosis: Current findings and future directions. Ther. Adv. Chronic Dis. 2018, 9, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Chen, E.S.; et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef]
- Judson, M.A. Environmental Risk Factors for Sarcoidosis. Front. Immunol. 2020, 11, 1340. [Google Scholar] [CrossRef] [PubMed]
- Culver, D.A.; Judson, M.A. New advances in the management of pulmonary sarcoidosis. BMJ 2019, 367, l5553. [Google Scholar] [CrossRef]
- Newman, L.S.; Rose, C.S.; Bresnitz, E.A.; Rossman, M.D.; Barnard, J.; Frederick, M.; Terrin, M.L.; Weinberger, S.E.; Moller, D.R.; McLennan, G.; et al. A case control etiologic study of sarcoidosis: Environmental and occupational risk factors. Am. J. Respir. Crit. Care Med. 2004, 170, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Prezant, D.J.; Dhala, A.; Goldstein, A.; Janus, D.; Ortiz, F.; Aldrich, T.K.; Kelly, K.J. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest 1999, 116, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.E.; Herbert, R.; Moline, J.M.; Wallenstein, S.; Shukla, G.; Schechter, C.; Skloot, G.S.; Udasin, I.; Luft, B.J.; Harrison, D.; et al. “Sarcoid like” granulomatous pulmonary disease in World Trade Center disaster responders. Am. J. Ind. Med. 2011, 54, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Izbicki, G.; Chavko, R.; Banauch, G.I.; Weiden, M.D.; Berger, K.I.; Aldrich, T.K.; Hall, C.; Kelly, K.J.; Prezant, D.J. World Trade Center “Sarcoid-Like” Granulomatous Pulmonary Disease in New York City Fire Department Rescue Workers. Chest 2007, 131, 1414–1423. [Google Scholar] [CrossRef]
- Sunil, V.R.; Radbel, J.; Hussain, S.; Vayas, K.N.; Cervelli, J.; Deen, M.; Kipen, H.; Udasin, I.; Laumbach, R.; Sunderram, J.; et al. Sarcoid-Like Granulomatous Disease: Pathologic Case Series in World Trade Center Dust Exposed Rescue and Recovery Workers. Int J. Environ. Res. Public Health 2019, 16, 815. [Google Scholar] [CrossRef]
- Amenta, V.; Aschberger, K. Carbon nanotubes: Potential medical applications and safety concerns. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 371–386. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon nanotubes: Applications in pharmacy and medicine. Biomed. Res. Int. 2013, 2013, 578290. [Google Scholar] [CrossRef] [PubMed]
- Huizar, I.; Malur, A.; Midgette, Y.A.; Kukoly, C.; Chen, P.; Ke, P.C.; Podila, R.; Rao, A.M.; Wingard, C.J.; Dobbs, L.; et al. Novel Murine Model of Chronic Granulomatous Lung Inflammation Elicited by Carbon Nanotubes. Am. J. Respir. Cell Mol. Biol. 2011, 45, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Looney, M.; Robriquet, L.; Fang, X.; Matthay, M.A. Direct visual instillation as a method for efficient delivery of fluid into the distal airspaces of anesthetized mice. Exp. Lung Res. 2004, 30, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-G.; Li, W.-L.; Xu, J.-Y.; Cai, X.-Q.; Liu, R.-L.; Li, Y.-J.; Zhao, Q.-F.; Li, Q.N. Comparative study of pathological lesions induced by multiwalled carbon nanotubes in lungs of mice by intrtracheal instillation and inhalation. Environ. Toxicol. 2007, 22, 415–421. [Google Scholar] [CrossRef]
- Costa, D.L.; Lehmann, J.R.; Winsett, D.; Richards, J.; Ledbetter, A.D.; Dreher, K.L. Comparative pulmonary toxicological assessment of oil combustion particles following inhalation or instillation exposure. Toxicol. Sci. 2006, 91, 237–246. [Google Scholar] [CrossRef]
- Driscoll, K.E.; Costa, D.L.; Hatch, G.; Henderson, R.; Oberdorster, G.; Salem, H.; Schlesinger, R.B. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: Uses and limitations. Toxicol. Sci. 2000, 55, 24–35. [Google Scholar] [CrossRef]
- Mohan, A.; Malur, A.; McPeek, M.; Barna, B.P.; Schnapp, L.M.; Thomassen, M.J.; Gharib, S.A. Transcriptional survey of alveolar macrophages in a murine model of chronic granulomatous inflammation reveals common themes with human sarcoidosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L617–L625. [Google Scholar] [CrossRef]
- O’Regan, A.W.; Hayden, J.M.; Body, S.; Liaw, L.; Mulligan, N.; Goetschkes, M.; Berman, J.S. Abnormal pulmonary granuloma formation in osteopontin-deficient mice. Am. J. Respir. Crit. Care Med. 2001, 164, 2243–2247. [Google Scholar] [CrossRef]
- Fingleton, B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2036–2042. [Google Scholar] [CrossRef]
- Helming, L.; Gordon, S. The molecular basis of macrophage fusion. Immunobiology 2007, 212, 785–793. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Straus, D.S.; Glass, C.K. Anti-inflammatory actions of PPAR ligands: New insights on cellular and molecular mechanisms. Trends Immunol. 2007, 28, 551–558. [Google Scholar] [CrossRef]
- Smith, M.R.; Standiford, T.J.; Reddy, R.C. PPARs in alveolar macrophage biology. PPAR Res. 2007, 2007, 23812. [Google Scholar] [CrossRef] [PubMed]
- Croasdell, A.; Duffney, P.F.; Kim, N.; Lacy, S.H.; Sime, P.J.; Phipps, R.P. PPARgamma and the Innate Immune System Mediate the Resolution of Inflammation. PPAR Res. 2015, 2015, 549691. [Google Scholar] [CrossRef]
- Schachtrup, C.; Malcharek, S.; Haitsma, J.J.; Lachmann, B.; Owada, Y.; Binas, B.; Kondo, H.; Rustow, B.; Galla, H.J.; Spener, F. Activation of PPARgamma reverses a defect of surfactant synthesis in mice lacking two types of fatty acid binding protein. Biochim. Biophys. Acta 2008, 1781, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Malur, A.; Mccoy, A.J.; Arce, S.; Barna, B.P.; Kavuru, M.S.; Malur, A.G.; Thomassen, M.J. Deletion of PPARg in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J. Immunol. 2009, 182, 5816–5822. [Google Scholar] [CrossRef] [PubMed]
- Huizar, I.; Malur, A.; Patel, J.; McPeek, M.; Dobbs, L.; Wingard, C.; Barna, B.P.; Thomassen, M.J. The role of PPARgamma in carbon nanotube-elicited granulomatous lung inflammation. Respir. Res. 2013, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Culver, D.A.; Barna, B.P.; Raychaudhuri, B.; Bonfield, T.L.; Abraham, S.; Malur, A.; Farver, C.F.; Kavuru, M.S.; Thomassen, M.J. Peroxisome proliferator-activated receptor gamma activity is deficient in alveolar macrophages in pulmonary sarcoidosis. Am. J. Respir. Cell Mol. Biol. 2004, 30, 1–5. [Google Scholar] [CrossRef]
- Barna, B.P.; Culver, D.A.; Abraham, S.; Malur, A.; Bonfield, T.L.; John, N.; Farver, C.F.; Drazba, J.A.; Raychaudhuri, B.; Kavuru, M.S.; et al. Depressed peroxisome proliferator-activated receptor gamma (PPARgamma) is indicative of severe pulmonary sarcoidosis: Possible involvement of interferon gamma (IFN-gamma). Sarcoidosis. Vasc. Diffuse. Lung Dis. 2006, 23, 93–100. [Google Scholar] [PubMed]
- Robinson, B.W.; McLemore, T.L.; Crystal, R.G. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J. Clin. Investig. 1985, 75, 1488–1495. [Google Scholar] [CrossRef]
- Asano, M.; Minagawa, T.; Ohmichi, M.; Hiraga, Y. Detection of endogenous cytokines in sera or in lymph nodes obtained from patients with sarcoidosis. Clin. Exp. Immunol. 1991, 84, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Ramstein, J.; Broos, C.E.; Simpson, L.J.; Ansel, K.M.; Sun, S.A.; Ho, M.E.; Woodruff, P.G.; Bhakta, N.R.; Christian, L.; Nguyen, C.P.; et al. Interferon-γ-producing Th17.1 Cells are Increased in Sarcoidosis and More Prevalent Than Th1 Cells. Am. J. Respir. Crit. Care Med. 2016, 193, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Cunard, R.; Eto, Y.; Muljadi, J.T.; Glass, C.K.; Kelly, C.J.; Ricote, M. Repression of IFN-gamma expression by peroxisome proliferator-activated receptor gamma. J. Immunol. 2004, 172, 7530–7536. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Neequaye, N.; Malur, A.; Soliman, E.; McPeek, M.; Leffler, N.; Ogburn, D.; Tokarz, D.A.; Knudson, W.; Gharib, S.A.; et al. Matrix Metalloproteinase-12 Is Required for Granuloma Progression. Front. Immunol. 2020, 11, 553949. [Google Scholar] [CrossRef]
- McPeek, M.; Malur, A.; Tokarz, D.A.; Murray, G.; Barna, B.P.; Thomassen, M.J. PPAR-gamma pathways attenuate pulmonary granuloma formation in a carbon nanotube induced murine model of sarcoidosis. Biochem. Biophys. Res. Commun. 2018, 503, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Cardarelli, P.M.; Parry, G.C.; Felts, K.A.; Cobb, R.R. Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-kappa B and AP-1. Eur. J. Immunol. 1997, 27, 1091–1097. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, L.; Zhang, M.; Wang, P.; Zhang, L.; Yuan, C.; Qi, J.; Qiao, Y.; Kuo, P.C.; Gao, C. NF-kappaB- and AP-1-mediated DNA looping regulates osteopontin transcription in endotoxin-stimulated murine macrophages. J. Immunol. 2011, 186, 3173–3179. [Google Scholar] [CrossRef]
- Bonham, C.A.; Strek, M.E.; Patterson, K.C. From granuloma to fibrosis: Sarcoidosis associated pulmonary fibrosis. Curr. Opin. Pulm. Med. 2016, 22, 484–491. [Google Scholar] [CrossRef]
- Malur, A.; Barna, B.P.; Patel, J.; McPeek, M.; Wingard, C.J.; Dobbs, L.; Thomassen, M.J. Exposure to a Mycobacterial Antigen, ESAT-6, Exacerbates Granulomatous and Fibrotic Changes in a Multiwall Carbon Nanotube Model of Chronic Pulmonary Disease. J. Nanomed. Nanotechnol. 2015, 6, 340. [Google Scholar] [CrossRef]
- Malur, A.; Mohan, A.; Barrington, R.A.; Leffler, N.; Malur, A.; Muller-Borer, B.; Murray, G.; Kew, K.; Zhou, C.; Russell, J.; et al. PPARgamma Deficiency Exacerbates Fibrotic Response to Mycobacteria Peptide in Murine Sarcoidosis Model. Am. J. Respir. Cell Mol. Biol. 2019. [Google Scholar] [CrossRef]
- Carlisle, J.; Evans, W.; Hajizadeh, R.; Nadaf, M.; Shepherd, B.; Ott, R.D.; Richter, K.; Drake, W. Multiple Mycobacterium antigens induce interferon-gamma production from sarcoidosis peripheral blood mononuclear cells. Clin. Exp. Immunol. 2007, 150, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Drake, W.P.; Dhason, M.S.; Nadaf, M.; Shepherd, B.E.; Vadivelu, S.; Hajizadeh, R.; Newman, L.S.; Kalams, S.A. Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect. Immun. 2007, 75, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Huang, H.; Xu, Z. Immunolgical evidence for the role of mycobacteria in sarcoidosis: A meta-analysis. PLoS ONE 2016, 11, e0154716. [Google Scholar] [CrossRef] [PubMed]
- Gautier, E.L.; Chow, A.; Spanbroek, R.; Marcelin, G.; Greter, M.; Jakubzick, C.; Bogunovic, M.; Leboeuf, M.; Van, R.N.; Habenicht, A.J.; et al. Systemic analysis of PPARgamma in mouse macrophage populations reveals marked diversity in expression with critical roles in resolution of inflammation and airway immunity. J. Immunol. 2012, 189, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Sanderford, V.; Barna, B.P.; Barrington, R.A.; Malur, A.; Mohan, A.; Leffler, N.; Soliman, E.; Thomassen, M.J. PPARgamma Deficiency in Carbon Nanotube-elicited Granulomatous Inflammation Promotes a Th17 Response to a Microbial Antigen. J. Nanomed. Nanotechnol. 2020, 11. [Google Scholar] [CrossRef]
- Crouser, E.D. Role of imbalance between Th17 and regulatory T-cells in sarcoidosis. Curr. Opin. Pulm. Med. 2018, 24, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.; Bargagli, E.; Refini, R.M.; Rottoli, P. New concepts in the pathogenesis of sarcoidosis. Expert Rev. Respir. Med. 2019, 13, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.R.; Tao, J.Q.; Collins, H.L.; Francone, O.L.; Rothblat, G.H. Pulmonary abnormalities due to ABCA1 deficiency in mice. AJP Lung Cell. Mol. Physiol. 2005, 289, L980–L989. [Google Scholar] [CrossRef]
- Baldan, A.; Tarr, P.; Lee, R.; Edwards, P.A. ATP-binding cassette transporter G1 and lipid homeostasis. Curr. Opin. Lipidol. 2006, 17, 227–232. [Google Scholar] [CrossRef]
- Thomassen, M.J.; Barna, B.P.; Malur, A.; Bonfield, T.L.; Farver, C.F.; Malur, A.; Dalrymple, H.; Kavuru, M.S.; Febbraio, M. ABCG1 is deficient in alveolar macrophages of GM-CSF knock-out mice and patients with pulmonary alveolar proteinsosis. J. Lipid Res. 2007, 48, 2762–2768. [Google Scholar] [CrossRef]
- Barna, B.P.; McPeek, M.; Malur, A.; Fessler, M.B.; Wingard, C.J.; Dobbs, L.; Verbanac, K.M.; Bowling, M.; Judson, M.A.; Thomassen, M.J. Elevated MicroRNA-33 in Sarcoidosis and a Carbon Nanotube Model of Chronic Granulomatous Disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.D.; Malur, A.; Barna, B.P.; Ghosh, S.; Kavuru, M.S.; Malur, A.G.; Thomassen, M.J. Targeted PPAR{gamma} deficiency in alveolar macrophages disrupts surfactant catabolism. J. Lipid Res. 2010, 51, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- McPeek, M.; Malur, A.; Tokarz, D.A.; Lertpiriyapong, K.; Gowdy, K.M.; Murray, G.; Wingard, C.J.; Fessler, M.B.; Barna, B.P.; Thomassen, M.J. Alveolar Macrophage ABCG1 Deficiency Promotes Pulmonary Granulomatous Inflammation. Am. J. Respir. Cell Mol. Biol. 2019, 61, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Samokhin, A.O.; Gauthier, J.Y.; Percival, M.D.; Bromme, D. Lack of cathepsin activities alter or prevent the development of lung granulomas in a mouse model of sarcoidosis. Respir. Res. 2011, 12, 13. [Google Scholar] [CrossRef]
- Nguyen, K.B.; Watford, W.T.; Salomon, R.; Hofmann, S.R.; Pien, G.C.; Morinobu, A.; Gadina, M.; O’Shea, J.J.; Biron, C.A. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science 2002, 297, 2063–2066. [Google Scholar] [CrossRef]
- O’Regan, A. The role of osteopontin in lung disease. Cytokine Growth Factor Rev. 2003, 14, 479–488. [Google Scholar] [CrossRef]
- Crouser, E.D.; Culver, D.A.; Knox, K.S.; Julian, M.W.; Shao, G.; Abraham, S.; Liyanarachchi, S.; Macre, J.E.; Wewers, M.D.; Gavrilin, M.A.; et al. Gene Expression Profiling Identifies MMP-12 and ADAMDEC1 as Potential Pathogenic Mediators of Pulmonary Sarcoidosis. Am. J. Respir. Crit. Care Med. 2009, 179, 929–938. [Google Scholar] [CrossRef]
- O’Regan, A.W.; Chupp, G.L.; Lowry, J.A.; Goetschkes, M.; Mulligan, N.; Berman, J.S. Osteopontin is associated with T cells in sacroid granulomas and has T cell adhesive and cytokin-like properties in vitro. J. Immunol. 1999, 162, 1024–1031. [Google Scholar]
- Palchevskiy, V.; Hashemi, N.; Weigt, S.S.; Xue, Y.Y.; Derhovanessian, A.; Keane, M.P.; Strieter, R.M.; Fishbein, M.C.; Deng, J.C.; Lynch, J.P., III; et al. Immune response CC chemokines CCL2 and CCL5 are associated with pulmonary sarcoidosis. Fibrogenesis. Tissue Repair. 2011, 4, 10. [Google Scholar] [CrossRef]
- Barna, B.P.; Huizar, I.; Malur, A.; McPeek, M.; Marshall, I.; Jacob, M.; Dobbs, L.; Kavuru, M.S.; Thomassen, M.J. Carbon nanotube-induced pulmonary granulomatous disease: Twist1 and alveolar macrophage m1 activation. Int. J. Mol. Sci. 2013, 14, 23858–23871. [Google Scholar] [CrossRef]
- Patterson, K.C.; Chen, E.S. The Pathogenesis of Pulmonary Sarcoidosis and Implications for Treatment. Chest 2018, 153, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Katwa, P.; Podila, R.; Chen, P.; Ke, P.C.; Rao, A.M.; Walters, D.M.; Wingard, C.J.; Brown, J.M. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Part. Fibre. Toxicol. 2011, 8, 24. [Google Scholar] [CrossRef]
- Lu, B.; Rutledge, B.J.; Gu, L.; Fiorillo, J.; Lukacs, N.W.; Kunkel, S.L.; North, R.; Gerard, C.; Rollins, B.J. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 1998, 187, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, H.; Zissel, G.; Goldmann, T.; Tschernig, T.; Vollmer, E.; Pabst, R.; Muller-Quernheim, J. Alveolar macrophages are the main source for tumour necrosis factor-alpha in patients with sarcoidosis. Eur. Respir. J. 2003, 21, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Prasse, A.; Georges, C.G.; Biller, H.; Hamm, H.; Matthys, H.; Luttmann, W.; Virchow, J.C., Jr. Th1 cytokine pattern in sarcoidosis is expressed by bronchoalveolar CD4+ and CD8+ T cells. Clin. Exp. Immunol. 2000, 122, 241–248. [Google Scholar] [CrossRef]
- Rayner, K.J.; Sheedy, F.J.; Esau, C.C.; Hussain, F.N.; Temel, R.E.; Parathath, S.; van Gils, J.M.; Rayner, A.J.; Chang, A.N.; Suarez, Y.; et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J. Clin. Investig. 2011, 121, 2921–2931. [Google Scholar] [CrossRef]
- Besnard, V.; Jeny, F. Models Contribution to the Understanding of Sarcoidosis Pathogenesis: “Are There Good Models of Sarcoidosis?”. J. Clin. Med. 2020, 9, 2445. [Google Scholar] [CrossRef]
| Mediators and Status | Role in Pathophysiology | Pulmonary Locations | |
|---|---|---|---|
| MWCNT MODEL | SARCOIDOSIS | ||
| Peroxisome proliferator-activated receptor gamma (PPARγ)—Deficiency | Granuloma Formation [27] | Bronchoalveolar lavage (BAL) cells [27] | BAL cells [28] |
| Lipid Dysregulation [22] | |||
| Fibrosis [40] | |||
| Inflammatory Profiles [26] | |||
| Osteopontin—Elevation | Granuloma Formation [18] | Granuloma tissue, BAL cells, BAL fluids [12,62] | Granuloma tissue [58] |
| Matrix-metalloproteinase 12 (MMP-12)—Elevation | Granuloma Formation [34] | Granuloma tissue, BAL cells (11) [34] | Granuloma tissue, BAL cells [57] |
| CCL2 (MCP-1)—Elevation | Granuloma Formation [63] | Granuloma tissue [12,62] | Granuloma tissue [59] |
| ABCG1—Deficiency | Granuloma Formation [53] | BAL cells [51] | BAL cells [51] |
| Fibrosis [53] | |||
| Lipid Dysregulation [51] | |||
| Tumor Necrosis Factor alpha (TNFα)—Elevation | Macrophage M1 Inflammatory Profile [64] | Granuloma tissue (11) | BAL cells [64] |
| Interferon-gamma (IFN-γ)—Elevation | T Cell Inflammatory Profile [65] | Granuloma tissue, BAL cells [17] | BAL cells [30] |
| Signal Transducer and Activator of Transcription (STAT 4)—Elevation | T Cell Inflammatory Profile [55] | BAL cells [17] | BAL cells [17] |
| Cathepsin K (CTSK)—Elevation | Granuloma Formation [54] | BAL cells [17] | BAL cells [17] |
| TWIST 1—Elevation | Macrophage M1 Inflammatory Profile [60] | BAL cells [60] | BAL cells [60] |
| Th17 cells—Elevation | Inflammatory Profile [32] | BAL cells [45] | BAL cells [32] |
| Granuloma Formation [47] | |||
| T cells—Elevation | Inflammatory Profile [12,26] | Granuloma tissue [27,40,45] | Granuloma tissue [47] |
| ABCA1—Deficiency | Lipid Dysregulation [48] | BAL cells [51] | BAL cells [51] |
| MicroRNA 33 (Mir-33)—Elevation | Lipid Dysregulation [66] | Granuloma tissue, BAL cells [51] | Granuloma tissue, BAL cells [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barna, B.P.; Malur, A.; Thomassen, M.J. Studies in a Murine Granuloma Model of Instilled Carbon Nanotubes: Relevance to Sarcoidosis. Int. J. Mol. Sci. 2021, 22, 3705. https://doi.org/10.3390/ijms22073705
Barna BP, Malur A, Thomassen MJ. Studies in a Murine Granuloma Model of Instilled Carbon Nanotubes: Relevance to Sarcoidosis. International Journal of Molecular Sciences. 2021; 22(7):3705. https://doi.org/10.3390/ijms22073705
Chicago/Turabian StyleBarna, Barbara P., Anagha Malur, and Mary Jane Thomassen. 2021. "Studies in a Murine Granuloma Model of Instilled Carbon Nanotubes: Relevance to Sarcoidosis" International Journal of Molecular Sciences 22, no. 7: 3705. https://doi.org/10.3390/ijms22073705
APA StyleBarna, B. P., Malur, A., & Thomassen, M. J. (2021). Studies in a Murine Granuloma Model of Instilled Carbon Nanotubes: Relevance to Sarcoidosis. International Journal of Molecular Sciences, 22(7), 3705. https://doi.org/10.3390/ijms22073705






