BODIPY Conjugate of Epibrassinolide as a Novel Biologically Active Probe for In Vivo Imaging
Abstract
1. Introduction
2. Results
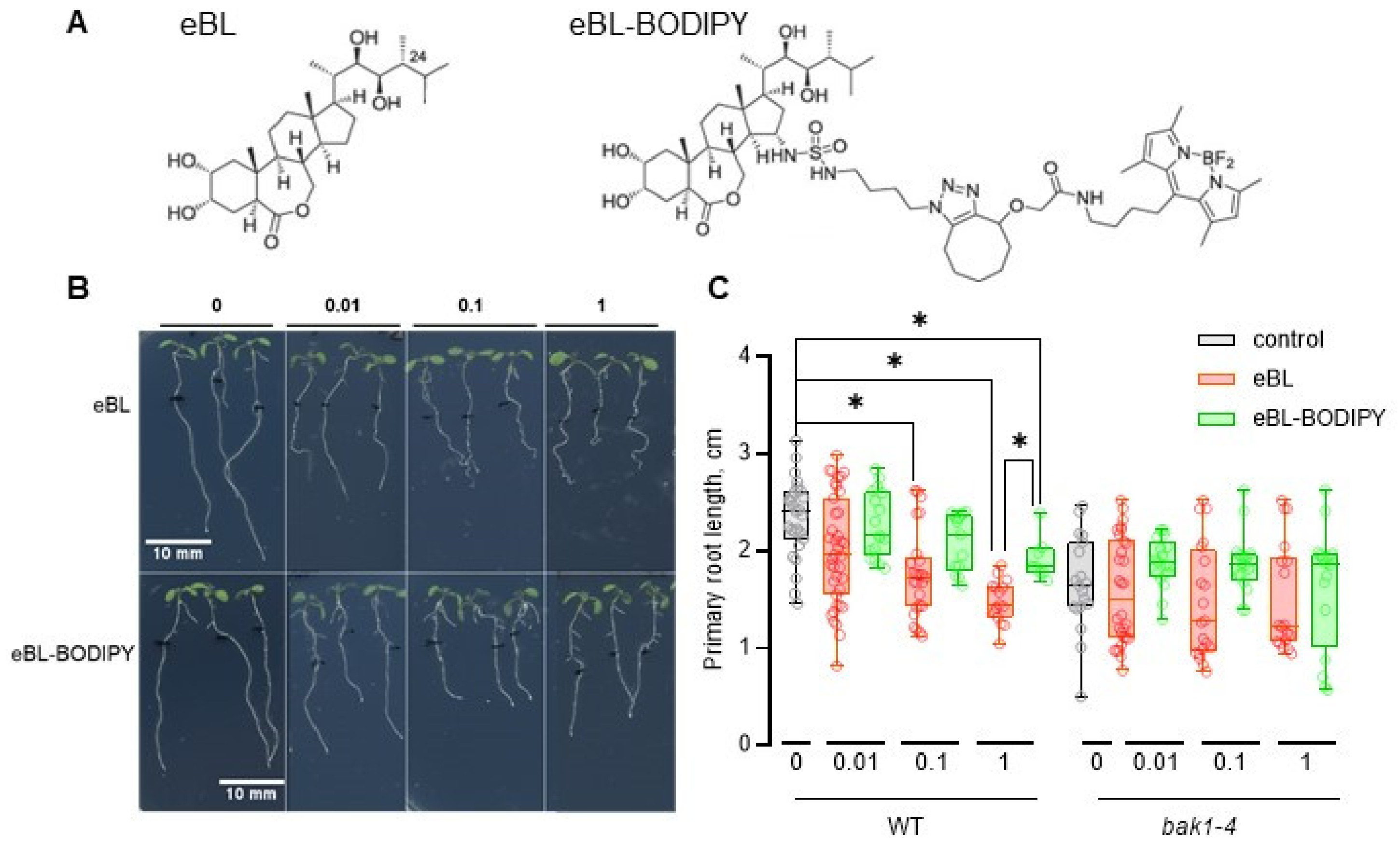
2.1. eBL-BODIPY Affects Root Growth Similarly to eBL
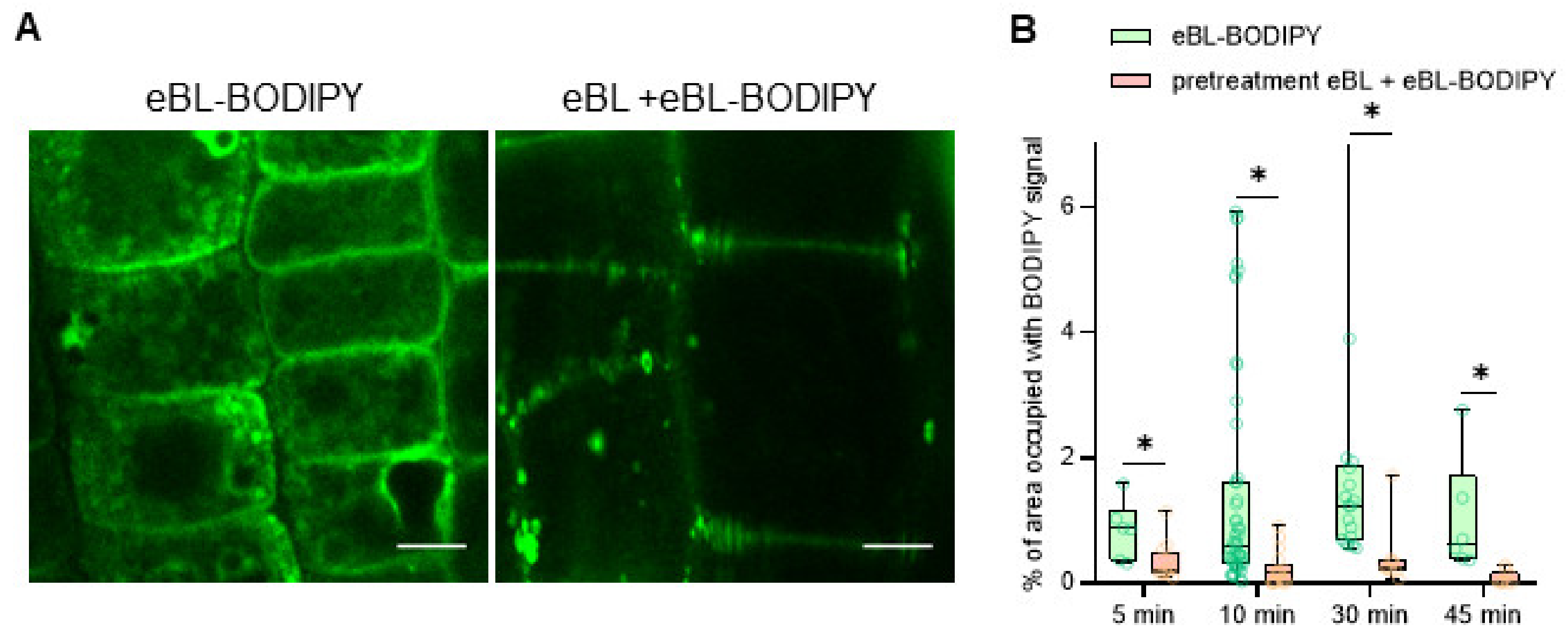
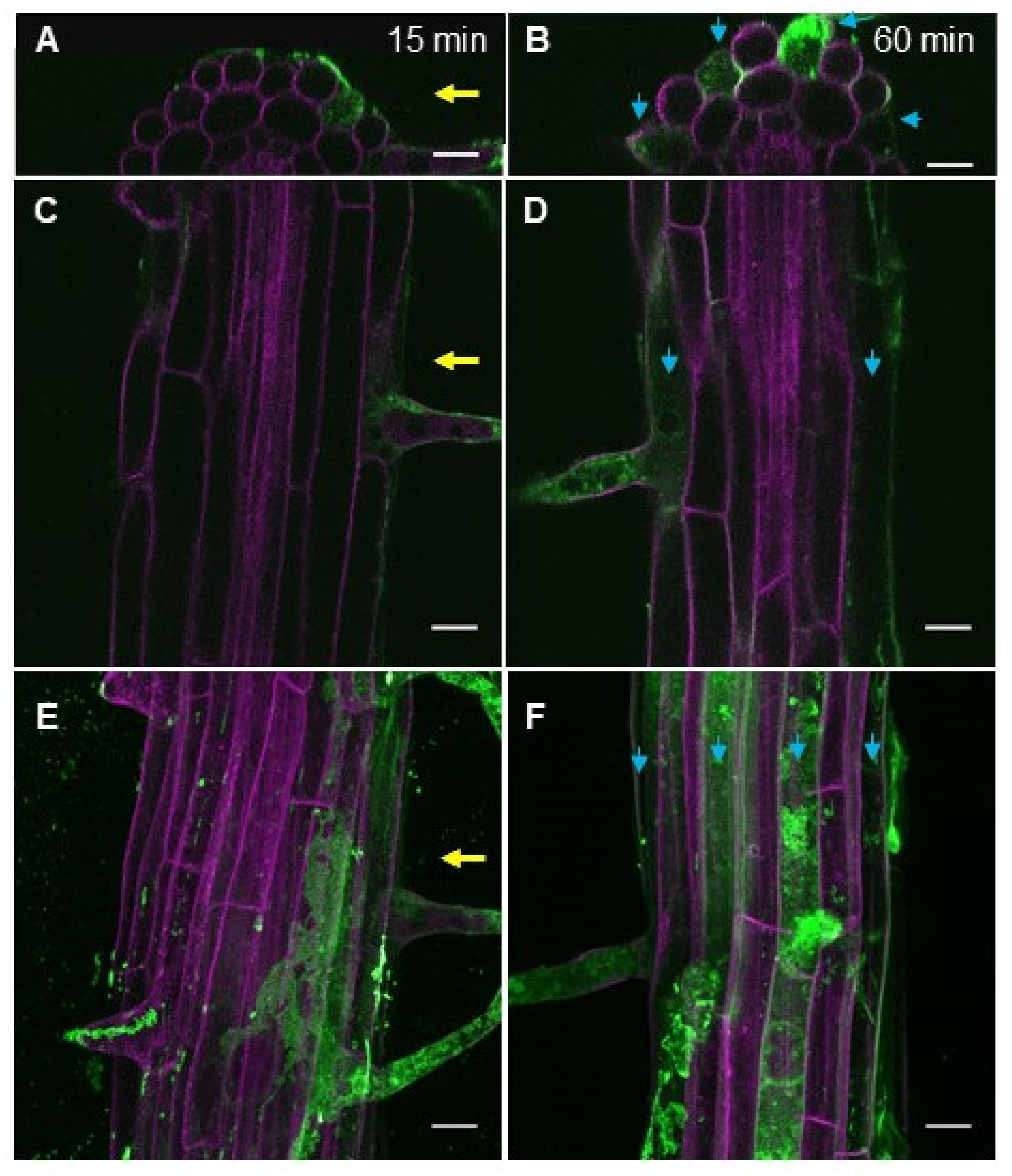
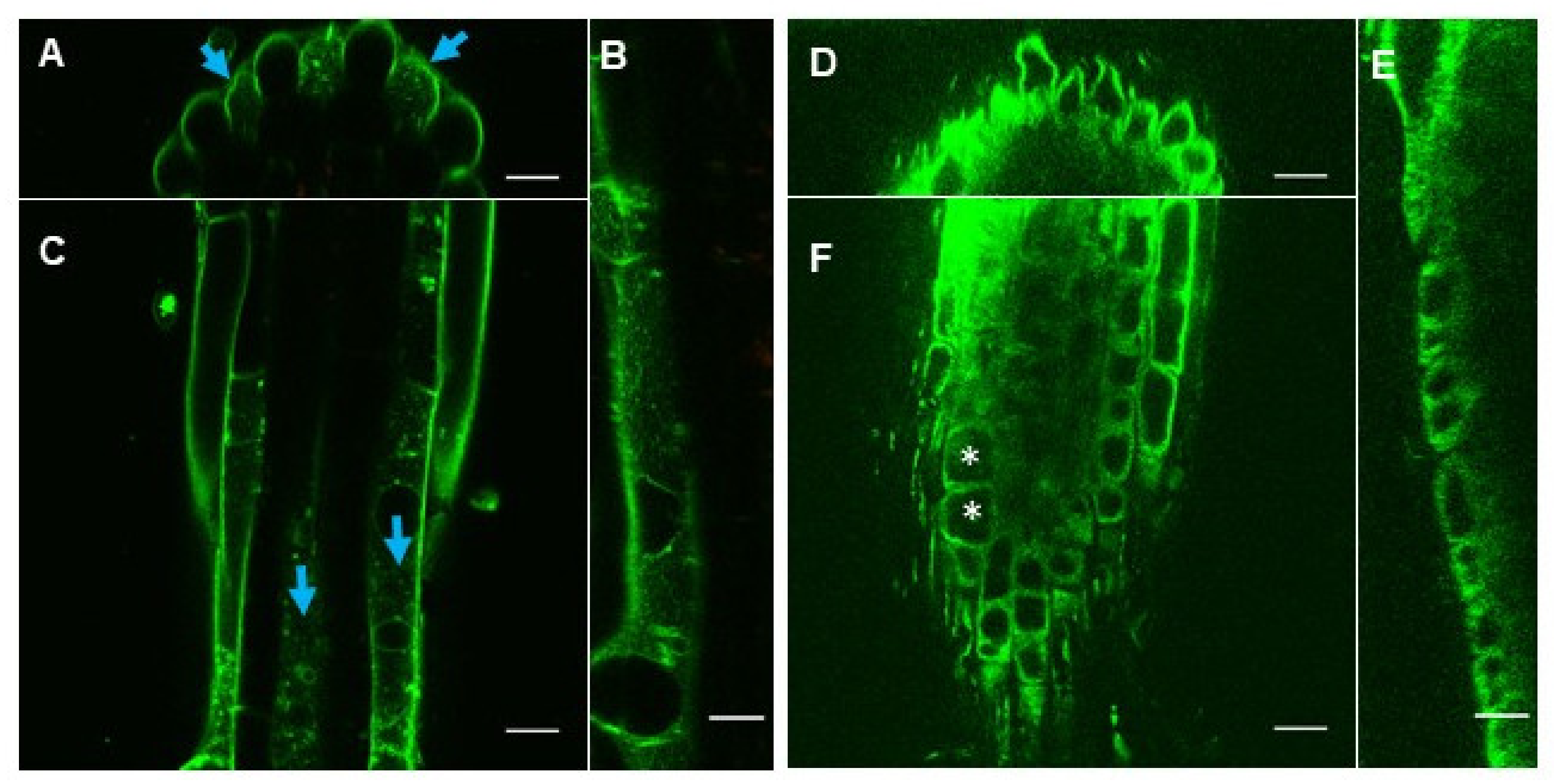
2.2. eBL-BODIPY Associates with Plant Cell Wall and Penetrates the Cytoplasm
2.3. eBL-BODIPY Partly Mimics Transcriptomic Response to eBL
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Plant Material
4.3. Root Growth Analysis
4.4. Transcript Accumulation Measurement
4.5. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-J.; Zhang, C.; Lu, Y.-N.; Jin, J.-Q.; Wang, X.-L. The mechanisms of brassinosteroids’ action: From signal transduction to plant development. Mol. Plant 2011, 4, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Li, J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 2002, 110, 203–212. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Lease, K.A.; Doke, J.T.; Tax, F.E.; Walker, J.C. BAK1, an arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 2002, 110, 213–222. [Google Scholar] [CrossRef]
- The Molecular Circuitry of Brassinosteroid Signaling-Belkhadir-2015-New Phytologist-Wiley Online Library. Available online: https://nph.onlinelibrary.wiley.com/doi/full/10.1111/nph.13269 (accessed on 17 February 2021).
- Russinova, E.; Borst, J.-W.; Kwaaitaal, M.; Caño-Delgado, A.; Yin, Y.; Chory, J.; de Vries, S.C. Heterodimerization and endocytosis of arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 2004, 16, 3216–3229. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nam, K.H. Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science 2002, 295, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, G.; He, P.; Shan, L. SERKing coreceptors for receptors. Trends Plant Sci. 2016, 21, 1017–1033. [Google Scholar] [CrossRef]
- Wang, X.; Kota, U.; He, K.; Blackburn, K.; Li, J.; Goshe, M.B.; Huber, S.C.; Clouse, S.D. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell 2008, 15, 220–235. [Google Scholar] [CrossRef]
- Wang, X.; Chory, J. Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science 2006. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Meisenhelder, J.; Hunter, T.; Yoshida, S.; Asami, T.; Chory, J. Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev. Cell 2005, 8, 855–865. [Google Scholar] [CrossRef]
- Lin, W.; Lu, D.; Gao, X.; Jiang, S.; Ma, X.; Wang, Z.; Mengiste, T.; He, P.; Shan, L. Inverse modulation of plant immune and brassinosteroid signaling pathways by the receptor-like cytoplasmic kinase BIK1. Proc. Nat. Aada. Sci. USA 2013, 110, 12114–12119. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Guan, S.; Burlingame, A.L.; Wang, Z.-Y. The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol. Cell 2011, 43, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Kim, T.-W.; Oses-Prieto, J.A.; Sun, Y.; Deng, Z.; Zhu, S.; Wang, R.; Burlingame, A.L.; Wang, Z.-Y. BSKs mediate signal transduction from the receptor kinase BRI1 in arabidopsis. Science 2008, 321, 557–560. [Google Scholar] [CrossRef]
- Kim, T.-W.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.-X.; Sun, Y.; Burlingame, A.L.; Wang, Z.-Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009, 11, 1254–1260. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Z.-Y.; Mora-Garcia, S.; Li, J.; Yoshida, S.; Asami, T.; Chory, J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 2002, 109, 181–191. [Google Scholar] [CrossRef]
- Tang, W.; Yuan, M.; Wang, R.; Yang, Y.; Wang, C.; Oses-Prieto, J.A.; Kim, T.-W.; Zhou, H.-W.; Deng, Z.; Gampala, S.S.; et al. PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat. Cell Biol. 2011, 13, 124–131. [Google Scholar] [CrossRef] [PubMed]
- He, J.-X.; Gendron, J.M.; Sun, Y.; Gampala, S.S.L.; Gendron, N.; Sun, C.Q.; Wang, Z.-Y. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 2005, 307, 1634–1638. [Google Scholar] [CrossRef]
- Yin, Y.; Vafeados, D.; Tao, Y.; Yoshida, S.; Asami, T.; Chory, J. A new class of transcription factors mediates brassinosteroid-regulated gene expression in arabidopsis. Cell 2005, 120, 249–259. [Google Scholar] [CrossRef]
- Aerts, N.; Mendes, M.P.; Wees, S.C.M.V. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Sokołowska, K.; Kizińska, J.; Szewczuk, Z.; Banasiak, A. Auxin conjugated to fluorescent dyes—A tool for the analysis of auxin transport pathways. Plant Biol. 2014, 16, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Auxin Transport Sites are Visualized in Planta Using Fluorescent Auxin Analogs|PNAS. Available online: https://www.pnas.org/content/111/31/11557.abstract (accessed on 17 February 2021).
- Benson, C.L.; Kepka, M.; Wunschel, C.; Rajagopalan, N.; Nelson, K.M.; Christmann, A.; Abrams, S.R.; Grill, E.; Loewen, M.C. Abscisic acid analogs as chemical probes for dissection of abscisic acid responses in arabidopsis thaliana. Phytochemistry 2015, 113, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Fluorescence-Labeled Abscisic Acid Possessing Abscisic Acid-Like Activity in Barley Aleurone Protoplasts: Bioscience, Biotechnology, and Biochemistry: Volume 61, No. 7. Available online: https://www.tandfonline.com/doi/abs/10.1271/bbb.61.1198 (accessed on 17 February 2021).
- Lace, B.; Prandi, C. Shaping small bioactive molecules to untangle their biological function: A focus on fluorescent plant hormones. Mol. Plant 2016, 9, 1099–1118. [Google Scholar] [CrossRef]
- Synthesis and Cytokinin Activity of Fluorescent 7-Phenylethynylimidazo[4,5-b]Pyridine and Its Riboside. J. Agric. Food Chem. Available online: https://pubs.acs.org/doi/abs/10.1021/jf0000225 (accessed on 17 February 2021).
- Liu, S.; Wang, W.-H.; Dang, Y.-L.; Fu, Y.; Sang, R. Rational design and efficient synthesis of a fluorescent-labeled jasmonate. Tetrahedron Lett. 2012, 53, 4235–4239. [Google Scholar] [CrossRef]
- Gamoh, K.; Takatsuto, S. A boronic acid derivative as a highly sensitive fluorescence derivatization reagent for brassinosteroids in liquid chromatography. Anal. Chim. Acta 1989, 222, 201–204. [Google Scholar] [CrossRef]
- Gamoh, K.; Omote, K.; Okamoto, N.; Takatsuto, S. High-Performance liquid chromatography of brassinosteroids in plants with derivatization using 9-phenanthreneboronic acid. J. Chromatogr. A 1989, 469, 424–428. [Google Scholar] [CrossRef]
- Winter, J.; Schneider, B.; Meyenburg, S.; Strack, D.; Adam, G. Monitoring brassinosteroid biosynthetic enzymes by fluorescent tagging and HPLC analysis of their substrates and products. Phytochemistry 1999, 51, 237–242. [Google Scholar] [CrossRef]
- Borisevich, N.A.; Raichenok, T.F.; Khripach, V.A.; Zhabinskii, V.N.; Ivanova, G.V. Solution electronic spectra of brassinosteroid and a synthesized conjugate of a steroid and a fluorescent label. J. Appl. Spectrosc. 2008, 75, 75–79. [Google Scholar] [CrossRef]
- Borisevich, N.A.; Bagnich, S.A.; Raichenok, T.F.; Knyukshto, V.N.; Baranovskii, A.V.; Zhabinskii, V.N. Luminescence of biologically active 24-epicastasterone and a model compound. J. Appl. Spectrosc. 2008, 75, 187–191. [Google Scholar] [CrossRef]
- Raichenok, T.F.; Litvinovskaya, R.P.; Zhabinskii, V.N.; Raiman, M.E.; Kurtikova, A.L.; Minin, P.S. Synthesis and spectral and luminescence properties of new conjugates of brassinosteroids for immunofluorescence analysis. Chem. Nat. Compd. 2012, 48, 267–271. [Google Scholar] [CrossRef]
- Lv, T.; Zhao, X.-E.; Zhu, S.; Ji, Z.; Chen, G.; Sun, Z.; Song, C.; You, J.; Suo, Y. Development of an efficient HPLC fluorescence detection method for brassinolide by ultrasonic-assisted dispersive liquid–liquid microextraction coupled with derivatization. Chromatographia 2014, 77, 1653–1660. [Google Scholar] [CrossRef]
- Malachowska-Ugarte, M.; Sperduto, C.; Ermolovich, Y.V.; Sauchuk, A.L.; Jurášek, M.; Litvinovskaya, R.P.; Straltsova, D.; Smolich, I.; Zhabinskii, V.N.; Drašar, P.; et al. Brassinosteroid-BODIPY conjugates: Design, synthesis, and properties. Steroids 2015, 102, 53–59. [Google Scholar] [CrossRef]
- Synthetic Protocol for AFCS: A Biologically Active Fluorescent Castasterone Analog Conjugated to an Alexa Fluor 647 Dye.-Abstract-Europe PMC. Available online: https://europepmc.org/article/med/28124242 (accessed on 23 February 2021).
- Litvinovskaya, R.P.; Savchuk, A.L.; Kuprienko, O.S.; Sviridov, O.V.; Khripach, V.A. Competitive lanthanide immunofluorescent assay of endogenous brassinosteroids in plants. Chem. Nat. Compd. 2018, 54, 1106–1113. [Google Scholar] [CrossRef]
- Khripach, V.; Zhabinskii, V.; Antonchick, A.; Litvinovskaya, R.; Drach, S.; Sviridov, O.; Pryadko, A.; Novik, T.; Matveentsev, V.; Schneider, B. A new type of modified brassinosteroids for enzyme-linked immunosorbent assay. Nat. Prod. Commun. 2008, 3, 735–748. [Google Scholar] [CrossRef]
- Irani, N.; Rubbo, S.; Mylle, E.; Begin, J.; Schneider-Pizoń, J.; Hniliková, J.; Sisa, M.; Buyst, D.; Vilarrasa-Blasi, J.; Szatmári, A.-M.; et al. Fluorescent castasterone reveals bri1 signaling from the plasma membrane. Nat. Chem. Biol. 2012, 8, 583–589. [Google Scholar] [CrossRef]
- Hurski, A.L.; Kukel, A.G.; Liubina, A.I.; Baradzenka, A.G.; Straltsova, D.; Demidchik, V.; Drašar, P.; Zhabinskii, V.N.; Khripach, V.A. Regio- and stereoselective C–H functionalization of brassinosteroids. Steroids 2019, 146, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Paës, G. Fluorescent probes for exploring plant cell wall deconstruction: A review. Molecules 2014, 19, 9380–9402. [Google Scholar] [CrossRef]
- He, K.; Gou, X.; Yuan, T.; Lin, H.; Asami, T.; Yoshida, S.; Russell, S.D.; Li, J. BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr. Biol. 2007, 17, 1109–1115. [Google Scholar] [CrossRef]
- Divi, U.K.; Rahman, T.; Krishna, P. Gene expression and functional analyses in brassinosteroid-mediated stress tolerance. Plant Biotechnol. J. 2016, 14, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Besseau, S.; Li, J.; Palva, E.T. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in arabidopsis thaliana. J. Exp. Bot. 2012, 63, 2667–2679. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, R.; Palva, E.T. WRKY70 and its homolog WRKY54 negatively modulate the cell wall-associated defenses to necrotrophic pathogens in Arabidopsis. PLoS ONE 2017, 12, e0183731. [Google Scholar]
- Irani, N.G.; Di Rubbo, S.; Russinova, E. In vivo imaging of brassinosteroid endocytosis in arabidopsis. In Plant Endosomes: Methods and Protocols; Otegui, M.S., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2014; pp. 107–117. ISBN 978-1-4939-1420-3. [Google Scholar]
- Hacham, Y.; Holland, N.; Butterfield, C.; Ubeda-Tomas, S.; Bennett, M.J.; Chory, J.; Savaldi-Goldstein, S. Brassinosteroid perception in the epidermis controls root meristem size. Development 2011, 138, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Fridman, Y.; Elkouby, L.; Holland, N.; Vragović, K.; Elbaum, R.; Savaldi-Goldstein, S. Root growth is modulated by differential hormonal sensitivity in neighboring cells. Genes Dev. 2014, 28, 912–920. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, W.; Chen, Y.; Ito, S.; Asami, T.; Wang, X. Brassinosteroids control root epidermal cell fate via direct regulation of a MYB-BHLH-WD40 complex by GSK3-like kinases. Elife 2014, 3, e02525. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, K.T.; Chen, A.Y.; Nemhauser, J.L. Steroids are required for epidermal cell fate establishment in arabidopsis roots. Proc. Nat. Aada. Sci. USA 2009, 106, 8073–8076. [Google Scholar] [CrossRef]
- Lanza, M.; Garcia-Ponce, B.; Castrillo, G.; Catarecha, P.; Sauer, M.; Rodriguez-Serrano, M.; Páez-García, A.; Sánchez-Bermejo, E.; Tc, M.; Leo del Puerto, Y.; et al. Role of actin cytoskeleton in brassinosteroid signaling and in its integration with the auxin response in plants. Dev. Cell 2012, 22, 1275–1285. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Xu, Z.-H.; Xue, H.-W. Brassinosteroids stimulate plant tropisms through modulation of polar auxin transport in brassica and arabidopsis. Plant Cell 2005, 17, 2738–2753. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Retzer, K.; Akhmanova, M.; Konstantinova, N.; Malínská, K.; Leitner, J.; Petrášek, J.; Luschnig, C. Brassinosteroid signaling delimits root gravitropism via sorting of the arabidopsis PIN2 auxin transporter. Nat. Commun. 2019, 10, 5516. [Google Scholar] [CrossRef] [PubMed]
- Durbak, A.; Yao, H.; McSteen, P. Hormone signaling in plant development. Curr. Opin. Plant Biol. 2012, 15, 92–96. [Google Scholar] [CrossRef]
- Ibañes, M.; Fàbregas, N.; Chory, J.; Caño-Delgado, A.I. Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of arabidopsis shoot vascular bundles. Proc. Nat. Aada. Sci. USA 2009. [Google Scholar] [CrossRef]
- Brassinosteroids Interact with Auxin to Promote Lateral Root Development in Arabidopsis. Plant Physiol. Available online: http://www.plantphysiol.org/content/134/4/1624 (accessed on 17 February 2021).
- Goda, H.; Sawa, S.; Asami, T.; Fujioka, S.; Shimada, Y.; Yoshida, S. Comprehensive comparison of auxin-regulated and brassinosteroid-regulated genes in arabidopsis. Plant Physiol. 2004, 134, 1555–1573. [Google Scholar] [CrossRef]
- Nemhauser, J.L.; Mockler, T.C.; Chory, J. Interdependency of brassinosteroid and auxin signaling in arabidopsis. PLoS Biol. 2004, 2, e258. [Google Scholar] [CrossRef]
- Kretynin, S.V.; Kolesnikov, Y.S.; Derevyanchuk, M.V.; Kalachova, T.A.; Blume, Y.B.; Khripach, V.A.; Kravets, V.S. Brassinosteroids application induces phosphatidic acid production and modify antioxidant enzymes activity in tobacco in calcium-dependent manner. Steroids 2019, 108444. [Google Scholar] [CrossRef]
- Schaller, F.; Biesgen, C.; Müssig, C.; Altmann, T.; Weiler, E.W. 12-oxophytodienoate reductase 3 (opr3) is the isoenzyme involved in jasmonate biosynthesis. Planta 2000, 210, 979–984. [Google Scholar] [CrossRef]
- Kitanaga, Y.; Jian, C.; Hasegawa, M.; Yazaki, J.; Kishimoto, N.; Kikuchi, S.; Nakamura, H.; Ichikawa, H.; Asami, T.; Yoshida, S.; et al. Sequential regulation of gibberellin, brassinosteroid, and jasmonic acid biosynthesis occurs in rice coleoptiles to control the transcript levels of anti-microbial thionin genes. Biosci. Biotechnol. Biochem. 2006, 70, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wu, H.; Wu, D.; Zhang, Z.; Guo, Z.; Yang, N.; Xia, K.; Zhou, X.; Oh, K.; Matsuoka, M.; et al. Methyl jasmonate inhibits lamina joint inclination by repressing brassinosteroid biosynthesis and signaling in rice. Plant Sci. 2015, 241, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Han, C.; Yuan, L.; Zhang, K.; Huang, H.; Ren, C. Brassinosteroid enhances jasmonate-induced anthocyanin accumulation in arabidopsis seedlings. J. Integr. Plant Biol. 2011, 53, 632–640. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, Y.; Lu, Y.; Wang, X. Mechanisms of brassinosteroids interacting with multiple hormones. Plant Signal Behav. 2009, 4, 1117–1120. [Google Scholar] [CrossRef]
- The Antagonistic Regulation of Abscisic Acid-Inhibited Root Growth by Brassinosteroids is Partially Mediated via Direct Suppression of ABSCISIC ACID INSENSITIVE 5 Expression by BRASSINAZOLE RESISTANT 1-Yang-2016-Plant, Cell &Environment-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/pce.12763 (accessed on 17 February 2021).
- Divi, U.K.; Rahman, T.; Krishna, P. Brassinosteroid-mediated stress tolerance in arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. Bmc. Plant Biol. 2010, 10, 151. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Krishnamoorthy, P.; Schubert, V.; Hause, G.; Heilmann, M.; Heilmann, I. A dual role for cell plate-associated PI4Kβ in endocytosis and phragmoplast dynamics during plant somatic cytokinesis. EMBO J. 2019, 38, e100303. [Google Scholar] [CrossRef] [PubMed]
- Leontovyčová, H.; Kalachova, T.; Trdá, L.; Pospíchalová, R.; Lamparová, L.; Dobrev, P.I.; Malínská, K.; Burketová, L.; Valentová, O.; Janda, M. Actin depolymerization is able to increase plant resistance against pathogens via activation of salicylic acid signalling pathway. Sci. Rep. 2019, 9, 10397. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starodubtseva, A.; Kalachova, T.; Iakovenko, O.; Stoudková, V.; Zhabinskii, V.; Khripach, V.; Ruelland, E.; Martinec, J.; Burketová, L.; Kravets, V. BODIPY Conjugate of Epibrassinolide as a Novel Biologically Active Probe for In Vivo Imaging. Int. J. Mol. Sci. 2021, 22, 3599. https://doi.org/10.3390/ijms22073599
Starodubtseva A, Kalachova T, Iakovenko O, Stoudková V, Zhabinskii V, Khripach V, Ruelland E, Martinec J, Burketová L, Kravets V. BODIPY Conjugate of Epibrassinolide as a Novel Biologically Active Probe for In Vivo Imaging. International Journal of Molecular Sciences. 2021; 22(7):3599. https://doi.org/10.3390/ijms22073599
Chicago/Turabian StyleStarodubtseva, Anastasiia, Tetiana Kalachova, Oksana Iakovenko, Vera Stoudková, Vladimir Zhabinskii, Vladimir Khripach, Eric Ruelland, Jan Martinec, Lenka Burketová, and Volodymyr Kravets. 2021. "BODIPY Conjugate of Epibrassinolide as a Novel Biologically Active Probe for In Vivo Imaging" International Journal of Molecular Sciences 22, no. 7: 3599. https://doi.org/10.3390/ijms22073599
APA StyleStarodubtseva, A., Kalachova, T., Iakovenko, O., Stoudková, V., Zhabinskii, V., Khripach, V., Ruelland, E., Martinec, J., Burketová, L., & Kravets, V. (2021). BODIPY Conjugate of Epibrassinolide as a Novel Biologically Active Probe for In Vivo Imaging. International Journal of Molecular Sciences, 22(7), 3599. https://doi.org/10.3390/ijms22073599








