Genome-wide Identification and Characterization of FCS-Like Zinc Finger (FLZ) Family Genes in Maize (Zea mays) and Functional Analysis of ZmFLZ25 in Plant Abscisic Acid Response
Abstract
1. Introduction
2. Results
2.1. Identification of FLZ Family Genes in Maize Genome
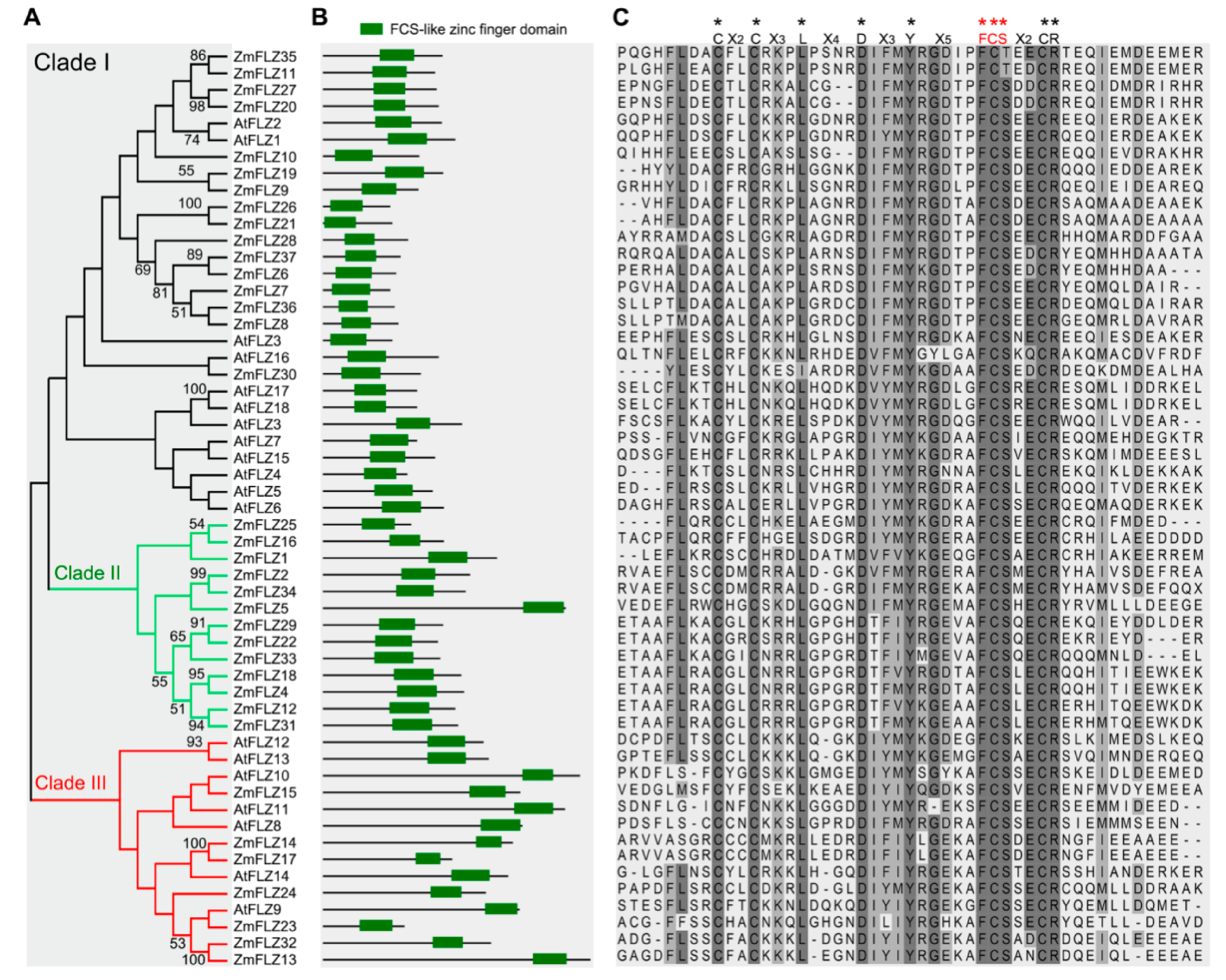
2.2. Phylogenetic and Structural Analysis of FLZ Proteins
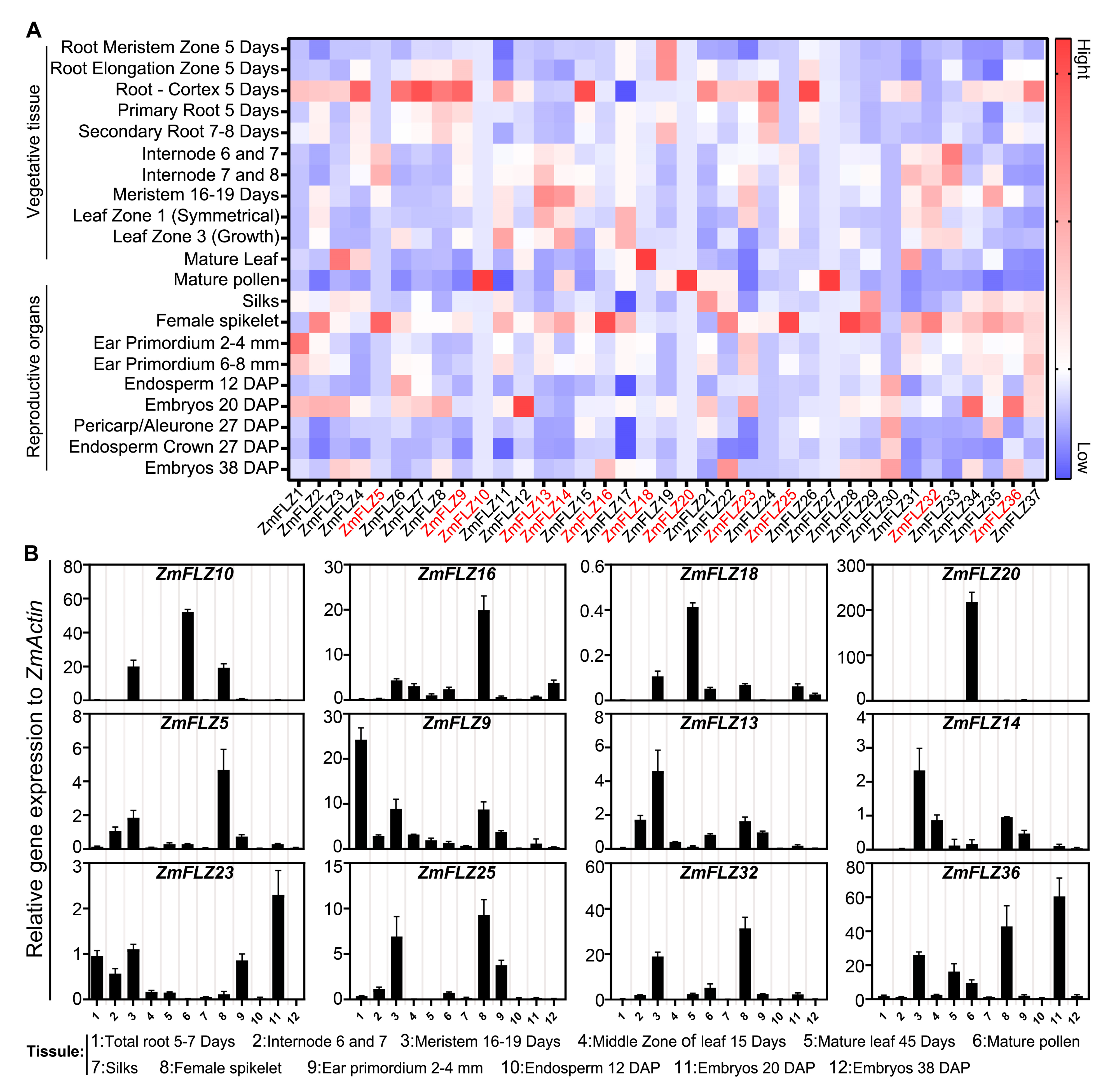
2.3. Tissue Specific Expression Pattern of ZmFLZ Genes
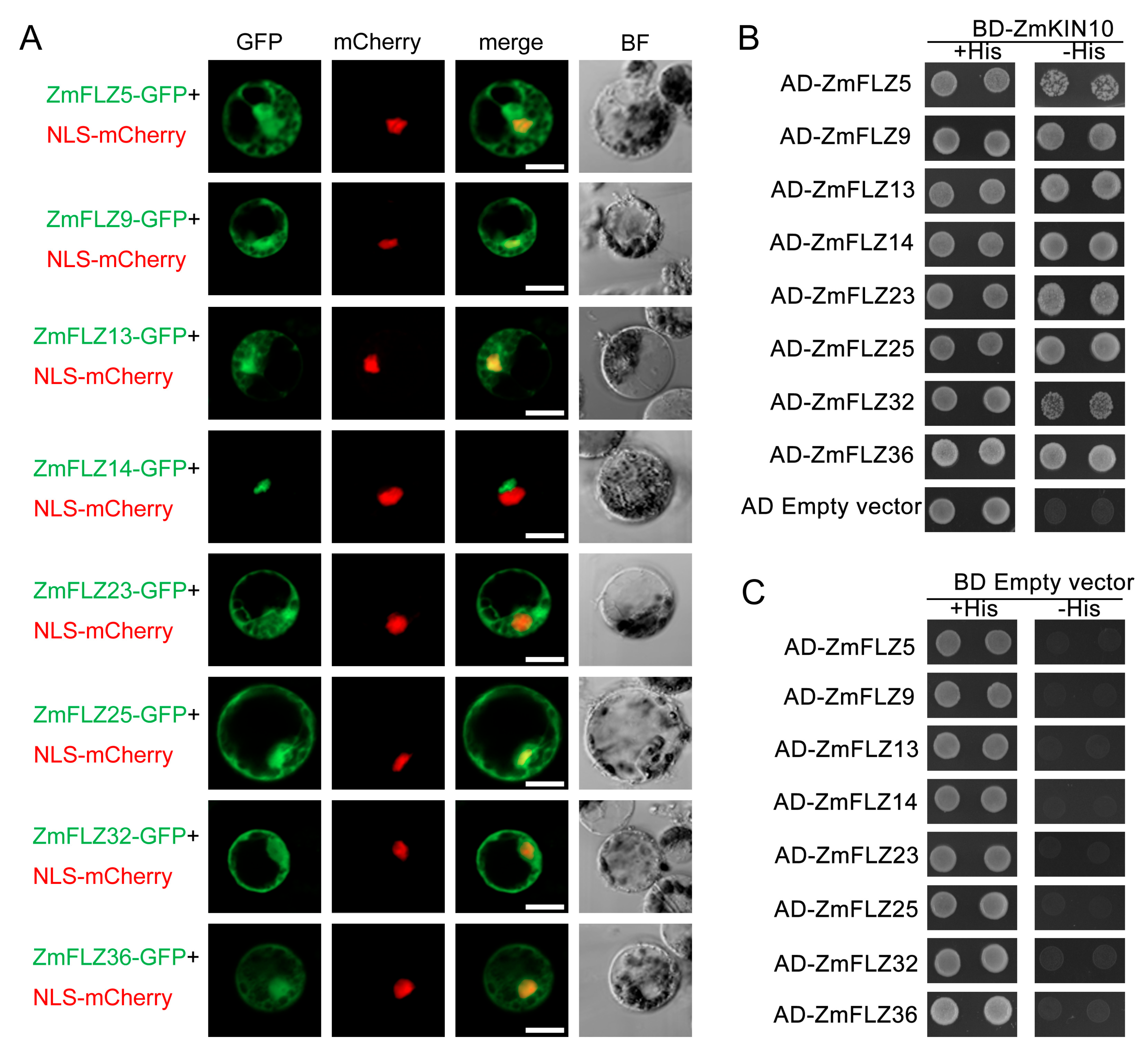
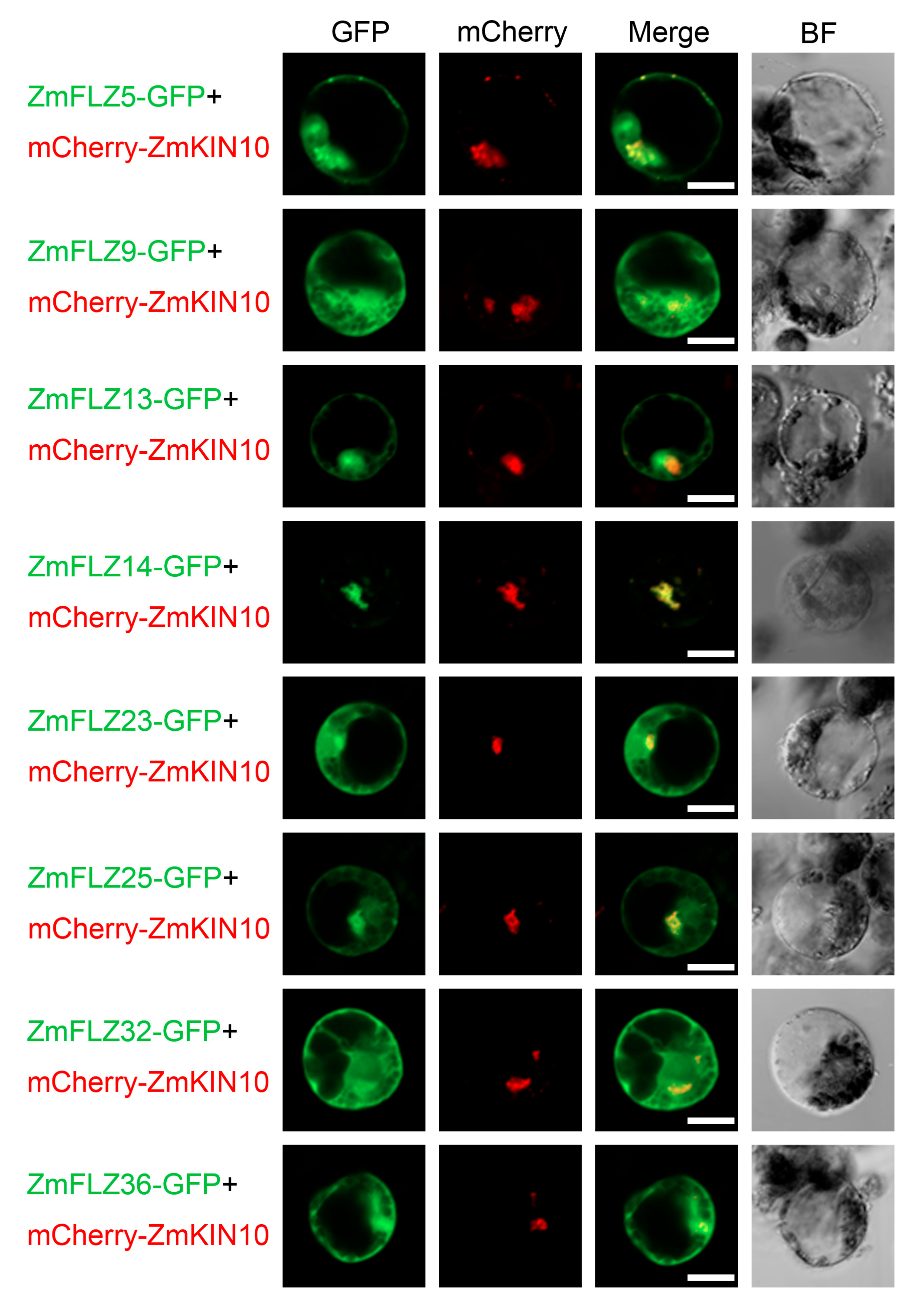
2.4. Subcellular Localizations of Eight Typical ZmFLZ Proteins and Their Interactions with ZmKIN10
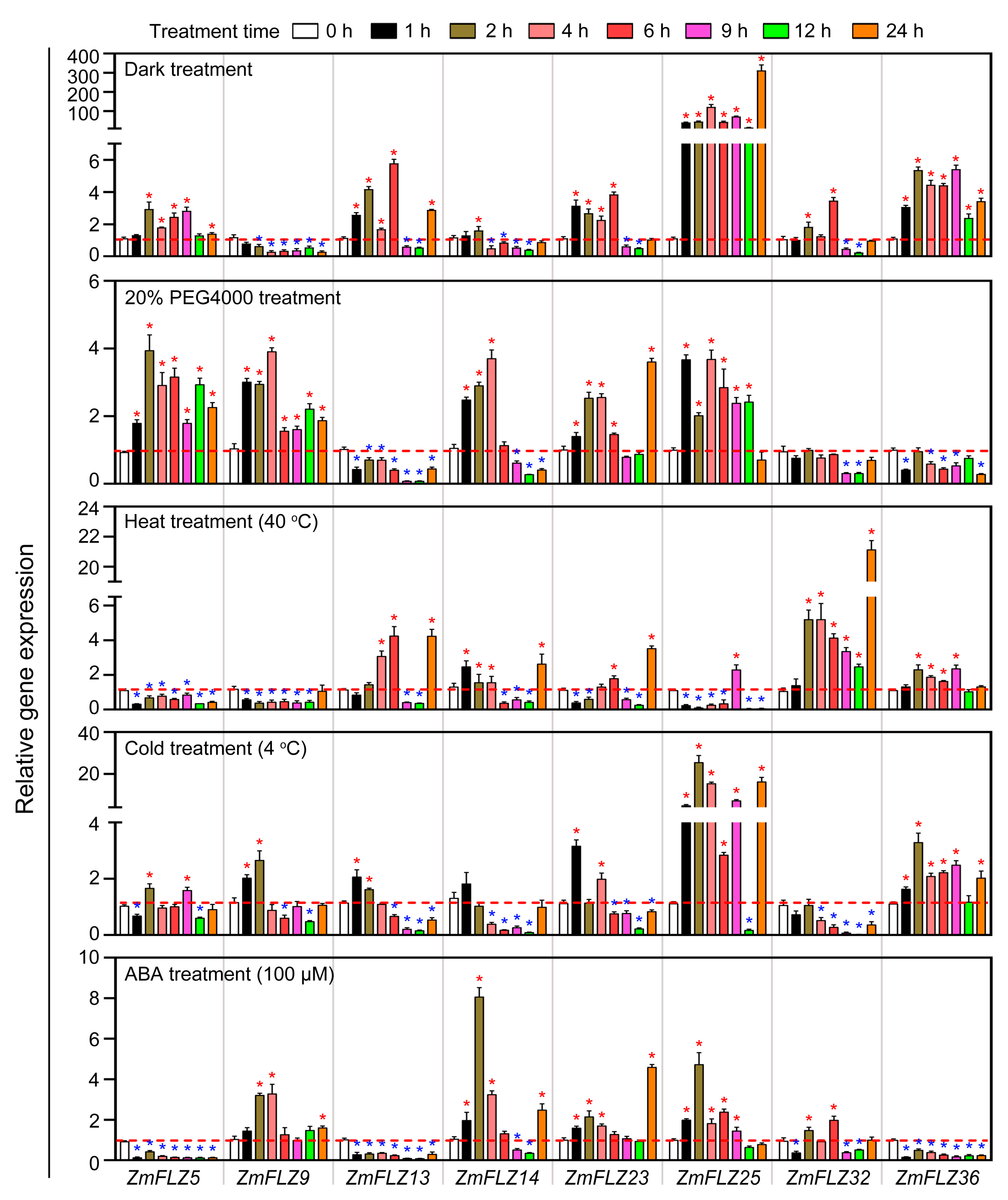
2.5. Stress-Responsive Expression Profiles of ZmFLZ Genes
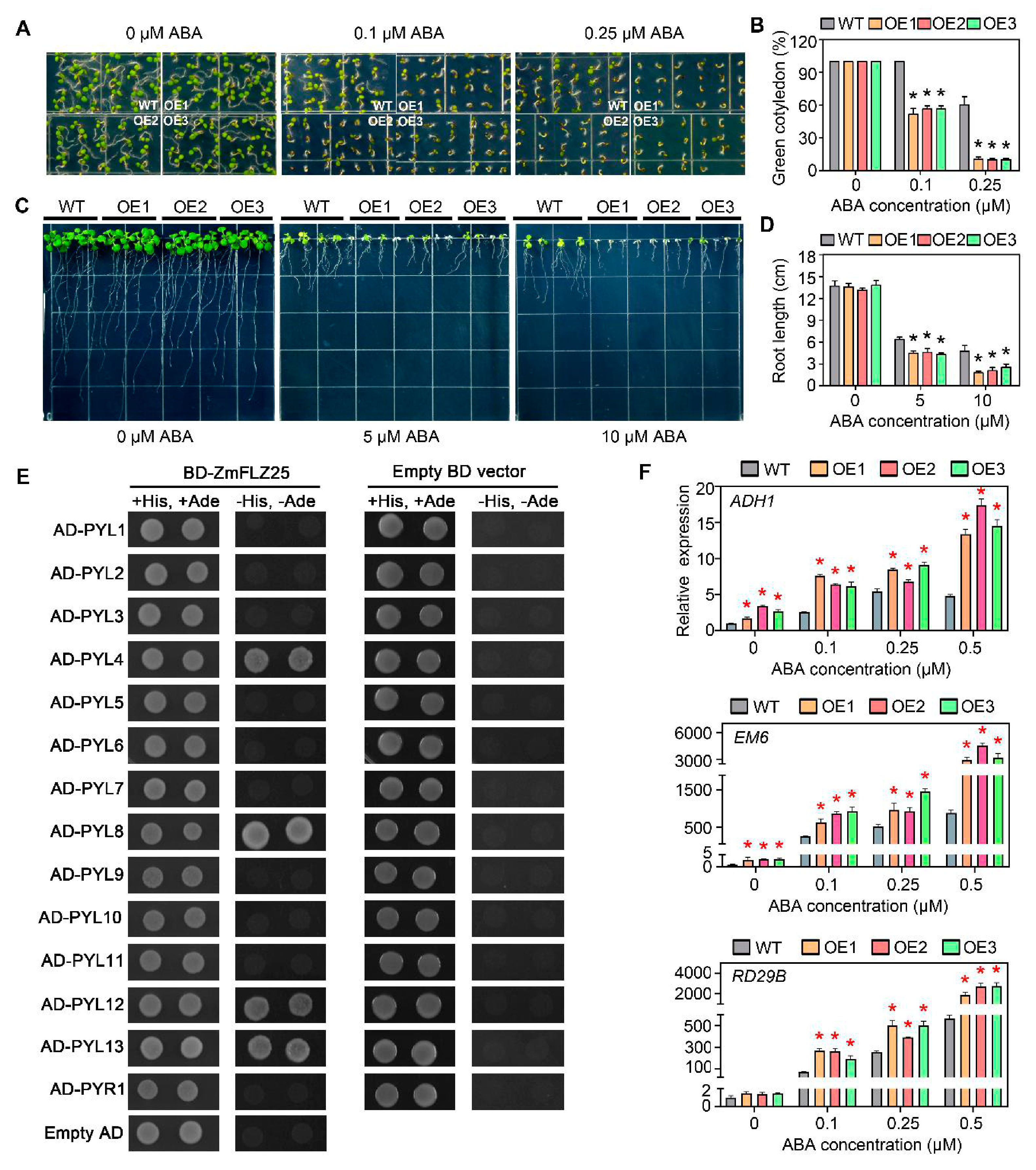
2.6. Ectopic Expression of ZmFLZ25 in Arabidopsis Confers Plant the Sensitivity to ABA Treatment
3. Discussion
4. Materials and Methods
4.1. Data Search and Analyses
4.2. Maize Growth Conditions and Treatments
4.3. Generation of ZmFLZ25 Overexpressed Arabidopsis and ABA Sensitivity Test
4.4. Subcellular Localization Assays
4.5. Yeast-Two-Hybrid Assay
4.6. RNA Isolation and Quantitative Real-Time PCR
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Y.; Tang, W.; Swain, J.D.; Green, A.L.; Jack, T.; Gan, S. Networking Senescence-Regulating Pathways by Using Arabidopsis Enhancer Trap Lines. Plant Physiol. 2001, 126, 707–716. [Google Scholar] [CrossRef]
- He, Y.; Gan, S. A novel zinc-finger protein with a proline-rich domain mediates ABA-regulated seed dormancy in Arabidopsis. Plant Mol. Biol. 2004, 54, 1–9. [Google Scholar] [CrossRef]
- Laxmi, A. DUF581 Is Plant Specific FCS-Like Zinc Finger Involved in Protein-Protein Interaction. PLOS ONE 2014, 9, e99074. [Google Scholar]
- Jamsheer, K.M.; Laxmi, A. Expression of Arabidopsis FCS-Like Zinc finger genes is differentially regulated by sugars, cellular energy level, and abiotic stress. Front Plant Sci. 2015, 6, 746. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Visser, R.G.F.; Broekgaarden, C.; Vosman, B. Overexpression of IRM1 Enhances Resistance to Aphids in Arabidopsis thaliana. PLoS ONE 2013, 8, e70914. [Google Scholar] [CrossRef]
- Hou, X.; Liang, Y.; He, X.; Shen, Y.; Huang, Z. A Novel ABA-Responsive TaSRHP Gene from Wheat Contributes to Enhanced Resistance to Salt Stress in Arabidopsis thaliana. Plant Mol. Biol. Rep. 2013, 31, 791–801. [Google Scholar] [CrossRef]
- Jamsheer, K.M.; Sharma, M.; Singh, D.; Mannully, C.T.; Jindal, S.; Shukla, B.N.; Laxmi, A. FCS-like zinc finger 6 and 10 repress SnRK1 signalling in Arabidopsis. Plant J. 2018, 94, 232–245. [Google Scholar] [CrossRef]
- Jamsheer K, M.; Jindal, S.; Laxmi, A. Evolution of TOR-SnRK dynamics in green plants and its integration with phytohormone signaling networks. J. Exp. Bot. 2019, 70, 2239–2259. [Google Scholar] [CrossRef]
- Nietzsche, M.; Schiesl, I.; Bornke, F. The complex becomes more complex: Protein-protein interactions of SnRK1 with DUF581 family proteins provide a framework for cell- and stimulus type-specific SnRK1 signaling in plants. Front. Plant Sci. 2014, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Nietzsche, M.; Landgraf, R.; Tohge, T.; Brnke, F. A protein-protein interaction network linking the energy-sensor kinase SnRK1 to multiple signaling pathways in Arabidopsis thaliana. Curr. Plant Biol. 2015, 5, 36–44. [Google Scholar] [CrossRef]
- Crepin, N.; Rolland, F. SnRK1 activation, signaling, and networking for energy homeostasis. Curr. Opin. Plant Biol. 2019, 51, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Margalha, L.; Confraria, A.; Baena-González, E. SnRK1 and TOR: Modulating growth-defense trade-offs in plant stress responses. J. Exp. Bot. 2019, 70, 2261–2274. [Google Scholar] [CrossRef]
- Shukla, B.N.; Jindal, S.; Gopan, N.; Mannully, C.T.; Laxmi, A. The FCS-like zinc finger scaffold of the kinase SnRK1 is formed by the coordinated actions of the FLZ domain and intrinsically disordered regions. J. Biol. Chem. 2018, 293, 13134–13150. [Google Scholar]
- Jinge, T.; Chenglong, W.; Jinliang, X.; Lishuan, W.; Guanghui, X.; Weihao, W.; Dan, L.; Wenchao, Q.; Xu, H.; Qiuyue, C. Teosinte ligule allele narrows plant architecture and enhances high-density maize yields. Science 2019, 365, 658–664. [Google Scholar]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.; Graves, T.A. The B73 Maize Genome: Complexity, Diversity, and Dynamics. Science 2009, 326, 1112–1115. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, Y.; Chen, J.; Shi, J.; Zhao, H.; Zhao, H.; Song, W.; Zhang, M.; Cui, Y.; Dong, X.; et al. Extensive intraspecific gene order and gene structural variations between Mo17 and other maize genomes. Nat. Genet. 2018, 50, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Springer, N.M.; Anderson, S.N.; Andorf, C.M.; Ahern, K.R.; Bai, F.; Barad, O.; Barbazuk, W.B.; Bass, H.W.; Baruch, K.; Ben-Zvi, G.; et al. The maize W22 genome provides a foundation for functional genomics and transposon biology. Nat. Genet. 2018, 50, 1282–1288. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Rodriguez, L.; Gonzalez-Guzman, M.; Diaz, M.; Rodrigues, A.; Izquierdo-Garcia, A.C.; Peirats-Llobet, M.; Fernandez, M.A.; Antoni, R.; Fernandez, D.; Marquez, J.A.; et al. C2-domain abscisic acid-related proteins mediate the interaction of PYR/PYL/RCAR abscisic acid receptors with the plasma membrane and regulate abscisic acid sensitivity in Arabidopsis. Plant Cell 2014, 26, 4802–4820. [Google Scholar] [CrossRef]
- Bueso, E.; Rodriguez, L.; Lorenzo-Orts, L.; Gonzalez-Guzman, M.; Sayas, E.; Munoz-Bertomeu, J.; Ibanez, C.; Serrano, R.; Rodriguez, P.L. The single-subunit RING-type E3 ubiquitin ligase RSL1 targets PYL4 and PYR1 ABA receptors in plasma membrane to modulate abscisic acid signaling. Plant J. 2014, 80, 1057–1071. [Google Scholar] [CrossRef]
- Schneider, H.M.; Klein, S.P.; Hanlon, M.T.; Nord, E.A.; Shawn, K.; Brown, K.M.; Andrew, W.; Rahul, B.; Lynch, J.P. Genetic control of root architectural plasticity in maize. J. Exp. Bot. 2020, 71, 3185–3197. [Google Scholar] [CrossRef]
- Han, C.; Liu, Y.; Shi, W.; Qiao, Y.; Wang, L.; Tian, Y.; Fan, M.; Deng, Z.; Lau, O.S.; De Jaeger, G.; et al. KIN10 promotes stomatal development through stabilization of the SPEECHLESS transcription factor. Nat. Commun. 2020, 11, 4214. [Google Scholar] [CrossRef]
- Ramon, M.; Dang, T.; Broeckx, T.; Hulsmans, S.; Crepin, N.; Sheen, J.; Rolland, F. Default Activation and Nuclear Translocation of the Plant Cellular Energy Sensor SnRK1 Regulate Metabolic Stress Responses and Development. Plant Cell 2019, 31, 1614–1632. [Google Scholar] [CrossRef]
- Bitrián, M.; Roodbarkelari, F.; Horváth, M.; Koncz, C. BAC-recombineering for studying plant gene regulation: Developmental control and cellular localization of SnRK1 kinase subunits. Plant J. 2011, 65, 829–842. [Google Scholar] [CrossRef]
- Williams, S.P.; Rangarajan, P.; Donahue, J.L.; Hess, J.E.; Gillaspy, G.E. Regulation of Sucrose non-Fermenting Related Kinase 1 genes in Arabidopsis thaliana. Front Plant Sci. 2014, 5, 324. [Google Scholar] [CrossRef]
- O’Brien, M.; Kaplan-Levy, R.N.; Quon, T.; Sappl, P.G.; Smyth, D.R. PETAL LOSS, a trihelix transcription factor that represses growth in Arabidopsis thaliana, binds the energy-sensing SnRK1 kinase AKIN10. J. Exp. Bot. 2015, 66, 2475–2485. [Google Scholar] [CrossRef]
- Fragoso, S.; Espíndola, L.; Páez-Valencia, J.; Gamboa, A.; Camacho, Y.; Martínez-Barajas, E.; Coello, P. SnRK1 isoforms AKIN10 and AKIN11 are differentially regulated in Arabidopsis plants under phosphate starvation. Plant Physiol. 2009, 149, 1906–1916. [Google Scholar] [CrossRef]
- Blanco, N.E.; Liebsch, D.; Guinea Díaz, M.; Strand, Å.; Whelan, J. Dual and dynamic intracellular localization of Arabidopsis thaliana SnRK1.1. J. Exp. Bot. 2019, 70, 2325–2338. [Google Scholar] [CrossRef]
- Yang, C.; Shen, W.; Yang, L.; Sun, Y.; Li, X.; Lai, M.; Wei, J.; Wang, C.; Xu, Y.; Li, F. HY5-HDA9 Module Transcriptionally Regulates Plant Autophagy in Response to Light-to-dark Conversion and Nitrogen Starvation. Mol. Plant 2020, 13, 515–531. [Google Scholar] [CrossRef]
- Wang, X.F.; Xu, M.; Gao, C.J.; Zeng, Y.L.; Cui, Y.; Shen, W.J.; Jiang, L.W. The roles of endomembrane trafficking in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 55–69. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Liu, S.; Ferjani, A.; Li, J.; Yan, J.; Yang, X.; Qin, F. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat. Genet. 2016, 48, 1233–1241. [Google Scholar] [CrossRef]
- Qin, F.; Shinozaki, K.; Yamaguchishinozaki, K. Achievements and Challenges in Understanding Plant Abiotic Stress Responses and Tolerance. Plant Cell Physiol. 2011, 52, 1569–1582. [Google Scholar] [CrossRef]
- Julia, K.; Claudia, J. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Yang, C.; Shen, W.; Chen, H.; Chu, L.; Xu, Y.; Zhou, X.; Liu, C.; Chen, C.; Zeng, J.; Liu, J. Characterization and subcellular localization of histone deacetylases and their roles in response to abiotic stresses in soybean. BMC Plant Biol. 2018, 18, 226. [Google Scholar] [CrossRef]
- Lawrence, C.J.; Qunfeng, D.; Polacco, M.L.; Seigfried, T.E.; Volker, B. MaizeGDB, the community database for maize genetics and genomics. Nucleic Acids Res. 2004, 32, D393–D397. [Google Scholar] [CrossRef]
- Finn, R.D.; Alex, B.; Jody, C.; Penelope, C.; Eberhardt, R.Y.; Eddy, S.R.; Andreas, H.; Kirstie, H.; Liisa, H.; Jaina, M. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Zhang, X.; Mi, Y.; Mao, H.; Liu, S.; Chen, L.; Qin, F. Genetic variation in ZmTIP1 contributes to root hair elongation and drought tolerance in maize. Plant Biotechnol. J. 2019, 18, 1271–1283. [Google Scholar] [CrossRef]
- Ma, H.; Liu, C.; Li, Z.; Ran, Q.; Xie, G.; Wang, B.; Fang, S.; Chu, J.; Zhang, J. ZmbZIP4 Contributes to Stress Resistance in Maize by Regulating ABA Synthesis and Root Development. Plant Physiol. 2018, 178, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Luo, M.; Zhao, Q.; Yang, R.; Cui, Y.; Zeng, Y.; Xia, J.; Jiang, L. A Unique Plant ESCRT Component, FREE1, Regulates Multivesicular Body Protein Sorting and Plant Growth. Curr. Biol. 2014, 24, 2556–2563. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zhao, Q.; Li, T.; Wei, J.; Li, B.; Shen, W.; Yang, C.; Zeng, Y.; Rodriguez, P.L. The plant ESCRT component FREE1 shuttles to the nucleus to attenuate abscisic acid signalling. Nat. Plants 2019, 5, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yang, C.; Liu, C.; Yang, L.; Shen, W. SINAT E3 ligases regulate the stability of the ESCRT component FREE1 in response to iron deficiency in plants. J. Integr. Plant Biol. 2020, 62, 1399–1417. [Google Scholar] [CrossRef]
- Emanuelsson, O.; Brunak, S.R.; Von Heijne, G.; Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protocols 2007, 2, 953–971. [Google Scholar] [CrossRef]
- Brameier, M.; Krings, A.; Maccallum, R.M. NucPred - Predicting Nuclear Localization of Proteins. Bioinformatics 2007, 23, 1159–1160. [Google Scholar] [CrossRef]
- Gao, L.; Shen, G.; Zhang, L.; Qi, J.; Zhang, C.; Ma, C.; Li, J.; Wang, L.; Malook, S.U.; Wu, J. An efficient system composed of maize protoplast transfection and HPLC–MS for studying the biosynthesis and regulation of maize benzoxazinoids. Plant Methods 2019, 15, 144. [Google Scholar] [CrossRef]
- Shen, W.; Xiao, Z.; Shen, J.; Gao, C. Analysis of Golgi-Mediated Protein Traffic in Plant Cells. Methods Mol. Biol. 2017, 1662, 75–86. [Google Scholar]
- Walley, J.W.; Sartor, R.C.; Shen, Z.; Schmitz, R.J.; Wu, K.J.; Urich, M.A.; Nery, J.R.; Smith, L.G.; Schnable, J.C.; Ecker, J.R. Integration of omic networks in a developmental atlas of maize. Science 2016, 353, 814–818. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Li, X.; Yang, C.; Yan, W.; Liu, C.; Tang, X.; Gao, C. Genome-wide Identification and Characterization of FCS-Like Zinc Finger (FLZ) Family Genes in Maize (Zea mays) and Functional Analysis of ZmFLZ25 in Plant Abscisic Acid Response. Int. J. Mol. Sci. 2021, 22, 3529. https://doi.org/10.3390/ijms22073529
Chen S, Li X, Yang C, Yan W, Liu C, Tang X, Gao C. Genome-wide Identification and Characterization of FCS-Like Zinc Finger (FLZ) Family Genes in Maize (Zea mays) and Functional Analysis of ZmFLZ25 in Plant Abscisic Acid Response. International Journal of Molecular Sciences. 2021; 22(7):3529. https://doi.org/10.3390/ijms22073529
Chicago/Turabian StyleChen, Shunquan, Xibao Li, Chao Yang, Wei Yan, Chuanliang Liu, Xiaoyan Tang, and Caiji Gao. 2021. "Genome-wide Identification and Characterization of FCS-Like Zinc Finger (FLZ) Family Genes in Maize (Zea mays) and Functional Analysis of ZmFLZ25 in Plant Abscisic Acid Response" International Journal of Molecular Sciences 22, no. 7: 3529. https://doi.org/10.3390/ijms22073529
APA StyleChen, S., Li, X., Yang, C., Yan, W., Liu, C., Tang, X., & Gao, C. (2021). Genome-wide Identification and Characterization of FCS-Like Zinc Finger (FLZ) Family Genes in Maize (Zea mays) and Functional Analysis of ZmFLZ25 in Plant Abscisic Acid Response. International Journal of Molecular Sciences, 22(7), 3529. https://doi.org/10.3390/ijms22073529





