Chimeric Antigen Receptor Design and Efficacy in Ovarian Cancer Treatment
Abstract
1. Introduction
2. What Is CAR?
3. How Are CARs Engineered?
4. In Vitro and In Vivo Studies
5. Clinical Trials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | antibody-dependent cytotoxicity |
| ANXA2 | annexin 2 |
| APCs | antigen-presenting cells |
| CAR | chimeric antigen receptors |
| CCC | clear cell carcinomas |
| CCC | clear cell carcinoma |
| CSC | cancer stem cell |
| CTLA-4 | cytotoxic T lymphocyte-associated antigen-4 |
| CXCR1 | interleukin-8 receptor alpha |
| dCas9 | catalytically inactive Cas9 |
| EC | endometrioid carcinomas |
| ECM | extracellular matrix |
| EGFR | epidermal growth factor receptor |
| Fab | antigen-binding fragment |
| FBP | folate binding protein |
| FIGO | International Federation of Gynaecology and Obstetrics |
| FR-α | folate receptor-α |
| FSHR | follicle-stimulating hormone receptor |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GPI | glycosylphosphatidylinositol |
| HE4 | human epididymis protein 4 |
| HER2/neu | receptor tyrosine-protein kinase erbB-2 |
| HGSC | high-grade serous carcinoma |
| HPV | Human Papillomavirus |
| HSV-TK | herpes simplex virus I-derived thymidine kinase |
| iCasp9 | caspase 9 |
| IDO | indoleamine 2,3-dioxygenase |
| L1-CAM | L1 cell adhesion molecule |
| LGSC | low-grade serous carcinoma |
| M1SMC | stimulated mononuclear cells |
| mAb | antigen-specific monoclonal antibody |
| MC | mucinous carcinoma |
| MDSCs | myeloid-derived suppressor cells |
| MHC | major histocompatibility complex |
| MMPs | matrix metalloproteinases |
| MSLN | mesothelin |
| MUC16 | mucin 16 |
| NK | natural killer |
| OC | ovarian cancer |
| OCSCs | ovarian cancer stem cells |
| OSE | ovarian surface epithelium |
| PARP1 | poly [ADP-ribose] polymerase 1 |
| PCR | polymerase chain reaction |
| PD-1 | Programmed cell death-1 |
| PD-L1/L2 | programmed death-ligand 1/2 |
| SB | sleeping beauty transposon system |
| scFv | single-chain variable fragment |
| TAMs | tumour-associated macrophages |
| TGFβ | transforming growth factor beta |
| TIR | terminal inverted repeats |
| TME | tumour microenvironment |
| TNF | tumour necrosis factor |
| Tregs | regulatory T cells |
| uPAR | urokinase plasminogen activator receptor |
| VEGF-A | vascular endothelial growth factor A |
| VNTR | mucin 1 variable number tandem repeat |
| Z-VAD | pan-caspase inhibitor carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Jelovac, D.; Armstrong, D.K. Recent Progress in the Diagnosis and Treatment of Ovarian Cancer. CA Cancer J. Clin. 2011, 61, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; Karpinskyj, C.; Gentry-Maharaj, A. Ovarian Cancer Prevention and Screening. Obstet. Gynecol. 2018, 131, 909–927. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Elias, K.M.; Guo, J.; Bast, R.C., Jr. Early Detection of Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018, 32, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of Ovarian Cancer: A Review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; Jordan, S.J. Epidemiology of Epithelial Ovarian Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial Ovarian Cancer: Evolution of Management in the Era of Precision Medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Lengyel, E. Ovarian Cancer Development and Metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rueger, R. Mechanisms and Targets Involved in Dissemination of Ovarian Cancer. Cancer Genom. Proteom. 2016, 13, 407–423. [Google Scholar] [CrossRef]
- Kaku, T.; Ogawa, S.; Kawano, Y.; Ohishi, Y.; Kobayashi, H.; Hirakawa, T.; Nakano, H. Histological Classification of Ovarian Cancer. Med. Electron Microsc. 2003, 36, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, P. Morphologic, Immunophenotypic, and Molecular Features of Epithelial Ovarian Cancer. Oncology 2016, 30, 166–176. [Google Scholar]
- Murali, R.; Davidson, B.; Fadare, O.; Carlson, J.A.; Crum, C.P.; Gilks, C.B.; Irving, J.A.; Malpica, A.; Matias-Guiu, X.; McCluggage, W.G.; et al. High-grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. 1), S40–S63. [Google Scholar] [CrossRef] [PubMed]
- Prat, J. Ovarian Carcinomas: Five Distinct Diseases with Different Origins, Genetic Alterations, and Clinicopathological Features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef]
- Prat, J.; D’Angelo, E.; Espinosa, I. Ovarian Carcinomas: At Least Five Different Diseases with Distinct Histological Features and Molecular Genetics. Hum. Pathol. 2018, 80, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.M.; Barry, S.C.; Ricciardelli, C.; Oehler, M.K. Epithelial Ovarian Cancer and the Immune System: Biology, Interactions, Challenges and Potential Advances for Immunotherapy. J. Clin. Med. 2020, 9, 2967. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Jammal, M.P.; Crispim, P.C.A.; Murta, E.F.C.; Nomelini, R.S. The Role of Stroma in Ovarian Cancer. Immunol. Invest. 2020, 49, 406–424. [Google Scholar] [CrossRef]
- Chen, G.M.; Kannan, L.; Geistlinger, L.; Kofia, V.; Safikhani, Z.; Gendoo, D.M.A.; Parmigiani, G.; Birrer, M.; Haibe-Kains, B.; Waldron, L. Consensus on Molecular Subtypes of High-Grade Serous Ovarian Carcinoma. Clin. Cancer Res. 2018, 24, 5037–5047. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The Concept of Immune Surveillance against Tumors. The First Theories. Oncotarget 2017, 8, 7175–7180. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Rosenberg, S.A. Treating B-cell Cancer with T Cells Expressing Anti-CD19 Chimeric Antigen Receptors. Nat. Rev. Clin. Oncol. 2013, 10, 267–276. [Google Scholar] [CrossRef]
- Yang, C.; Xia, B.R.; Zhang, Z.C.; Zhang, Y.J.; Lou, G.; Jin, W.L. Immunotherapy for Ovarian Cancer: Adjuvant, Combination, and Neoadjuvant. Front. Immunol. 2020, 11, 577869. [Google Scholar] [CrossRef] [PubMed]
- Scarlett, U.K.; Conejo-Garcia, J.R. Modulating the Tumor Immune Microenvironment as an Ovarian Cancer Treatment Strategy. Expert Rev. Obstet. Gynecol. 2012, 7, 413–419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A Framework for Advancing Our Understanding of Cancer-associated Fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Hagemann, T.; Wilson, J.; Burke, F.; Kulbe, H.; Li, N.F.; Plüddemann, A.; Charles, K.; Gordon, S.; Balkwill, F.R. Ovarian Cancer Cells Polarize Macrophages Toward a Tumor-associated Phenotype. J. Immunol. 2006, 176, 5023–5032. [Google Scholar] [CrossRef]
- Chen, F.; Hou, M.; Ye, F.; Lv, W.; Xie, X. Ovarian Cancer Cells Induce Peripheral Mature Dendritic Cells to Differentiate into Macrophagelike Cells in vitro. Int. J. Gynecol. Cancer 2009, 19, 1487–1493. [Google Scholar] [CrossRef]
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic Analysis of Immune Infiltrates in High-grade Serous Ovarian Cancer Reveals CD20, FoxP3 and TIA-1 as Positive Prognostic Factors. PLoS ONE 2009, 4, e6412. [Google Scholar] [CrossRef] [PubMed]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van den Eynde, B.J. Evidence for a Tumoral Immune Resistance Mechanism Based on Tryptophan Degradation by Indoleamine 2,3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef]
- Terraneo, N.; Jacob, F.; Dubrovska, A.; Grünberg, J. Novel Therapeutic Strategies for Ovarian Cancer Stem Cells. Front Oncol. 2020, 10, 319. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Hombach, A.; Hombach, A.A.; Abken, H. Adoptive Immunotherapy with Genetically Engineered T Cells: Modification of the IgG1 Fc ’Spacer’ Domain in the Extracellular Moiety of Chimeric Antigen Receptors Avoids ’Off-target’ Activation and Unintended Initiation of an Innate Immune Response. Gene Ther. 2010, 17, 1206–1213. [Google Scholar] [CrossRef]
- Sadelain, M.; Brentjens, R.; Rivière, I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, S.; Ying, T. From Therapeutic Antibodies to Chimeric Antigen Receptors (CARs): Making Better CARs Based on Antigen-binding Domain. Expert Opin. Biol. Ther. 2016, 16, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Hudecek, M.; Sommermeyer, D.; Kosasih, P.L.; Silva-Benedict, A.; Liu, L.; Rader, C.; Jensen, M.C.; Riddell, S.R. The Nonsignaling Extracellular Spacer Domain of Chimeric Antigen Receptors is Decisive for in vivo Antitumor Activity. Cancer Immunol. Res. 2015, 3, 125–135. [Google Scholar] [CrossRef]
- Janda, A.; Eryilmaz, E.; Nakouzi, A.; Cowburn, D.; Casadevall, A. Variable Region Identical Immunoglobulins Differing in Isotype Express Different Paratopes. J. Biol. Chem. 2012, 287, 35409–35417. [Google Scholar] [CrossRef]
- Imai, C.; Mihara, K.; Andreansky, M.; Nicholson, I.C.; Pui, C.H.; Geiger, T.L.; Campana, D. Chimeric Receptors with 4-1BB Signaling Capacity Provoke Potent Cytotoxicity Against Acute Lymphoblastic Leukemia. Leukemia 2004, 18, 676–684. [Google Scholar] [CrossRef]
- Yeku, O.O.; Purdon, T.J.; Koneru, M.; Spriggs, D.; Brentjens, R.J. Armored CAR T Cells Enhance Antitumor Efficacy and Overcome the Tumor Microenvironment. Sci. Rep. 2017, 7, 10541. [Google Scholar] [CrossRef]
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific Activation and Targeting of Cytotoxic Lymphocytes through Chimeric Single Chains Consisting of Antibody-binding Domains and the Gamma or Zeta Subunits of the Immunoglobulin and T-cell Receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724. [Google Scholar] [CrossRef]
- Kershaw, M.H.; Westwood, J.A.; Parker, L.L.; Wang, G.; Eshhar, Z.; Mavroukakis, S.A.; White, D.E.; Wunderlich, J.R.; Canevari, S.; Rogers-Freezer, L.; et al. A Phase I Study on Adoptive Immunotherapy Using Gene-modified T Cells for Ovarian Cancer. Clin. Cancer Res. 2006, 12, 6106–6115. [Google Scholar] [CrossRef]
- O’Neill, R.E.; Du, W.; Mohammadpour, H.; Alqassim, E.; Qiu, J.; Chen, G.; McCarthy, P.L.; Lee, K.P.; Cao, X. T Cell-Derived CD70 Delivers an Immune Checkpoint Function in Inflammatory T Cell Responses. J. Immunol. 2017, 199, 3700–3710. [Google Scholar] [CrossRef]
- Savoldo, B.; Ramos, C.A.; Liu, E.; Mims, M.P.; Keating, M.J.; Carrum, G.; Kamble, R.T.; Bollard, C.M.; Gee, A.P.; Mei, Z.; et al. CD28 Costimulation Improves Expansion and Persistence of Chimeric Antigen Receptor-modified T Cells in Lymphoma Patients. J. Clin. Invest. 2011, 121, 1822–1826. [Google Scholar] [CrossRef]
- Till, B.G.; Jensen, M.C.; Wang, J.; Qian, X.; Gopal, A.K.; Maloney, D.G.; Lindgren, C.G.; Lin, Y.; Pagel, J.M.; Budde, L.E.; et al. CD20-specific Adoptive Immunotherapy for Lymphoma Using a Chimeric Antigen Receptor with Both CD28 and 4-1BB Domains: Pilot Clinical Trial Results. Blood 2012, 119, 3940–3950. [Google Scholar] [CrossRef]
- Haso, W.; Lee, D.W.; Shah, N.N.; Stetler-Stevenson, M.; Yuan, C.M.; Pastan, I.H.; Dimitrov, D.S.; Morgan, R.A.; FitzGerald, D.J.; Barrett, D.M.; et al. Anti-CD22-chimeric Antigen Receptors Targeting B-cell Precursor Acute Lymphoblastic Leukemia. Blood 2013, 121, 1165–1174. [Google Scholar] [CrossRef]
- Chmielewski, M.; Hombach, A.A.; Abken, H. Of CARs and TRUCKs: Chimeric Antigen Receptor (CAR) T Cells Engineered with an Inducible Cytokine to Modulate the Tumor Stroma. Immunol. Rev. 2014, 257, 83–90. [Google Scholar] [CrossRef]
- Rajan, T.S.; Gugliandolo, A.; Bramanti, P.; Mazzon, E. In Vitro-Transcribed mRNA Chimeric Antigen Receptor T Cell (IVT mRNA CAR T) Therapy in Hematologic and Solid Tumor Management: A Preclinical Update. Int. J. Mol. Sci. 2020, 21, 6514. [Google Scholar] [CrossRef]
- Pang, Y.; Hou, X.; Yang, C.; Liu, Y.; Jiang, G. Advances on Chimeric Antigen Receptor-modified T-cell Therapy for Oncotherapy. Mol. Cancer. 2018, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.S.; Liu, D.P.; Liang, C.C. Challenges and Strategies: The Immune Responses in Gene Therapy. Med. Res. Rev. 2004, 24, 748–761. [Google Scholar] [CrossRef]
- Aronovich, E.L.; McIvor, R.S.; Hackett, P.B. The Sleeping Beauty Transposon System: A Non-viral Vector for Gene Therapy. Hum. Mol. Genet. 2011, 20, R14–R20. [Google Scholar] [CrossRef] [PubMed]
- Link, C.J., Jr.; Moorman, D.; Seregina, T.; Levy, J.P.; Schabold, K.J. A Phase I Trial of in vivo Gene Therapy with the Herpes Simplex Thymidine Kinase/Ganciclovir System for the Treatment of Refractory or Recurrent Ovarian Cancer. Hum. Gene Ther. 1996, 7, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.S.; Pathak, V.K. Design of Retroviral Vectors and Helper Cells for Gene Therapy. Pharmacol. Rev. 2000, 52, 493–511. [Google Scholar]
- Li, Q.; Kay, M.A.; Finegold, M.; Stratford-Perricaudet, L.D.; Woo, S.L. Assessment of Recombinant Adenoviral Vectors for Hepatic Gene Therapy. Hum. Gene Ther. 1993, 4, 403–409. [Google Scholar] [CrossRef]
- Isayeva, T.; Ren, C.; Ponnazhagan, S. Recombinant Adeno-associated Virus 2-mediated Antiangiogenic Prevention in a Mouse Model of Intraperitoneal Ovarian Cancer. Clin. Cancer Res. 2005, 11, 1342–1347. [Google Scholar]
- Fraley, R.; Subramani, S.; Berg, P.; Papahadjopoulos, D. Introduction of Liposome-encapsulated SV40 DNA into Cells. J. Biol. Chem. 1980, 255, 10431–10435. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Z.; Cohen, C.J.; Gattinoni, L.; Palmer, D.C.; Restifo, N.P.; Rosenberg, S.A.; Morgan, R.A. High-efficiency Transfection of Primary Human and Mouse T Lymphocytes Using RNA Electroporation. Mol. Ther. 2006, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Schüßler-Lenz, M.; Bondanza, A.; Buchholz, C.J. Clinical Development of CAR T Cells-challenges and Opportunities in Translating Innovative Treatment Concepts. EMBO Mol. Med. 2017, 9, 1183–1197. [Google Scholar] [CrossRef]
- Hamm, A.; Krott, N.; Breibach, I.; Blindt, R.; Bosserhoff, A.K. Efficient Transfection Method for Primary Cells. Tissue Eng. 2002, 8, 235–245. [Google Scholar] [CrossRef]
- Stevens, J.M.; Galyov, E.E.; Stevens, M.P. Actin-dependent Movement of Bacterial Pathogens. Nat. Rev. Microbiol. 2006, 4, 91–101. [Google Scholar] [CrossRef]
- Monjezi, R.; Miskey, C.; Gogishvili, T.; Schleef, M.; Schmeer, M.; Einsele, H.; Ivics, Z.; Hudecek, M. Enhanced CAR T-cell Engineering Using Non-viral Sleeping Beauty Transposition from Minicircle Vectors. Leukemia 2017, 31, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Amberger, M.; Ivics, Z. Latest Advances for the Sleeping Beauty Transposon System: 23 Years of Insomnia but Prettier than Ever: Refinement and Recent Innovations of the Sleeping Beauty Transposon System Enabling Novel, Nonviral Genetic Engineering Applications. Bioessays 2020, 42, e2000136. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Kovač, A.; Miskey, C.; Menzel, M.; Grueso, E.; Gogol-Döring, A.; Ivics, Z. RNA-guided Retargeting of Sleeping Beauty Transposition in Human Cells. Elife 2020, 9, e53868. [Google Scholar] [CrossRef] [PubMed]
- Belur, L.R.; Podetz-Pedersen, K.M.; Sorenson, B.S.; Hsu, A.H.; Parker, J.B.; Carlson, C.S.; Saltzman, D.A.; Ramakrishnan, S.; McIvor, R.S. Inhibition of Angiogenesis and Suppression of Colorectal Cancer Metastatic to the Liver Using the Sleeping Beauty Transposon System. Mol. Cancer 2011, 10, 14. [Google Scholar] [CrossRef]
- Magnani, C.F.; Turazzi, N.; Benedicenti, F.; Calabria, A.; Tenderini, E.; Tettamanti, S.; Giordano Attianese, G.M.; Cooper, L.J.; Aiuti, A.; Montini, E.; et al. Immunotherapy of Acute Leukemia by Chimeric Antigen Receptor-modified Lymphocytes Using an Improved Sleeping Beauty Transposon Platform. Oncotarget 2016, 7, 51581–51597. [Google Scholar] [CrossRef]
- Ohlfest, J.R.; Demorest, Z.L.; Motooka, Y.; Vengco, I.; Oh, S.; Chen, E.; Scappaticci, F.A.; Saplis, R.J.; Ekker, S.C.; Low, W.C.; et al. Combinatorial Antiangiogenic Gene Therapy by Nonviral Gene Transfer Using the Sleeping Beauty Transposon Causes Tumor Regression and Improves Survival in Mice Bearing Intracranial Human Glioblastoma. Mol. Ther. 2005, 12, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qiu, J.; Sun, Y. The Basics of CAR T Design and Challenges in Immunotherapy of Solid Tumors—Ovarian Cancer as a Model. Hum. Vaccin. Immunother. 2017, 13, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, K.C.; Duncan, B.B.; Lee, D.W. CAR-T Cell Therapy for Acute Lymphoblastic Leukemia: Transforming the Treatment of Relapsed and Refractory Disease. Curr. Hematol. Malig. Rep. 2018, 13, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, H.; Chen, S.Y.; Liu, C.J.; Hu, F.F.; Yu, J.; Wu, Y.; Guo, A.Y. Transcriptome and Regulatory Network Analyses of CD19-CAR-T Immunotherapy for B-ALL. Genom. Proteom. Bioinform. 2019, 17, 190–200. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Bagley, S.J.; O’Rourke, D.M. Clinical Investigation of CAR T Cells for Solid Tumors: Lessons Learned and Future Directions. Pharmacol. Ther. 2020, 205, 107419. [Google Scholar] [CrossRef]
- Ma, S.; Li, X.; Wang, X.; Cheng, L.; Li, Z.; Zhang, C.; Ye, Z.; Qian, Q. Current Progress in CAR-T Cell Therapy for Solid Tumors. Int. J. Biol. Sci. 2019, 15, 2548–2560. [Google Scholar] [CrossRef]
- Abbott, R.C.; Cross, R.S.; Jenkins, M.R. Finding the Keys to the CAR: Identifying Novel Target Antigens for T Cell Redirection Immunotherapies. Int. J. Mol. Sci. 2020, 21, 515. [Google Scholar] [CrossRef]
- Titov, A.; Valiullina, A.; Zmievskaya, E.; Zaikova, E.; Petukhov, A.; Miftakhova, R.; Bulatov, E.; Rizvanov, A. Advancing CAR T-Cell Therapy for Solid Tumors: Lessons Learned from Lymphoma Treatment. Cancers 2020, 12, 125. [Google Scholar] [CrossRef]
- Anderson, K.G.; Stromnes, I.M.; Greenberg, P.D. Obstacles Posed by the Tumor Microenvironment to T-cell Activity: A Case for Synergistic Therapies. Cancer Cell 2017, 31, 311–325. [Google Scholar] [CrossRef]
- Straathof, K.C.; Pulè, M.A.; Yotnda, P.; Dotti, G.; Vanin, E.F.; Brenner, M.K.; Heslop, H.E.; Spencer, D.M.; Rooney, C.M. An Inducible Caspase 9 Safety Switch for T-cell Therapy. Blood 2005, 105, 4247–4254. [Google Scholar] [CrossRef]
- Hung, C.F.; Xu, X.; Li, L.; Ma, Y.; Jin, Q.; Viley, A.; Allen, C.; Natarajan, P.; Shivakumar, R.; Peshwa, M.V.; et al. Development of Anti-Human Mesothelin-Targeted Chimeric Antigen Receptor Messenger RNA-Transfected Peripheral Blood Lymphocytes for Ovarian Cancer Therapy. Hum. Gene Ther. 2018, 29, 614–625. [Google Scholar] [CrossRef]
- Klapdor, R.; Wang, S.; Morgan, M.; Dörk, T.; Hacker, U.; Hillemanns, P.; Büning, H.; Schambach, A. Characterization of a Novel Third-Generation Anti-CD24-CAR against Ovarian Cancer. Int. J. Mol. Sci. 2019, 20, 660. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23, 181–192.e5. [Google Scholar] [CrossRef] [PubMed]
- Leong, L.; Tan, H.L.; Cua, S.; Yong, K.S.M.; Chen, Q.; Choo, A. Preclinical Activity of Embryonic Annexin A2-Specific Chimeric Antigen Receptor T Cells against Ovarian Cancer. Int. J. Mol. Sci. 2020, 21, 381. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Yang, Y.; Li, W.; Tan, Y.; Guo, W.; Ao, L.; He, X.; Wu, X.; Xia, J.; Xu, X.; et al. Anti-αFR CAR-engineered NK-92 Cells Display Potent Cytotoxicity against αFR-positive Ovarian Cancer. J. Immunother. 2019, 42, 284–296. [Google Scholar] [CrossRef]
- Ng, Y.Y.; Tay, J.C.K.; Wang, S. CXCR1 Expression to Improve Anti-Cancer Efficacy of Intravenously Injected CAR-NK Cells in Mice with Peritoneal Xenografts. Mol. Ther. Oncolytics 2019, 16, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, J. Therapeutic Effect of Dual CAR-T Targeting PDL1 and MUC16 Antigens on Ovarian Cancer Cells in Mice. BMC Cancer 2020, 20, 678. [Google Scholar] [CrossRef]
- Hong, H.; Brown, C.E.; Ostberg, J.R.; Priceman, S.J.; Chang, W.C.; Weng, L.; Lin, P.; Wakabayashi, M.T.; Jensen, M.C.; Forman, S.J. L1 Cell Adhesion Molecule-Specific Chimeric Antigen Receptor-Redirected Human T Cells Exhibit Specific and Efficient Antitumor Activity against Human Ovarian Cancer in Mice. PLoS ONE 2016, 11, e0146885. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Shi, H.; Liu, C.; Liu, J.; Liu, X.; Sun, Y. Construction and Evaluation of a Novel Humanized HER2-specific Chimeric Receptor. Breast Cancer Res. 2014, 16, R61. [Google Scholar] [CrossRef]
- Song, D.G.; Ye, Q.; Carpenito, C.; Poussin, M.; Wang, L.P.; Ji, C.; Figini, M.; June, C.H.; Coukos, G.; Powell, D.J., Jr. In vivo Persistence, Tumor Localization, and Antitumor Activity of CAR-engineered T Cells is Enhanced by Costimulatory Signaling through CD137 (4-1BB). Cancer Res. 2011, 71, 4617–4627. [Google Scholar] [CrossRef]
- Perales-Puchalt, A.; Svoronos, N.; Rutkowski, M.R.; Allegrezza, M.J.; Tesone, A.J.; Payne, K.K.; Wickramasinghe, J.; Nguyen, J.M.; O’Brien, S.W.; Gumireddy, K.; et al. Follicle-Stimulating Hormone Receptor Is Expressed by Most Ovarian Cancer Subtypes and Is a Safe and Effective Immunotherapeutic Target. Clin. Cancer Res. 2017, 23, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.L.; Sheard, V.E.; Kalaitsidou, M.; Blount, D.; Lad, Y.; Cheadle, E.J.; Edmondson, R.J.; Kooner, G.; Gilham, D.E.; Harrop, R. Preclinical Assessment of CAR T-Cell Therapy Targeting the Tumor Antigen 5T4 in Ovarian Cancer. J. Immunother. 2018, 41, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Murad, J.P.; Kozlowska, A.K.; Lee, H.J.; Ramamurthy, M.; Chang, W.C.; Yazaki, P.; Colcher, D.; Shively, J.; Cristea, M.; Forman, S.J.; et al. Effective Targeting of TAG72+ Peritoneal Ovarian Tumors via Regional Delivery of CAR-Engineered T Cells. Front. Immunol. 2018, 9, 2268. [Google Scholar] [CrossRef]
- Hilliard, T.S. The Impact of Mesothelin in the Ovarian Cancer Tumor Microenvironment. Cancers 2018, 10, 277. [Google Scholar] [CrossRef]
- Hassan, R.; Thomas, A.; Alewine, C.; Le, D.T.; Jaffee, E.M.; Pastan, I. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J. Clin. Oncol. 2016, 34, 4171–4179. [Google Scholar] [CrossRef]
- Oble, D.A.; Loewe, R.; Yu, P.; Mihm, M.C., Jr. Focus on TILs: Prognostic Significance of Tumor Infiltrating Lymphocytes in Human Melanoma. Cancer Immun. 2009, 9, 3. [Google Scholar]
- Jelovac, D.; Armstrong, D.K. Role of Farletuzumab in Epithelial Ovarian Carcinoma. Curr. Pharm. Des. 2012, 18, 3812–3815. [Google Scholar] [CrossRef]
- Dobrzycka, B.; Mackowiak-Matejczyk, B.; Terlikowska, K.M.; Kulesza-Bronczyk, B.; Kinalski, M.; Terlikowski, S.J. Serum Levels of IL-6, IL-8 and CRP as Prognostic Factors in Epithelial Ovarian Cancer. Eur. Cytokine Netw. 2013, 24, 106–113. [Google Scholar] [CrossRef]
- Rodrigues, I.S.S.; Martins-Filho, A.; Micheli, D.C.; Lima, C.A.; Tavares-Murta, B.M.; Murta, E.F.C.; Nomelini, R.S. IL-6 and IL-8 as Prognostic Factors in Peritoneal Fluid of Ovarian Cancer. Immunol. Invest. 2020, 49, 510–521. [Google Scholar] [CrossRef]
- Whilding, L.M.; Halim, L.; Draper, B.; Parente-Pereira, A.C.; Zabinski, T.; Davies, D.M.; Mahe, J. CAR T-Cells Targeting the Integrin αvβ6 and Co-Expressing the Chemokine Receptor CXCR2 Demonstrate Enhanced Homing and Efficacy against Several Solid Malignancies. Cancers 2019, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Tao, H.; Karachi, A.; Long, Y.; Hou, A.Y.; Na, M.; Dyson, K.A.; Grippin, A.J.; Deleyrolle, L.P.; Zhang, W.; et al. CXCR1- or CXCR2-modified CAR T Cells Co-opt IL-8 for Maximal Antitumor Efficacy in Solid Tumors. Nat. Commun. 2019, 10, 4016. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Anders, R.A.; Young, G.D.; Xu, H.; Sharma, R.; McMiller, T.L.; Chen, S.; Klein, A.P.; Pardoll, D.M.; Topalian, S.L.; et al. Colocalization of Inflammatory Response with B7-h1 Expression in Human Melanocytic Lesions Supports an Adaptive Resistance Mechanism of Immune Escape. Sci. Transl. Med. 2012, 4, 127ra37. [Google Scholar] [CrossRef]
- Fan, C.A.; Reader, J.; Roque, D.M. Review of Immune Therapies Targeting Ovarian Cancer. Curr. Treat. Options Oncol. 2018, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Strickland, K.C.; Howitt, B.E.; Shukla, S.A.; Rodig, S.; Ritterhouse, L.L.; Liu, J.F.; Garber, J.E.; Chowdhury, D.; Wu, C.J.; D’Andrea, A.D.; et al. Association and Prognostic Significance of BRCA1/2-mutation Status with Neoantigen Load, Number of Tumor-infiltrating Lymphocytes and Expression of PD-1/PD-L1 in High Grade Serous Ovarian Cancer. Oncotarget 2016, 22, 13587–13598. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Ma, J.; Han, W.; Wang, X.; Fang, Y.; Li, D.; Pan, H.; Zhang, L. The Anticancer Immune Response of Anti-PD-1/PD-L1 and the Genetic Determinants of Response to Anti-PD-1/PD-L1 Antibodies in Cancer Patients. Oncotarget 2015, 6, 19393–19404. [Google Scholar] [CrossRef] [PubMed]
- Wahba, J.; Natoli, M.; Whilding, L.M.; Parente-Pereira, A.C.; Jung, Y.; Zona, S.; Lam, E.W.-F.; Smith, J.R.; Maher, J.; Ghaem-Magham, S. Chemotherapy-induced Apoptosis, Autophagy and Cell Cycle Arrest are Key Drivers of Synergy in Chemo-immunotherapy of Epithelial Ovarian Cancer. Cancer Immunol. Immunother. 2018, 67, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- RodrÍguez, E.; Schetters, S.T.T.; van Kooyk, Y. The Tumour Glyco-code as a Novel Immune Checkpoint for Immunotherapy. Nat. Rev. Immunol. 2018, 18, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.; Marcos-Silva, L.; Ricardo, S.; Ponte, F.; Costa, A.; Lopes, J.M.; David, L. Peritoneal Dissemination of Ovarian Cancer: Role of MUC16 - Mesothelin Interaction and Implications for Treatment. Expert Rev. Anticancer Ther. 2018, 18, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Meldolesi, J. L1-CAM and N-CAM: From Adhesion Proteins to Pharmacological Targets. Trends Pharmacol. Sci. 2015, 36, 769–781. [Google Scholar] [CrossRef]
- Hua, T.; Liu, S.; Xin, X.; Jin, Z.; Liu, Q.; Chi, S.; Wang, X.; Wang, H. Prognostic Significance of L1 Cell Adhesion Molecule in Cancer Patients: A Systematic Review and Meta-analysis. Oncotarget 2016, 7, 85196–85207. [Google Scholar] [CrossRef]
- Oh, D.Y.; Bang, Y.J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, H.; Ye, Q.; Wang, C.; Xu, Y. Prognostic Value of KIF2A and HER2-Neu Overexpression in Patients with Epithelial Ovarian Cancer. Medicine 2016, 95, e2803. [Google Scholar] [CrossRef]
- Ding, H.; Yang, X.; Wei, Y. Fusion Proteins of NKG2D/NKG2DL in Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 177. [Google Scholar] [CrossRef]
- Zingoni, A.; Vulpis, E.; Loconte, L.; Santoni, A. NKG2D Ligand Shedding in Response to Stress: Role of ADAM10. Front. Immunol. 2020, 11, 447. [Google Scholar] [CrossRef]
- Kang, T.H.; Mao, C.P.; He, L.; Tsai, Y.C.; Liu, K.; La, V.; Wu, T.C.; Hung, C.F. Tumor-targeted Delivery of IL-2 by NKG2D Leads to Accumulation of Antigen-specific CD8+ T Cells in the Tumor Loci and Enhanced Anti-tumor Effects. PLoS ONE 2012, 7, e35141. [Google Scholar] [CrossRef]
- Kohrt, H.E.; Houot, R.; Marabelle, A.; Cho, H.J.; Osman, K.; Goldstein, M.; Levy, R.; Brody, J. Combination Strategies to Enhance Antitumor ADCC. Immunotherapy 2012, 4, 511–527. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Zhao, L.; Zhang, X.; Xu, T.; Cui, M. Basing on uPAR-binding Fragment to Design Chimeric Antigen Receptors Triggers Antitumor Efficacy against uPAR Expressing Ovarian Cancer Cells. Biomed. Pharmacother. 2019, 117, 109173. [Google Scholar] [CrossRef]
- van Dam, P.A.; Coelho, A.; Rolfo, C. Is There a Role for Urokinase-type Plasminogen Activator Inhibitors as Maintenance Therapy in Patients with Ovarian Cancer? Eur. J. Surg. Oncol. 2017, 43, 252–257. [Google Scholar] [CrossRef]
- Yadav, A.S.; Pandey, P.R.; Butti, R.; Radharani, N.N.V.; Roy, S.; Bhalara, S.R.; Gorain, M.; Kundu, G.C.; Kumar, D. The Biology and Therapeutic Implications of Tumor Dormancy and Reactivation. Front Oncol. 2018, 8, 72. [Google Scholar] [CrossRef]
- Stern, P.L.; Harrop, R. 5T4 Oncofoetal Antigen: An Attractive Target for Immune Intervention in Cancer. Cancer Immunol. Immunother. 2017, 66, 415–426. [Google Scholar] [CrossRef]
- Wan, Y.L.; Sapra, P.; Bolton, J.; Chua, J.X.; Durrant, L.G.; Stern, P.L. Combination Treatment with an Antibody-Drug Conjugate (A1mcMMAF) Targeting the Oncofetal Glycoprotein 5T4 and Carboplatin Improves Survival in a Xenograft Model of Ovarian Cancer. Target. Oncol. 2019, 14, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Hu, H.; Tang, B. Advances of Chimeric Antigen Receptor T Cell Therapy in Ovarian Cancer. OncoTargets Ther. 2019, 12, 8015–8022. [Google Scholar] [CrossRef] [PubMed]
- Jindal, V.; Arora, E.; Gupta, S.; Lal, A.; Masab, M.; Potdar, R. Prospects of Chimeric Antigen Receptor T Cell Therapy in Ovarian Cancer. Med. Oncol. 2018, 35, 70. [Google Scholar] [CrossRef]
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T Cells in Solid Tumors: Challenges and Opportunities. Stem Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.E.; Rewers-Felkins, K.A.; Quinlin, I.S.; Phillips, C.A.; Townsend, M.; Philip, R.; Dobrzanski, M.J.; Lockwood-Cooke, P.R.; Robinson, W. Cytotoxic T-lymphocyte Immunotherapy for Ovarian Cancer: A Pilot Study. J. Immunother. 2012, 35, 196–204. [Google Scholar] [CrossRef]
- Haas, A.R.; Tanyi, J.L.; O’Hara, M.H.; Gladney, W.L.; Lacey, S.F.; Torigian, D.A.; Soulen, M.C.; Tian, L.; McGarvey, M.; Nelson, A.M.; et al. Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers. Mol. Ther. 2019, 27, 1919–1929. [Google Scholar] [CrossRef]
- Morgan, M.A.; Schambach, A. Engineering CAR-T Cells for Improved Function Against Solid Tumors. Front. Immunol. 2018, 9, 2493. [Google Scholar] [CrossRef] [PubMed]
- Benard, E.; Casey, N.P.; Inderberg, E.M.; Wälchli, S. SJI 2020 Special Issue: A Catalogue of Ovarian Cancer Targets for CAR Therapy. Scand. J. Immunol. 2020, 92, e12917. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Kumagai, A.; Iriguchi, S.; Yasui, Y.; Miyasaka, T.; Nakagoshi, K.; Nakane, K.; Saito, K.; Takahashi, M.; Sasaki, A.; et al. Non-clinical Efficacy, Safety and Stable Clinical Cell Processing of Induced Pluripotent Stem Cell-derived Anti-glypican-3 Chimeric Antigen Receptor-expressing Natural Killer/Innate Lymphoid Cells. Cancer Sci. 2020, 111, 1478–1490. [Google Scholar] [CrossRef]
- Wang, L.; Xu, T.; Cui, M. Are Ovarian Cancer Stem Cells the Target for Innovative Immunotherapy? OncoTargets Ther. 2018, 11, 2615–2626. [Google Scholar] [CrossRef]
- Parte, S.C.; Batra, S.K.; Kakar, S.S. Characterization of Stem Cell and Cancer Stem Cell Populations in Ovary and Ovarian Tumors. J. Ovarian Res. 2018, 11, 69. [Google Scholar] [CrossRef]
- Zhao, L.; Li, J.; Liu, M.; Zhou, H.; Zou, H.; Wei, Y.; Sun, K.; Li, G.; Li, S.; Pang, L. The Clinicopathological Parameters Significance of CD133 and Nestin in Epithelial Ovarian Cancer: A Meta-analysis. Future Oncol. 2017, 13, 2555–2570. [Google Scholar] [CrossRef] [PubMed]
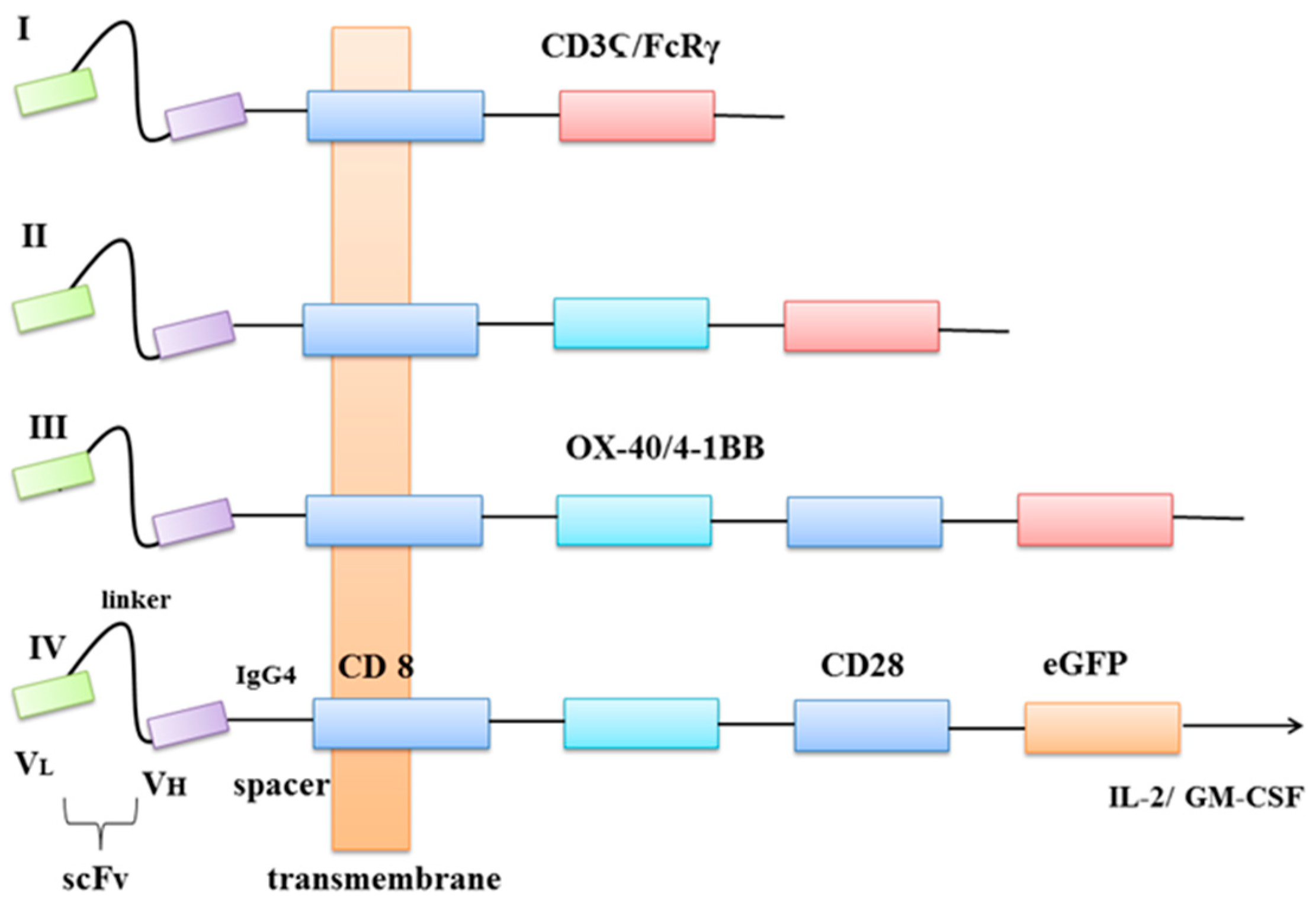
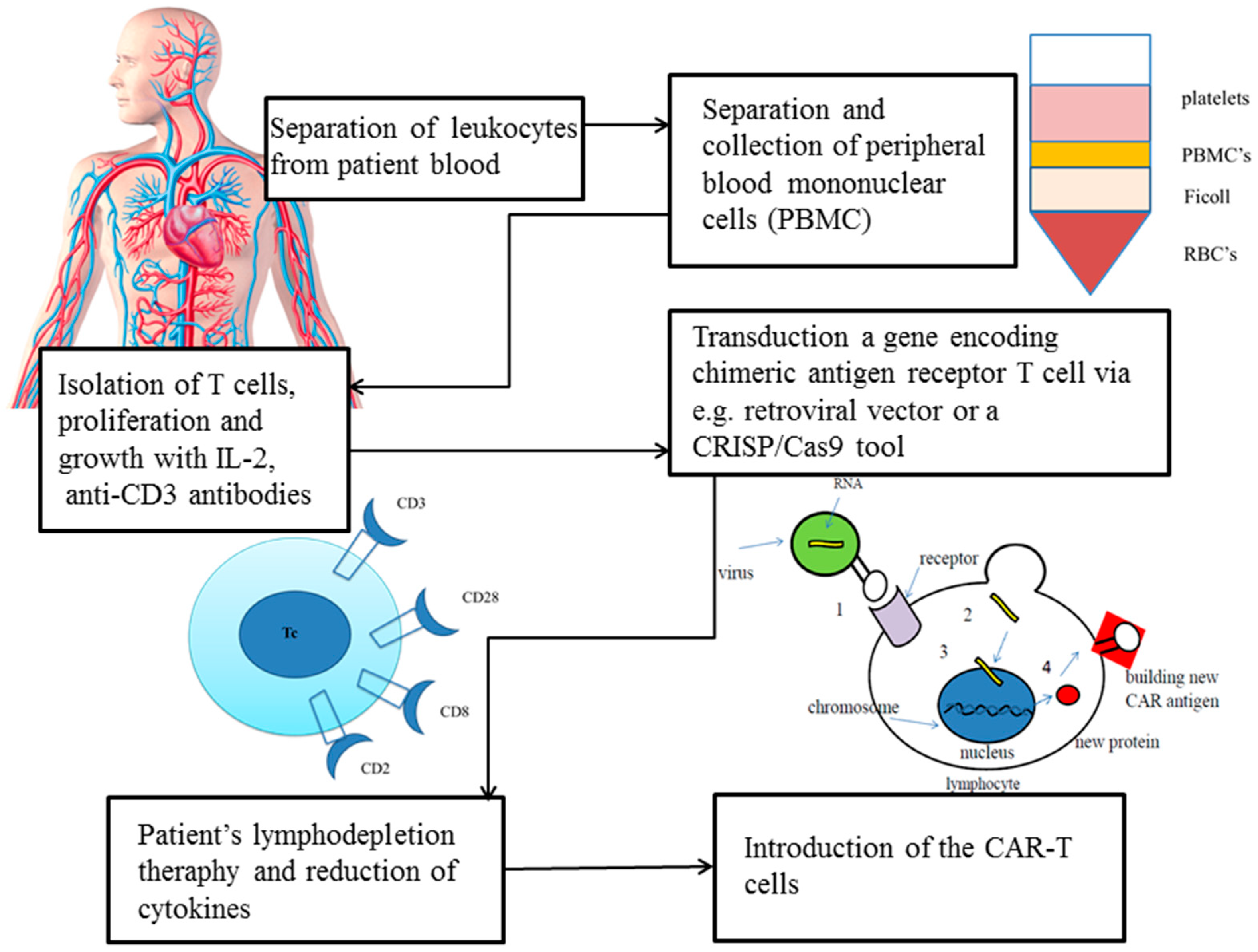
| Genetic Approach | Methods | Structure | Study | Target | Advantages | Disadvantages | Source |
|---|---|---|---|---|---|---|---|
| Viral | Retroviral vectors | ssRNA | in vivo | only mitosis | substitutability↑ | insertional mutagenesis↑ titre vector production ↑ | [50] |
| Lentiviral vectors | ssRNA | in vivo | entire cycle | integration ↑ risk of insertional mutagenesis ↓ | possible insertional mutagenesis↑ presence of regulatory proteins in the packaging construct transient expression of the transgene with integration-defective vector↑ | [51] | |
| Adenoviruses | dsDNA | in vivo | entire cycle | toxicity↓ | integrity↓ | [52] | |
| Adeno-associated viral vectors | ssDNA | in vivo | entire cycle | infection efficiency ↑ gene expression ↑ | internalisation ↓ endosomal trafficking↓ nuclear import↓ | [53] | |
| Nonviral | Liposome-mediated gene transfer | lipid n-layer | in vitro | entire cycle | condensation of DNA ↑ infection efficiency ↑ | transfection efficiency↓ | [54] |
| Messenger RNA-mediated gene transduction | ssRNA | in vitro | entire cycle | insertional mutations ↓ potential malignant transformation/ genotoxicity ↓ off-tumour, on-target side effects ↓ | instable, non-biocompatible↓ low biodegradability, low efficacy↓ toxicity at high dose, difficult preparation, low transformation efficiency↓ | [46,55] | |
| Sleeping Beauty transposon/transposase system | plasmid-plasmid | in vivo | entire cycle | integration ↑ | insertional mutagenesis ↓ | [50] |
| Target Antigen | Cells | Gene Transfer | Intervention/ Monitoring | Study | Studied Material | Outcomes | Source |
|---|---|---|---|---|---|---|---|
| MSLN | T/NK | messenger RNA-mediated gene transduction | i.p. 1 × 108 cells/ up to 6 weeks | in vivo | Defb29 Vegf-luc/Hmeso Platinum resistant OC | ↓tumour burdens ↑mice survival | [77] |
| CD24 MSLN | NK-29 | lentiviral transduction | 5 × 104 cells/ 24 h | in vitro | A2780, OVCAR3, SKOV3, Primary OC | ↑cytotoxicity | [78] |
| MSLN | NK | transposon vector | i.p. 1.5 × 107 cells/ up to 7 weeks | in vivo | A1847, MA 148 | ↑inhibition of tumour growth ↑survival | [79] |
| ANXA2 | T | lentiviral vector | i.p. 5 × 106 cells/ up to 5 days | in vivo | IGROV-1, SKOV3 | ↑cytokine release ↑cytotoxicity ↑survival ↓tumour burdens | [80] |
| FRα | NK | lentiviral vector | i.p. 1 × 106 cells/ up to 10 days | in vivo | SKOV3, A2780, HTC116, A431 | ↑elimination of cancer cells ↑survival | [81] |
| CXCR1 | NK | mRNA transfection | i.v. 5 × 106 cells/twice a week/2 weeks | in vivo | SKOV3, CaOV3, SW626 | ascites generation↓ ↓tumour cells in ascites samples complete metabolic response ↑survival | [82] |
| PDL1 MUC16 | T | lentiviral infection | i.p. 1 × 106 cells/ up to 4 weeks | in vivo | SKOV3 | ↑IL-2, IFN-γ, TNF-α ↑regression of ovarian cells ↑survival | [83] |
| L1-CAM | Tcm | lentiviral vector | i.p. 5 × 106/up to 17 weeks | in vivo | CAOV-3, OVCAR-3, SKOV-3, MADH2780, A2780 | regression of tumours in the peritoneal cavity and massive ascites | [84] |
| HER2/ neu | T | lentiviral vector | 1 × 105 CAR/1 × 105 tumour cells | in vitro | SKOV3, OVCAR3, A2780, A1847 | ↑expression of CARs ↑cytotoxicity ↓tumour cells | [85] |
| FRα | T | retroviral vector | i.v. up to 5 × 105 cells/48 h | in vivo | 14 patients with recurrent, resected recurrent, or residual epithelial FR+ ovarian cancer | The treatment was well tolerated, but no antitumour effect was observed. | [40] |
| FRα | T | lentiviral vector | i.v. up to 1 × 106 cells/4 weeks | in vivo | SKOV-3, OVCAR3, A1847, C30, PEO-1 | tumour regression T-cell persistence↓ | [86] |
| FSHR | T | retroviral vector | i.p. 2 injections up to 1.5 × 106 cells/up to 50 days | in vivo | mouse xenografts OVCAR-3, CaOV3, RNG1, OVTOKO and TOV-21G | increased survival no toxicity | [87] |
| 5T4 | T | lentiviral vector | i.p. up to 6 × 104 cells/100 days | in vivo | SKOV-3 | 5T4-specific CAR can recognise and respond physiologically to autologous tumour cells | [88] |
| TAG72 | lentiviral vector | i.p.,i.v. 5 × 106 cells/up to 8 weeks | in vivo | mouse xenografts SKOV-3, OVCAR-3, OVCAR-3 | ↓tumour growth ↑survival | [89] |
| Study Title | Summary | Intervention | Phase | Locations |
|---|---|---|---|---|
| The Fourth Generation CART-cell Therapy for Refractory-Relapsed OC | The goal of this clinical trial is to study the safety and feasibility of anti-Mesothelin Chimeric Antigen Receptor T-Cell (MESO CAR-T cells) therapy for Refractory-Relapsed OC | Autologous genetically modified anti-MESO CAR transduced T cells | Early 1 | Shanghai 6th People’s Hospital Shanghai, China |
| Safety and Effectiveness of MESO-CAR T-Cells Therapy for Relapsed and Refractory Epithelial Ovarian Cancer | The goal of this clinical trial is to study the feasibility and efficacy of anti-MESO antigen receptors (CARs) T-cell therapy for relapsed and refractory epithelial ovarian cancer | Retroviral vector-transduced autologous T cells to express anti-MESO CARs Fludarabine 30 mg/m2/d Cyclophosphamide 300 mg/m2/d | 1 and 2 | The Second Affiliated hospital of Zhejiang University School of Medicine Hangzhou, China |
| A Clinical Trial of MESO-CAR T-Cells Therapy for Relapsed and Refractory Ovarian Cancer MESO-CAR T Cells | The goal of this clinical trial is to study the feasibility and efficacy of anti-MESO antigen receptors (CARs) T-cell therapy for relapsed and refractory ovarian cancer | Retroviral vector-transduced autologous T cells to express anti-MESO CARs Fludarabine 30 mg/m2/d; Cyclophosphamide 300 mg/m2/d | Early 1 | The Second Affiliated hospital of Zhejiang University School of Medicine Hangzhou, China |
| A Single-Center, Phase I Clinical Study to Evaluate the Safety, Tolerability and Efficacy of LCAR-M23, a CAR-T-Cell Therapy Targeting MSLN in Patients With Relapsed and Refractory Epithelial Ovarian Cancer | This study is a prospective, single-arm, single-centre, open-label, single-dose dose finding and extension study to evaluate the safety, tolerability, pharmacokinetics and anti-tumour efficacy profiles of the LCAR-M23 CAR-T-cell therapy in subjects with relapsed and refractory epithelial ovarian cancer after prior adequate standard of care | LCAR-M23 cells Prior to infusion of LCAR-M23, subjects will receive a premedication regimen (IV of cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 once daily for 3 days) | 1 | Shanghai East Hospital Shanghai, China |
| A Single-Arm, Single-Center, Open-Label Pilot Study of Anti-ALPP CART-cells in Patient With Alkaline Phosphatase, Placental (ALPP)-Positive Metastatic Ovarian and Endometrial Cancer | The goal of this clinical trial is to evaluate the safety and efficacy of anti-ALPP chimeric antigen receptor (CAR)-modified T (CAR-T) cells in treating patients with ALPP-positive metastatic ovarian and endometrial cancer. | CART treatment Retroviral vector-transduced autologous T cells to express anti-ALPP CARs Cyclophosphamide will be administered at dose of 20 mg/kg for 1 day and then fludarabine will be given for the next 3 days with 35 mg/m2 and then the CAR-T cells will be administered | 1 and 2 | Xinqiao Hospital of Chongqing’ China |
| An Exploratory Study of αPD1-MSLN-CAR T Cells Secreting PD-1 Nanobodies for the Treatment of MSLN-positive Advanced Solid Tumours | This is a single arm, open-label, dose escalation clinical study to evaluate the safety and tolerability of autologous mesothelin (MSLN)-targeted chimeric antigen receptor (MSLN-CAR) T cells secreting PD-1 nanobodies (αPD1-MSLN-CAR T cells) in patients with solid tumours | αPD1-MSLN-CAR T cells Subjects will undergo leukapheresis to isolate peripheral blood mononuclear cells (PBMCs) for the production of αPD1-MSLN-CAR T cells. The initial dose of 1 × 105 CAR+ T cells/kg will be infused on day 0. | Early 1 | Shanghai Tenth people’s Hospital Shanghai, China |
| Phase I Study Evaluating Benefit of PRGN-3005 UltraCAR-T™ (Autologous CAR T Cells) in Advanced Stage Platinum Resistant Ovarian Cancer Patients | This is a study to identify the best dose and side effects of modified immune cells PRGN-3005 (autologous chimeric antigen receptor (CAR) T cells developed by Precigen, Inc) in treating patients with ovarian, fallopian tube, or primary peritoneal cancer that has spread to other places in the body, that has come back and is resistant to platinum chemotherapy. | PRGN-3005 UltraCAR-T cells given IP or IV | 1 | Fred Hutch/University of Washington Cancer Consortium Seattle, United States |
| A Phase 1 Study of Autologous Activated T-cells Targeting the B7-H3 Antigen in Subjects With Recurrent Epithelial Ovarian Cancer | This is single centre, open-label phase 1 dose escalation trial that uses modified 3+3 design to identify a recommended phase 2 dose (RP2D) of CAR.B7-H3 T cell. An expansion cohort will enrol additional subjects at the RP2D for a total enrolment of up to 21 subjects on the protocol. | CAR.B7-H3 Two dose levels will be evaluated: Dose Level 1 (7.5 × 107 cells/infusion), dose Level 2 (2 × 108 cells/infusion). | 1 | Lineberger Comprehensive Cancer Center Chapel Hill, United States |
| Phase I Clinical Trial of Adoptive Transfer of Autologous Folate Receptor-Alpha Redirected T Cells for Recurrent High Grade Serous Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | Phase I study to establish safety and feasibility of IP(L) administered lentiviral transduced MOv19-BBz CAR T cells with or without cyclophosphamide + fludarabine as lymphodepleting chemotherapy. | MOv19-BBz CAR T cells IP administered lentiviral transduced MOv19-BBz CAR T cells with or without cyclophosphamide + fludarabine as lymphodepleting chemotherapy | 1 | University of Pennsylvania Health System Philadelphia, United States |
| Innovative Treatment of Ovarian Cancer Based on Immunogene-modified T Cells (IgT) | The primary objectives are to evaluate the safety and efficacy of infusion of autologous OC immunogene-modified T cells (OC-IgT cells) | OC-IgT cells. Autologous human OC-IgT cells | 1 and 2 | Shenzhen Geno-immune Medical Institute Shenzhen, China |
| A Phase 1 Open-Label, Multi-Center First in Human Study of TnMUC1-Targeted Genetically-Modified Chimeric Antigen Receptor T Cells in Patients With Advanced TnMUC1-Positive Solid Tumours and Multiple Myeloma | Phase 1 study of the safety, tolerability, feasibility and preliminary efficacy of the administration of genetically modified autologous T cells (CART-TnMUC1 cells) engineered to express a chimeric antigen receptor (CAR) capable of recognizing the tumour antigen, TnMUC1 and activating the T cell (CART- TnMUC1 cells) | CART-TnMUC1 Single IV administration of genetically modified autologous T cells engineered to express a TnMUC1-Targeted Genetically-Modified Chimeric Antigen (CAR) Drug: Cyclophosphamide | 1 | The Angeles Clinic and Research Institute Los Angeles and 7 others, United States |
| Autologous Immunotherapy With Multi-target Gene-modified CAR-T/TCR-T Cell for Malignancies | This is a single arm, open-label, uni-center, phase I-II study to evaluate the safety and effectiveness of CAR-T/TCR-T-cell immunotherapy in treating with different malignancies patients (OC and 13 more) | CAR-T-cell immunotherapy According to tumour burden and other conditions, patients will be treated with cyclophosphamide or fludarabine, then, CAR-T. cells will be infused 48-72 h later | 1 and 2 | The First Affiliated Hospital of Zhengzhou University Zhengzhou, China |
| Phase I Study of Human Chimeric Antigen Receptor Modified T Cells in Patients With Mesothelin Expressing Cancers | Phase I study to establish safety and feasibility of IV or IP(L) administered lentiviral transduced huCART-meso cells with or without lymphodepletion by way of administering cyclophosphamide | huCART-meso cells IV or IP(L) lentiviral transduced huCART-meso cells in 6 cohorts with and without cyclophosphamide in a 3+3 dose escalation design. | 1 | University of Pennsylvania Philadelphia, United States |
| A Phase I Trial to Assess Safety, Tolerability and Anti-tumour Activity of Autologous T Cell Modified Chimeric Antigen Receptor (CAR) (CCT303-406) in Patients With Relapsed or Refractory HER2 Positive Solid Tumours | This clinical study is to investigate the safety and tolerability of CCT303-406 CAR modified autologous T cells (CCT303-406) in subjects with relapsed or refractory stage IV metastatic HER2-positive solid tumours | CCT303-406 Blood will be collected from subjects to isolate peripheral blood mononuclear cells for the production of CCT303-406. cyclophosphamide and fludarabine for lymphodepletion followed by a single dose of CCT303-406 via IV. | 1 | Zhongshan Hospital Affiliated to Fudan University Shanghai, China |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terlikowska, K.M.; Dobrzycka, B.; Terlikowski, S.J. Chimeric Antigen Receptor Design and Efficacy in Ovarian Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 3495. https://doi.org/10.3390/ijms22073495
Terlikowska KM, Dobrzycka B, Terlikowski SJ. Chimeric Antigen Receptor Design and Efficacy in Ovarian Cancer Treatment. International Journal of Molecular Sciences. 2021; 22(7):3495. https://doi.org/10.3390/ijms22073495
Chicago/Turabian StyleTerlikowska, Katarzyna M., Bożena Dobrzycka, and Sławomir J. Terlikowski. 2021. "Chimeric Antigen Receptor Design and Efficacy in Ovarian Cancer Treatment" International Journal of Molecular Sciences 22, no. 7: 3495. https://doi.org/10.3390/ijms22073495
APA StyleTerlikowska, K. M., Dobrzycka, B., & Terlikowski, S. J. (2021). Chimeric Antigen Receptor Design and Efficacy in Ovarian Cancer Treatment. International Journal of Molecular Sciences, 22(7), 3495. https://doi.org/10.3390/ijms22073495






