The Molecular Basis of Ubiquitin-Conjugating Enzymes (E2s) as a Potential Target for Cancer Therapy
Abstract
1. Introduction
2. The Position of E2 in UPP
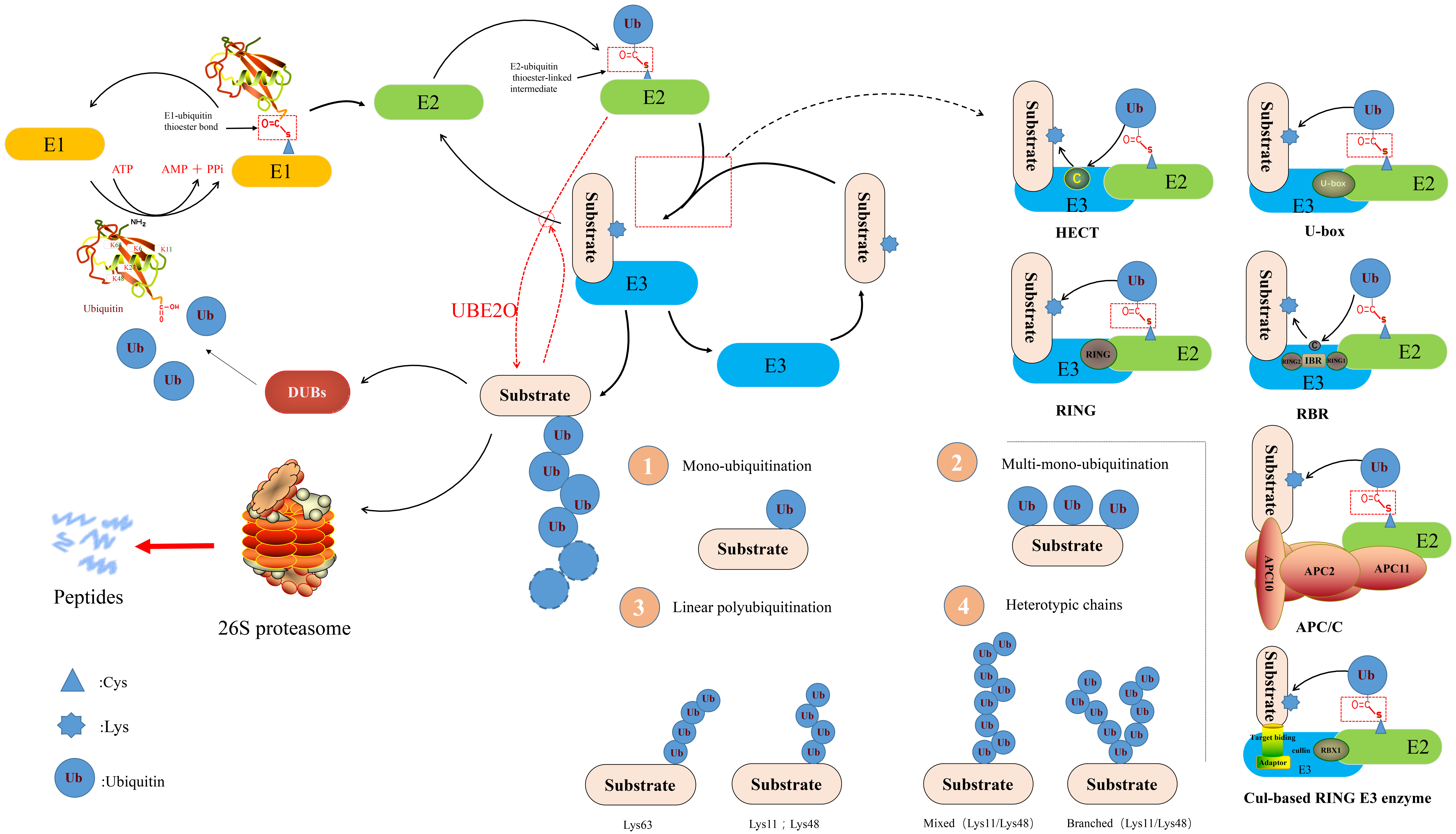
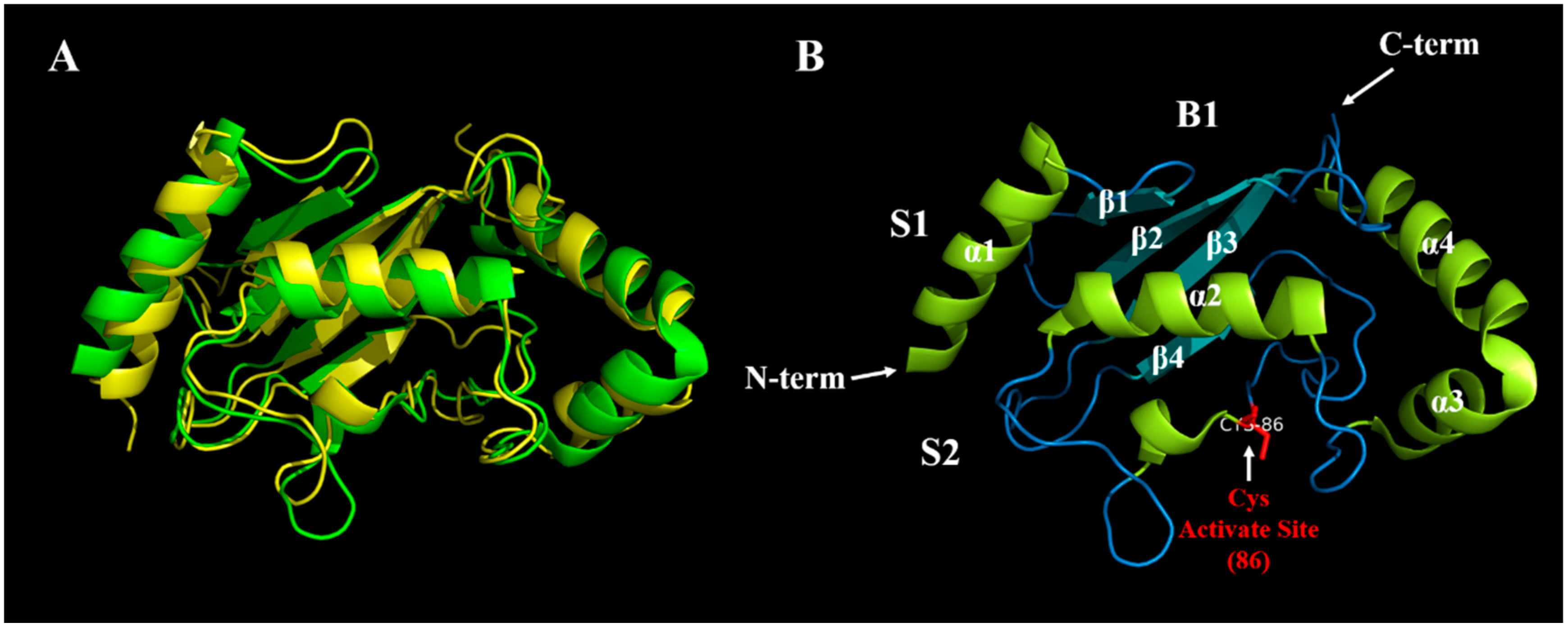
3. The Structure and Classification of E2
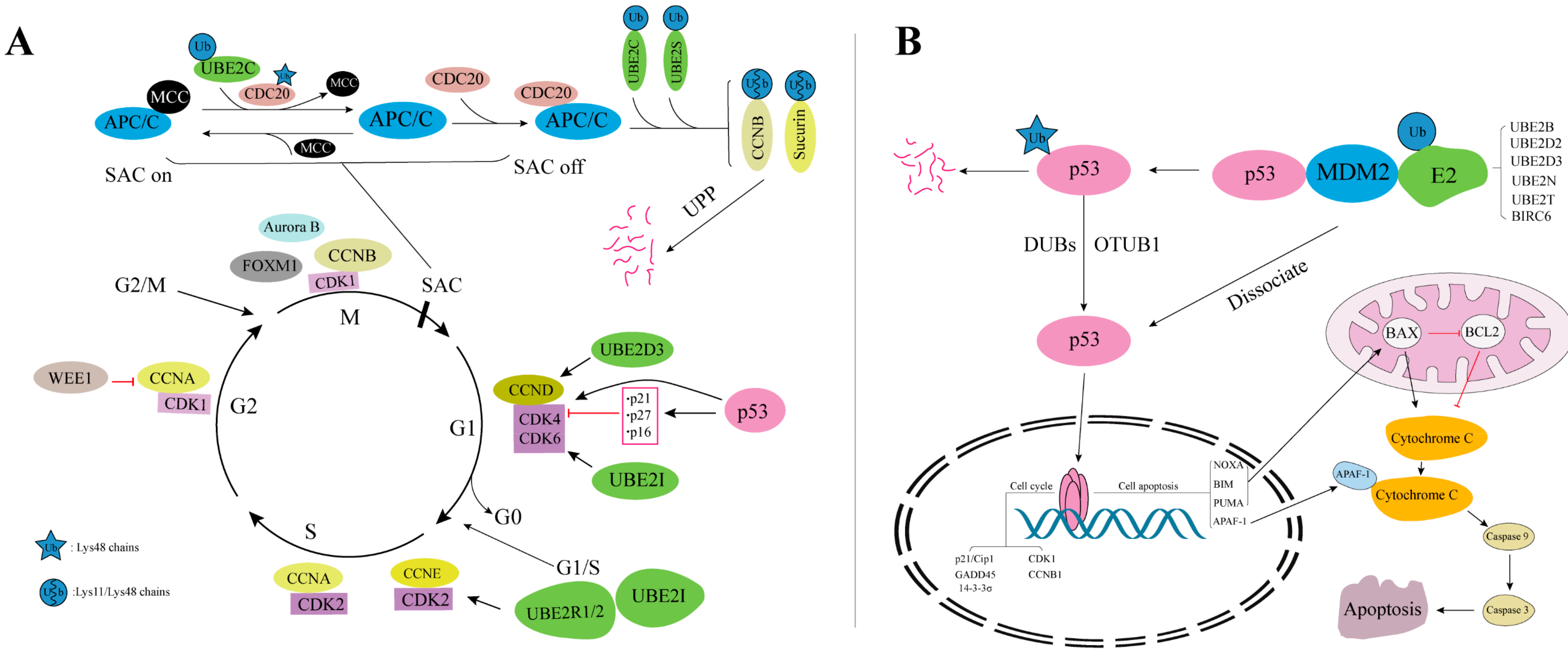
4. Biological Processes Involving E2s
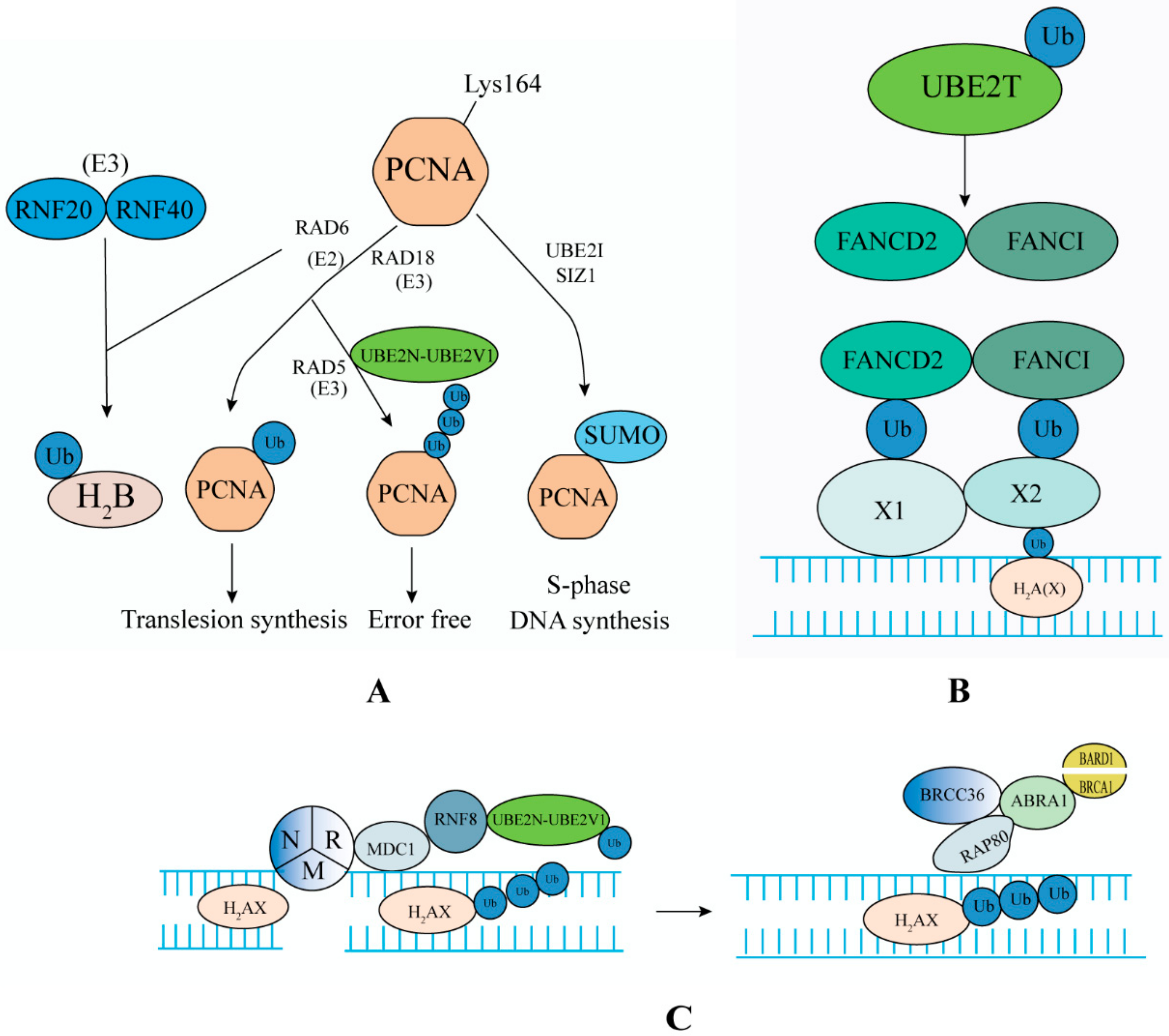
4.1. DNA Repair Pathway
4.2. Cell Cycle
4.3. Apoptosis
4.4. The Wnt/β-Catenin Pathway
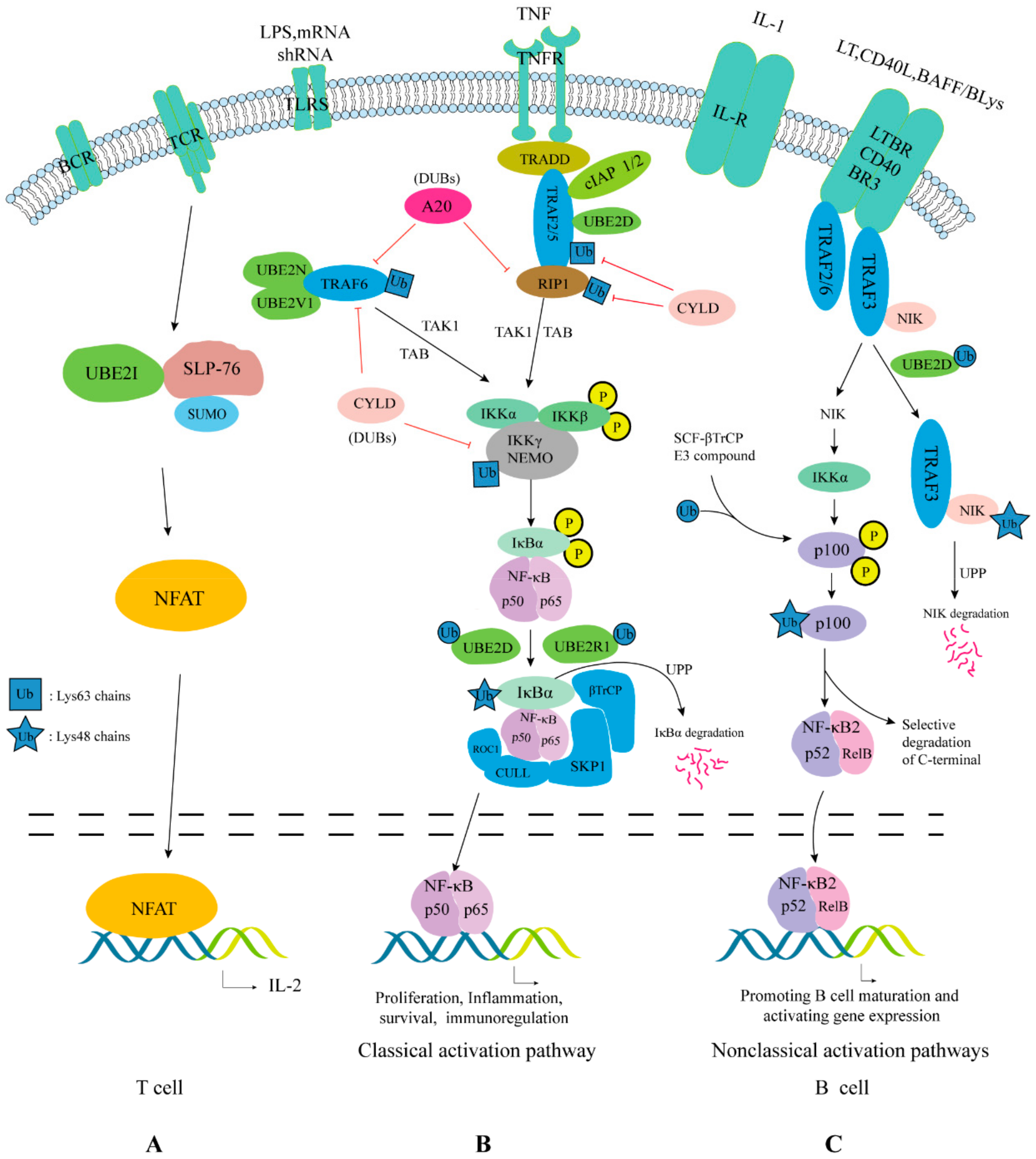
4.5. Nuclear Factor-Kappa B (NF-κB) Pathway
4.6. Other Cases
5. Inhibitors and miRNAs Targeting E2s
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | Acute Lymphoblastic Leukemia |
| BC | Breast cancer |
| BMAL1 | Brain and muscle arnt-like 1 |
| C- | C-terminal |
| CCN (A/B/D/E) | Cell cyclin (A/B/D/E) |
| CDK | Cyclin-dependent kinase |
| CLPs | Common lymphatic progenitor cells |
| CRLs | Cullin-RING ligases |
| DLBCL | Diffuse large B-cell lymphoma |
| DUB | Deubiquitinating enzyme |
| E2 | Ubiquitin-conjugating enzyme |
| EBV | Epstein-Barr virus |
| ERAD | Endoplasmic reticulum-associated degradation |
| FA | Fanconi |
| GBM | Glioblastoma |
| HCC | Hepatocellular carcinoma |
| HR | Homologous recombination |
| ICL | Interstrand cross-link |
| IKK | IκB kinase |
| LC | Lung cancer |
| LUBAC | Linear ubiquitin chain assembly complex |
| mES | Mouse embryonic stem cells |
| MM | Multiple myeloma |
| MMP | Matrix metalloprotinase |
| N- | N-terminal |
| NF-κB | Nuclear factor-kappa B |
| NIK | NF-κB inducing kinase |
| NSCLC | Non-small cell lung cancer |
| OSCC | Oral squamous cell carcinoma |
| PCa | Prostate cancer |
| PCNA | Proliferating cell nuclear antigen |
| polyUb | Polyubiquitin |
| RING | Really interesting new gene |
| RPs | Ribosomal proteins |
| SCF | SKP1/CUL1/F-box protein complex |
| TLS | Translesion synthesis |
| TNF-α | Tumor necrosis factor α |
| βTrCP | β-transducin repeat-containing protein |
| Ub | Ubiquitin |
| Ubl | Ub-like |
| APC/C | Anaphase-promoting complex/cyclosome |
| BCL2 | B-cell leukemia/lymphoma 2 |
| BRCA1 | Breast cancer 1 |
| CC | Colon cancer |
| CDC20 | Cell division cycle 20 |
| CDKN1A/p21/Cip1 | Cyclin dependent kinase inhibitor 1A |
| CRC | Colorectal cancer |
| DDP | Cisplatin |
| DSBR | DNA Double strand break repair |
| E1 | Ubiquitin activating enzyme |
| E3 | Ubiquitin ligase |
| EMT | Epithelial to mesenchyme transition |
| ERK | Extracellular regulated protein kinases |
| FOXM1 | Forkhead box protein M1 |
| GC | Gastric cancer |
| HNSCC | Head and neck squamous cell carcinoma |
| IAPs | Inhibitor of apoptosis proteins |
| ICT | Icaritin |
| IκB | Inhibitor of nuclear factor kappa B |
| LCSCs | Liver cancer stem cell |
| MDM2 | Mouse double minute 2 homolog |
| miRNAs | microRNAs |
| MMC | Mitomycin C |
| MXI1 | Max-interacting protein 1 |
| NB | Neuroblastoma |
| NHEJ | Non-homologous end joining |
| NPC | Nasopharyngeal carcinoma |
| OC | Ovarian cancer |
| p27Kip1 | Kinase inhibition protein p27 |
| PCGF2 | Polycomb group ring finger 2 |
| PML-RARA | Promyelocytic leukemia (PML) and the retinoic acid receptor-α (RARA) |
| RC | Renal carcinoma |
| RIP1 | Receptor-interacting protein 1 |
| SAC | Spindle-assembly checkpoint |
| SUMO | Small ubiquitin-like modifier |
| TNBC | Triple negative breast cancer |
| TRAF | TNF receptor associated factor |
| TSCC | Tongue Squamous Cell Carcinoma |
| UBC | Ubiquitin-conjugating |
| UPP | Ubiquitin-Proteasome Pathway |
References
- Wilkinson, K.D. The discovery of ubiquitin-dependent proteolysis. Proc. Natl. Acad. Sci. USA 2005, 102, 15280–15282. [Google Scholar] [CrossRef] [PubMed]
- Hicke, L.; Dunn, R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 2003, 19, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, G.; Agriesti, F.; Mazzoccoli, C.; Tataranni, T.; Costantino, V.; Piccoli, C. Clogging the Ubiquitin-Proteasome Machinery with Marine Natural Products: Last Decade Update. Mar. Drugs 2018, 16, 467. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Rape, M. Building ubiquitin chains: E2 enzymes at work. Nat. Rev. Mol. Cell Biol. 2009, 10, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Zubia, E.; Narayan, M.; Yang, J.; Xu, G. Diverse roles of the E2/E3 hybrid enzyme UBE2O in the regulation of protein ubiquitination, cellular functions, and disease onset. FEBS J. 2019, 286, 2018–2034. [Google Scholar] [CrossRef]
- Xie, C.; Powell, C.; Yao, M.; Wu, J.; Dong, Q. Ubiquitin-conjugating enzyme E2C: A potential cancer biomarker. Int. J. Biochem. Cell Biol. 2014, 47, 113–117. [Google Scholar] [CrossRef]
- Wang, R.; Song, Y.; Liu, X.; Wang, Q.; Wang, Y.; Li, L.; Kang, C.; Zhang, Q. UBE2C induces EMT through Wnt/β-catenin and PI3K/Akt signaling pathways by regulating phosphorylation levels of Aurora-A. Int. J. Oncol. 2017, 50, 1116–1126. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Zhao, G.; Ke, B.; Ma, G.; Liu, G.L.; Liang, H.; Liu, L.R.; Hao, X.S. Overexpression of UBE2C correlates with poor prognosis in gastric cancer patients. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1665–1671. [Google Scholar] [CrossRef]
- Yang, M.; Qu, Y.; Shi, R.; Wu, X.; Su, C.; Hu, Z.; Chang, Q.; Liu, S.; Pan, G.; Lei, M.; et al. Ubiquitin-conjugating enzyme UbcH10 promotes gastric cancer growth and is a potential biomarker for gastric cancer. Oncol. Rep. 2016, 36, 779–786. [Google Scholar] [CrossRef]
- Van Ree, J.H.; Jeganathan, K.B.; Malureanu, L.; van Deursen, J.M. Overexpression of the E2 ubiquitin-conjugating enzyme UbcH10 causes chromosome missegregation and tumor formation. J. Cell Biol. 2010, 188, 83–100. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Font-Burgada, J.; Palmer, T.; Hamil, A.S.; Biswas, S.K.; Poidinger, M.; Borcherding, N.; Xie, Q.; Ellies, L.G.; et al. Ubiquitin-conjugating enzyme Ubc13 controls breast cancer metastasis through a TAK1-p38 MAP kinase cascade. Proc. Natl. Acad. Sci. USA 2014, 111, 13870–13875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Linder, S.; Bazzaro, M. Drug Development Targeting the Ubiquitin-Proteasome System (UPS) for the Treatment of Human Cancers. Cancers 2020, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tang, X.; Qi, X.; Fu, X.; Ghimire, S.; Ma, R.; Li, S.; Zhang, N.; Si, H. The Ubiquitin Conjugating Enzyme: An Important Ubiquitin Transfer Platform in Ubiquitin-Proteasome System. Int. J. Mol. Sci 2020, 21, 2894. [Google Scholar] [CrossRef]
- Xu, F.Q.; Xue, H.W. The ubiquitin-proteasome system in plant responses to environments. Plant. Cell Environ. 2019, 42, 2931–2944. [Google Scholar] [CrossRef]
- Sadanandom, A.; Bailey, M.; Ewan, R.; Lee, J.; Nelis, S. The ubiquitin-proteasome system: Central modifier of plant signalling. New Phytol. 2012, 196, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.; Tahiliani, P.; Kumar, A.; Chandu, D. The ubiquitin-proteasome system. J. Biosci. 2006, 31, 137–155. [Google Scholar] [CrossRef] [PubMed]
- McGinty, R.K.; Henrici, R.C.; Tan, S. Crystal structure of the PRC1 ubiquitylation module bound to the nucleosome. Nature 2014, 514, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Pruneda, J.N.; Smith, F.D.; Daurie, A.; Swaney, D.L.; Villén, J.; Scott, J.D.; Stadnyk, A.W.; Le Trong, I.; Stenkamp, R.E.; Klevit, R.E.; et al. E2~Ub conjugates regulate the kinase activity of Shigella effector OspG during pathogenesis. EMBO J. 2014, 33, 437–449. [Google Scholar] [CrossRef]
- Spit, M.; Rieser, E.; Walczak, H. Linear ubiquitination at a glance. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef]
- Grice, G.L.; Lobb, I.T.; Weekes, M.P.; Gygi, S.P.; Antrobus, R.; Nathan, J.A. The Proteasome Distinguishes between Heterotypic and Homotypic Lysine-11-Linked Polyubiquitin Chains. Cell Rep. 2015, 12, 545–553. [Google Scholar] [CrossRef]
- Damgaard, R.B.; Walker, J.A.; Marco-Casanova, P.; Morgan, N.V.; Titheradge, H.L.; Elliott, P.R.; McHale, D.; Maher, E.R.; McKenzie, A.N.J.; Komander, D. The Deubiquitinase OTULIN Is an Essential Negative Regulator of Inflammation and Autoimmunity. Cell 2016, 166, 1215–1230.e1220. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.J.; Rape, M. Enhanced protein degradation by branched ubiquitin chains. Cell 2014, 157, 910–921. [Google Scholar] [CrossRef]
- Haglund, K.; Dikic, I. Ubiquitylation and cell signaling. EMBO J. 2005, 24, 3353–3359. [Google Scholar] [CrossRef]
- Kuang, P.; Tan, M.; Zhou, W.; Zhang, Q.; Sun, Y. SAG/RBX2 E3 ligase complexes with UBCH10 and UBE2S E2s to ubiquitylate β-TrCP1 via K11-linkage for degradation. Sci. Rep. 2016, 6, 37441. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Elmira, E.; Rohondia, S.; Wang, J.; Liu, J.; Dou, Q.P. A patent review of the ubiquitin ligase system: 2015–2018. Expert Opin. Ther. Pat. 2018, 28, 919–937. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, S.J.; Timmers, H.T. The family of ubiquitin-conjugating enzymes (E2s): Deciding between life and death of proteins. FASEB J. 2010, 24, 981–993. [Google Scholar] [CrossRef]
- Stewart, M.D.; Ritterhoff, T.; Klevit, R.E.; Brzovic, P.S. E2 enzymes: More than just middle men. Cell Res. 2016, 26, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Sancho, E.; Vilá, M.R.; Sánchez-Pulido, L.; Lozano, J.J.; Paciucci, R.; Nadal, M.; Fox, M.; Harvey, C.; Bercovich, B.; Loukili, N.; et al. Role of UEV-1, an inactive variant of the E2 ubiquitin-conjugating enzymes, in in vitro differentiation and cell cycle behavior of HT-29-M6 intestinal mucosecretory cells. Mol. Cell Biol. 1998, 18, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, D.M.; Stoll, K.E.; Klevit, R.E. E2s: Structurally economical and functionally replete. Biochem. J. 2011, 433, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Hormaechea-Agulla, D.; Kim, Y.; Song, M.S.; Song, S.J. New Insights into the Role of E2s in the Pathogenesis of Diseases: Lessons Learned from UBE2O. Mol. Cells 2018, 41, 168–178. [Google Scholar] [CrossRef]
- Garg, P.; Ceccarelli, D.F.; Keszei, A.F.A.; Kurinov, I.; Sicheri, F.; Sidhu, S.S. Structural and Functional Analysis of Ubiquitin-based Inhibitors That Target the Backsides of E2 Enzymes. J. Mol. Biol. 2020, 432, 952–966. [Google Scholar] [CrossRef]
- Cappadocia, L.; Lima, C.D. Ubiquitin-like Protein Conjugation: Structures, Chemistry, and Mechanism. Chem. Rev. 2018, 118, 889–918. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Okoye, I.; Chaleshtari, M.G.; Hazhirkarzar, B.; Mohamadnejad, J.; Azizi, G.; Hojjat-Farsangi, M.; Mohammadi, H.; Shotorbani, S.S.; Jadidi-Niaragh, F. E2 ubiquitin-conjugating enzymes in cancer: Implications for immunotherapeutic interventions. Clin. Chim. Acta 2019, 498, 126–134. [Google Scholar] [CrossRef]
- Magistroni, V.; Mauri, M.; D’Aliberti, D.; Mezzatesta, C.; Crespiatico, I.; Nava, M.; Fontana, D.; Sharma, N.; Parker, W.; Schreiber, A.; et al. De novo UBE2A mutations are recurrently acquired during chronic myeloid leukemia progression and interfere with myeloid differentiation pathways. Haematologica 2019, 104, 1789–1797. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.J.; Hou, J.; Wang, Y.Y.; Tang, W.Q.; Shen, X.Z.; Tu, R.Q. Expression and clinical significance of BIRC6 in human epithelial ovarian cancer. Tumour Biol. 2014, 35, 4891–4896. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, J.; Pan, B.; Ma, G.; Liu, L. UBE2C overexpression in melanoma and its essential role in G2/M transition. J. Cancer 2019, 10, 2176–2184. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Y.; Qiao, S.; Li, X.; Li, Q.; Zhao, J.; Hu, J.; Wei, Z.; Shan, A.; Sun, X.; et al. Identification of the potential therapeutic target gene UBE2C in human hepatocellular carcinoma: An investigation based on GEO and TCGA databases. Oncol. Lett. 2019, 17, 5409–5418. [Google Scholar] [CrossRef]
- Jin, Z.; Zhao, X.; Cui, L.; Xu, X.; Zhao, Y.; Younai, F.; Messadi, D.; Hu, S. UBE2C promotes the progression of head and neck squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2020, 523, 389–397. [Google Scholar] [CrossRef]
- Cacciola, N.A.; Calabrese, C.; Malapelle, U.; Pellino, G.; De Stefano, A.; Sepe, R.; Sgariglia, R.; Quintavalle, C.; Federico, A.; Bianco, A.; et al. UbcH10 expression can predict prognosis and sensitivity to the antineoplastic treatment for colorectal cancer patients. Mol. Carcinog. 2016, 55, 793–807. [Google Scholar] [CrossRef]
- Ma, R.; Kang, X.; Zhang, G.; Fang, F.; Du, Y.; Lv, H. High expression of UBE2C is associated with the aggressive progression and poor outcome of malignant glioma. Oncol. Lett. 2016, 11, 2300–2304. [Google Scholar] [CrossRef]
- Liu, P.F.; Chen, C.F.; Shu, C.W.; Chang, H.M.; Lee, C.H.; Liou, H.H.; Ger, L.P.; Chen, C.L.; Kang, B.H. UBE2C is a Potential Biomarker for Tumorigenesis and Prognosis in Tongue Squamous Cell Carcinoma. Diagnostics 2020, 10, 674. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, P.; Wang, J.; Gong, T.; Zhang, F.; Ma, J.; Han, N. Ubiquitin-conjugating enzyme E2C regulates apoptosis-dependent tumor progression of non-small cell lung cancer via ERK pathway. Med. Oncol. 2015, 32, 149. [Google Scholar] [CrossRef]
- Li, J.; Zhi, X.; Shen, X.; Chen, C.; Yuan, L.; Dong, X.; Zhu, C.; Yao, L.; Chen, M. Depletion of UBE2C reduces ovarian cancer malignancy and reverses cisplatin resistance via downregulating CDK1. Biochem. Biophys. Res. Commun. 2020, 523, 434–440. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tokumoto, M.; Fujiwara, Y.; Hasegawa, T.; Seko, Y.; Shimada, A.; Satoh, M. Accumulation of p53 via down-regulation of UBE2D family genes is a critical pathway for cadmium-induced renal toxicity. Sci. Rep. 2016, 6, 21968. [Google Scholar] [CrossRef]
- Guan, G.G.; Wang, W.B.; Lei, B.X.; Wang, Q.L.; Wu, L.; Fu, Z.M.; Zhou, F.X.; Zhou, Y.F. UBE2D3 is a positive prognostic factor and is negatively correlated with hTERT expression in esophageal cancer. Oncol. Lett. 2015, 9, 1567–1574. [Google Scholar] [CrossRef]
- Luo, H.; Qin, Y.; Reu, F.; Ye, S.; Dai, Y.; Huang, J.; Wang, F.; Zhang, D.; Pan, L.; Zhu, H.; et al. Microarray-based analysis and clinical validation identify ubiquitin-conjugating enzyme E2E1 (UBE2E1) as a prognostic factor in acute myeloid leukemia. J. Hematol. Oncol. 2016, 9, 125. [Google Scholar] [CrossRef]
- Desai, S.D.; Reed, R.E.; Burks, J.; Wood, L.M.; Pullikuth, A.K.; Haas, A.L.; Liu, L.F.; Breslin, J.W.; Meiners, S.; Sankar, S. ISG15 disrupts cytoskeletal architecture and promotes motility in human breast cancer cells. Exp. Biol. Med. 2012, 237, 38–49. [Google Scholar] [CrossRef]
- Plafker, K.S.; Zyla, K.; Berry, W.; Plafker, S.M. Loss of the ubiquitin conjugating enzyme UBE2E3 induces cellular senescence. Redox Biol. 2018, 17, 411–422. [Google Scholar] [CrossRef]
- Debonneville, C.; Staub, O. Participation of the ubiquitin-conjugating enzyme UBE2E3 in Nedd4-2-dependent regulation of the epithelial Na+ channel. Mol. Cell Biol. 2004, 24, 2397–2409. [Google Scholar] [CrossRef]
- Liu, R.; Cheng, Q.; Song, X.; Wang, H.; Wang, X.; Wang, L.; Zhu, B.; Song, L. A vital ubiquitin-conjugating enzyme CgUbe2g1 participated in regulation of immune response of Pacific oyster Crassostrea gigas. Dev. Comp. Immunol. 2019, 91, 132–142. [Google Scholar] [CrossRef]
- Lu, G.; Weng, S.; Matyskiela, M.; Zheng, X.; Fang, W.; Wood, S.; Surka, C.; Mizukoshi, R.; Lu, C.C.; Mendy, D.; et al. UBE2G1 governs the destruction of cereblon neomorphic substrates. eLife 2018, 7. [Google Scholar] [CrossRef]
- Van de Weijer, M.L.; Schuren, A.B.C.; van den Boomen, D.J.H.; Mulder, A.; Claas, F.H.J.; Lehner, P.J.; Lebbink, R.J.; Wiertz, E. Multiple E2 ubiquitin-conjugating enzymes regulate human cytomegalovirus US2-mediated immunoreceptor downregulation. J. Cell Sci. 2017, 130, 2883–2892. [Google Scholar] [CrossRef]
- Zhao, X.; Yongchun, Z.; Qian, H.; Sanhui, G.; Jie, L.; Hong, Y.; Yanfei, Z.; Guizhen, W.; Yunchao, H.; Guangbiao, Z. Identification of a potential tumor suppressor gene, UBL3, in non-small cell lung cancer. Cancer Biol. Med. 2020, 17, 76–87. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, H. Identification of prognosis biomarkers of prostatic cancer in a cohort of 498 patients from TCGA. Curr. Probl. Cancer 2019, 43, 100503. [Google Scholar] [CrossRef]
- Vourc’h, P.; Martin, I.; Bonnet-Brilhault, F.; Marouillat, S.; Barthélémy, C.; Pierre Müh, J.; Andres, C. Mutation screening and association study of the UBE2H gene on chromosome 7q32 in autistic disorder. Psychiatry Genet. 2003, 13, 221–225. [Google Scholar] [CrossRef]
- Li, Y.P.; Lecker, S.H.; Chen, Y.; Waddell, I.D.; Goldberg, A.L.; Reid, M.B. TNF-alpha increases ubiquitin-conjugating activity in skeletal muscle by up-regulating UbcH2/E220k. FASEB J. 2003, 17, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Deng, L.; Lu, X.; Pan, W.; Wu, Q.; Dai, J. Ubiquitin-conjugating enzyme UBE2J1 negatively modulates interferon pathway and promotes RNA virus infection. Virol. J. 2018, 15, 132. [Google Scholar] [CrossRef]
- Palmer, C.J.; Galan-Caridad, J.M.; Weisberg, S.P.; Lei, L.; Esquilin, J.M.; Croft, G.F.; Wainwright, B.; Canoll, P.; Owens, D.M.; Reizis, B. Zfx facilitates tumorigenesis caused by activation of the Hedgehog pathway. Cancer Res. 2014, 74, 5914–5924. [Google Scholar] [CrossRef]
- Chen, S.; Tan, Y.; Deng, H.; Shen, Z.; Liu, Y.; Wu, P.; Tan, C.; Jiang, Y. UBE2J2 promotes hepatocellular carcinoma cell epithelial-mesenchymal transition and invasion in vitro. Oncotarget 2017, 8, 71736–71749. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.N.; Zhang, Z.Z.; Ren, J.H.; Zhang, J.; Zhou, Y.J.; Wai Wong, V.K.; Kwan Law, B.Y.; Cheng, S.T.; Zhou, H.Z.; Chen, W.X.; et al. Overexpression of ubiquitin-conjugating enzyme E2 L3 in hepatocellular carcinoma potentiates apoptosis evasion by inhibiting the GSK3β/p65 pathway. Cancer Lett. 2020, 481, 1–14. [Google Scholar] [CrossRef]
- Yi, S.A.; Kim, G.W.; Yoo, J.; Han, J.W.; Kwon, S.H. HP1γ Sensitizes Cervical Cancer Cells to Cisplatin through the Suppression of UBE2L3. Int. J. Mol. Sci. 2020, 21, 5976. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, J.; Yang, F.; Liu, H.; Qi, W. Ubiquitin conjugating enzyme E2 L3 promoted tumor growth of NSCLC through accelerating p27kip1 ubiquitination and degradation. Oncotarget 2017, 8, 84193–84203. [Google Scholar] [CrossRef]
- Whitcomb, E.A.; Tsai, Y.C.; Basappa, J.; Liu, K.; Le Feuvre, A.K.; Weissman, A.M.; Taylor, A. Stabilization of p27(Kip1)/CDKN1B by UBCH7/UBE2L3 catalyzed ubiquitinylation: A new paradigm in cell-cycle control. FASEB J. 2019, 33, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Li, S.; Wang, L.; Berman, M.A.; Dorf, M.E. The ubiquitin conjugating enzyme UBE2L3 regulates TNFα-induced linear ubiquitination. Cell Res. 2014, 24, 376–379. [Google Scholar] [CrossRef]
- Alpi, A.F.; Chaugule, V.; Walden, H. Mechanism and disease association of E2-conjugating enzymes: Lessons from UBE2T and UBE2L3. Biochem. J. 2016, 473, 3401–3419. [Google Scholar] [CrossRef] [PubMed]
- Hodge, C.D.; Edwards, R.A.; Markin, C.J.; McDonald, D.; Pulvino, M.; Huen, M.S.; Zhao, J.; Spyracopoulos, L.; Hendzel, M.J.; Glover, J.N. Covalent Inhibition of Ubc13 Affects Ubiquitin Signaling and Reveals Active Site Elements Important for Targeting. ACS Chem. Biol. 2015, 10, 1718–1728. [Google Scholar] [CrossRef]
- Song, T.T.; Xu, F.; Wang, W. Inhibiting ubiquitin conjugating enzyme E2 N by microRNA-590-3p reduced cell growth of cervical carcinoma. Kaohsiung J. Med. Sci. 2020, 36, 501–507. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, Q.; Wang, Y.; Wang, H.; He, L.; Jin, X.; Li, N. MicroRNA miR-147b promotes tumor growth via targeting UBE2N in hepatocellular carcinoma. Oncotarget 2017, 8, 114072–114080. [Google Scholar] [CrossRef]
- Dikshit, A.; Jin, Y.J.; Degan, S.; Hwang, J.; Foster, M.W.; Li, C.Y.; Zhang, J.Y. UBE2N Promotes Melanoma Growth via MEK/FRA1/SOX10 Signaling. Cancer Res. 2018, 78, 6462–6472. [Google Scholar] [CrossRef]
- Shen, T.; Cai, L.D.; Liu, Y.H.; Li, S.; Gan, W.J.; Li, X.M.; Wang, J.R.; Guo, P.D.; Zhou, Q.; Lu, X.X.; et al. Ube2v1-mediated ubiquitination and degradation of Sirt1 promotes metastasis of colorectal cancer by epigenetically suppressing autophagy. J. Hematol. Oncol. 2018, 11, 95. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Zhang, Y.; Duan, C.; Liu, Q.; Huang, Z.; Xu, Y.; Zhou, L.; Xu, G. Ubiquitin-conjugating enzyme UBE2O regulates cellular clock function by promoting the degradation of the transcription factor BMAL1. J. Biol. Chem. 2018, 293, 11296–11309. [Google Scholar] [CrossRef]
- Liu, X.; Ma, F.; Liu, C.; Zhu, K.; Li, W.; Xu, Y.; Li, G.; Niu, Z.; Liu, J.; Chen, D.; et al. UBE2O promotes the proliferation, EMT and stemness properties of breast cancer cells through the UBE2O/AMPKα2/mTORC1-MYC positive feedback loop. Cell Death Dis. 2020, 11, 10. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, S.; Liu, C.; Li, G.; Lu, S.; Wang, Y.; Zhang, X.; Huang, D.; Qiu, Y.; Liu, Y. UBE2O Promotes Progression and Epithelial-Mesenchymal Transition in Head and Neck Squamous Cell Carcinoma. OncoTargets Ther. 2020, 13, 6191–6202. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Lu, Y.; Zhao, Y.; Meng, R.; Zhang, S.; Dong, X.; Xu, S.; Wu, G. UBE2O targets Mxi1 for ubiquitination and degradation to promote lung cancer progression and radioresistance. Cell Death Differ. 2021, 28, 671–684. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Prado, M.A.; Schmidt, P.J.; Sendamarai, A.K.; Wilson-Grady, J.T.; Min, M.; Campagna, D.R.; Tian, G.; Shi, Y.; Dederer, V.; et al. UBE2O remodels the proteome during terminal erythroid differentiation. Science 2017, 357. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Bauer, A.; Zhang, L.; Selinger, D.W.; Lu, C.X.; Ten Dijke, P. Fine-tuning BMP7 signalling in adipogenesis by UBE2O/E2-230K-mediated monoubiquitination of SMAD6. EMBO J. 2013, 32, 996–1007. [Google Scholar] [CrossRef]
- Shafiee, S.M.; Rasti, M.; Seghatoleslam, A.; Azimi, T.; Owji, A.A. UBE2Q1 in a Human Breast Carcinoma Cell Line: Overexpression and Interaction with p53. Asian Pac. J. Cancer Prev. 2015, 16, 3723–3727. [Google Scholar] [CrossRef]
- Seghatoleslam, A.; Bozorg-Ghalati, F.; Monabati, A.; Nikseresht, M.; Owji, A.A. UBE2Q1, as a Down Regulated Gene in Pediatric Acute Lymphoblastic Leukemia. Int. J. Mol. Cell Med. 2014, 3, 95–101. [Google Scholar]
- Shafiee, S.M.; Seghatoleslam, A.; Nikseresht, M.; Hosseini, S.V.; Alizadeh-Naeeni, M.; Safaei, A.; Owji, A.A. UBE2Q1 expression in human colorectal tumors and cell lines. Mol. Biol. Rep. 2013, 40, 7045–7051. [Google Scholar] [CrossRef]
- Kravic, B.; Behrends, C.; Meyer, H. Regulation of lysosome integrity and lysophagy by the ubiquitin-conjugating enzyme UBE2QL1. Autophagy 2020, 16, 179–180. [Google Scholar] [CrossRef]
- Maeda, H.; Miyajima, N.; Kano, S.; Tsukiyama, T.; Okumura, F.; Fukuda, S.; Hatakeyama, S. Ubiquitin-conjugating enzyme UBE2Q2 suppresses cell proliferation and is down-regulated in recurrent head and neck cancer. Mol. Cancer Res. 2009, 7, 1553–1562. [Google Scholar] [CrossRef]
- Shafiee, S.M.; Seghatoleslam, A.; Nikseresht, M.; Hosseini, S.V.; Alizadeh-Naeeni, M.; Safaei, A.; Owji, A.A. Expression Status of UBE2Q2 in Colorectal Primary Tumors and Cell Lines. Iran. J. Med. Sci. 2014, 39, 196–202. [Google Scholar]
- Williams, K.M.; Qie, S.; Atkison, J.H.; Salazar-Arango, S.; Alan Diehl, J.; Olsen, S.K. Structural insights into E1 recognition and the ubiquitin-conjugating activity of the E2 enzyme Cdc34. Nat. Commun. 2019, 10, 3296. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Hu, Z.; Li, Q.; Yang, L.; Xu, G. The Catalytically Inactive Mutation of the Ubiquitin-Conjugating Enzyme CDC34 Affects its Stability and Cell Proliferation. Protein J. 2018, 37, 132–143. [Google Scholar] [CrossRef]
- Zhao, X.C.; Wang, G.Z.; Wen, Z.S.; Zhou, Y.C.; Hu, Q.; Zhang, B.; Qu, L.W.; Gao, S.H.; Liu, J.; Ma, L.; et al. Systematic identification of CDC34 that functions to stabilize EGFR and promote lung carcinogenesis. eBioMedicine 2020, 53, 102689. [Google Scholar] [CrossRef]
- Eliseeva, E.; Pati, D.; Diccinanni, M.B.; Yu, A.L.; Mohsin, S.K.; Margolin, J.F.; Plon, S.E. Expression and localization of the CDC34 ubiquitin-conjugating enzyme in pediatric acute lymphoblastic leukemia. Cell Growth Differ. 2001, 12, 427–433. [Google Scholar]
- Yoshimura, S.; Kasamatsu, A.; Nakashima, D.; Iyoda, M.; Kasama, H.; Saito, T.; Takahara, T.; Endo-Sakamoto, Y.; Shiiba, M.; Tanzawa, H.; et al. UBE2S associated with OSCC proliferation by promotion of P21 degradation via the ubiquitin-proteasome system. Biochem. Biophys. Res. Commun. 2017, 485, 820–825. [Google Scholar] [CrossRef]
- Ayesha, A.K.; Hyodo, T.; Asano, E.; Sato, N.; Mansour, M.A.; Ito, S.; Hamaguchi, M.; Senga, T. UBE2S is associated with malignant characteristics of breast cancer cells. Tumour Biol. 2016, 37, 763–772. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Hou, J.; Qian, X.; Zhang, H.; Zhang, Z.; Li, M.; Wang, R.; Liao, K.; Wang, Y.; et al. Ube2s regulates Sox2 stability and mouse ES cell maintenance. Cell Death Differ. 2016, 23, 393–404. [Google Scholar] [CrossRef]
- Kelsall, I.R.; Langenick, J.; MacKay, C.; Patel, K.J.; Alpi, A.F. The Fanconi anaemia components UBE2T and FANCM are functionally linked to nucleotide excision repair. PLoS ONE 2012, 7, e36970. [Google Scholar] [CrossRef]
- Hira, A.; Yoshida, K.; Sato, K.; Okuno, Y.; Shiraishi, Y.; Chiba, K.; Tanaka, H.; Miyano, S.; Shimamoto, A.; Tahara, H.; et al. Mutations in the gene encoding the E2 conjugating enzyme UBE2T cause Fanconi anemia. Am. J. Hum. Genet. 2015, 96, 1001–1007. [Google Scholar] [CrossRef]
- Yu, H.; Xiang, P.; Pan, Q.; Huang, Y.; Xie, N.; Zhu, W. Ubiquitin-Conjugating Enzyme E2T is an Independent Prognostic Factor and Promotes Gastric Cancer Progression. Tumour Biol. 2016, 37, 11723–11732. [Google Scholar] [CrossRef]
- Wang, Y.; Leng, H.; Chen, H.; Wang, L.; Jiang, N.; Huo, X.; Yu, B. Knockdown of UBE2T Inhibits Osteosarcoma Cell Proliferation, Migration, and Invasion by Suppressing the PI3K/Akt Signaling Pathway. Oncol Res. 2016, 24, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Kwon, Y.; Wang, Y.; Mao, J.H.; Wei, G. Elevated expression of UBE2T exhibits oncogenic properties in human prostate cancer. Oncotarget 2015, 6, 25226–25239. [Google Scholar] [CrossRef]
- Wu, Z.H.; Zhang, Y.J.; Sun, H.Y. High ubiquitin conjugating enzyme E2 T mRNA expression and its prognostic significance in lung adenocarcinoma: A study based on the TCGA database. Medicine 2020, 99, e18543. [Google Scholar] [CrossRef]
- Perez-Peña, J.; Corrales-Sánchez, V.; Amir, E.; Pandiella, A.; Ocana, A. Ubiquitin-conjugating enzyme E2T (UBE2T) and denticleless protein homolog (DTL) are linked to poor outcome in breast and lung cancers. Sci. Rep. 2017, 7, 17530. [Google Scholar] [CrossRef]
- Liu, L.L.; Zhu, J.M.; Yu, X.N.; Zhu, H.R.; Shi, X.; Bilegsaikhan, E.; Guo, H.Y.; Wu, J.; Shen, X.Z. UBE2T promotes proliferation via G2/M checkpoint in hepatocellular carcinoma. Cancer Manag. Res. 2019, 11, 8359–8370. [Google Scholar] [CrossRef]
- Yu, H.; Wang, H.; Dong, W.; Cao, Z.Y.; Li, R.; Yang, C.; Cong, W.M.; Dong, H.; Jin, G.Z. The diagnostic and prognostic value of UBE2T in intrahepatic cholangiocarcinoma. PeerJ 2020, 8, e8454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, T.; Niu, X.; Chen, L.; Ge, C. Identification of UBE2T as an independent prognostic biomarker for gallbladder cancer. Oncol. Lett. 2020, 20, 44. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Yang, Z.; Liu, X.; Yang, P.; Wang, J.; Hu, K.; He, X.; Zhang, X.; Jing, H. High expression of UBE2T predicts poor prognosis and survival in multiple myeloma. Cancer Gene Ther. 2019, 26, 347–355. [Google Scholar] [CrossRef]
- Zou, R.; Xu, H.; Li, F.; Wang, S.; Zhu, L. Increased Expression of UBE2T Predicting Poor Survival of Epithelial Ovarian Cancer: Based on Comprehensive Analysis of UBE2s, Clinical Samples, and the GEO Database. DNA Cell Biol. 2021, 40, 36–60. [Google Scholar] [CrossRef]
- Liu, L.P.; Yang, M.; Peng, Q.Z.; Li, M.Y.; Zhang, Y.S.; Guo, Y.H.; Chen, Y.; Bao, S.Y. UBE2T promotes hepatocellular carcinoma cell growth via ubiquitination of p53. Biochem. Biophys. Res. Commun. 2017, 493, 20–27. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuang, Y.; Zhang, Y.; Yang, X.; Zhang, H.; Wang, G.; Yin, W.; Wang, R.; Zhang, Z.; Xiao, W. Uev1A facilitates osteosarcoma differentiation by promoting Smurf1-mediated Smad1 ubiquitination and degradation. Cell Death Dis. 2017, 8, e2974. [Google Scholar] [CrossRef]
- Lei, B.; Xie, L.; Zhang, S.; Lv, D.; Shu, F.; Deng, Y. UBE2W down-regulation promotes cell apoptosis and correlates with hypospermatogenesis. Andrologia 2020, 52, e13474. [Google Scholar] [CrossRef]
- Ikeda, F. The anti-apoptotic ubiquitin conjugating enzyme BIRC6/BRUCE regulates autophagosome-lysosome fusion. Autophagy 2018, 14, 1283–1284. [Google Scholar] [CrossRef]
- Tang, W.; Xue, R.; Weng, S.; Wu, J.; Fang, Y.; Wang, Y.; Ji, L.; Hu, T.; Liu, T.; Huang, X.; et al. BIRC6 promotes hepatocellular carcinogenesis: Interaction of BIRC6 with p53 facilitating p53 degradation. Int. J. Cancer 2015, 136, E475–E487. [Google Scholar] [CrossRef]
- Yang, G.; Wang, X.; Liu, B.; Lu, Z.; Xu, Z.; Xiu, P.; Liu, Z.; Li, J. circ-BIRC6, a circular RNA, promotes hepatocellular carcinoma progression by targeting the miR-3918/Bcl2 axis. Cell Cycle 2019, 18, 976–989. [Google Scholar] [CrossRef]
- Lamers, F.; Schild, L.; Koster, J.; Speleman, F.; Øra, I.; Westerhout, E.M.; van Sluis, P.; Versteeg, R.; Caron, H.N.; Molenaar, J.J. Identification of BIRC6 as a novel intervention target for neuroblastoma therapy. BMC Cancer 2012, 12, 285. [Google Scholar] [CrossRef]
- Zhuang, W.; Zhang, C.; Hao, F.; Sun, X. Baculoviral IAP Repeat Containing 6 (BIRC6) Is a Predictor of Prognosis in Prostate Cancer. Med. Sci. Monit. 2018, 24, 839–845. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, J.; Li, H.; Xu, M.; Chen, Z.J.; Wei, W.; Pan, Z.; Sun, Y. Neddylation E2 UBE2F Promotes the Survival of Lung Cancer Cells by Activating CRL5 to Degrade NOXA via the K11 Linkage. Clin. Cancer Res. 2017, 23, 1104–1116. [Google Scholar] [CrossRef]
- Wang, A.; Ding, X.; Demarque, M.; Liu, X.; Pan, D.; Xin, H.; Zhong, B.; Wang, X.; Dejean, A.; Jin, W.; et al. Ubc9 Is Required for Positive Selection and Late-Stage Maturation of Thymocytes. J. Immunol. 2017, 198, 3461–3470. [Google Scholar] [CrossRef]
- Wang, F.; Sun, F.; Luo, J.; Yue, T.; Chen, L.; Zhou, H.; Zhang, J.; Yang, C.; Luo, X.; Zhou, Q.; et al. Loss of ubiquitin-conjugating enzyme E2 (Ubc9) in macrophages exacerbates multiple low-dose streptozotocin-induced diabetes by attenuating M2 macrophage polarization. Cell Death Dis. 2019, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Varadaraj, A.; Mattoscio, D.; Chiocca, S. SUMO Ubc9 enzyme as a viral target. IUBMB Life 2014, 66, 27–33. [Google Scholar] [CrossRef]
- Fang, S.; Qiu, J.; Wu, Z.; Bai, T.; Guo, W. Down-regulation of UBC9 increases the sensitivity of hepatocellular carcinoma to doxorubicin. Oncotarget 2017, 8, 49783–49795. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Wu, P.; Nai, C.; Luo, Y.; Hu, F.; Gao, W.; Zhai, N.; Xu, T.; Li, D. Effect of MicroRNA-30e on the Behavior of Vascular Smooth Muscle Cells via Targeting Ubiquitin-Conjugating Enzyme E2I. Circ. J. 2017, 81, 567–576. [Google Scholar] [CrossRef]
- McManus, F.P.; Bourdeau, V.; Acevedo, M.; Lopes-Paciencia, S.; Mignacca, L.; Lamoliatte, F.; Rojas Pino, J.W.; Ferbeyre, G.; Thibault, P. Quantitative SUMO proteomics reveals the modulation of several PML nuclear body associated proteins and an anti-senescence function of UBC9. Sci. Rep. 2018, 8, 7754. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, H.; Mo, Y.Y. Regulation of bcl-2 expression by Ubc9. Exp. Cell Res. 2006, 312, 1865–1875. [Google Scholar] [CrossRef]
- Jo, S.; Lee, Y.L.; Kim, S.; Lee, H.; Chung, H. PCGF2 negatively regulates arsenic trioxide-induced PML-RARA protein degradation via UBE2I inhibition in NB4 cells. Biochim. Biophys. Acta 2016, 1863, 1499–1509. [Google Scholar] [CrossRef]
- Edrees, M.A.H.; Luo, J.; Sun, F.; Wang, F.; He, L.; Yue, T.; Chen, L.; Zhang, J.; Zhou, H.; Yang, C.; et al. Ubc9 deficiency selectively impairs the functionality of common lymphoid progenitors (CLPs) during bone marrow hematopoiesis. Mol. Immunol. 2019, 114, 314–322. [Google Scholar] [CrossRef]
- Cukras, S.; Morffy, N.; Ohn, T.; Kee, Y. Inactivating UBE2M impacts the DNA damage response and genome integrity involving multiple cullin ligases. PLoS ONE 2014, 9, e101844. [Google Scholar] [CrossRef]
- Zhang, G.C.; Yu, X.N.; Sun, J.L.; Xiong, J.; Yang, Y.J.; Jiang, X.M.; Zhu, J.M. UBE2M promotes cell proliferation via the β-catenin/cyclin D1 signaling in hepatocellular carcinoma. Aging 2020, 12, 2373–2392. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Deng, Y.; Bi, R.; Guo, H.; Shu, C.; Shah, N.K.; Chang, J.; Liu, G.; Du, Y.; Wei, W.; et al. A first-in-class inhibitor, MLN4924 (pevonedistat), induces cell-cycle arrest, senescence, and apoptosis in human renal cell carcinoma by suppressing UBE2M-dependent neddylation modification. Cancer Chemother. Pharmacol. 2018, 81, 1083–1093. [Google Scholar] [CrossRef]
- Zhao, B.; Gao, C.; Shi, D.; Mao, J.; Zhao, J.; Guo, L.; Guo, J.; Jiao, Z. Knockdown of Nedd8-conjugating enzyme UBE2M suppresses the proliferation and induces the apoptosis of intrahepatic cholangiocarcinoma cells. Oncol. Rep. 2019, 42, 2670–2679. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, J.; Tan, M.; Li, H.; Li, H.; Wei, W.; Sun, Y. UBE2M Is a Stress-Inducible Dual E2 for Neddylation and Ubiquitylation that Promotes Targeted Degradation of UBE2F. Mol. Cell 2018, 70, 1008–1024.e1006. [Google Scholar] [CrossRef]
- Li, L.; Kang, J.; Zhang, W.; Cai, L.; Wang, S.; Liang, Y.; Jiang, Y.; Liu, X.; Zhang, Y.; Ruan, H.; et al. Validation of NEDD8-conjugating enzyme UBC12 as a new therapeutic target in lung cancer. eBioMedicine 2019, 45, 81–91. [Google Scholar] [CrossRef]
- Shi, X.; Wang, B.; Chen, X.; Zheng, Y.; Ding, Y.; Wang, C. Upregulation of ubiquitin-conjugating enzyme E2Z is associated with human hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2020, 523, 25–32. [Google Scholar] [CrossRef]
- Hofmann, K. Ubiquitin-binding domains and their role in the DNA damage response. DNA Repair 2009, 8, 544–556. [Google Scholar] [CrossRef]
- Akita, M.; Tak, Y.S.; Shimura, T.; Matsumoto, S.; Okuda-Shimizu, Y.; Shimizu, Y.; Nishi, R.; Saitoh, H.; Iwai, S.; Mori, T.; et al. SUMOylation of xeroderma pigmentosum group C protein regulates DNA damage recognition during nucleotide excision repair. Sci. Rep. 2015, 5, 10984. [Google Scholar] [CrossRef]
- Sun, Y.; Miller Jenkins, L.M.; Su, Y.P.; Nitiss, K.C.; Nitiss, J.L.; Pommier, Y. A conserved SUMO pathway repairs topoisomerase DNA-protein cross-links by engaging ubiquitin-mediated proteasomal degradation. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Kaiser, P.; Mansour, H.A.; Greeten, T.; Auer, B.; Schweiger, M.; Schneider, R. The human ubiquitin-conjugating enzyme UbcH1 is involved in the repair of UV-damaged, alkylated and cross-linked DNA. FEBS Lett. 1994, 350, 1–4. [Google Scholar] [CrossRef][Green Version]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef]
- Torres-Ramos, C.A.; Prakash, S.; Prakash, L. Requirement of RAD5 and MMS2 for postreplication repair of UV-damaged DNA in Saccharomyces cerevisiae. Mol. Cell Biol. 2002, 22, 2419–2426. [Google Scholar] [CrossRef]
- Hoege, C.; Pfander, B.; Moldovan, G.L.; Pyrowolakis, G.; Jentsch, S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 2002, 419, 135–141. [Google Scholar] [CrossRef]
- Lentucci, C.; Belkina, A.C.; Cederquist, C.T.; Chan, M.; Johnson, H.E.; Prasad, S.; Lopacinski, A.; Nikolajczyk, B.S.; Monti, S.; Snyder-Cappione, J.; et al. Inhibition of Ubc13-mediated Ubiquitination by GPS2 Regulates Multiple Stages of B Cell Development. J. Biol. Chem. 2017, 292, 2754–2772. [Google Scholar] [CrossRef]
- Burma, S.; Chen, B.P.; Chen, D.J. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair 2006, 5, 1042–1048. [Google Scholar] [CrossRef]
- Hu, L.; Li, X.; Liu, Q.; Xu, J.; Ge, H.; Wang, Z.; Wang, H.; Wang, Z.; Shi, C.; Xu, X.; et al. UBE2S, a novel substrate of Akt1, associates with Ku70 and regulates DNA repair and glioblastoma multiforme resistance to chemotherapy. Oncogene 2017, 36, 1145–1156. [Google Scholar] [CrossRef]
- An, H.; Yang, L.; Wang, C.; Gan, Z.; Gu, H.; Zhang, T.; Huang, X.; Liu, Y.; Li, Y.; Chang, S.J.; et al. Interactome Analysis Reveals a Novel Role for RAD6 in the Regulation of Proteasome Activity and Localization in Response to DNA Damage. Mol. Cell Biol. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Machida, Y.J.; Machida, Y.; Chen, Y.; Gurtan, A.M.; Kupfer, G.M.; D’Andrea, A.D.; Dutta, A. UBE2T is the E2 in the Fanconi anemia pathway and undergoes negative autoregulation. Mol. Cell 2006, 23, 589–596. [Google Scholar] [CrossRef]
- Nijman, S.M.; Huang, T.T.; Dirac, A.M.; Brummelkamp, T.R.; Kerkhoven, R.M.; D’Andrea, A.D.; Bernards, R. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol. Cell 2005, 17, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Mamrak, N.E.; Shimamura, A.; Howlett, N.G. Recent discoveries in the molecular pathogenesis of the inherited bone marrow failure syndrome Fanconi anemia. Blood Rev. 2017, 31, 93–99. [Google Scholar] [CrossRef]
- Rickman, K.A.; Lach, F.P.; Abhyankar, A.; Donovan, F.X.; Sanborn, E.M.; Kennedy, J.A.; Sougnez, C.; Gabriel, S.B.; Elemento, O.; Chandrasekharappa, S.C.; et al. Deficiency of UBE2T, the E2 Ubiquitin Ligase Necessary for FANCD2 and FANCI Ubiquitination, Causes FA-T Subtype of Fanconi Anemia. Cell Rep. 2015, 12, 35–41. [Google Scholar] [CrossRef]
- Lyakhovich, A.; Surralles, J. FANCD2 depletion sensitizes cancer cells repopulation ability in vitro. Cancer Lett. 2007, 256, 186–195. [Google Scholar] [CrossRef]
- Ramaekers, C.H.; van den Beucken, T.; Meng, A.; Kassam, S.; Thoms, J.; Bristow, R.G.; Wouters, B.G. Hypoxia disrupts the Fanconi anemia pathway and sensitizes cells to chemotherapy through regulation of UBE2T. Radiother. Oncol. 2011, 101, 190–197. [Google Scholar] [CrossRef]
- Alagpulinsa, D.A.; Kumar, S.; Talluri, S.; Nanjappa, P.; Buon, L.; Chakraborty, C.; Samur, M.K.; Szalat, R.; Shammas, M.A.; Munshi, N.C. Amplification and overexpression of E2 ubiquitin conjugase UBE2T promotes homologous recombination in multiple myeloma. Blood Adv. 2019, 3, 3968–3972. [Google Scholar] [CrossRef]
- Tarsounas, M.; Sung, P. The antitumorigenic roles of BRCA1-BARD1 in DNA repair and replication. Nat. Rev. Mol. Cell Biol. 2020, 21, 284–299. [Google Scholar] [CrossRef]
- Kothayer, H.; Spencer, S.M.; Tripathi, K.; Westwell, A.D.; Palle, K. Synthesis and in vitro anticancer evaluation of some 4,6-diamino-1,3,5-triazine-2-carbohydrazides as Rad6 ubiquitin conjugating enzyme inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 2030–2034. [Google Scholar] [CrossRef] [PubMed]
- Somasagara, R.R.; Spencer, S.M.; Tripathi, K.; Clark, D.W.; Mani, C.; Madeira da Silva, L.; Scalici, J.; Kothayer, H.; Westwell, A.D.; Rocconi, R.P.; et al. RAD6 promotes DNA repair and stem cell signaling in ovarian cancer and is a promising therapeutic target to prevent and treat acquired chemoresistance. Oncogene 2017, 36, 6680–6690. [Google Scholar] [CrossRef]
- Clark, D.W.; Mani, C.; Palle, K. RAD6 promotes chemoresistance in ovarian cancer. Mol. Cell. Oncol. 2018, 5, e1392403. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Chen, S.H.; Kuo, C.C.; Chang, J.Y. Ubiquitin-conjugating enzyme E2 B regulates the ubiquitination of O(6)-methylguanine-DNA methyltransferase and BCNU sensitivity in human nasopharyngeal carcinoma cells. Biochem. Pharmacol. 2018, 158, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, L.; Ke, S.; Wang, W.; Yang, L.; Gao, X.; Fang, H.; Yu, H.; Zhong, Y.; Xie, C.; et al. Downregulation of Ubiquitin-conjugating Enzyme UBE2D3 Promotes Telomere Maintenance and Radioresistance of Eca-109 Human Esophageal Carcinoma Cells. J. Cancer 2016, 7, 1152–1162. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, R.; Luo, X.; Zhang, W.D.; Qin, J.J. The E2 ubiquitin-conjugating enzyme UbcH5c: An emerging target in cancer and immune disorders. Drug Discov. Today 2020, 25, 1988–1997. [Google Scholar] [CrossRef]
- Lydeard, J.R.; Schulman, B.A.; Harper, J.W. Building and remodelling Cullin-RING E3 ubiquitin ligases. EMBO Rep. 2013, 14, 1050–1061. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, M.; Jang, K.; Shin, M.; Besser, A.; Xiao, X.; Zhao, D.; Wander, S.A.; Briegel, K.; Morey, L.; et al. p27 transcriptionally coregulates cJun to drive programs of tumor progression. Proc. Natl. Acad. Sci. USA 2019, 116, 7005–7014. [Google Scholar] [CrossRef]
- Mittal, M.K.; Singh, K.; Misra, S.; Chaudhuri, G. SLUG-induced elevation of D1 cyclin in breast cancer cells through the inhibition of its ubiquitination. J. Biol. Chem. 2011, 286, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Pierce, N.W.; Lee, J.E.; Liu, X.; Sweredoski, M.J.; Graham, R.L.; Larimore, E.A.; Rome, M.; Zheng, N.; Clurman, B.E.; Hess, S.; et al. Cand1 promotes assembly of new SCF complexes through dynamic exchange of F box proteins. Cell 2013, 153, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.; Acquaviva, C.; Matsusaka, T.; Koop, L.; Pines, J. UbcH10 has a rate-limiting role in G1 phase but might not act in the spindle checkpoint or as part of an autonomous oscillator. J. Cell Sci. 2008, 121, 2319–2326. [Google Scholar] [CrossRef]
- Cai, F.; Chen, P.; Chen, L.; Biskup, E.; Liu, Y.; Chen, P.C.; Chang, J.F.; Jiang, W.; Jing, Y.; Chen, Y.; et al. Human RAD6 promotes G1-S transition and cell proliferation through upregulation of cyclin D1 expression. PLoS ONE 2014, 9, e113727. [Google Scholar] [CrossRef]
- Eifler, K.; Vertegaal, A.C.O. SUMOylation-Mediated Regulation of Cell Cycle Progression and Cancer. Trends Biochem. Sci. 2015, 40, 779–793. [Google Scholar] [CrossRef]
- Bellail, A.C.; Olson, J.J.; Hao, C. SUMO1 modification stabilizes CDK6 protein and drives the cell cycle and glioblastoma progression. Nat. Commun. 2014, 5, 4234. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Boyer, T.G.; Yew, P.R. Phosphorylation of the human ubiquitin-conjugating enzyme, CDC34, by casein kinase. J. Biol. Chem. 2001, 276, 41049–41058. [Google Scholar] [CrossRef]
- Ciliberto, A.; Shah, J.V. A quantitative systems view of the spindle assembly checkpoint. EMBO J. 2009, 28, 2162–2173. [Google Scholar] [CrossRef]
- Wild, T.; Larsen, M.S.; Narita, T.; Schou, J.; Nilsson, J.; Choudhary, C. The Spindle Assembly Checkpoint Is Not Essential for Viability of Human Cells with Genetically Lowered APC/C Activity. Cell Rep. 2016, 14, 1829–1840. [Google Scholar] [CrossRef]
- Reddy, S.K.; Rape, M.; Margansky, W.A.; Kirschner, M.W. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature 2007, 446, 921–925. [Google Scholar] [CrossRef]
- Ben-Eliezer, I.; Pomerantz, Y.; Galiani, D.; Nevo, N.; Dekel, N. Appropriate expression of Ube2C and Ube2S controls the progression of the first meiotic division. FASEB J. 2015, 29, 4670–4681. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, H.; Cowell, J. Ubiquitin-conjugating enzyme UBE2C: Molecular biology, role in tumorigenesis, and potential as a biomarker. Tumour. Biol. 2012, 33, 723–730. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Ubiquitin- and ubiquitin-like proteins-conjugating enzymes (E2s) in breast cancer. Mol. Biol. Rep. 2013, 40, 2019–2034. [Google Scholar] [CrossRef]
- Bremm, A.; Komander, D. Emerging roles for Lys11-linked polyubiquitin in cellular regulation. Trends. Biochem. Sci. 2011, 36, 355–363. [Google Scholar] [CrossRef]
- Saville, M.K.; Sparks, A.; Xirodimas, D.P.; Wardrop, J.; Stevenson, L.F.; Bourdon, J.C.; Woods, Y.L.; Lane, D.P. Regulation of p53 by the ubiquitin-conjugating enzymes UbcH5B/C in vivo. J. Biol. Chem. 2004, 279, 42169–42181. [Google Scholar] [CrossRef]
- Wu, M.; Li, X.; Huang, W.; Chen, Y.; Wang, B.; Liu, X. Ubiquitin-conjugating enzyme E2T(UBE2T) promotes colorectal cancer progression by facilitating ubiquitination and degradation of p53. Clin. Res. Hepatol. Gastroenterol. 2020, 101493. [Google Scholar] [CrossRef]
- Taylor, W.R.; Stark, G.R. Regulation of the G2/M transition by p53. Oncogene 2001, 20, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; O’Neill, E.; Gordon-Weeks, A.; Mukherjee, S.; McKenna, W.G.; Muschel, R.J. Pancreatic ductal adenocarcinoma: From genetics to biology to radiobiology to oncoimmunology and all the way back to the clinic. Biochim. Biophys. Acta 2015, 1855, 61–82. [Google Scholar] [CrossRef]
- Pan, Y.H.; Yang, M.; Liu, L.P.; Wu, D.C.; Li, M.Y.; Su, S.G. UBE2S enhances the ubiquitination of p53 and exerts oncogenic activities in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2018, 503, 895–902. [Google Scholar] [CrossRef]
- Hong, N.H.; Tak, Y.J.; Rhim, H.; Kang, S. Hip2 ubiquitin-conjugating enzyme has a role in UV-induced G1/S arrest and re-entry. Genes Genom. 2019, 41, 159–166. [Google Scholar] [CrossRef]
- Bae, Y.; Jung, S.H.; Kim, G.Y.; Rhim, H.; Kang, S. Hip2 ubiquitin-conjugating enzyme overcomes radiation-induced G2/M arrest. Biochim. Biophys. Acta 2013, 1833, 2911–2921. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, C.; Wang, L.; Zhou, F.; Zhang, S.; Kang, W.; Zhan, P.; Chen, J.; Shen, S.; Guo, H.; et al. Upregulation of UBE2Q1 via gene copy number gain in hepatocellular carcinoma promotes cancer progression through β-catenin-EGFR-PI3K-Akt-mTOR signaling pathway. Mol. Carcinog. 2018, 57, 201–215. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Ma, Y.; Zhang, X.; Li, Z.; Yu, W.; Zhu, M.; Wang, J.; Xu, Y.; Xu, A. Comprehensive Investigation into the Role of Ubiquitin-Conjugating Enzyme E2S in Melanoma Development. J. Invest. Dermatol. 2021, 141, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Q.; Peng, D.; Ning, X.H.; Yang, X.Y.; Li, X.S.; Zhou, L.Q.; Guo, Y.L. UBE2T silencing suppresses proliferation and induces cell cycle arrest and apoptosis in bladder cancer cells. Oncol. Lett. 2016, 12, 4485–4492. [Google Scholar] [CrossRef]
- Luo, C.; Yao, Y.; Yu, Z.; Zhou, H.; Guo, L.; Zhang, J.; Cao, H.; Zhang, G.; Li, Y.; Jiao, Z. UBE2T knockdown inhibits gastric cancer progression. Oncotarget 2017, 8, 32639–32654. [Google Scholar] [CrossRef]
- Palumbo, A., Jr.; Da Costa, N.M.; De Martino, M.; Sepe, R.; Pellecchia, S.; de Sousa, V.P.; Nicolau Neto, P.; Kruel, C.D.; Bergman, A.; Nasciutti, L.E.; et al. UBE2C is overexpressed in ESCC tissues and its abrogation attenuates the malignant phenotype of ESCC cell lines. Oncotarget 2016, 7, 65876–65887. [Google Scholar] [CrossRef] [PubMed]
- Nicolau-Neto, P.; Palumbo, A.; De Martino, M.; Esposito, F.; de Almeida Simão, T.; Fusco, A.; Nasciutti, L.E.; Meireles Da Costa, N.; Ribeiro Pinto, L.F. UBE2C Is a Transcriptional Target of the Cell Cycle Regulator FOXM. Genes 2018, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, R.; Chi, S.; Zhang, W.; Xiao, C.; Zhou, X.; Zhao, Y.; Wang, H. UBE2C Is Upregulated by Estrogen and Promotes Epithelial-Mesenchymal Transition via p53 in Endometrial Cancer. Mol. Cancer Res. 2020, 18, 204–215. [Google Scholar] [CrossRef]
- Wang, X.; Yin, L.; Yang, L.; Zheng, Y.; Liu, S.; Yang, J.; Cui, H.; Wang, H. Silencing ubiquitin-conjugating enzyme 2C inhibits proliferation and epithelial-mesenchymal transition in pancreatic ductal adenocarcinoma. FEBS J. 2019, 286, 4889–4909. [Google Scholar] [CrossRef]
- Huang, P.; Guo, Y.; Zhao, Z.; Ning, W.; Wang, H.; Gu, C.; Zhang, M.; Qu, Y.; Zhang, H.; Song, Y. UBE2T promotes glioblastoma invasion and migration via stabilizing GRP78 and regulating EMT. Aging 2020, 12, 10275–10289. [Google Scholar] [CrossRef]
- Bisol, Â.; de Campos, P.S.; Lamers, M.L. Flavonoids as anticancer therapies: A systematic review of clinical trials. Phytother. Res. 2020, 34, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Van Cruchten, S.; Van Den Broeck, W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat. Histol. Embryol. 2002, 31, 214–223. [Google Scholar] [CrossRef]
- Jesenberger, V.; Jentsch, S. Deadly encounter: Ubiquitin meets apoptosis. Nat. Rev. Mol. Cell Biol. 2002, 3, 112–121. [Google Scholar] [CrossRef]
- Aharinejad, S.; Andrukhova, O.; Lucas, T.; Zuckermann, A.; Wieselthaler, G.; Wolner, E.; Grimm, M. Programmed cell death in idiopathic dilated cardiomyopathy is mediated by suppression of the apoptosis inhibitor Apollon. Ann. Thorac. Surg. 2008, 86, 109–114, discussion 114. [Google Scholar] [CrossRef]
- Bartke, T.; Pohl, C.; Pyrowolakis, G.; Jentsch, S. Dual role of BRUCE as an antiapoptotic IAP and a chimeric E2/E3 ubiquitin ligase. Mol. Cell 2004, 14, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Weng, S.; Tang, W.; Xue, R.; Chen, S.; Cai, G.; Cai, Y.; Shen, X.; Zhang, S.; Dong, L. Overexpression of BIRC6 Is a Predictor of Prognosis for Colorectal Cancer. PLoS ONE 2015, 10, e0125281. [Google Scholar] [CrossRef] [PubMed]
- Low, C.G.; Luk, I.S.; Lin, D.; Fazli, L.; Yang, K.; Xu, Y.; Gleave, M.; Gout, P.W.; Wang, Y. BIRC6 protein, an inhibitor of apoptosis: Role in survival of human prostate cancer cells. PLoS ONE 2013, 8, e55837. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Lin, D.; Low, C.; Vucic, E.A.; English, J.C.; Yee, J.; Murray, N.; Lam, W.L.; Ling, V.; Lam, S.; et al. Elevated expression of BIRC6 protein in non-small-cell lung cancers is associated with cancer recurrence and chemoresistance. J. Thorac. Oncol. 2013, 8, 161–170. [Google Scholar] [CrossRef]
- Ismail, E.A.; Mahmoud, H.M.; Tawfik, L.M.; Habashy, D.M.; Adly, A.A.; El-Sherif, N.H.; Abdelwahab, M.A. BIRC6/Apollon gene expression in childhood acute leukemia: Impact on therapeutic response and prognosis. Eur. J. Haematol. 2012, 88, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Luk, S.U.; Xue, H.; Cheng, H.; Lin, D.; Gout, P.W.; Fazli, L.; Collins, C.C.; Gleave, M.E.; Wang, Y. The BIRC6 gene as a novel target for therapy of prostate cancer: Dual targeting of inhibitors of apoptosis. Oncotarget 2014, 5, 6896–6908. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Z.; Li, J.; Tong, J.; Cao, B.; Taylor, P.; Tang, X.; Wu, D.; Moran, M.F.; Zeng, Y.; et al. The ubiquitin-conjugating enzyme UBE2O modulates c-Maf stability and induces myeloma cell apoptosis. J. Hematol. Oncol. 2017, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Ba, C.; Ni, X.; Yu, J.; Zou, G.; Zhu, H. Ubiquitin conjugating enzyme E2 M promotes apoptosis in osteoarthritis chondrocytes via Wnt/β-catenin signaling. Biochem. Biophys. Res. Commun. 2020, 529, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Challagundla, K.B.; Dai, M.S. Positive regulation of p53 stability and activity by the deubiquitinating enzyme Otubain. EMBO J. 2012, 31, 576–592. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Shi, M.; Liu, R.; Yang, Q.H.; Johnson, T.; Skarnes, W.C.; Du, C. The Birc6 (Bruce) gene regulates p53 and the mitochondrial pathway of apoptosis and is essential for mouse embryonic development. Proc. Natl. Acad. Sci. USA 2005, 102, 565–570. [Google Scholar] [CrossRef]
- Zhou, C.; Bi, F.; Yuan, J.; Yang, F.; Sun, S. Gain of UBE2D1 facilitates hepatocellular carcinoma progression and is associated with DNA damage caused by continuous IL-6. J. Exp. Clin. Cancer Res. 2018, 37, 290. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, K.N.; Peifer, M. Wnt/Beta-Catenin Signaling Regulation and a Role for Biomolecular Condensates. Dev. Cell 2019, 48, 429–444. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Li, Y.; Yin, W.; Mo, L.; Qian, X.; Zhang, Y.; Wang, G.; Bu, F.; Zhang, Z.; et al. Ube2s stabilizes β-Catenin through K11-linked polyubiquitination to promote mesendoderm specification and colorectal cancer development. Cell Death Dis. 2018, 9, 456. [Google Scholar] [CrossRef]
- Shekhar, M.P.; Gerard, B.; Pauley, R.J.; Williams, B.O.; Tait, L. Rad6B is a positive regulator of beta-catenin stabilization. Cancer Res. 2008, 68, 1741–1750. [Google Scholar] [CrossRef]
- Shekhar, M.P.; Tait, L.; Gerard, B. Essential role of T-cell factor/beta-catenin in regulation of Rad6B: A potential mechanism for Rad6B overexpression in breast cancer cells. Mol. Cancer Res. 2006, 4, 729–745. [Google Scholar] [CrossRef]
- Shin, S.; Im, H.J.; Kwon, Y.J.; Ye, D.J.; Baek, H.S.; Kim, D.; Choi, H.K.; Chun, Y.J. Human steroid sulfatase induces Wnt/β-catenin signaling and epithelial-mesenchymal transition by upregulating Twist1 and HIF-1α in human prostate and cervical cancer cells. Oncotarget 2017, 8, 61604–61617. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Du, J.; Fan, C. Ube2S regulates Wnt/β-catenin signaling and promotes the progression of non-small cell lung cancer. Int. J. Med. Sci. 2020, 17, 274–279. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.R.; Hwang, K.S.; Yoo, J.; Cho, W.K.; Kim, J.M.; Kim, W.H.; Im, D.S. E2-EPF UCP targets pVHL for degradation and associates with tumor growth and metastasis. Nat. Med. 2006, 12, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Lei, T.; Zheng, J.; Chen, S.; Du, L.; Xie, H. UBE2S mediates tumor progression via SOX6/β-Catenin signaling in endometrial cancer. Int. J. Biochem. Cell Biol. 2019, 109, 17–22. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X. UBE2T silencing inhibited non-small cell lung cancer cell proliferation and invasion by suppressing the wnt/β-catenin signaling pathway. Int. J. Clin. Exp. Pathol. 2017, 10, 9482–9488. [Google Scholar]
- Hu, W.; Xiao, L.; Cao, C.; Hua, S.; Wu, D. UBE2T promotes nasopharyngeal carcinoma cell proliferation, invasion, and metastasis by activating the AKT/GSK3β/β-catenin pathway. Oncotarget 2016, 7, 15161–15172. [Google Scholar] [CrossRef]
- Deng, L.; Meng, T.; Chen, L.; Wei, W.; Wang, P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal. Transduct. Target. Ther. 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Magnani, M.; Crinelli, R.; Bianchi, M.; Antonelli, A. The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-kB (NF-kB). Curr. Drug Targets 2000, 1, 387–399. [Google Scholar] [CrossRef]
- Wu, X.; Karin, M. Emerging roles of Lys63-linked polyubiquitylation in immune responses. Immunol. Rev. 2015, 266, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, E.; Goncharov, T.; Fedorova, A.V.; Dynek, J.N.; Zobel, K.; Deshayes, K.; Fairbrother, W.J.; Vucic, D. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J. Biol. Chem. 2008, 283, 24295–24299. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wertz, I.; O’Rourke, K.; Ultsch, M.; Seshagiri, S.; Eby, M.; Xiao, W.; Dixit, V.M. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature 2004, 427, 167–171. [Google Scholar] [CrossRef]
- Deng, L.; Wang, C.; Spencer, E.; Yang, L.; Braun, A.; You, J.; Slaughter, C.; Pickart, C.; Chen, Z.J. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 2000, 103, 351–361. [Google Scholar] [CrossRef]
- Shi, C.S.; Kehrl, J.H. Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2). J. Biol. Chem. 2003, 278, 15429–15434. [Google Scholar] [CrossRef]
- Ea, C.K.; Deng, L.; Xia, Z.P.; Pineda, G.; Chen, Z.J. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 2006, 22, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Kovacev, J.; Pan, Z.Q. Priming and extending: A UbcH5/Cdc34 E2 handoff mechanism for polyubiquitination on a SCF substrate. Mol. Cell 2010, 37, 784–796. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Z.J. Expanding role of ubiquitination in NF-κB signaling. Cell Res. 2011, 21, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Yang, X.; Wang, J.; Wang, R.; Qian, X.; Zhang, W.; Xiao, W. Uev1A-Ubc13 catalyzes K63-linked ubiquitination of RHBDF2 to promote TACE maturation. Cell. Signal. 2018, 42, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Liu, Y.; Wang, W.; Li, A.; Wan, P.; Liu, W.; Shereen, M.A.; Liu, F.; Zhang, W.; Tan, Q.; et al. SUMO1 SUMOylates and SENP3 deSUMOylates NLRP3 to orchestrate the inflammasome activation. FASEB J. 2020, 34, 1497–1515. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Prasad, S.; Ravindran, J.; Aggarwal, B.B. NF-kappaB and cancer: How intimate is this relationship. Mol. Cell Biochem. 2010, 336, 25–37. [Google Scholar] [CrossRef]
- Xiao, W.; Lin, S.L.; Broomfield, S.; Chow, B.L.; Wei, Y.F. The products of the yeast MMS2 and two human homologs (hMMS2 and CROC-1) define a structurally and functionally conserved Ubc-like protein family. Nucleic Acids Res. 1998, 26, 3908–3914. [Google Scholar] [CrossRef]
- Syed, N.A.; Andersen, P.L.; Warrington, R.C.; Xiao, W. Uev1A, a ubiquitin conjugating enzyme variant, inhibits stress-induced apoptosis through NF-kappaB activation. Apoptosis 2006, 11, 2147–2157. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, S.; Zhang, Z.; Zhang, W.; Xiao, W. Ubiquitin-conjugating enzyme complex Uev1A-Ubc13 promotes breast cancer metastasis through nuclear factor-κB mediated matrix metalloproteinase-1 gene regulation. Breast Cancer Res. 2014, 16, R75. [Google Scholar] [CrossRef] [PubMed]
- Dynek, J.N.; Goncharov, T.; Dueber, E.C.; Fedorova, A.V.; Izrael-Tomasevic, A.; Phu, L.; Helgason, E.; Fairbrother, W.J.; Deshayes, K.; Kirkpatrick, D.S.; et al. c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 2010, 29, 4198–4209. [Google Scholar] [CrossRef]
- Ditsworth, D.; Zong, W.X. NF-kappaB: Key mediator of inflammation-associated cancer. Cancer Biol. Ther. 2004, 3, 1214–1216. [Google Scholar] [CrossRef]
- Xiong, Y.; Yi, Y.; Wang, Y.; Yang, N.; Rudd, C.E.; Liu, H. Ubc9 Interacts with and SUMOylates the TCR Adaptor SLP-76 for NFAT Transcription in T Cells. J. Immunol. 2019, 203, 3023–3036. [Google Scholar] [CrossRef]
- Hattori, K.; Hatakeyama, S.; Shirane, M.; Matsumoto, M.; Nakayama, K. Molecular dissection of the interactions among IkappaBalpha, FWD1, and Skp1 required for ubiquitin-mediated proteolysis of IkappaBalpha. J. Biol. Chem. 1999, 274, 29641–29647. [Google Scholar] [CrossRef] [PubMed]
- Yamoah, K.; Oashi, T.; Sarikas, A.; Gazdoiu, S.; Osman, R.; Pan, Z.Q. Autoinhibitory regulation of SCF-mediated ubiquitination by human cullin 1’s C-terminal tail. Proc. Natl. Acad. Sci. USA 2008, 105, 12230–12235. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Matsuzawa, A.; Zhang, W.; Tseng, P.H.; Keats, J.J.; Wang, H.; Vignali, D.A.; Bergsagel, P.L.; Karin, M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat. Immunol. 2008, 9, 1364–1370. [Google Scholar] [CrossRef]
- Xiao, G.; Harhaj, E.W.; Sun, S.C. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p-100. Mol. Cell 2001, 7, 401–409. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Senftleben, U.; Cao, Y.; Xiao, G.; Greten, F.R.; Krähn, G.; Bonizzi, G.; Chen, Y.; Hu, Y.; Fong, A.; Sun, S.C.; et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 2001, 293, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Niu, T.; Xiao, W. Uev1A promotes breast cancer cell survival and chemoresistance through the AKT-FOXO1-BIM pathway. Cancer Cell Int. 2019, 19, 331. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Kang, B.; Li, Y.; Hao, W.; Ma, F. UBE2T promotes proliferation and regulates PI3K/Akt signaling in renal cell carcinoma. Mol. Med. Rep. 2019, 20, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Duncan, L.M.; Piper, S.; Dodd, R.B.; Saville, M.K.; Sanderson, C.M.; Luzio, J.P.; Lehner, P.J. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 2006, 25, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Allam, U.S.; Ahsan, A.; Chen, G.; Krishnamurthy, P.M.; Marsh, K.; Rumschlag, M.; Shankar, S.; Whitehead, C.; Schipper, M.; et al. KRAS protein stability is regulated through SMURF2: UBCH5 complex-mediated β-TrCP1 degradation. Neoplasia 2014, 16, 115–128. [Google Scholar] [CrossRef]
- Vila, I.K.; Yao, Y.; Kim, G.; Xia, W.; Kim, H.; Kim, S.J.; Park, M.K.; Hwang, J.P.; González-Billalabeitia, E.; Hung, M.C.; et al. A UBE2O-AMPKα2 Axis that Promotes Tumor Initiation and Progression Offers Opportunities for Therapy. Cancer Cell 2017, 31, 208–224. [Google Scholar] [CrossRef]
- Wang, L.; Ji, S. Inhibition of Ubc9-Induced CRMP2 SUMOylation Disrupts Glioblastoma Cell Proliferation. J. Mol. Neurosci. 2019, 69, 391–398. [Google Scholar] [CrossRef]
- Vij, R.; Wang, M.; Kaufman, J.L.; Lonial, S.; Jakubowiak, A.J.; Stewart, A.K.; Kukreti, V.; Jagannath, S.; McDonagh, K.T.; Alsina, M.; et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood 2012, 119, 5661–5670. [Google Scholar] [CrossRef]
- Pal, A.; Young, M.A.; Donato, N.J. Emerging potential of therapeutic targeting of ubiquitin-specific proteases in the treatment of cancer. Cancer Res. 2014, 74, 4955–4966. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, W.M.; Lountos, G.T.; Zlotkowski, K.; Dahlhauser, S.D.; Saunders, L.B.; Needle, D.; Tropea, J.E.; Zhan, C.; Wei, G.; Ma, B.; et al. Insights into the Allosteric Inhibition of the SUMO E2 Enzyme Ubc9. Angew. Chem. Int. Ed. Engl. 2016, 55, 5703–5707. [Google Scholar] [CrossRef] [PubMed]
- Ramatenki, V.; Dumpati, R.; Vadija, R.; Vellanki, S.; Potlapally, S.R.; Rondla, R.; Vuruputuri, U. Targeting the ubiquitin-conjugating enzyme E2D4 for cancer drug discovery-a structure-based approach. J. Chem. Biol. 2017, 10, 51–67. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L. MicroRNAs in amyotrophic lateral sclerosis: From pathogenetic involvement to diagnostic biomarker and therapeutic agent development. Neurol. Sci. 2020, 41, 3569–3577. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.Y.; Huang, L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef]
- Sharma, P.; Dando, I.; Strippoli, R.; Kumar, S.; Somoza, A.; Cordani, M.; Tafani, M. Nanomaterials for Autophagy-Related miRNA-34a Delivery in Cancer Treatment. Front. Pharmacol. 2020, 11, 1141. [Google Scholar] [CrossRef]
- Liu, L.; Hua, Y.; Wang, D.; Shan, L.; Zhang, Y.; Zhu, J.; Jin, H.; Li, H.; Hu, Z.; Zhang, W. A sesquiterpene lactone from a medicinal herb inhibits proinflammatory activity of TNF-α by inhibiting ubiquitin-conjugating enzyme UbcH5. Chem. Biol. 2014, 21, 1341–1350. [Google Scholar] [CrossRef]
- Chen, H.; Wu, G.; Gao, S.; Guo, R.; Zhao, Z.; Yuan, H.; Liu, S.; Wu, J.; Lu, X.; Yuan, X.; et al. Discovery of Potent Small-Molecule Inhibitors of Ubiquitin-Conjugating Enzyme UbcH5c from α-Santonin Derivatives. J. Med. Chem. 2017, 60, 6828–6852. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, X. Molecular Simulation Elaborating the Mechanism of 1β-Hydroxy Alantolactone Inhibiting Ubiquitin-Conjugating Enzyme UbcH5s. Sci. Rep. 2020, 10, 141. [Google Scholar] [CrossRef]
- Wang, C.; Shi, G.; Ji, X. Design, synthesis, and anticancer activity evaluation of irreversible allosteric inhibitors of the ubiquitin-conjugating enzyme Ube2g2. Med. Chem. Commun. 2018, 9, 1818–1825. [Google Scholar] [CrossRef]
- Sanders, M.A.; Brahemi, G.; Nangia-Makker, P.; Balan, V.; Morelli, M.; Kothayer, H.; Westwell, A.D.; Shekhar, M.P.V. Novel inhibitors of Rad6 ubiquitin conjugating enzyme: Design, synthesis, identification, and functional characterization. Mol. Cancer Ther. 2013, 12, 373–383. [Google Scholar] [CrossRef]
- Ceccarelli, D.F.; Tang, X.; Pelletier, B.; Orlicky, S.; Xie, W.; Plantevin, V.; Neculai, D.; Chou, Y.C.; Ogunjimi, A.; Al-Hakim, A.; et al. An allosteric inhibitor of the human Cdc34 ubiquitin-conjugating enzyme. Cell 2011, 145, 1075–1087. [Google Scholar] [CrossRef]
- Kim, Y.S.; Keyser, S.G.; Schneekloth, J.S., Jr. Synthesis of 2′,3′,4′-trihydroxyflavone (2-D08), an inhibitor of protein sumoylation. Bioorg. Med. Chem. Lett. 2014, 24, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Zlotkowski, K.; Hewitt, W.M.; Sinniah, R.S.; Tropea, J.E.; Needle, D.; Lountos, G.T.; Barchi, J.J., Jr.; Waugh, D.S.; Schneekloth, J.S., Jr. A Small-Molecule Microarray Approach for the Identification of E2 Enzyme Inhibitors in Ubiquitin-Like Conjugation Pathways. SLAS Discov. 2017, 22, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Takeuchi, T.; Rotinsulu, H.; Mangindaan, R.E.; van Soest, R.W.; Ukai, K.; Kobayashi, H.; Namikoshi, M.; Ohta, T.; Yokosawa, H. Leucettamol A: A new inhibitor of Ubc13-Uev1A interaction isolated from a marine sponge, Leucetta aff. microrhaphis. Bioorg. Med. Chem. Lett. 2008, 18, 6319–6320. [Google Scholar] [CrossRef] [PubMed]
- Ushiyama, S.; Umaoka, H.; Kato, H.; Suwa, Y.; Morioka, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.; de Voogd, N.J.; Yokosawa, H.; et al. Manadosterols A and B, sulfonated sterol dimers inhibiting the Ubc13-Uev1A interaction, isolated from the marine sponge Lissodendryx fibrosa. J. Nat. Prod. 2012, 75, 1495–1499. [Google Scholar] [CrossRef]
- Cheng, J.; Fan, Y.H.; Xu, X.; Zhang, H.; Dou, J.; Tang, Y.; Zhong, X.; Rojas, Y.; Yu, Y.; Zhao, Y.; et al. A small-molecule inhibitor of UBE2N induces neuroblastoma cell death via activation of p53 and JNK pathways. Cell Death Dis. 2014, 5, e1079. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Hsu, W.H.; Tsai, P.H.; Huang, Y.T.; Lin, C.W.; Chen, K.C.; Tsai, I.H.; Kandaswami, C.C.; Huang, C.J.; Chang, G.D.; et al. Dietary flavonoids, luteolin and quercetin, inhibit invasion of cervical cancer by reduction of UBE2S through epithelial-mesenchymal transition signaling. Food Funct. 2017, 8, 1558–1568. [Google Scholar] [CrossRef]
- Cornwell, M.J.; Thomson, G.J.; Coates, J.; Belotserkovskaya, R.; Waddell, I.D.; Jackson, S.P.; Galanty, Y. Small-Molecule Inhibition of UBE2T/FANCL-Mediated Ubiquitylation in the Fanconi Anemia Pathway. ACS Chem. Biol. 2019, 14, 2148–2154. [Google Scholar] [CrossRef]
- Jin, D.; Guo, J.; Wu, Y.; Du, J.; Wang, X.; An, J.; Hu, B.; Kong, L.; Di, W.; Wang, W. Retraction of “UBE2C, Directly Targeted by miR-548e-5p, Increases the Cellular Growth and Invasive Abilities of Cancer Cells Interacting with the EMT Marker Protein Zinc Finger E-box Binding Homeobox 1/2 in NSCLC”. Theranostics 2020, 10, 9619. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Gu, X.; Liu, F.; Rui, Z.; Liu, M.; Zhao, L. Antitumor effects of hsa-miR661-3p on non-small cell lung cancer in vivo and in vitro. Oncol. Rep. 2019, 41, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, X.; Yang, C.; Rashid, K.; Ma, C.; Hu, M.; Ding, Q.; Jiang, H. Anticancer effect of icaritin on prostate cancer via regulating miR-381-3p and its target gene UBE2C. Cancer Med. 2019, 8, 7833–7845. [Google Scholar] [CrossRef]
- Wei, X.; You, X.; Zhang, J.; Zhou, C. MicroRNA-1305 Inhibits the Stemness of LCSCs and Tumorigenesis by Repressing the UBE2T-Dependent Akt-Signaling Pathway. Mol. Ther. Nucleic Acids 2019, 16, 721–732. [Google Scholar] [CrossRef]
- Zhao, Z.; Tan, X.; Zhao, A.; Zhu, L.; Yin, B.; Yuan, J.; Qiang, B.; Peng, X. microRNA-214-mediated UBC9 expression in glioma. BMB Rep. 2012, 45, 641–646. [Google Scholar] [CrossRef]
| Name (Human) | Synonyms | Classification | Biological Roles | Relevant Cancers |
|---|---|---|---|---|
| UBE2A | RAD6(A) | Class I | Transcriptional regulation | Chronic myeloid leukemia |
| DNA repair [33] | ||||
| Regulating myeloid differentiation [34] | ||||
| UBE2B | RAD6(B) | Class I | Ubiquitinating H2A/B and MGMT to participate in DNA repair | MM, BC |
| Monoubiquitinating H2B to participate in transcriptional activation | ||||
| UBE2C | UBCH10 | Class II | Ubiquitinating p53 and Ki67 [35] to participate in G2/M transition [36] | MM of uterus, Melanoma, HCC [37], HNSCC [38], CRC [39], Glioma [40], TSCC [41], Cerebral cancer, LC, Leukemia, Lymphoma, GC [6,7,8,9], BC, Esophageal cancer, CC, Endometrial carcinoma, OC |
| Regulating the level of phosphorylated ERK1/2 to participate in cell apoptosis [42] | ||||
| Its depletion reduced OC malignancy and reversed DDP resistance via downregulating CDK1 [43] | ||||
| UBE2D(1/2/3) | UBCH5(a/b/c) | Class I | Regulating the level of p53 protein [44] | Esophageal cancer [45], PCa |
| Ubiquitinating MDM2 and CCND1 | ||||
| UBE2E1 | UBCH6 | Class II | Affects the patient’s response to induction chemotherapy [46] | Acute myelogenous leukemia, PCa |
| UBE2E2 | UBCH8 | Class II | Stable substrate protein with ISG15 can promote cancer cell movement and invasion [47] | BC |
| UBE2E3 | UBCH9 | Class II | Maintaining mitochondrial homeostasis [48], participating in NEDD4− dependent epithelial Na+ channel regulation [49] | - |
| UBE2G1 | UBE2G/E217K | Class I | Regulating inflammation and innate immune response [50], ubiquitinating and degrading of IKZF1 and IKZF3 [51] | Myeloma |
| UBE2G2 | UBC7 | Class I | Co-regulating immune receptor downregulation mediated by human cytomegalovirus US2 with TRC8 [52] | NSCLC [53], PCa [54] |
| UBE2H | UBC8/UBCH2 | Class III | Participating in neurodevelopment [55] | - |
| TNF-α promotes the binding of the UBE2H promoter region to NF-κB [56] | ||||
| UBE2J1 | UBC6 | Class III | It negatively regulates interferon to promote RNA virus infection [57] | Medulloblastoma [58], PCa |
| Participating in spermatogenesis and growth and development | ||||
| UBE2J2 | NCUBE2 | Class III | Regulates ERAD induced by human cytomegalovirus US2 through TRC8 [52] | HCC [59] |
| UBE2K | UBCH1/E2-25k | Class III | Regulating the cell cycle | - |
| UBE2L3 | UBCH7 | Class I | Participating in DSB repair | HCC [60], Cervical Cancer [61], NSCLC [62], B-cell lymphoma |
| Ubiquitinating p53 and p27Kip1 to regulate the cell cycle [63] | ||||
| Regulating the NF-κB signal driven by TNF-α [64], Rate limiting factors and therapeutic targets of LUBAC activity [65] | ||||
| UBE2N | UBC13 | Class I | UBE2N-UBE2V1 complex regulates innate immunity and participates in the activation of NF-κB [66] | BC, Cervical Cancer [67], HCC [68], DLBCL, LC, Malignant melanoma [69] |
| UBE2N-UBE2V2 ubiquitinates PCNA and H2A | ||||
| Ubiquitinating and degrading Sirt1 and inhibiting histone H4 lysine 16 acetylation [70] | ||||
| Activating MAPKs | ||||
| Involved in the internalization of cell surface receptors | ||||
| UBE2O | E2-230K | Class IV | Ubiquitinates BMAL1 to regulate transcriptional activity and circadian rhythm function [71] | BC [72], GC, RC [5], Anemia, MM, OC, HNSCC [73], LC [74] |
| Participating in erythropoiesis, ubiquitinating RPs to participate terminal erythroid differentiation [75] | ||||
| Regulating apoptosis | ||||
| Monoubiquitinating SMAD6 to participate in bone morphogenesis [76] | ||||
| Ubiquitinating and degrading MXI1 at the Lys46 residue | ||||
| UBE2Q1 | UBE2Q/NICE5 | Class II | Regulating p53 [77] | HCC, BC, ALL [78], CRC [79] |
| Regulation of lysosome integrity and lysophagy [80] | ||||
| UBE2Q2 | Nothing | Class II | Regulating apoptosis | HNSCC [81], CRC [82] |
| UBE2R1 | CDC34/UBC3/UBCH3 | Class III | Ubiquitinating and degrading p27Kip1 [83] and IκBα | MM, HCC [84], NSCLC [85], ALL [86] |
| UBE2S | E2EPF/EPF5 | Class III | Ubiquitinating CDKN1A, CCNB1, CDC20, and p53 (Lys11/Lys48 polyUb chain) to regulate apoptosis | HCC [68], BC, OSCC [87], NSCLC [88], Melanoma [89], CRC |
| Ubiquitinating β-catenin to maintain its stability | ||||
| Ubiquitinating SOX2 to regulate neuroectodermal differentiation and maintaining mES cells [90] | ||||
| UBE2T | FANCT/PIG50/ | Class III | Nucleic acid excision repair for UV damage [91] | FA [65,92], GC [93], Osteosarcoma [94], PCa [95], LC [96], BC [97], HCC [98], Intrahepatic cholangiocarcinoma [99], Gallbladder cancer [100], NPC, CRC, MM [101], OC [102] |
| Ubiquitinating and degrading p53 [101] | ||||
| Participating in Wnt/β-catenin signaling and P13K/AKT signaling, regulating BRCA1 degradation | ||||
| UBE2V1 | UEV1A | Class II | It participates in the activation of NF-κB together with UBE2N [71] | Metastatic CRC, BC, Osteosarcoma [103] |
| UBE2V2 | MMS2 | Class I | Participates in DNA repair together with UBE2N | - |
| UBE2W | UBC16 | Class I | UBE2W downregulation promotes cell apoptosis and correlates with hypospermatogenesis [104] | - |
| BIRC6 | Appolon/BRUCE | Class IV | A positive regulator of macroautophagy/autophagy [105] | HCC [106,107], NB [35,108], CRC, PCa [109], OC [35] |
| Ub-like | ||||
| UBE2F | NCE2 | Class II | Promoting the survival of lung cancer cells [110] | LC |
| UBE2I | UBC9 | Class I | Promoting the development of T cells [111], SUMOylation of IRF4 promotes the M2 process of macrophages [112], SUMOylation of IRF7 limits its transcriptional activity [113] | HCC [114], BC |
| SUMOylation of (SUMO1) NLRP3 activates the inflammasome, Regulating the NF-κB signaling [115] | ||||
| Participating in the formation of Lys49 polyUb chain to resist senescence [116] | ||||
| Affects BCL2 expression through the ER signaling pathway [117] | ||||
| UBE2I-PCGF2 complex inhibits the SUMOylation of PML-RARA [118] | ||||
| Participating in the development and survival of CLPs [119] | ||||
| UBE2M | UBC12/UBC-RS2 | Class II | DNA repair [120] | HCC [121], RC [122], LC, Intrahepatic cholangiocarcinoma [123] |
| Ubiquitinating and degrading UBE2F [124] | ||||
| Participating in the cell cycle [125] | ||||
| UBE2Z | USE1/HOYS7 | Class IV | Participating in the ERK and STAT3 signal pathway [126] | HCC |
| Name | Target | Origin | Inhibition Mechanisms | Test Diseases | Characteristics |
|---|---|---|---|---|---|
| Inhibitors | |||||
| IJ-5 [249] | UBE2D3 | Herb | Combines with Cys85 of UBE2D3 to inhibit NF-κB signaling | Arthritis, Hepatitis | Difficulty in synthesis |
| Compound 6d [250] | UBE2D3 | α-Santonin derivatives | Same as above | Arthritis | The efficacy of 6d is greater than IJ-5, but 6d is unstable |
| 1β-hydroxy alantolactone [251] | UBE2D | Herbal medicine | Same as above | Inflammation | It is more efficient in combination with UBE2D3 |
| CW3 [252] | UBE2G2 | Synthesis | The vinyl group of CW3 inhibits E2 by forming a covalent bond with the thiol group of Cys48 of UBE2G2 | Melanoma | - |
| TZ9 [253] | UBE2B | Synthesis | - | BC | Selective suppression |
| New triazine drugs (6a-c) [151] | UBE2B | Based on TZ9 synthesis | It incorporates deep inside the UBE2B binding pocket by interaction with UBE2B active site residues Cys88 and Asp90. | OC, LC, BC, CC | Inhibitory activity > TZ9, Selective suppression |
| CC0651 [254] | UBE2R1 | Synthesis | It inserts into the hidden binding pocket of the non-catalytic site of UBE2R1 and interferes with the release of Ub to the Lys residue of the substrate | - | Allosteric inhibition |
| 2-D08 [255] | UBE2I | Synthesis | Preventing transfer of SUMO from the UBE2I-SUMO thioester to the substrate | - | In vitro biochemical test |
| Compound 2 [256] | UBE2I | Synthesis | Binding near the active site of UBE2I | - | Low potency, low selectivity |
| Leucettamol A [257] | UBE2N | Leucetta aff. microrhaphis | Inhibiting the formation of the UBE2N-UBE2V1 complex | - | Its hydrogenation increased its inhibitory activity |
| Manadosterols A and B [258] | UBE2N | Lissodendoryx fibrosa manadosterols | Same as above | - | The activities are more potent than those of Leucettamol A |
| NSC697923 [259] | UBE2N | Synthesis | Impeding the formation of the UBE2N and Ub thioester conjugate. | NB | Efficacy > Doxorubicin and Etoposide |
| Luteolin and Quercetin [260] | UBE2S | Plants | - | Cervical cancer | - |
| CU2 [261] | UBE2T | Synthesis | Inhibiting UBE2T/FANCL-mediated FANCD2 monoubiquitylation | - | Cell and biochemical tests |
| miRNAs | |||||
| miR-548e-5p [262] | UBE2C | Human LC organization | Binding to the 3′-UTR of UBE2C | NSCLC | In vitro test |
| miR661-3p [263] | UBE2C | Human 293 cells | Binding to the 3′-UTR of UBE2C | NSCLC | In vivo and in vitro tests |
| miR-381-3p [264] | UBE2C | Human PCa cells | ICT upregulates the level of miR-381-3p to downregulate the expression of UBE2C in human PCa cells | PCa | In vivo and in vitro tests |
| miR-147b [69] | UBE2N | HC organization | Binding to 3′-UTR of UBE2N | HC | In vivo and in vitro tests |
| miR-1305 [265] | UBE2T | Synthesis | Binding to 3′-UTR of UBE2T | LC | In vivo and in vitro tests |
| miR-214 [266] | UBE2I | Synthesis | Binding to 3′-UTR of UBE2I | Glioma | In vitro test |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Song, H.; Shen, N.; Hua, R.; Yang, G. The Molecular Basis of Ubiquitin-Conjugating Enzymes (E2s) as a Potential Target for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 3440. https://doi.org/10.3390/ijms22073440
Du X, Song H, Shen N, Hua R, Yang G. The Molecular Basis of Ubiquitin-Conjugating Enzymes (E2s) as a Potential Target for Cancer Therapy. International Journal of Molecular Sciences. 2021; 22(7):3440. https://doi.org/10.3390/ijms22073440
Chicago/Turabian StyleDu, Xiaodi, Hongyu Song, Nengxing Shen, Ruiqi Hua, and Guangyou Yang. 2021. "The Molecular Basis of Ubiquitin-Conjugating Enzymes (E2s) as a Potential Target for Cancer Therapy" International Journal of Molecular Sciences 22, no. 7: 3440. https://doi.org/10.3390/ijms22073440
APA StyleDu, X., Song, H., Shen, N., Hua, R., & Yang, G. (2021). The Molecular Basis of Ubiquitin-Conjugating Enzymes (E2s) as a Potential Target for Cancer Therapy. International Journal of Molecular Sciences, 22(7), 3440. https://doi.org/10.3390/ijms22073440






