Amyloid Structural Changes Studied by Infrared Microspectroscopy in Bigenic Cellular Models of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
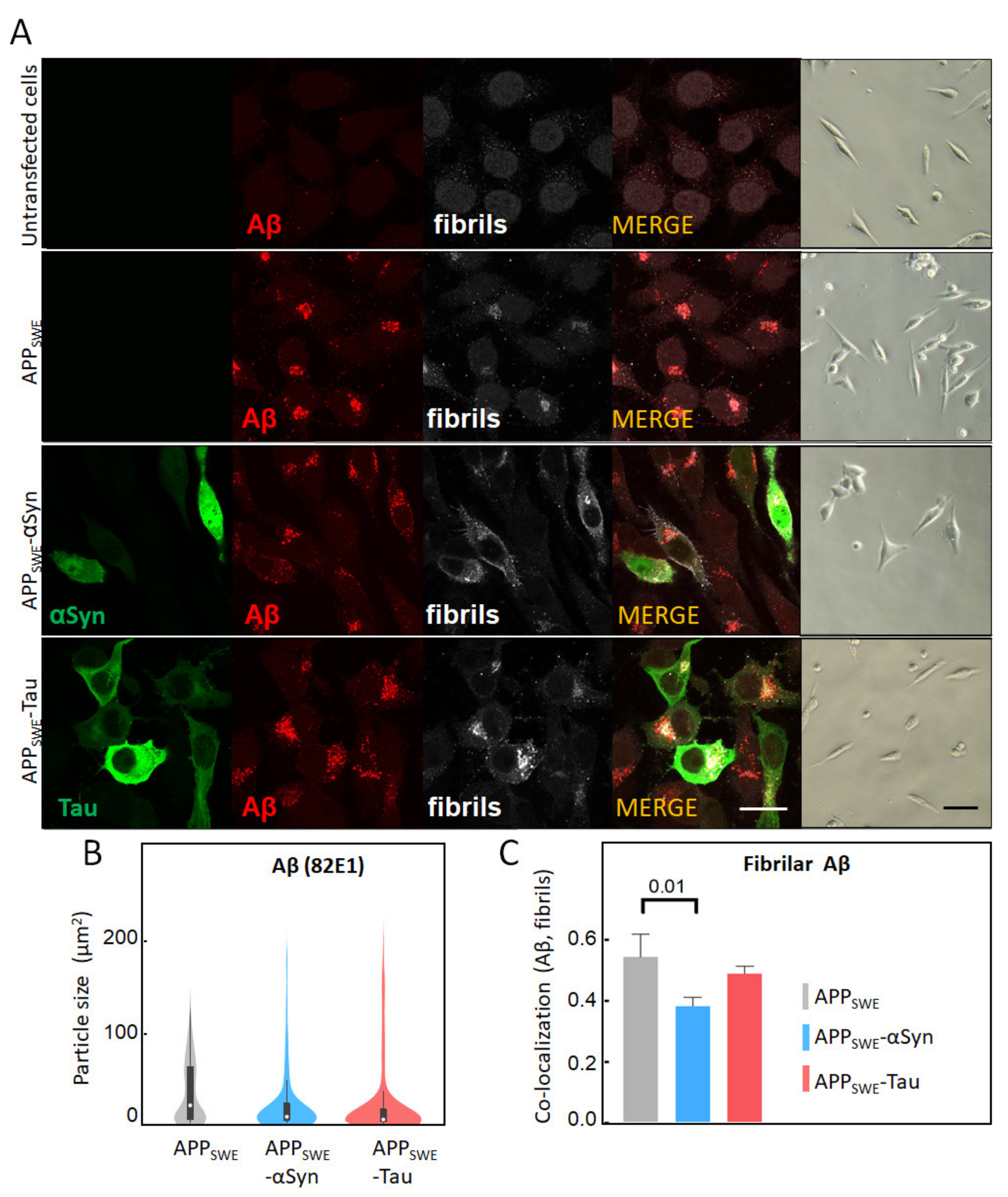
2.1. αSyn and Tau Affect the Quality of Intracellular Aβ Aggregates
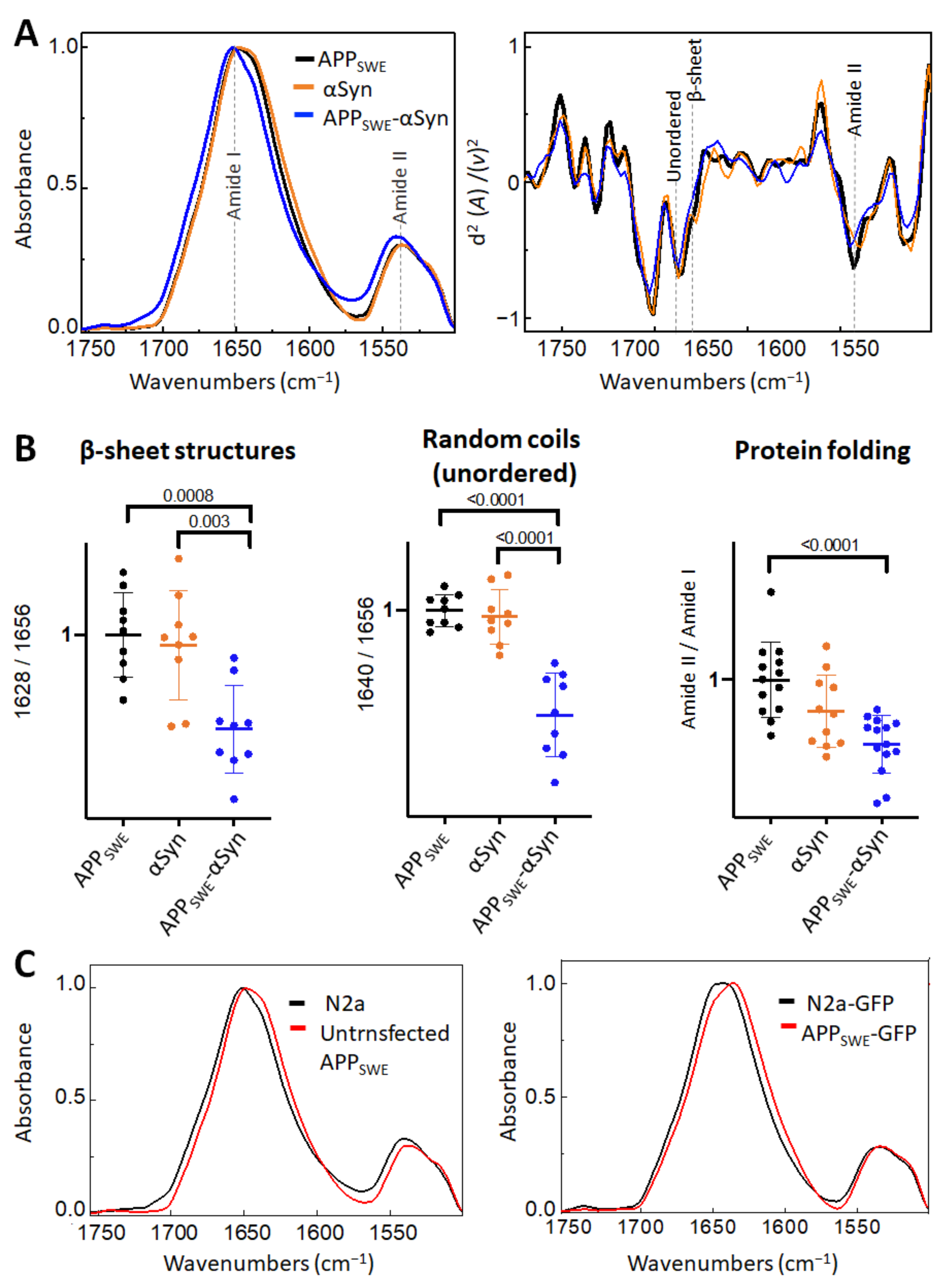
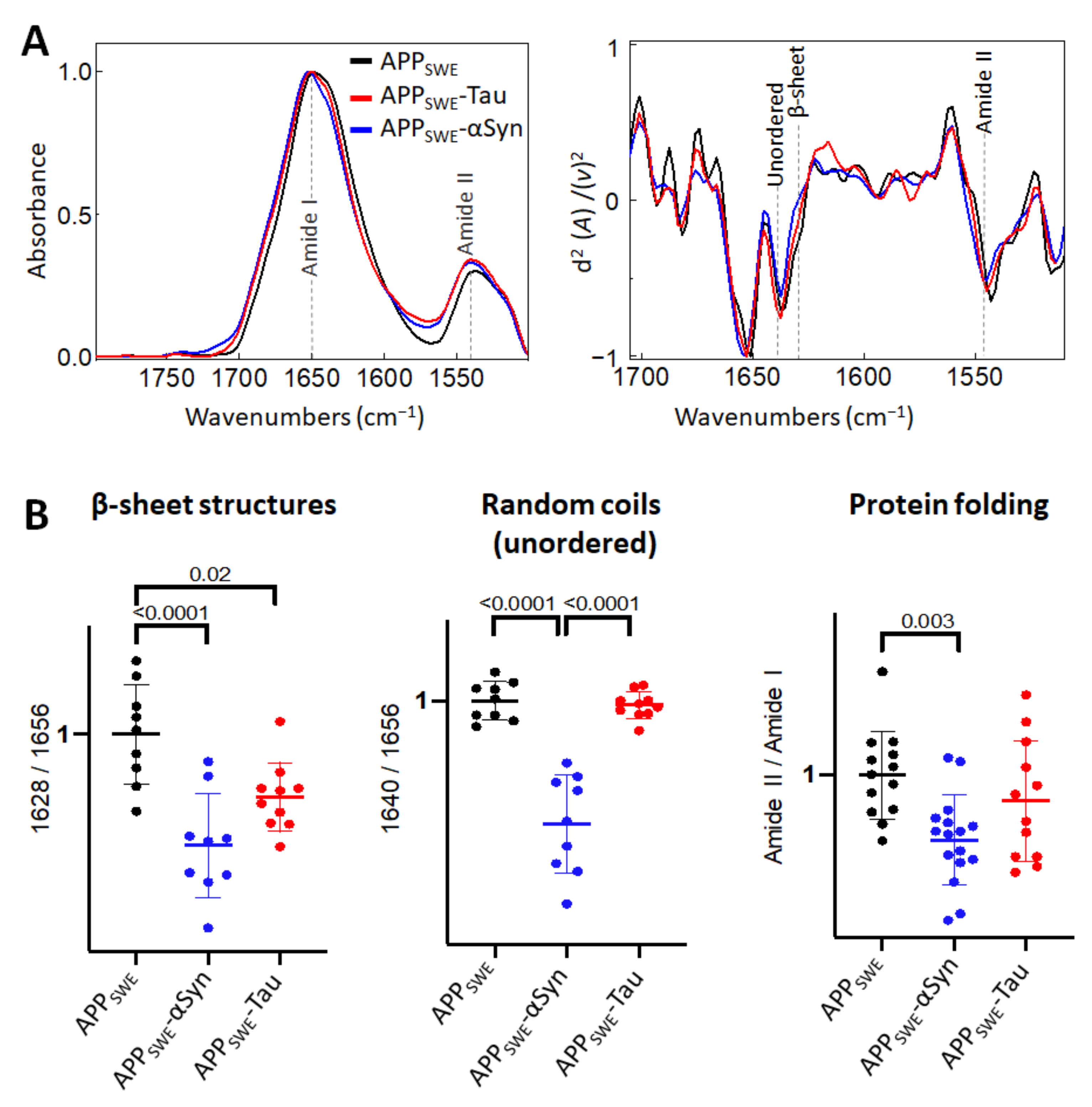
2.2. αSyn Reduces β-Sheet Load in a Bigenic Aβ/αSyn Cellular Model
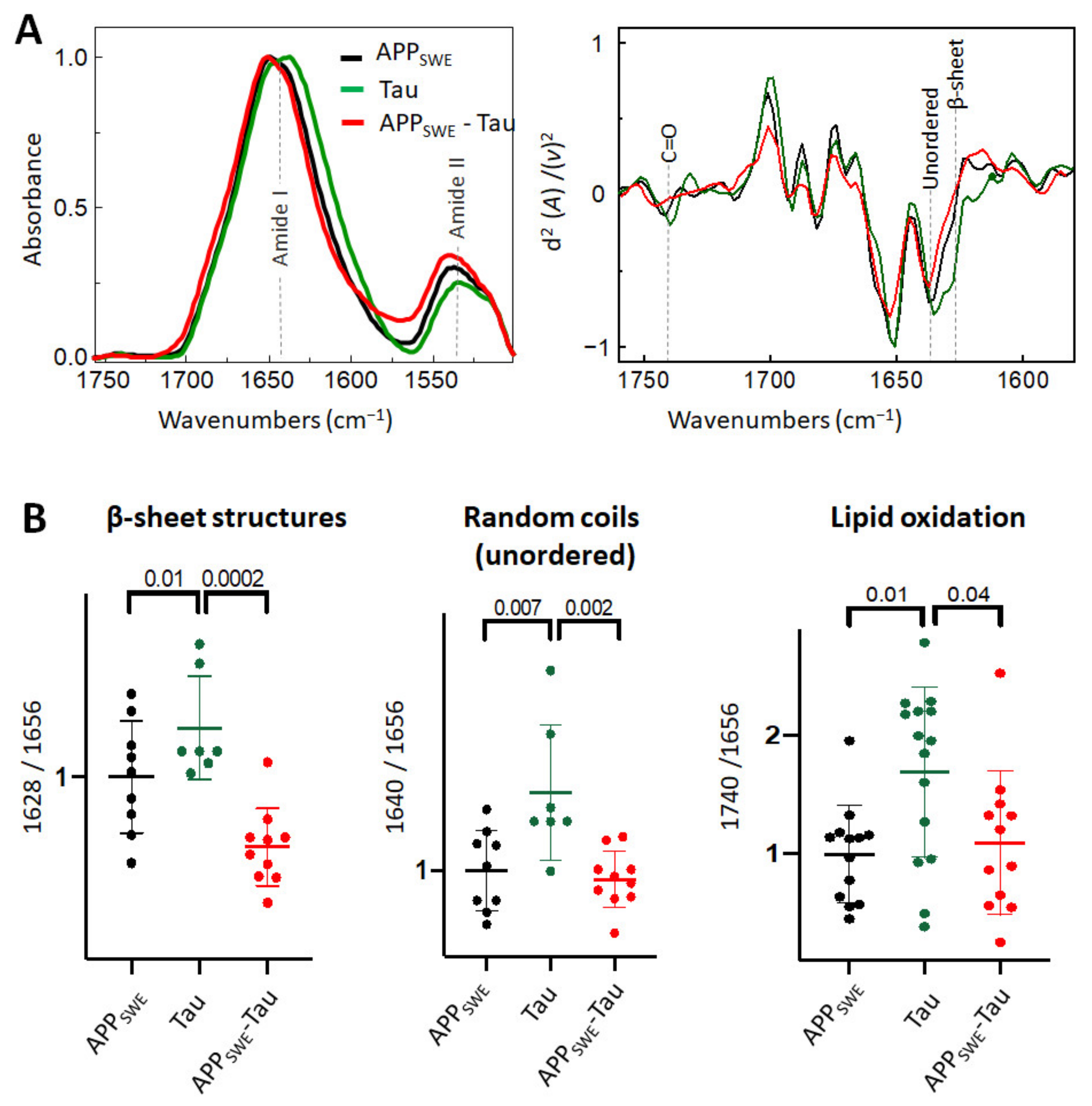
2.3. Aβ Reduces the Level of β-Sheet Aggregates in the Bigenic Aβ/Tau Cellular Model
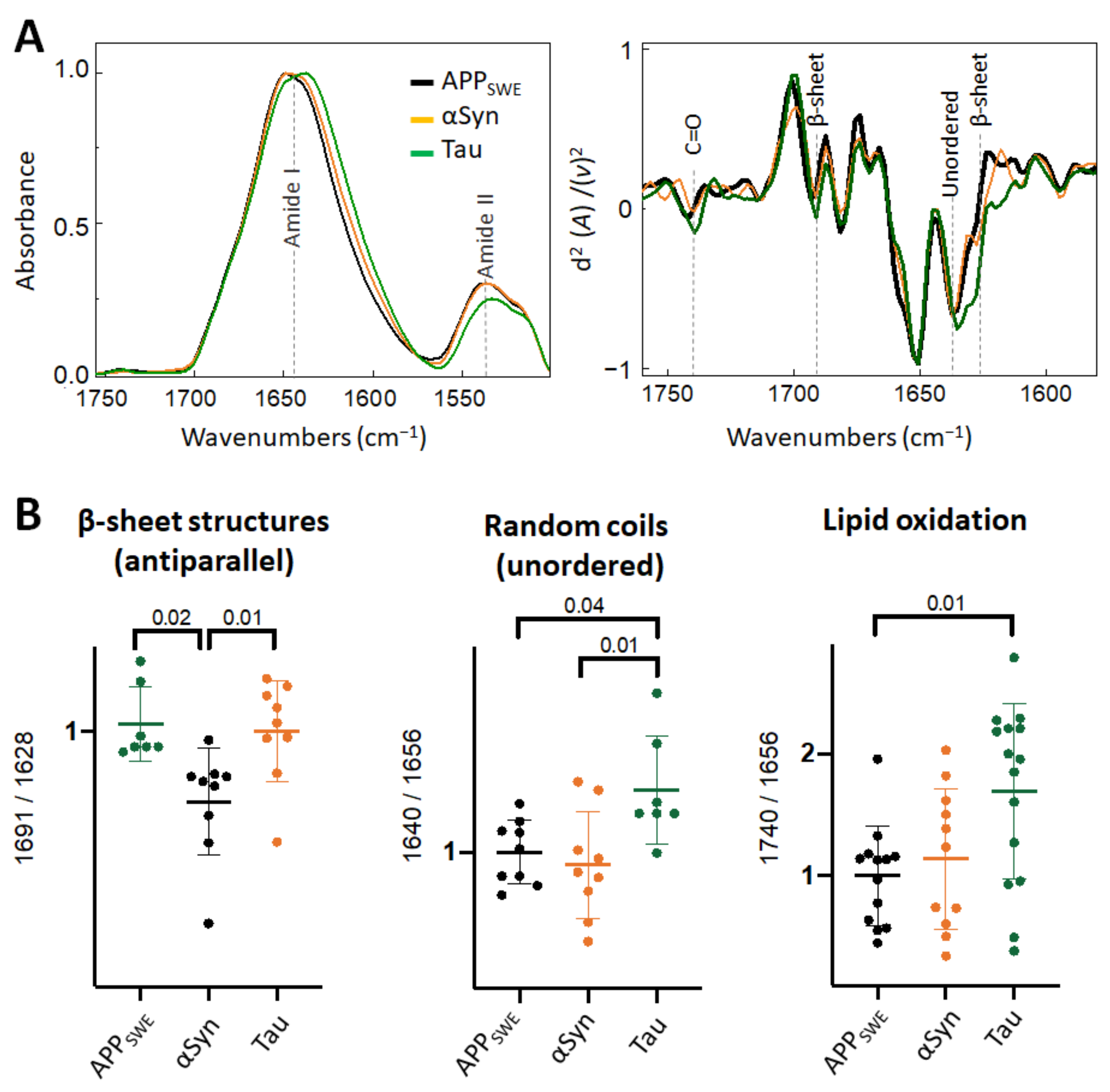
2.4. Diverging Patterns of Amyloid Aggregation in Monogenic and Bigenic Cellular Models
3. Discussion
4. Materials and Methods
4.1. Plasmids
4.2. Cell Lines
4.3. Synchrotron-Based FTIR Microspectroscopy
4.4. Data Analysis
4.5. Aβ Immunofluorescent Labeling and Confocal Imaging
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amar, F.; Sherman, M.A.; Rush, T.; Larson, M.; Boyle, G.; Chang, L.; Götz, J.; Buisson, A.; Lesné, S.E. The Amyloid-β Oligomer Aβ*56 Induces Specific Alterations in Neuronal Signaling That Lead to Tau Phosphorylation and Aggregation. Sci. Signal. 2017, 10, eaal2021. [Google Scholar] [CrossRef] [PubMed]
- Gerson, J.E.; Farmer, K.M.; Henson, N.; Castillo-Carranza, D.L.; Carretero Murillo, M.; Sengupta, U.; Barrett, A.; Kayed, R. Tau Oligomers Mediate α-Synuclein Toxicity and Can Be Targeted by Immunotherapy. Mol. Neurodegener. 2018, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Dettmer, U.; Lesné, S.E. Soluble Endogenous Oligomeric α-Synuclein Species in Neurodegenerative Diseases: Expression, Spreading, and Cross-Talk. JPD 2020, 10, 791–818. [Google Scholar] [CrossRef] [PubMed]
- Bassil, F.; Brown, H.J.; Pattabhiraman, S.; Iwasyk, J.E.; Maghames, C.M.; Meymand, E.S.; Cox, T.O.; Riddle, D.M.; Zhang, B.; Trojanowski, J.Q.; et al. Amyloid-Beta (Aβ) Plaques Promote Seeding and Spreading of Alpha-Synuclein and Tau in a Mouse Model of Lewy Body Disorders with Aβ Pathology. Neuron 2020, 105, 260–275.e6. [Google Scholar] [CrossRef] [PubMed]
- Colom-Cadena, M.; Gelpi, E.; Charif, S.; Belbin, O.; Blesa, R.; Martí, M.J.; Clarimon, J.; Lleó, A. Confluence of α-Synuclein, Tau, and β-Amyloid Pathologies in Dementia With Lewy Bodies. J. Neuropathol. Exp. Neurol. 2013, 72, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef]
- Hamilton, R.L. Lewy Bodies in Alzheimer’s Disease: A Neuropathological Review of 145 Cases Using α-Synuclein Immunohistochemistry. Brain Pathol. 2006, 10, 378–384. [Google Scholar] [CrossRef]
- Irwin, D.J.; Lee, V.M.-Y.; Trojanowski, J.Q. Parkinson’s Disease Dementia: Convergence of α-Synuclein, Tau and Amyloid-β Pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef]
- Robinson, J.L.; Lee, E.B.; Xie, S.X.; Rennert, L.; Suh, E.; Bredenberg, C.; Caswell, C.; Van Deerlin, V.M.; Yan, N.; Yousef, A.; et al. Neurodegenerative Disease Concomitant Proteinopathies Are Prevalent, Age-Related and APOE4-Associated. Brain 2018, 141, 2181–2193. [Google Scholar] [CrossRef]
- He, Z.; Guo, J.L.; McBride, J.D.; Narasimhan, S.; Kim, H.; Changolkar, L.; Zhang, B.; Gathagan, R.J.; Yue, C.; Dengler, C.; et al. Amyloid-β Plaques Enhance Alzheimer’s Brain Tau-Seeded Pathologies by Facilitating Neuritic Plaque Tau Aggregation. Nat. Med. 2018, 24, 29–38. [Google Scholar] [CrossRef]
- Hardy, J.; Allsop, D. Amyloid Deposition as the Central Event in the Aetiology of Alzheimer’s Disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Kametani, F.; Hasegawa, M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef]
- Schneider, L. A Resurrection of Aducanumab for Alzheimer’s Disease. Lancet Neurol. 2020, 19, 111–112. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Seripa, D.; Imbimbo, B.P. Amyloid-β Immunotherapy for Alzheimer Disease: Is It Now a Long Shot? Aβ Immunotherapy Gamble. Ann. Neurol. 2019, 85, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Vlassenko, A.G.; Benzinger, T.L.S.; Morris, J.C. PET Amyloid-Beta Imaging in Preclinical Alzheimer’s Disease. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2012, 1822, 370–379. [Google Scholar] [CrossRef]
- Poduslo, J.F.; Wengenack, T.M.; Curran, G.L.; Wisniewski, T.; Sigurdsson, E.M.; Macura, S.I.; Borowski, B.J.; Jack, C.R. Molecular Targeting of Alzheimer’s Amyloid Plaques for Contrast-Enhanced Magnetic Resonance Imaging. Neurobiol. Dis. 2002, 11, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Dudeffant, C.; Vandesquille, M.; Herbert, K.; Garin, C.M.; Alves, S.; Blanchard, V.; Comoy, E.E.; Petit, F.; Dhenain, M. Contrast-Enhanced MR Microscopy of Amyloid Plaques in Five Mouse Models of Amyloidosis and in Human Alzheimer’s Disease Brains. Sci. Rep. 2017, 7, 4955. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.H.; Milner, T.A.; Li, F.; Nam, E.E.; Edgar, M.A.; Yamaguchi, H.; Beal, M.F.; Xu, H.; Greengard, P.; Gouras, G.K. Intraneuronal Alzheimer Abeta42 Accumulates in Multivesicular Bodies and Is Associated with Synaptic Pathology. Am. J. Pathol. 2002, 161, 1869–1879. [Google Scholar] [CrossRef]
- Dear, A.J.; Michaels, T.C.T.; Meisl, G.; Klenerman, D.; Wu, S.; Perrett, S.; Linse, S.; Dobson, C.M.; Knowles, T.P.J. Kinetic Diversity of Amyloid Oligomers. Proc. Natl. Acad. Sci. USA 2020, 117, 12087–12094. [Google Scholar] [CrossRef]
- Bansal, A.; Schmidt, M.; Rennegarbe, M.; Haupt, C.; Liberta, F.; Stecher, S.; Puscalau-Girtu, I.; Biedermann, A.; Fändrich, M. AA Amyloid Fibrils from Diseased Tissue Are Structurally Different from in Vitro Formed SAA Fibrils. Nat. Commun. 2021, 12, 1013. [Google Scholar] [CrossRef]
- Kayed, R. Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef]
- Kayed, R.; Head, E.; Sarsoza, F.; Saing, T.; Cotman, C.W.; Necula, M.; Margol, L.; Wu, J.; Breydo, L.; Thompson, J.L.; et al. Fibril Specific, Conformation Dependent Antibodies Recognize a Generic Epitope Common to Amyloid Fibrils and Fibrillar Oligomers That Is Absent in Prefibrillar Oligomers. Mol. Neurodegener. 2007, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Costantino, I.; Venugopalan, N.; Fischetti, R.F.; Hyman, B.T.; Frosch, M.P.; Gomez-Isla, T.; Makowski, L. Amyloid Structure Exhibits Polymorphism on Multiple Length Scales in Human Brain Tissue. Sci. Rep. 2016, 6, 33079. [Google Scholar] [CrossRef]
- Kollmer, M.; Close, W.; Funk, L.; Rasmussen, J.; Bsoul, A.; Schierhorn, A.; Schmidt, M.; Sigurdson, C.J.; Jucker, M.; Fändrich, M. Cryo-EM Structure and Polymorphism of Aβ Amyloid Fibrils Purified from Alzheimer’s Brain Tissue. Nat. Commun. 2019, 10, 4760. [Google Scholar] [CrossRef] [PubMed]
- Klementieva, O.; Sandt, C.; Martinsson, I.; Kansiz, M.; Gouras, G.K.; Borondics, F. Super-Resolution Infrared Imaging of Polymorphic Amyloid Aggregates Directly in Neurons. Adv. Sci. 2020, 7, 1903004. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier Transform IR Spectroscopy to Analyze Biological Materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef]
- Bombalska, A.; Mularczyk-Oliwa, M.; Kwaśny, M.; Włodarski, M.; Kaliszewski, M.; Kopczyński, K.; Szpakowska, M.; Trafny, E.A. Classification of the Biological Material with Use of FTIR Spectroscopy and Statistical Analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Prentice, B.M.; Caprioli, R.M.; Vuiblet, V. Label-Free Molecular Imaging of the Kidney. Kidney Int. 2017, 92, 580–598. [Google Scholar] [CrossRef]
- Klementieva, O.; Willén, K.; Martinsson, I.; Israelsson, B.; Engdahl, A.; Cladera, J.; Uvdal, P.; Gouras, G.K. Pre-Plaque Conformational Changes in Alzheimer’s Disease-Linked Aβ and APP. Nat. Commun. 2017, 8, 14726. [Google Scholar] [CrossRef]
- Miller, L.M.; Dumas, P. From Structure to Cellular Mechanism with Infrared Microspectroscopy. Curr. Opin. Struct. Biol. 2010, 20, 649–656. [Google Scholar] [CrossRef]
- Diem, M.; Romeo, M.; Boydston-White, S.; Miljković, M.; Matthäus, C. A Decade of Vibrational Micro-Spectroscopy of Human Cells and Tissue (1994–2004). Analyst 2004, 129, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Holman, H.-Y.N.; Martin, M.C.; McKinney, W.R. Synchrotron-Based FTIR Spectromicroscopy: Cytotoxicity and Heating Considerations. J. Biol. Phys. 2003, 29, 275–286. [Google Scholar] [CrossRef] [PubMed]
- DeFlores, L.P.; Ganim, Z.; Nicodemus, R.A.; Tokmakoff, A. Amide I′−II′ 2D IR Spectroscopy Provides Enhanced Protein Secondary Structural Sensitivity. J. Am. Chem. Soc. 2009, 131, 3385–3391. [Google Scholar] [CrossRef]
- Jackson, M.; Mantsch, H.H. The Use and Misuse of FTIR Spectroscopy in the Determination of Protein Structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the Secondary Structure of Proteins by Deconvolved FTIR Spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta BBA Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Cerf, E.; Sarroukh, R.; Tamamizu-Kato, S.; Breydo, L.; Derclaye, S.; Dufrêne, Y.F.; Narayanaswami, V.; Goormaghtigh, E.; Ruysschaert, J.-M.; Raussens, V. Antiparallel β-Sheet: A Signature Structure of the Oligomeric Amyloid β-Peptide. Biochem. J. 2009, 421, 415–423. [Google Scholar] [CrossRef]
- Roeters, S.J.; Iyer, A.; Pletikapić, G.; Kogan, V.; Subramaniam, V.; Woutersen, S. Evidence for Intramolecular Antiparallel Beta-Sheet Structure in Alpha-Synuclein Fibrils from a Combination of Two-Dimensional Infrared Spectroscopy and Atomic Force Microscopy. Sci. Rep. 2017, 7, 41051. [Google Scholar] [CrossRef]
- Benseny-Cases, N.; Klementieva, O.; Cladera, J. In vitro Oligomerization and Fibrillogenesis of Amyloid-beta Peptides. In Protein Aggregation and Fibrillogenesis in Cerebral and Systemic Amyloid Disease; Harris, J.R., Ed.; Springer: Dordrecht, The Netherlands, 2012; Volume 65, pp. 53–74. ISBN 978-94-007-5415-7. [Google Scholar]
- Benseny-Cases, N.; Klementieva, O.; Cotte, M.; Ferrer, I.; Cladera, J. Microspectroscopy (ΜFTIR) Reveals Co-Localization of Lipid Oxidation and Amyloid Plaques in Human Alzheimer Disease Brains. Anal. Chem. 2014, 86, 12047–12054. [Google Scholar] [CrossRef]
- Olsson, T.T.; Klementieva, O.; Gouras, G.K. Prion-like Seeding and Nucleation of Intracellular Amyloid-β. Neurobiol. Dis. 2018, 113, 1–10. [Google Scholar] [CrossRef]
- Mullan, M.; Crawford, F.; Axelman, K.; Houlden, H.; Lilius, L.; Winblad, B.; Lannfelt, L. A Pathogenic Mutation for Probable Alzheimer’s Disease in the APP Gene at the N–Terminus of β–Amyloid. Nat. Genet. 1992, 1, 345–347. [Google Scholar] [CrossRef]
- Haass, C.; Lemere, C.A.; Capell, A.; Citron, M.; Seubert, P.; Schenk, D.; Lannfelt, L.; Selkoe, D.J. The Swedish Mutation Causes Early-Onset Alzheimer’s Disease by β-Secretase Cleavage within the Secretory Pathway. Nat. Med. 1995, 1, 1291–1296. [Google Scholar] [CrossRef]
- Aho, L.; Pikkarainen, M.; Hiltunen, M.; Leinonen, V.; Alafuzoff, I. Immunohistochemical Visualization of Amyloid-β Protein Precursor and Amyloid-β in Extra- and Intracellular Compartments in the Human Brain. JAD 2010, 20, 1015–1028. [Google Scholar] [CrossRef]
- Hatami, A.; Albay, R.; Monjazeb, S.; Milton, S.; Glabe, C. Monoclonal Antibodies against Aβ42 Fibrils Distinguish Multiple Aggregation State Polymorphisms in Vitro and in Alzheimer Disease Brain. J. Biol. Chem. 2014, 289, 32131–32143. [Google Scholar] [CrossRef]
- Marcelli, A.; Cricenti, A.; Kwiatek, W.M.; Petibois, C. Biological Applications of Synchrotron Radiation Infrared Spectromicroscopy. Biotechnol. Adv. 2012, 30, 1390–1404. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, S.; Zhao, K.; Long, H.; Liu, Z.; Gao, J.; Zhang, Y.; Su, X.-D.; Li, D.; Liu, C. Cryo-EM Structure of Full-Length α-Synuclein Amyloid Fibril with Parkinson’s Disease Familial A53T Mutation. Cell Res. 2020, 30, 360–362. [Google Scholar] [CrossRef]
- Linse, S.; Scheidt, T.; Bernfur, K.; Vendruscolo, M.; Dobson, C.M.; Cohen, S.I.A.; Sileikis, E.; Lundqvist, M.; Qian, F.; O’Malley, T.; et al. Kinetic Fingerprints Differentiate the Mechanisms of Action of Anti-Aβ Antibodies. Nat. Struct. Mol. Biol. 2020, 27, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, P.; Di Giambattista, L.; Giordani, S.; Udroiu, I.; Pozzi, D.; Gaudenzi, S.; Bedini, A.; Giliberti, C.; Palomba, R.; Congiu Castellano, A. Ultrasound-Mediated Structural Changes in Cells Revealed by FTIR Spectroscopy: A Contribution to the Optimization of Gene and Drug Delivery. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 84, 74–85. [Google Scholar] [CrossRef]
- Liu, H.; Su, Q.; Wu, Q.; Fang, W.; Yang, D.; Zheng, W.; Wang, X. FTIR Spectroscopic Study on Apoptosis of Lung Cancer Cell Line A549 Induced by Arsenic Trioxide. Infrared Phys. Technol. 2018, 93, 340–345. [Google Scholar] [CrossRef]
- Miller, L.M.; Bourassa, M.W.; Smith, R.J. FTIR Spectroscopic Imaging of Protein Aggregation in Living Cells. Biochim. Biophys. Acta BBA Biomembr. 2013, 1828, 2339–2346. [Google Scholar] [CrossRef]
- Sorrentino, Z.A.; Goodwin, M.S.; Riffe, C.J.; Dhillon, J.-K.S.; Xia, Y.; Gorion, K.-M.; Vijayaraghavan, N.; McFarland, K.N.; Golbe, L.I.; Yachnis, A.T.; et al. Unique α-Synuclein Pathology within the Amygdala in Lewy Body Dementia: Implications for Disease Initiation and Progression. Acta Neuropathol. Commun. 2019, 7, 142. [Google Scholar] [CrossRef]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of Tau Protein in Health and Disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef]
- Spadea, A.; Denbigh, J.; Lawrence, M.J.; Kansiz, M.; Gardner, P. Analysis of Fixed and Live Single Cells Using Optical Photothermal Infrared with Concomitant Raman Spectroscopy. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Peng, C.; Gathagan, R.J.; Covell, D.J.; Medellin, C.; Stieber, A.; Robinson, J.L.; Zhang, B.; Pitkin, R.M.; Olufemi, M.F.; Luk, K.C.; et al. Cellular Milieu Imparts Distinct Pathological α-Synuclein Strains in α-Synucleinopathies. Nature 2018, 557, 558–563. [Google Scholar] [CrossRef]
- Khan, S.S.; LaCroix, M.; Boyle, G.; Sherman, M.A.; Brown, J.L.; Amar, F.; Aldaco, J.; Lee, M.K.; Bloom, G.S.; Lesné, S.E. Bidirectional Modulation of Alzheimer Phenotype by Alpha-Synuclein in Mice and Primary Neurons. Acta Neuropathol. 2018, 136, 589–605. [Google Scholar] [CrossRef]
- Boza-Serrano, A.; Ruiz, R.; Sanchez-Varo, R.; García-Revilla, J.; Yang, Y.; Jimenez-Ferrer, I.; Paulus, A.; Wennström, M.; Vilalta, A.; Allendorf, D.; et al. Galectin-3, a Novel Endogenous TREM2 Ligand, Detrimentally Regulates Inflammatory Response in Alzheimer’s Disease. Acta Neuropathol. 2019, 138, 251–273. [Google Scholar] [CrossRef]
- Honcharenko, D.; Juneja, A.; Roshan, F.; Maity, J.; Galán-Acosta, L.; Biverstål, H.; Hjorth, E.; Johansson, J.; Fisahn, A.; Nilsson, L.; et al. Amyloid-β Peptide Targeting Peptidomimetics for Prevention of Neurotoxicity. ACS Chem. Neurosci. 2019, 10, 1462–1477. [Google Scholar] [CrossRef]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia–Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef]
- Martinsson, I.; Capetillo-Zarate, E.; Faideau, M.; Willén, K.; Esteras, N.; Frykman, S.; Tjernberg, L.O.; Gouras, G.K. APP Depletion Alters Selective Pre- and Post-Synaptic Proteins. Mol. Cell. Neurosci. 2019, 95, 86–95. [Google Scholar] [CrossRef]
- Voortman, G.; Gerrits, J.; Altavilla, M.; Henning, M.; van Bergeijk, L.; Hessels, J. Quantitative Determination of Faecal Fatty Acids and Triglycerides by Fourier Transform Infrared Analysis with a Sodium Chloride Transmission Flow Cell. Clin. Chem. Lab. Med. 2002, 40. [Google Scholar] [CrossRef]
- Nara, M.; Okazaki, M.; Kagi, H. Infrared Study of Human Serum Very-Low-Density and Low-Density Lipoproteins. Implication of Esterified Lipid C-O Stretching Bands for Characterizing Lipoproteins. Chem. Phys. Lipids 2002, 117, 1–6. [Google Scholar] [CrossRef]
- Petibois, C.; Déléris, G. Analysis and Monitoring of Oxidative Stress in Exercise and Training by FTIR Spectrometry. Int. J. Sports Physiol. Perform. 2008, 3, 119–130. [Google Scholar] [CrossRef] [PubMed]
| Wavenumbers (cm−1) | Structure |
|---|---|
| 1656 cm−1 | C=O stretching, Amide I |
| 1550 cm−1 | C–N stretching; N–H bending, Amide II |
| 1628 cm−1 | β-sheet (main) |
| 1640 cm−1 | random coils (unordered) |
| 1656 cm−1 | α-helix |
| 1691 cm−1 | β-sheet (weak) |
| 1740 cm−1 | C=O bond of the ester peak |
| Ratio | Structure |
|---|---|
| 1550 cm−1/1656 cm−1 | protein folding |
| 1628 cm−1/1656 cm−1 | parallel β-sheet features |
| 1640 cm−1/1656 cm−1 | random coils (unordered) |
| 1691 cm−1/1628 cm−1 | anti-parallel β-sheet |
| 1740 cm−1/1656 cm−1 | C=O, lipid oxidation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulus, A.; Engdahl, A.; Yang, Y.; Boza-Serrano, A.; Bachiller, S.; Torres-Garcia, L.; Svanbergsson, A.; Garcia, M.G.; Gouras, G.K.; Li, J.-Y.; et al. Amyloid Structural Changes Studied by Infrared Microspectroscopy in Bigenic Cellular Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 3430. https://doi.org/10.3390/ijms22073430
Paulus A, Engdahl A, Yang Y, Boza-Serrano A, Bachiller S, Torres-Garcia L, Svanbergsson A, Garcia MG, Gouras GK, Li J-Y, et al. Amyloid Structural Changes Studied by Infrared Microspectroscopy in Bigenic Cellular Models of Alzheimer’s Disease. International Journal of Molecular Sciences. 2021; 22(7):3430. https://doi.org/10.3390/ijms22073430
Chicago/Turabian StylePaulus, Agnes, Anders Engdahl, Yiyi Yang, Antonio Boza-Serrano, Sara Bachiller, Laura Torres-Garcia, Alexander Svanbergsson, Megg G. Garcia, Gunnar K. Gouras, Jia-Yi Li, and et al. 2021. "Amyloid Structural Changes Studied by Infrared Microspectroscopy in Bigenic Cellular Models of Alzheimer’s Disease" International Journal of Molecular Sciences 22, no. 7: 3430. https://doi.org/10.3390/ijms22073430
APA StylePaulus, A., Engdahl, A., Yang, Y., Boza-Serrano, A., Bachiller, S., Torres-Garcia, L., Svanbergsson, A., Garcia, M. G., Gouras, G. K., Li, J.-Y., Deierborg, T., & Klementieva, O. (2021). Amyloid Structural Changes Studied by Infrared Microspectroscopy in Bigenic Cellular Models of Alzheimer’s Disease. International Journal of Molecular Sciences, 22(7), 3430. https://doi.org/10.3390/ijms22073430







