Abstract
Arachis hypogaea abscisic acid transporter like-1 (AhATL1) modulates abscisic acid (ABA) sensitivity by specifically influencing the importing of ABA into cells, and is a key player in plant stress responses. However, there is limited information on ABA transporters in crops. In this study, we found that the level of AhATL1 expression and AhATL1 distribution increased more rapidly in the second drought (D2) compared with in the first drought (D1). Compared with the first recovery (R1), the AhATL1 expression level and ABA content remained at a higher level during the second recovery (R2). The heterologous overexpression of AhATL1 in Arabidopsis changed the expression pattern of certain memory genes and changed the post response gene type into the memory gene type. Regarding the proline and water content of Col (Arabidopsis thaliana L. Heynh., Col-0), atabcg22, and AhATL1-OX during drought training, the second drought (D2) was more severe than the first drought (D1), which was more conducive to maintaining the cell osmotic balance and resisting drought. In summary, drought stress memory resulted in a rapid increase in the AhATL1 expression and AhATL1 distribution level, and then raised the endogenous ABA content and changed the post response gene type into the memory gene type, which enhanced the drought resistance and recovery ability.
1. Introduction
Plants will experience the same environmental stress many times in the process of natural growth [1]. Plants enter a “priming” state for the first stress time. When the plant faces the stress again, the response will be faster and stronger than the first time, and will store the information of the previous stress to form the stress memory [2,3]. Among the numerous environmental stresses, drought is one of the most influential and destructive [4].
Peanuts (Arachis hypogaea. L) are an important economic and oil crop in China. The peanut yield reduction caused by drought accounted for more than 20% of the total peanut production in China. In the actual production of peanuts, drought and water shortage environments are repeated. Plants can recognize and remember the initial stress and show more rapid and strong physiological defense when re-exposed to biotic or abiotic stress to increase their chances of survival [5]. Therefore, it is urgent to explore whether there is a drought memory in peanuts and the molecular mechanism of the peanut response to drought stress, which will provide new ideas and an alternative gene bank for improving the drought resistance of peanuts.
Abscisic acid (ABA) is not only a cell signal but also a system stress signal, which plays a key role in the plant stress resistance, in particular the drought resistance, which is reflected in the response to stress signals and the steady state of the ABA level [6]. The fine regulation of the ABA level in specific physiological processes and tissues and organs by multi-factor system coordinated regulation [7]. The ATP binding cassette (ABC) transporter family is one of the largest protein families, which can hydrolyze the energy released by ATP and complete the transmembrane transport of substrates. We further found that the rate of the ABA uptake by mutant cells was significantly lower than that of the wild type, indicating that Arabidopsis thaliana AtABCG40/AtPDR12 is involved in the process of the ABA transmembrane transport into cells [8]. Kuromori et al. [9] also found that AtABCG25 can transport ABA across the membrane. ABCG40 and ABCG25 are involved in the absorption and efflux of ABA in plant cells, which play an important role in the rapid and effective response of plants to stress. AhATL1 (Arachis hypogaea abscisic acid transporter like-1) gene was isolated and identified from Peanut in our laboratory.
Its homologous protein, AhATL1, is a member of the G subfamily of ATP binding transporters and is located in plasma membrane. AhATL1 transcription and protein expression was up regulated by water stress and exogenous ABA treatment [10]. The study showed that during the repeated process of drought stress (dehydration–rehydration–dehydration–rehydration), the expression level of AhATL1 was low during the first drought, and increased after rehydration, and the expression of AhATL1 gene in leaves decreased and then increased with the duration of drought stress [11].
Currently, there are few studies on the memory regulation mechanism of plant response to drought stress, in particular, the distribution of related proteins leading to changes in plant hormone levels in this process. The biosynthesis, degradation, and inactivation of ABA affect the partial level of local active ABA, and cause further changes in ABA level through conjugation and transport. [12,13]. ABA is rapidly synthesized in root vascular parenchyma under root stress, and is then rapidly transported to leaf tissues, where it induces stomatal closure in peanut and Arabidopsis [14,15,16].
The compensation effect of water stress on root water absorption was positively correlated with the degree and duration of water stress before rehydration. The recovery process of photosynthesis and transpiration during rehydration showed that the compensation effect was positively correlated with the degree and duration of stress [17,18]. Our previous research found that the wilting degree of leaves in the second drought recovery (R2) was significantly lower than that in the first drought (D1). The stem was straighter, and the drought resistance was clearly improved [11]. Therefore, this treatment system can simulate the growth of the peanut memory under drought stress; however, the specific mechanism of the response to stress and memory regulation is not clear. This study will explore the changes in the AhATL1 protein and ABA levels in peanuts during the process of stress memory and provide a new perspective for the molecular mechanism of the peanut drought stress memory process.
2. Results
2.1. High Level of AhATL1 Expression and AhATL1 Distribution Resulted in Rapid Increase in ABA Content in Peanut during Drought Stress Memory
In the process of drought stress memory acquisition, the expression level of AhATL1 in peanut leaves was increased slowly in the D1 process and increased sharply at 8 h. Compared with that in D1, AhATL1 expression level at 4 h (in D2) was higher than that in D1 process (the same drought time node was compared). At 4–48 h of the R2 process, the expression trend of the gene was the same as that of R1, which first increased and then gradually decreased to a low level. However, the overall expression level of the R2 process was higher than that of R1 (Figure 1a). Then, we further studied the distribution level of AhATL1 and found that, under normal conditions, the specific distribution level of the AhATL1 protein in leaves was very low.
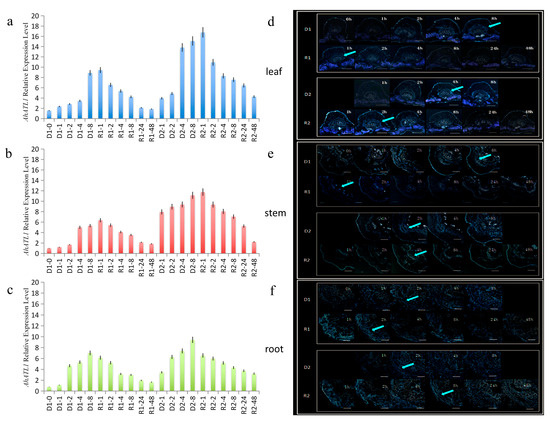
Figure 1.
The AhATL1 expression and AhATL1 distribution analyses in peanuts during the drought stress memory process evaluated with real-time quantitative PCR (RT-qPCR) and the immunofluorescence localization assays. The time points of the first drought (D1)-1 to the second drought recovery (R2)-48 were sampled to observe the expression and distribution-changing trend. The untreated group was used as the control (D1-0). (a–c) Expression analyses of AhATL1 in peanut leaves, stems, and roots during the drought stress memory process. UBQ10 was used as an internal control. The mean and SD (standard deviation) were obtained from more than three biological replicates. (d–f) AhATL1 immunostaining of the leaves, stems, and roots following the same treatment regime described for a. The control samples were treated with PBS; blue fluorescence (arrows) indicates the presence of AhATL1; and no color indicates a lack of staining.
However, in the D2 process, the AhATL1 distribution increased significantly at 4 h, faster than that in D1 (8 h). During the process of R1, the AhATL1 distribution level was maintained at a low level for 4 h. In the R2 process, the AhATL1 distribution level was increased first at 2 h, and then decreased gradually (Figure 1d). The distribution of AhATL1 in R2 was stronger than that in R1 (the same drought time mode was compared).
In stems, the expression level of AhATL1 in D1 increased remarkably at 4 h, while that in D2 increased at 1 h. During the process of R1, the expression level of AhATL1 increased slightly at 1–2 h and then decreased gradually; R2 increased at 4 h and then decreased gradually (Figure 1b). For the AhATL1 distribution level, those in the vascular tissue of the phloem of the stems of the D1 and D2 processes were increased gradually at 2 h. At the R1 and R2 stages of rehydration, the overall distribution level of AhATL1 was low. Among them, the AhATL1 distribution level was increased a little at 1–2 h, and then gradually decreased in R1 (Figure 1e), while the AhATL1 distribution level was increased at first (to 4 h).
In the root, the expression level of AhATL1 increased significantly at 2 h in the D1 process; however, the increasing trend became gradually flat. Meanwhile, in D2, the AhATL1 level gradually increased at 2–8 h, and was maintained at a high level. In the process of R1, the expression level of AhATL1 gradually decreased during the whole rehydration process, while R2 maintained a high level (1–4 h), and then gradually decreased (Figure 1c). At the protein level, the AhATL1 distribution level increased slowly and reached a higher level at 8 h in D1 and, in the D2 process, gradually increased at 2–8 h and maintained a higher level. At the R1 and R2 of rehydration, the AhATL1 distribution level decreased gradually in the whole rehydration process during the R1 process, while R2 maintained a high level (1–4 h) at first and then decreased gradually (Figure 1f).
In response to drought stress in peanuts, the expression of AhATL1 specifically affects ABA signal. We investigated the distribution and content of ABA in peanuts during the drought stress memory process. The content of ABA in peanut leaves increased continuously in the process of D1 and increased sharply at 4 h, which was 112.7% higher than at 0 h. With the drought treatment, the content of ABA increased slightly and then decreased after 4 h in R1 (Figure 2a). During D1 and D2, the distribution level of ABA showed a trend of increasing gradually, and the increasing speed of the ABA distribution level in the D2 process was faster than in the D1 process, which had obvious enhancement at 4 h (Figure 2d).
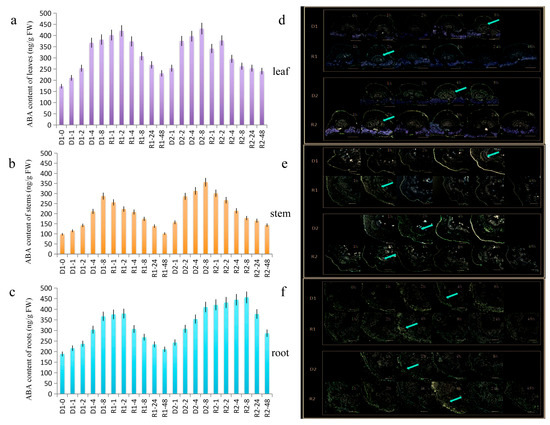
Figure 2.
The abscisic acid (ABA) content and distribution analyses in peanut during drought stress memory process using HPLC and immunofluorescence localization assays. The time points of D1-1 to R2-48 were sampled to observe the expression and distribution-changing trends. The untreated group was used as the control (D1-0). (a–c) The ABA content of the leaves, stems, and roots in peanuts during the drought stress memory process. The mean and SD were obtained from more than three biological replicates. (d–f) The ABA immunostaining of leaves, stems, and roots following the same treatment regime described for a. The control samples were treated with PBS; green fluorescence (arrows) indicates the presence of ABA; and no color indicates a lack of staining. Bars = 50 μm for leaf panels and 100 μm for the stem and root panels.
For the stem of peanuts, in the process of D1, the content of ABA increased gradually at 48 h. The ABA content was increased significantly at 2 h and then continued to increase in D2. The content of ABA maintained a decreased trend in R1, while the ABA content was increased (to 2 h) and then gradually decreased in R2 (Figure 2b). On the other hand, the distribution level of ABA equably increased with the stress in D1, while it was increased significantly at 2 h in D2. In the process of R1 and R2, the ABA distribution level increased slightly at first and then decreased gradually after 2 h; however, the overall ABA distribution level was stronger than that of R1 (Figure 2e).
In the roots, the increasing speed of the ABA content in D2 was faster than in D1, which was obviously enhanced at 2 h, while that in D1 was 4 h. In the process of R1, ABA content increased gradually at 4 h, while in the process of R2, it increased gradually at 8 h. The content of ABA increased first and then decreased in both processes (Figure 2c). For the level of the ABA distribution, those were increased in D2 faster than that in D1 and increased significantly at 2 h, and in D1 at 4 h. During the rehydration process, the ABA-specific distribution of R2 was stronger than that of R1 (Figure 2f).
2.2. Heterologous Overexpression of AhATL1 Alters the Expression Patterns of Some Memory Genes in Arabidopsis Thaliana
Ding et al. [19] proposed the standard of transcriptional memory in Zea mays and Arabidopsis thaliana, and believed that the transcriptional response must be different under similar stress conditions. Therefore, in the repeated dehydration stress/rehydration cycle, three types of genes were found: memory genes [+/+], [−/−], [−/+], and [+/−]; non-memory genes [+/=] and [−/=]; and post response genes [=/+] and [=/−]. The first symbol indicates that the transcriptional level of plants under the first stress was higher (+) or lower (−) than that under normal watering. The second symbol indicates that the transcriptional level of the second stress was higher (+) or lower (−) than that of the first stress. Non-memory genes [+/=] and [−/=] indicated that the transcriptional levels up or down regulated in the first stress were similar to those in the first stress. The post response genes [=/+] and [=/−] indicated that the transcriptional levels were similar in the first stress, but up-regulated or down-regulated in the second stress. In the process of rehydration, the genes that produced a similar level of transcriptional response to each stress are considered non-memory genes, and the genes showing significant differences from the first stress response in the subsequent stress are considered memory genes.
To further explore the response mechanism of AhATL1-mediated drought stress memory, Arabidopsis thaliana was used. After several short-term drought treatments, compared with the control group, the leaf cells of the experimental group in Arabidopsis plants had stronger water holding capacity and higher expression level of drought response genes. These results indicate that stress response genes can be “trained” by stress and show differential expression in response to repeated stress [19]. Therefore, plants can distinguish between single stress and repeated stress, and regulate the expression of stress response genes accordingly. In Arabidopsis and maize, after repeated drought stress, memory genes with functions in ABA and JA regulatory pathways were found by RNA SEQ analysis, which indicated that plant hormones were involved in short-term drought stress memory [19,20,21]. Drought stress can induce many signaling pathways [14]. Therefore, we will focus on the response to drought stress and ABA signaling pathway-related genes. These include: stress response marker gene, AtRD29A; ABA synthesis gene, AtNCED3 (9-cis-carotenoid dioxygenase gene); ABA degradation gene, AtCYP707A1 (cytochrome P450 monooxygenase gene); transformation of free ABA into binding ABA gene, AtUGT71C5 (ABA glycosyltransferase gene); the binding ABA was transformed into free ABA gene, AtBG2 (β—glucosidase gene); the downstream genes of ABA signaling pathway and regulation: AtPP2C5 (protein phosphatase gene), AtOST1 (atsnrk2.6, protein kinase gene), AtABI5 (ABA response binding factor), AtAREB1 (ABA response binding factor), AtSLAC1 (plasma membrane located anion channel gene), AtALMT12 (plasma membrane located anion channel gene); ABA transport genes: AtABCG11 (wax export gene), AtABCG22 (ABA input gene), AtABCG40 (ABA input gene), and AtABCG25 (ABA output gene).
We found that AtRD29A, AtNCED3, AtPP2C5, AtSLAC1, AtALMT12, AtABCG11, AtABCG22, AtABCG40, and AtABCG25 were memory gene types with [+/−] expression patterns, indicating that these genes were induced in the D1 process and down-regulated in the D2 process. AtUGT71C5 is a memory gene type, and its expression pattern is [−/+], indicating that the gene is down-regulated in the D1 process and up-regulated in the D2 process. AtCYP707A1 is a post response gene type with a [=/+] expression pattern, which indicates that the expression of AtCYP707A1 in the D1 process is similar to that in the D1-0 h process, and this is further induced in the D2 process (Figure 3). There were two types of gene expression change in Col Arabidopsis plants, including six expression patterns (Table 1). AtOST1, AtABI5, AtAREB1, and AtBG2 were memory gene types, which were [+/+] expression patterns, which indicated that these genes were induced to express in the D1 process and to a higher expression level in the D2 process.
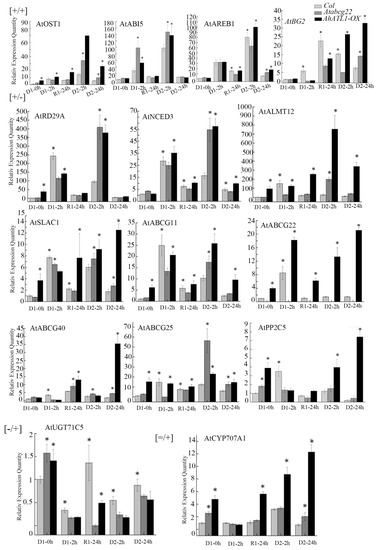
Figure 3.
Changes in the expression of ABA and stress response related genes under drought training. [+/+], [+/−], [−/+], and [=/+] indicate the expression patterns of each gene in wild-type Col. ‘*’ Indicates a significant difference between AhATL1-OX, Atabcg22, and Col plants under the same dehydration and rehydration treatment conditions (p < 0.05). Asterisks indicate significant differences from Col in D1 0h (Student’s t test p values, * p < 0.05).

Table 1.
Classification of the ABA and stress response-related genes under drought training.
On this basis, we found that AtOST1, AtABI5, and AtAREB1 were memory gene types and [+/+] expression patterns, while AtBG2 changed from the [+/+] expression mode to the [−/+] expression mode, which was a memory gene type in AhATL1-OX and atabcg22 plants. The memory gene expression patterns of AtRD29A, AtNCED3, AtSLAC1, AtALMT12, and AtABCG11 changed from [+/−] to [+/+]. The gene expression patterns of AtPP2C5, AtABCG40, and AtABCG25 changed from [+/−] to [−/+], which were memory gene types. The gene expression pattern of AtABCG22 was [+/−], which was the same as Col, while in the atabcg22 mutant, the gene expression pattern of AtUGT71C5 was [−/+], which was the same as Col. The gene expression pattern of AtCYP707A1 changed from [=/+] to [−/+], and the gene type changed from the post response gene to a memory gene (Table 1, Figure 3). These results indicate that the gene expression pattern of the AhATL1-OX strain was similar to that of the atabcg22 strain, but different from Col. Compared with Col, heterologous overexpression of AhATL1 in Arabidopsis changes the expression pattern of certain memory genes as well as the post response gene type into the memory gene type.
[+/+], [+/−], [−/+], and [=/+] indicate the expression patterns of each gene in wild-type Col. ‘*’ Indicates a significant difference between AhATL1-OX, atabcg22, and Col plants under the same dehydration and rehydration treatment conditions (p < 0.05). Asterisks indicate significant differences from Col in D1 0h (Student’s t test p values, * p < 0.05).
2.3. Overexpression of AhATL1 in Arabidopsis Enhances Tolerance Ability in the Drought Stress Memory Process
In order to further verify whether the expression changes of memory related genes under drought stress cause the changes in physiological indexes and plant phenotype, we conducted the following studies. ABA transporter ABCG22 has the function of ABA input, which is located on the plasma membrane of guard cells. The expression of AtABCG22 gene is induced by ABA, and the stomatal closure of atabcg22 mutant decreases under the conditions of low air humidity, high CO2 or exogenous ABA, indicating that it regulates ABA transport and may enhance ABA inflow into guard cells [9,20]. Therefore, since AtABCG22 is a homologous gene in Arabidopsis thaliana, which has ABA transport function and affects drought resistance, in order to study the function of AhATL1, atabcg22 mutant was used to observe whether it could complement each other. The localization of AhATL1—eGFP protein in cell membrane conforms to the characteristics of its membrane protein, which is consistent with the reported AtABCG22. Functional complementation experiments have also proved that AhATL1-eGFP has a function [10]. AhATL1 overexpression vector P35s::AhATL1-eGFP was transformed into Col wild type Arabidopsis thaliana. Compared with Col and P35S::AhATL1-eGFP, atabcg22 and P35S::AhATL1-eGFP/atabcg22 had a darker color and more developed roots. The petioles of plants were drooped, and the leaves were curled and wilted in the D1 process, then gradually recovered after rehydration. After the second dehydration stress (D2), the rate of petiole drooping and leaf wilting was slower. The wilting degree of Col and atabcg22 under dehydrated stress was more severe than that of P35S::AhATL1-eGFP and P35S::AhATL1-eGFP/atabcg22. After rehydration, the plants of Col, P35S::AhATL1-eGFP and P35S::AhATL1-eGFP/atabcg22 recovered well, while atabcg22 recovered poorly (Figure 4a).
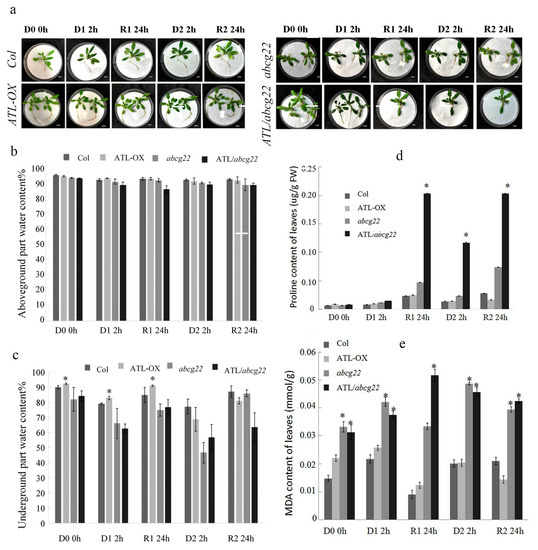
Figure 4.
Overexpression of AhATL1 in Arabidopsis enhanced the plant tolerance during the drought stress memory process. (a) Drought tolerance phenotypes of four lines (Col, P35S::AhATL1-eGFP, atabcg22, and P35S::AhATL1-eGFP/atabcg22) that were dehydrated for 2 h and rehydrated for 24 h two times. (b) The aboveground water content of the four lines that were dehydrated for 2 h and rehydrated for 24 h two times. (c) The belowground water content of the four lines that were dehydrated for 2 h and rehydrated for 24 h two times. (d) The proline content of the four lines that were dehydrated for 2 h and rehydrated for 24 h two times. (e) The MDA content of the four lines that were dehydrated for 2 h and rehydrated for 24 h two times. All experiments, means, and SDs were obtained from more than three biological replicates. ‘*’ indicates a significant difference between AhATL1-OX, atabcg22, P35S::AhATL1-eGFP/atabcg22, and Col plants under the same dehydration and rehydration treatment conditions (p < 0.05). Asterisks in (b) to (e) indicate significant differences from Col in D1 0h (Student’s t test p values, * p < 0.05).
After the first dehydrated stress (D1), the aboveground water content of the four lines decreased by 3.21%, 1.28%, 2.58%, and 4.80%, respectively, and changed little in the process of R1 and D2. The aboveground water content of Col and P35S::AhATL1-eGFP recovered to 91.03% and 90.55%, respectively, while atabcg22 and P35S::AhATL1-eGFP/atabcg22 did not recover and decreased to 87.53% and 87.49% after the second rehydration, respectively (R2) (Figure 4b). For the belowground part of the water content after D1, Col and P35S::AhATL1-eGFP decreased by 12.34% and 10.15%, and then that of P35S::AhATL1-eGFP/atabcg22 decreased significantly more than atabcg22, which decreased by 25.82% and 19.42%, respectively, After the first rehydration (R1), the recovery degrees of the belowground water contents of P35S::AhATL1-eGFP and P35S::AhATL1-eGFP/atabcg22 were greater than those of Col and atabcg22. Combined with the above ground water content, P35S::AhATL1-eGFP and P35S::AhATL1-/atabcg22, the plants that overexpressed AhATL1 may tend to distribute water to the belowground when rewatered. After the second dehydrated stress (D2), the belowground water content of atabcg22 was significantly greater than that of P35S::AhATL1-eGFP/atabcg22; compared with the first dehydrated stress, the belowground water content of the four Arabidopsis species decreased by 2.22%, 17.14%, 29.36%, and 9.30%, respectively (Figure 4c).
Proline is a kind of osmotic adjustment substance in plant cytoplasm. Under stress, the content of proline in plant increases significantly. The proline content in plants reflects the stress resistance of plants to a certain extent [22,23]. Four different Arabidopsis genotypes plants accumulated more proline, among which the proline content of P35S::AhATL1-eGFP/atabcg22 was the highest and was four times higher than that of atabcg22 after R1. Then, the proline content of the four lines (Col, P35S::AhATL1-eGFP, atabcg22, and P35S::AhATL1-eGFP/atabcg22) increased by 69.17%, 47.17%, 100.42%, and 681.36%, respectively, after D2 (Figure 4d).
The cell membrane was damaged by dehydrated stress, and the membrane lipid peroxidation produced malondialdehyde (MDA). After D1, the MDA contents of the four lines were increased by 46.95%, 16.72%, 27.01%, and 20.13%, respectively. During the first rehydration treatment (R1), the MDA contents of Col, P35S::AhATL1-eGFP, and atabcg22 plants were decreased, which were lower than that in D1. It indirectly indicated that the membrane damage was repaired to a certain extent after rehydration, while in D2, the MDA contents of Col, P35S::AhATL1-eGFP, and atabcg22 were increased, and those of P35S::AhATL1-eGFP/atabcg22 were decreased. Compared with the first dehydrated stress, the MDA content of Col and P35S::AhATL1-eGFP were decreased by 7.33% and 20.87%, the cell membrane damage was lighter, and the drought resistance was enhanced. After R2, the MDA content of P35S::AhATL1-eGFP, atabcg22, and P35S::AhATL1-eGFP/atabcg22 were decreased, during which the MDA content of the Col plants continued to accumulate (Figure 4e).
3. Discussion
During the growth cycle, plants will experience repeated dehydration stress, and there will be an intermittent water recovery period [19]. After repeated stress, pre-exposure to environmental stress will change the response to subsequent stresses [24,25,26,27], and the phenotypic plasticity during this process may improve the long-term survival rate and yield of plants [7,28]. Peanuts are an important economic and oil crop in China. The reduction in the peanut yield caused by drought accounted for more than 20% of the total peanut production in China. Research proved that drought training at the seedling stage could increase the yield of peanuts [14].
Crops can respond to the same stress more quickly and effectively through morphological adaptation, physiological, and hormonal changes and transcriptional modification, for example, by improving the transport and distribution of ABA, an important stress hormone, we found that both the AhATL1 protein and ABA existed in the roots, stems, and leaves of peanut plants during the process of drought stress memory.
The distribution level of the AhATL1 protein and ABA increased more rapidly and increased more rapidly in the second drought compared with in the first drought stress. In the process of rehydration, the distribution of the AhATL1 protein and ABA increased for a short time, and then decreased gradually. Compared with the first recovery, the distribution level of the AhATL1 protein and ABA remained at a higher level during the second recovery. The results show that the drought resistance ability of peanuts was enhanced after the first drought stress training.
ABA plays an important role in closing the stomata and reducing water transpiration in plants under drought stress [29]. Researchers found that the distribution level of ABA in a vascular bundle gradually increased during drought stress, and the distribution level of drought resistant peanuts was higher than that of drought sensitive peanuts [30]. For the site of ABA synthesis, previous research in the laboratory found that under drought stress, ABA in the leaves of peanut plants was mainly distributed in vascular tissue [31], which is also consistent with this point in this paper.
In addition, we also observed the ABA distribution in plant vascular tissues during the response of peanut leaves, roots, and stems to stress. After repeated drought stress on four leaf stage peanut seedlings, the leaf wilting degree, stem bending degree, and water content were compared. We found that the relative water content of the D2 peanut plants in the second drought was lower than that in the first drought, while the leaf wilting degree and stem bending degree of the peanut plants were lower than those of the first drought, and the recovery degree of leaves and stems was stronger than that of R1.
The drought resistance of peanut plants was enhanced after one drought and rehydration. This is possibly caused by the stomatal opening of peanut plants being less than in D1, suggesting that peanut plants had drought stress memory. The stomatal opening did not increase significantly, which promoted the rapid response of leaves, regulating stomatal closure, reducing transpiration, and maintaining the water content at D2 [11]. Therefore, peanut demonstrated a memory in the process of drought stress, and the expression of drought related genes increased, which may affect the ability of peanut plants to recover after drought.
Compared with the plants that experienced dehydration stress for the first time, the plants that repeatedly suffered dehydration stress and entered the water recovery stage alternately exhibited transcriptional and physiological memory responses during the subsequent dehydration stress [19,25]. For example, tobacco plants pretreated with methyl jasmonate showed “immune memory”, accumulating nicotine to cope with the second methyl jasmonate treatment [32].
Dehydration pretreatment increased the water holding capacity of the leaves of Arabidopsis thaliana [33] and maize [34]. In this study, we found that, in the leaf water content of Col, atabcg22, and AhATL1-OX during drought stress memory, the content of the second drought (D2 process) was similar to that of the first drought (D1 process), which was consistent with a previous report where the leaf relative water content was not affected by the previous drought stress [35], which may be related to the fact that the root system was not affected by drought training.
The proline contents of Col, atabcg22, and AhATL1-OX during drought training in the second drought (D2 process) were higher than in the first drought (D1 process), which was more conducive to maintaining the cell osmotic balance and resisting drought. We mainly focused on the comparison of proline content between Col and P35S::AhATL1-eGFP plants, and between P35S::AhATL1-eGFP/atabcg22 and atabcg22 plants. After second drought stress treatment (compared with the first drought stress treatment), the proline content of Col and P35S::AhATL1-eGFP plants were slightly increased. On the other hand, the proline content of P35S::AhATL1-eGFP/atabcg22 was higher than that of atabcg22, and the proline content of atabcg22 was higher than that of WT after first drought stress treatment. This may be because loss-of-function of AtABCG22 might cause an accumulation of proline but less sensitivity to proline. This may be due to the regulation of proline by some other signaling or metabolic pathways, which made proline easier to accumulate or not easy to degrade. Overexpression of AhATL1 can recover the loss of function of AtABCG22, resulting in insensitivity to proline. There is a high level of proline accumulation in the process of drought stress memory, and proline is a marker of drought degree, its metabolism and regulation mechanisms are relatively complex and proline may be under a positive feedback control. Proline accumulation is a significant metabolic adaptation mechanism of many organisms, including higher plants, under environmental stress, which is considered to play a protective role in plants under stress [36]. The proline content increased significantly after the first drought treatment and decreased with the rehydration treatment, reaching the second peak in the second recovery period and then remaining stable [37]. Regarding the ABA content of Col, atabcg22, and AhATL1-OX during drought training compared with the first drought (D1 process), the ABA contents of Col, atabcg22, and AhATL1-OX were increased during the second drought (D2 process), which was consistent with the reported changes in the endogenous ABA content in Arabidopsis thaliana [38].
The memory transcriptional pattern showed that the transcriptional behaviors of response genes under repeated stress were different from those under initial dehydration stress, indicating that stress memory is a complex phenotype, and is the result of multiple signal pathways [19,25,35]. Go analysis showed that [+/+] memory genes were involved in the ABA or abiotic stress response, [−/−] and [−/+] memory genes were involved in ribosome or protein synthesis, chloroplasts, and thylakoid membrane-related functions, and [+/−] memory genes were involved in a variety of signal pathway regulations, including the abscisic acid, ethylene, auxin, gibberellin, jasmonic acid, and salicylic acid pathway.
Transcriptional memory, similar to the activation of defense genes, can provide stronger or altered stress responses while reducing the consumption of readiness [18,26,36]. The overexpression of AhATL1 changed from a [+/+] memory gene to a [−/+] memory gene in Arabidopsis thaliana. AtRD29A, AtNCED3, AtSLAC1, AtALMT12, and AtABCG11 changed from [+/−] memory genes to [+/+] memory genes. AtPP2C5, AtABCG40, and AtABCG25 changed from [+/−] memory genes to [−/+] memory genes. AtUGT71C5 changed from a [−/+] memory gene to a [−/−] memory gene. AtCYP707A1 changed from a post response gene type [=/+] to a memory gene type [−/+].
The [+/+] memory gene is a marker gene involved in the ABA or abiotic stress response [21]. The above results indicate that ABA induction and stress-response-related genes were not always [+/+] memory genes in Col, atabcg22 mutants, or AhATL1-OX lines under drought training, and the mode transformation between memory genes might be more helpful for plant survival under repeated drought stress, especially in the case of the overexpression or knockout of a certain gene.
In summary, we found that the level of AhATL1 expression and AhATL1 distribution increased more rapidly in D2 compared with in D1. Compared with the first recovery (R1), the AhATL1 expression level and ABA content remained at a higher level during the second recovery (R2). The results show that the drought resistance ability of peanuts was enhanced after the first drought stress training. Overexpression of AhATL1 in Arabidopsis Col lines was also investigated in this paper to further study the response mechanism of AhATL1. ABA induction and stress-response-related genes were not always [+/+] memory genes in Col, atabcg22 mutants, or AhATL1-OX lines under drought training. The heterologous overexpression of AhATL1 in Arabidopsis changed the expression pattern of certain memory genes and changed the post response gene type into a memory gene type, and the mode transformation between memory genes might be more helpful for plant survival under repeated drought stress, particularly in the case of the overexpression or knockout of a certain gene. The proline and water contents of AhATL1-OX plants during drought training demonstrated that the second drought (D2) was less severe than the first drought (D1), which was more conducive to maintaining cell osmotic balance and resisting drought. In the future, we will further study the molecular mechanism of its regulation from the perspective of the transcriptional level and epigenetics.
4. Material and Methods
4.1. Plant Material and Growth Conditions
Peanut cultivar ‘Yueyou 7′, which is a drought-resistant variety, was provided by the Crop Research Institute, Guangdong Academy of Agricultural Sciences. Put the peanuts (in a drying basin) in a light incubator (GuangQi, GHP-160, Shanghai, China) to dehydrate (28 °C, 48% RH, with a 16 h light and 8 h dark cycle, Light intensity13,000 lx) and treated for 1, 2, 4, 8, and 24 h in the light. At each sampling point, samples (0.5 g) of leaf (first functional leaf), stem (middle of the stem) and root (taproot, 1 cm) were excised as sample (Figure 5).
Figure 5.
Schematic diagram of the peanut plant sampling.
The peanut seedlings were dehydrated for 8 h (D1), and then rehydrated with 1/8 MS solution (pH 6.0) for 48 h. In the same way, the peanut plants were subjected to the second drought (D2) and then rehydrated (R2). Aerial tissue was harvested at noon on the day specified. The excised samples were immediately frozen and stored at −80 °C until use. All treatments were performed in at least three independent experiments.
Drought training of Arabidopsis thaliana: the Arabidopsis plant was taken out from the soil. The residual soil in the root was washed with water, then placed on the drying filter paper to dry for 2 h, and then put into a humid room. The rehydration was conducted by dripping water to the root for 24 h. The treatment of the subsequent air-drying and rehydration were the same as the first process (Figure 6). The leaves of Arabidopsis thaliana were collected and preserved in different periods [35].

Figure 6.
Schematic diagram of the Arabidopsis drought training.
4.2. Measurement of Endogenous ABA Content
From the frozen samples described in 4.1, the endogenous ABA was extracted. Measurement of endogenous ABA content as described by Hu et al. [16]. After grinding, extracting, freezing centrifugation and C18 filtration, samples were added to 96-well enzyme plates, with 50 mm3 per well. We added the first antibody, incubated at 37 ℃ for 0.5 h, washed the plate 4 times and dried it. Then, we added the second antibody, incubated at 37 ℃ for 0.5 h, washed the plate 4 times, then added the substrate for color development. When the color development was appropriate, we added 50 mm3 2 mol/dm3 sulfuric acid to each well to terminate the reaction. The OD value of each sample at 490 nm was determined by enzyme-linked immunosorbent assay, and the content of ABA in the sample was calculated.
4.3. Immunofluorescence Localization Assays
Cryofixation and freeze-substitution of sample of leaf, stem and root in peanut were conducted as described by Kandasamy et al. [20]. Measurement of immunofluorescence localization assays was described by Hu et al. [16].
4.4. AhATL1 Overexpressing Arabidopsis Plants
AhATL1 overexpression vector P35S::AhATL1-eGFP was transformed into Col wild type Arabidopsis thaliana by Agrobacterium Eha105. Eight positive strains were screened (Figure S1A, Supplementary Materials). All the positive transgenic seedlings were transplanted into soil for 3 weeks. DNA was extracted from rosette leaves and identified by PCR with primers AhATL1-ORF-F, AhATL1-ORF-R, and five positive strains were identified. The expression level of AhATL1 gene was detected by RT-qPCR in five homozygous strains were screened by kanamycin resistance plate (Figure S1B). The results of RT-qPCR show that 5 strains were obtained. Among the positive transgenic plants, the expression level of AhATL1 gene in line 3 was higher than that in other lines, so it was selected for further studies. As for transgenic lines, P35S::AhATL1-eGFP/atabcg22 was obtained by crossing a wild-type P35S::AhATL1-eGFP line with atabcg22 to screen homozygotes, so as to ensure the same genetic background and adequately compare their physiological differences. The plants were grown on peat-containing soil with a daily cycle of 16 h light and 8 h dark in well-watered conditions at 22 ± 2 °C and 60–70% relative humidity.
4.5. Measurement of Water Content
The seedlings were separated from the above ground part and the belowground part, and the fresh weight was weighed, respectively; after 72 h of drying in the oven, the dry weight was weighed to a constant weight. According to the water content = (fresh weight dry weight)/fresh weight, the water contents of the upper part and the belowground part were calculated, respectively.
4.6. Measurement of Proline Content
A series of standard curves of the proline solution were drawn. We accurately weighed 0.2 g of a mixed blade sample, added liquid nitrogen to grind into powder, and then quickly transferred the sample to a test tube. We added 2 cm3 30 g/dm3 sulfosalicylic acid solution to each test tube, and the homogenate was extracted in a boiling water bath for 10 min after cooling. The sample was centrifuged at 3000× g for 10 min, and the supernatant was the extraction solution of proline.
We took 2 cm3 of supernatant into the tube with a plug, added 1 mL distilled water, 1 mL glacial acetic acid, and 2 cm3 acid ninhydrin reagent, and colored them in a boiling water bath for 1 h. After cooling, 2 cm3 toluene was added to each tube, and the tube was fully shaken for 30 s, and then placed for stratification. The upper toluene solution was absorbed into the colorimetric cup. Toluene was used as a blank control. The absorbance value was determined at 520 nm wavelength of the spectrophotometer (SHIMADZU, UV-1780, Tokyo, Japan). We determined the content of proline in the determination solution from the standard curve, and then calculated the percentage of the proline content in the sample.
4.7. Measurement of Malondialdehyde (MDA) Content
We accurately weighed 0.2 g of the blades, added liquid nitrogen to grind into powder, and then quickly moved the sample to the test tube. We added 2 cm3 10% Trichloroacetic acid into the mixture, and the homogenate was centrifuged at 4000× g for 10 min. The supernatant was malondialdehyde extract. We took 1 cm3 of supernatant, added 1 cm3 of distilled water to the control tube, and then added 1 mL of 0.6 Barbituric acid solution to each tube. After shaking, the mixture was reacted in a boiling water bath for 15 min, and then centrifuged after rapid cooling. The absorbance of the supernatant was measured at 532 and 450 nm. The content of malondialdehyde was calculated by substituting it into the formula.
4.8. Expression Analysis
A SYBR premix ex taqtm II Kit (Takara Bio, Dalian, China) was used for real-time quantitative PCR. An ABI 7500 prism real-time PCR system (Applied Biosystems) was used. The PCR reaction conditions were as follows: 95 ℃ for 10 s, 1 cycle; 95 ℃ for 10 s, 62 ℃ for 10 s (depending on the optimal reaction temperatures of the different primers), 72 ℃ for 20 s, a total of 45 cycles; each sample was repeated three times, and finally the average value was taken. UBQ10 was used as an internal control. The melting curve was analyzed by reading the fluorescence intensity every 0.2 s between 62 and 95 ℃. The primers used in Arabidopsis qPCR as Table 2. The relative gene expression was calculated using 2-ΔΔCT methods (Table 2).

Table 2.
The primers used in Arabidopsis qPCR.
4.9. Statistical Analysis
The quantitative data are expressed as the means ± SD. The statistical significance of the experimental data was assessed using Student’s t-test or ANOVA (one-way analysis of variance with a least significant difference (LSD) post hoc test), as appropriate, using the SPSS17.0 statistical package (Chicago, IL, USA).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22073398/s1, Figure S1: Filter of the transgenetic Arabidopsis in T1 generation and AhATL1 relative expression level in AhATL1 Overexpression Lines, Figure S2: Changes in the expression of AtABCG22 gene under drought training.
Author Contributions
M.Q., L.L. and B.H. designed the research. M.Q. and S.T. performed the research. M.Q., X.L. and B.H. analyzed the data and prepared figures. M.Q. wrote the manuscript in consultation with Y.H. and B.H., L.L. and B.H. contributed reagents/materials/analysis tools. All authors have read and agreed to the published version of the manuscript.
Funding
This research supported by the Natural Science Foundation of Guangdong Province (Grant No.2018A030313629 granted to BH), and Science and Technology Project of Guangzhou (Grant No 214201804010158 granted to BH).
Acknowledgments
This work was supported by the Natural Science Foundation of Guangdong Province (Grant No.2018A030313629 granted to BH), and Science and Technology Project of Guangzhou (Grant No 214201804010158 granted to BH).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, A.M.R. Priming for Enhanced Defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Kumar, A. Plant behavior: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Stief, A.; Altmann, S.; Hoffmann, K.; Pant, B.D.; Scheible, W.-R.; Bäurle, I. Arabidopsis miR156 Regulates Tolerance to Recurring Environmental Stress through SPL Transcription Factors. Plant Cell 2014, 26, 1792–1807. [Google Scholar] [CrossRef]
- Hilker, M.; Schmülling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar] [CrossRef]
- Lämke, J.; Bäurle, I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017, 18, 124. [Google Scholar] [CrossRef]
- Ebe, M.; Pirko, J.; Hannes, K. The Role of ABA Recycling and Transporter Proteins in Rapid Stomatal Responses to Reduced Air Humidity, Elevated CO2 and Exogenous ABA. Mol. Plant 2016, 8, 657–659. [Google Scholar]
- Kang, J.; Yim, S.; Choi, H.; Kim, A.; Lee, K.P.; Lopez-Molina, L.; Martinoia, E.; Lee, Y. Abscisic acid transporters cooperate to control seed germination. Nat. Commun. 2015, 6, 8113. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hwang, J.-U.; Lee, M.; Kim, Y.-Y.; Assmann, S.M.; Martinoia, E.; Lee, Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360. [Google Scholar] [CrossRef]
- Kuromori, T.; Miyaji, T.; Yabuuchi, H.; Shimizu, H.; Sugimoto, E.; Kamiya, A.; Moriyama, Y.; Shinozaki, K. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc. Natl. Acad. Sci. USA 2010, 107, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Ge, K.; Liu, X.; Li, X.; Hu, B.; Li, L. Isolation of an ABA Transporter-Like 1 Gene from Arachis hypogaea That Affects ABA Import and Reduces ABA Sensitivity in Arabidopsis. Front. Plant Sci. 2017, 8, 1150. [Google Scholar] [CrossRef]
- Chen, R.Q.; Shu, W.; Ge, K.; Hu, B.; Li, L. Effect on growth and expressions of stress-related genes in peanut under drought stress training. Plant Physiol. J. 2017, 10, 1921–1927. [Google Scholar]
- Nambara, E.; Marion, P.A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R. Abscisic Acid Synthesis and Response. Arab. Book 2013, 11, e0166. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Léran, S.; Corratgé-Faillie, C.; Gojon, A.; Krouk, G.; Lacombe, B. ABA transport and transporters. Trends Plant Sci. 2013, 18, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Puértolas, J.; Conesa, M.R.; Ballester, C.; Dodd, I.C. Local root abscisic acid (ABA) accumulation depends on the spatial distribution of soil moisture in potato: Implications for ABA signalling under heterogeneous soil drying. J. Exp. Bot. 2015, 66, 2325–2334. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Cao, J.; Ge, K.; Li, L. The site of water stress governs the pattern of ABA synthesis and transport in peanut. Sci. Rep. 2016, 6, 32143. [Google Scholar] [CrossRef]
- Colaneri, A.C.; Jones, A.M. Genome-wide quantitative identifi cation of DNA differentially methylated sites in Arabidopsis seedlings growing at different water potential. PLoS ONE 2013, 8, e59878. [Google Scholar] [CrossRef]
- Liao, W.-B.; Huang, G.-B.; Yu, J.-H.; Zhang, M.-L. Nitric oxide and hydrogen peroxide alleviate drought stress in marigold explants and promote its adventitious root development. Plant Physiol. Biochem. 2012, 58, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Virlouvet, L.; Liu, N.; Riethoven, J.-J.; Fromm, M.; Avramova, Z. Dehydration stress memory genes of Zea mays; comparison with Arabidopsis thaliana. BMC Plant Biol. 2014, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Kuromori, T.; Sugimoto, E.; Shinozaki, K. Arabidopsis mutants of AtABCG22, an ABC transporter gene, increase water transpiration and drought susceptibility. Plant J. 2011, 67, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fromm, M.E.; Avramova, Z. Multiple exposures to drought ’train’ transcriptional responses in Arabidopsis. Nat. Commun. 2012, 3, 740. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Bertazzini, M.; Cagnano, G. Stress-driven increase in proline levels, and not proline levels themselves, correlates with the ability to withstand excess salt in a group of 17 Italian rice genotypes. Plant Biol. 2019, 21, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Tajti, J.; Szalai, G.; Peeva, V.; Végh, B.; Janda, T. Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci. Rep. 2018, 8, 12839. [Google Scholar] [CrossRef] [PubMed]
- Slatyer, R.O.; Markus, D.K. Plant-Water Relationships. Soil Sci. 1967, 106, 478. [Google Scholar] [CrossRef]
- Byun, Y.-J.; Koo, M.-Y.; Joo, H.-J.; Ha-Lee, Y.-M.; Lee, D.-H. Comparative analysis of gene expression under cold acclimation, deacclimation and reacclimation in Arabidopsis. Physiol. Plant. 2014, 152, 256–274. [Google Scholar] [CrossRef]
- Fleta-Soriano, E.; Munné-Bosch, S. Stress memory and the inevitable effects of drought: A physiological perspective. Front. Plant Sci. 2016, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.B.; Davidson, A. Adaptive phenotypic plasticity and plant water use. Funct. Plant Biol. 2010, 37, 117–127. [Google Scholar] [CrossRef]
- Cavanagh, A.P.; Kubien, D.S. Can phenotypic plasticity in Rubisco performance contribute to photosynthetic acclimation? Photosynth. Res. 2014, 119, 203–214. [Google Scholar] [CrossRef]
- Cao, J.J.; Lü, Y.; Hu, B.; Li, L. Changers of AhCYP707A1 proteins and ABA distribution in peanut leaves under water stress. Plant Physiol. J. 2016, 52, 436–444. [Google Scholar]
- Hu, B.; Xiao, S.; Lü, Y.; Li, L. Distribution of ABA and AhNCED 1 in peanut leaves of different drought resistant cultivars subjected to drought stress. Chin. J. Cell Biol. 2012, 34, 992–997. [Google Scholar]
- Baldwin, I.T.; Schmelz, E.A. Immunological “Memory” in the Induced Accumulation of Nicotine in Wild Tobacco. Ecology 1996, 77, 236–246. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, N.; Virlouvet, L.; Riethoven, J.-J.; Fromm, M.; Avramova, Z. Four distinct types of dehydration stress memory genes in Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 229. [Google Scholar] [CrossRef]
- Virlouvet, L.; Fromm, M. Physiological and transcriptional memory in guard cells during repetitive dehydration stress. New Phytol. 2015, 205, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Nagy, L.; Hein, R.; Rascher, U.; Beierkuhnlein, C.; Willner, E.; Jentsch, A. Do plants remember drought? Hints towards a drought-memory in grasses. Environ. Exp. Bot. 2011, 71, 34–40. [Google Scholar] [CrossRef]
- Avramova, Z. Transcriptional ‘memory’ of a stress: Transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. 2015, 83, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Q.; Feng, H.; Deng, J.; Zhang, R.; Wen, J.; Dong, J.; Wang, T. Dehydrin MtCAS31 promotes autophagic degradation under drought stress. Autophagy 2019, 16, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lv, Y.; Wan, X.R.; Li, L.M.; Hu, B.; Li, L. Cloning and expression analysis of cDNAs encodingABA 8′-hydroxylase in peanut plants in response to osmotic stress. PLoS ONE 2014, 9, e97025. [Google Scholar]
- Kandasamy, M.K.; McKinney, E.C.; Meagher, R.B. Functional Nonequivalency of Actin Isovariants inArabidopsis. Mol. Biol. Cell 2002, 13, 251–261. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).