Effect of Chronic Administration of 5-(3-chlorophenyl)-4-Hexyl-2,4 -Dihydro-3H-1,2,4-Triazole-3-Thione (TP-315)—A New Anticonvulsant Drug Candidate—On Living Organisms
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of 1,2,4-Triazole-3-Thione Derivative as a Potential Antiepileptic Drug to Determine the Effects of Chronic Administration to a Living Organism
2.2. Effects of Chronic Administration of TP-315 on Living Organisms
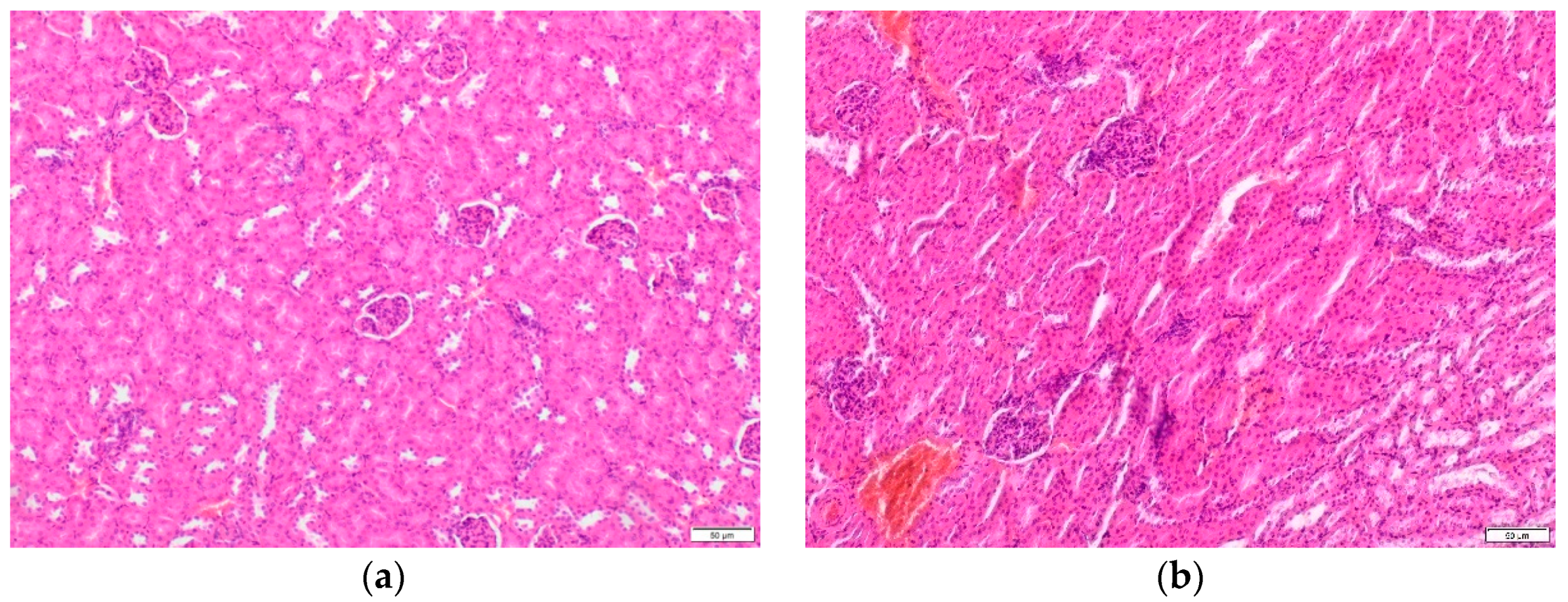
2.2.1. Histopathological Examination
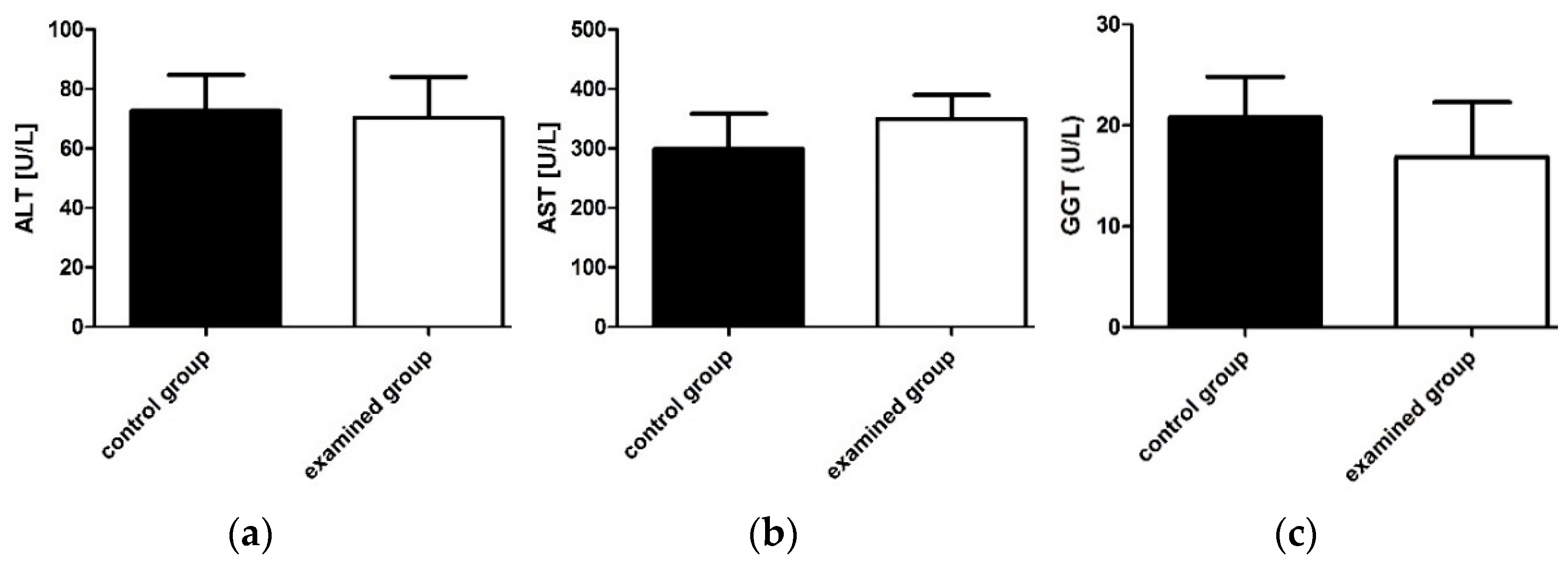
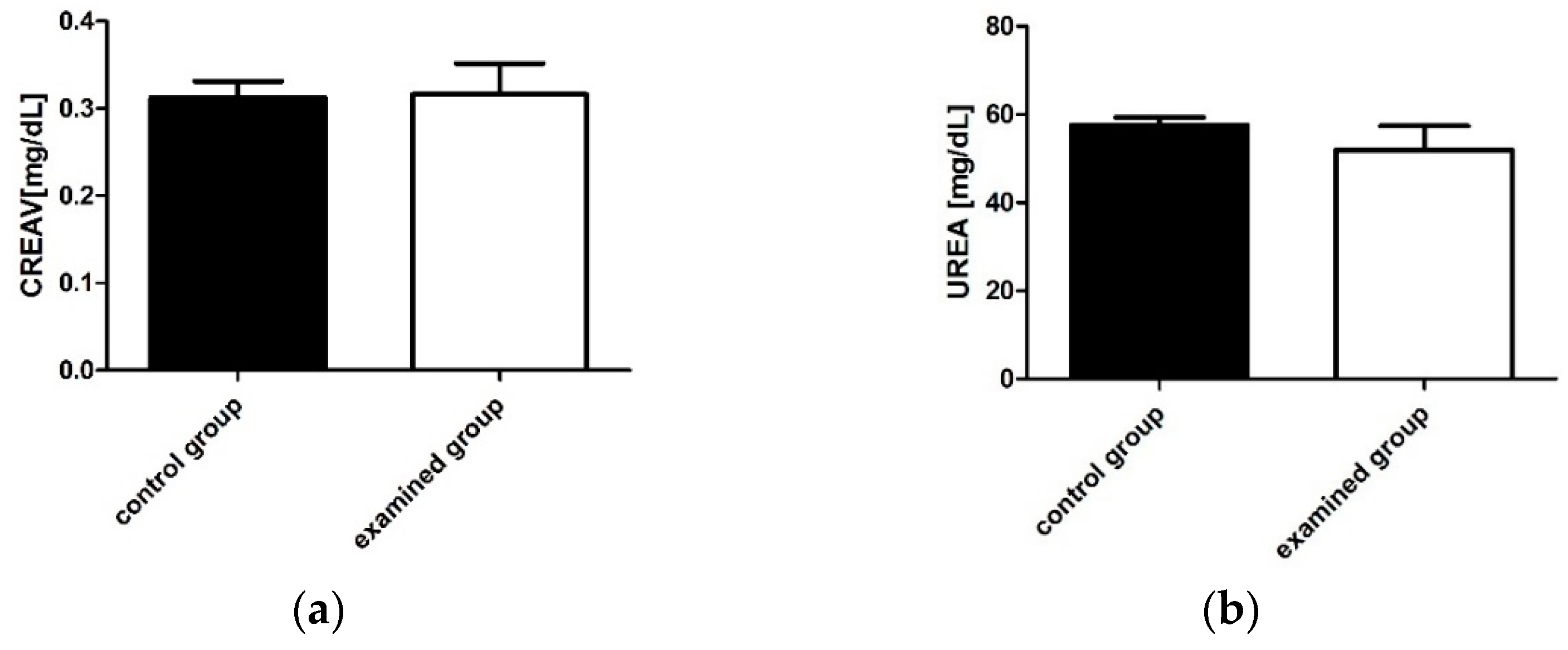
2.2.2. Determination of Biochemical Parameters of Liver and Renal Function
2.2.3. Determination of Morphological Blood Parameters
2.3. Effect of TP-315 on the Inhibition of Selected CYP450 Isoforms
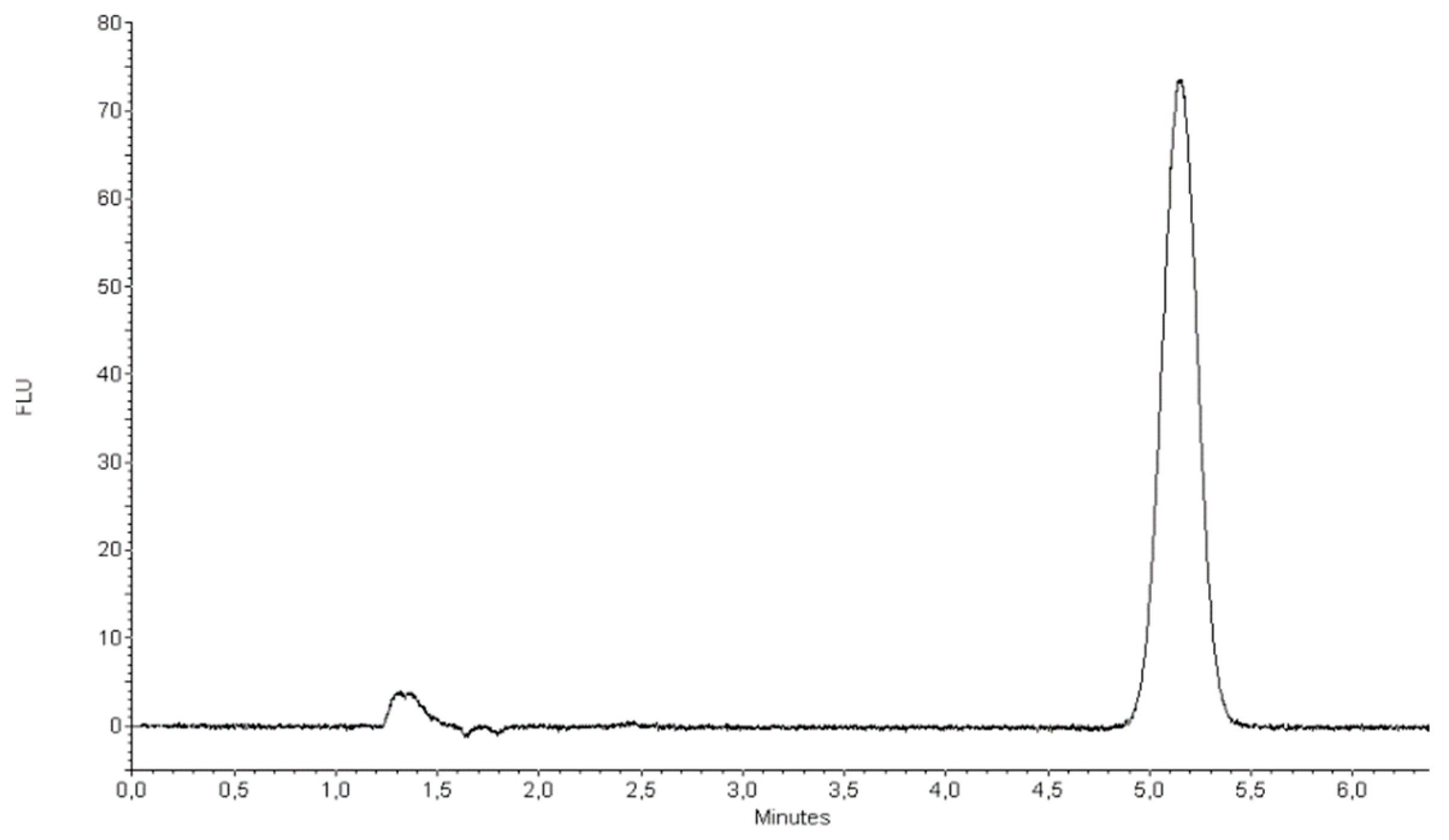
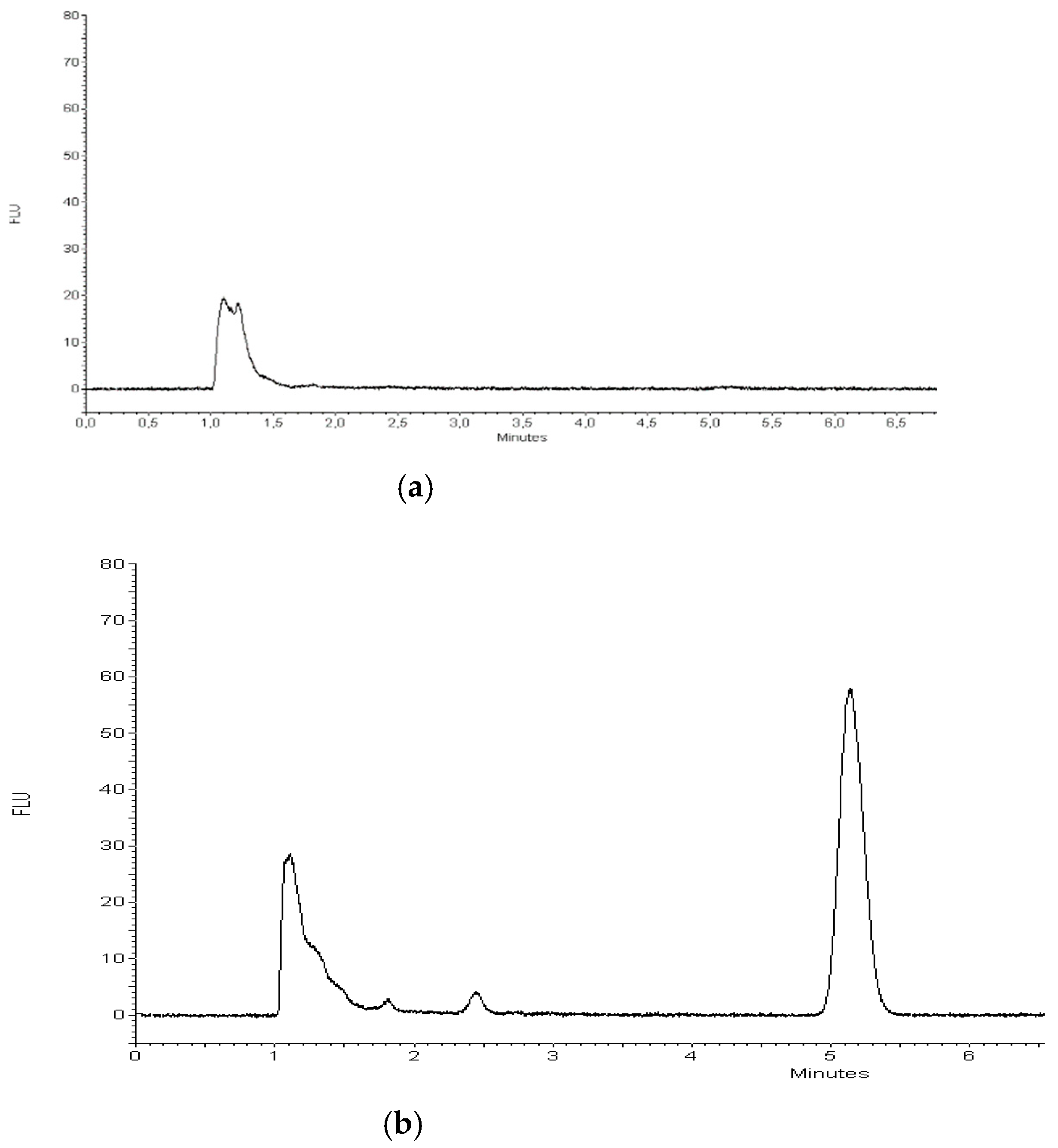
2.3.1. Quantification of TP-315 by HPLC-FL Method
- Recovery examination
- Analysis of serum samples
2.3.2. Concentration-Dependent Screening of TP-315 on Enzyme Activity
3. Materials and Methods
3.1. Synthesis of 1,2,4-Triazole-3-Thione Derivatives
3.2. Parallel Artificial Membrane Permeability ASSAY (PAMPA BBB)
3.3. Experiments on Adult Male Albino Swiss Mice
3.4. Quantification of TP-315 by HPLC-FL Method
- Stock and working standard solutions
- Sample preparation
- Sample extraction
- HPLC-FL conditions
- Validation procedure
3.5. Histopathological Examinations of Liver and Kidney Samples Collected from the Examined Mice
3.6. Determination of Morphological and Biochemical Parameters
3.7. Determination of CYP450 Activity with VIVID CYP450 Assay Kits
3.8. Methods of Statistical Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández-Suárez, E.; Villa-Estébanez, R.; Garcia-Martinez, A.; Fidalgo-González, J.A.; Zanabili Al-Sibbai, A.A.; Salas-Puig, J. Prevalence, type of epilepsy and use of antiepileptic drugs in primary care. Rev. Neurol. 2015, 60, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Ernst, L.D.; Boudreau, E.A. Recent advances in epilepsy management. Curr. Opin. Anaesthesiol. 2016, 29, 558–566. [Google Scholar] [CrossRef]
- Behr, C.; Goltzene, M.A.; Kosmalski, G.; Hirsch, E.; Ryvlin, P. Epidemiology of epilepsy. Rev. Neurol. 2016, 172, 27–36. [Google Scholar] [CrossRef]
- Filipska, K. Epilepsy-the Social Disease of the 21st Century. Innow. Pielęgniarstwie Naukach Zdrowiu 2016, 3, 72–78. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef]
- Beleza, P. Refractory epilepsy: A clinically oriented review. Eur. Neurol. 2009, 62, 65–71. [Google Scholar] [CrossRef]
- Braga, P.; Hosny, H.; Kakooza-Mwesige, A.; Rider, F.; Tripathi, M.; Guekht, A. How to understand and address the cultural aspects and consequences of diagnosis of epilepsy, including stigma. Epileptic Disord. 2020, 22, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Kaproń, B.; Łuszczki, J.J.; Siwek, A.; Karcz, T.; Nowak, G.; Zagaja, M.; Andres-Mach, M.; Stasiłowicz, A.; Cielecka-Piontek, J.; Kocki, J.; et al. Preclinical evaluation of 1,2,4-triazole-based compounds targeting voltage-gated sodium channels (VGSCs) as promising anticonvulsant drug candidates. Bioorg. Chem. 2020, 94. [Google Scholar] [CrossRef]
- Łuszczki, J.J.; Lepiech, J.; Zagaja, M.; Wróblewska-Łuczka, P.; Florek-Łuszczki, M.; Bojar, H.; Walczak, A.; Plech, T. Anticonvulsant and neurotoxic effects of a novel 1,2,4-triazole-3-thione derivative (TPF-34) and its isobolographic interaction profile with classical antiepileptic drugs in mice. Pharm. Rep. 2020, 72, 87–95. [Google Scholar] [CrossRef]
- Plech, T.; Łuszczki, J.J.; Wujec, M.; Flieger, J.; Pizoń, M. Synthesis, characterization and preliminary anticonvulsant evaluation of some 4-alkyl-1,2,4-triazoles. Eur. J. Med. Chem. 2013, 60, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Kaproń, B.; Łuszczki, J.J.; Paneth, A.; Siwek, A.; Kołaczkowski, M.; Żołnierek, M.; Nowak, G. Studies on the anticonvulsant activity of 4-alkyl-1,2,4-triazole-3-thiones and their effect on GABAergic system. Eur. J. Med. Chem. 2014, 86, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Kaproń, B.; Czarnomysy, R.; Wysokiński, M.; Andrys, R.; Musilek, K.; Angeli, A.; Supuran, T.C.; Plech, T. 1,2,4-Triazole-based anticonvulsant agents with additional ROS scavenging activity are effective in a model of pharmacoresistant epilepsy. J. Enzym. Inhib. Med. Chem. 2020, 35, 993–1002. [Google Scholar] [CrossRef]
- Plech, T.; Kaproń, B.; Łuszczki, J.J.; Wujec, M.; Paneth, A.; Siwek, A.; Kołaczkowski, M.; Żołnierek, M.; Nowak, G. Studies on the anticonvulsant activity and influence on GABA-ergic neurotransmission of 1,2,4-triazole-3-thione- based compounds. Molecules 2014, 19, 11279–11299. [Google Scholar] [CrossRef] [PubMed]
- Łuszczki, J.J.; Plech, T.; Wujec, M. Effect of 4-(4-bromophenyl)-5-(3-chlorophenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione on the anticonvulsant action of different classical antiepileptic drugs in the mouse maximal electroshock-induced seizure model. Eur. J. Pharm. 2012, 690, 99–106. [Google Scholar] [CrossRef]
- Łuszczki, J.J.; Plech, T.; Wujec, M. Influence of 5-(3-chlorophenyl)-4-(4-methylphenyl)-2,-dihydro-3H-1,2,4-triazole-3-thione on the anticonvulsant action of four classical antiepileptic drugs in the mouse maximal electroshock-induced seizure model. Pharm. Rep. 2012, 64, 970–978. [Google Scholar] [CrossRef]
- Ayati, A.; Emami, S.; Foroumadi, A. The importance of triazole scaffold in the development of anticonvulsant agents. Eur. J. Med. Chem. 2016, 109, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Song, M.X.; Deng, X.Q. Recent developments on triazole nucleus in anticonvulsant compounds: A review. J. Enzym. Inhib. Med. Chem. 2018, 33, 453–478. [Google Scholar] [CrossRef] [PubMed]
- Kaproń, B.; Łuszczki, J.J.; Płazińska, A.; Siwek, A.; Karcz, T.; Gryboś, A.; Nowak, G.; Makuch-Kocka, A.; Walczak, K.; Langner, E.; et al. Development of the 1,2,4-triazole-based anticonvulsant drug candidates acting on the voltage-gated sodium channels. Insights from in-vivo, in-vitro, and in-silico studies. Eur. J. Pharm. Sci. 2019, 129, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Mann, A.; Ekstein, D.; Eyal, S. Breaking bad: The structure and function of the blood-brain barrier in epilepsy. AAPS J. 2017, 19, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, L.; Fan, L.; Zha, Y.; Guo, L.; Zhang, Q.; Chen, J.; Pang, Z.; Wang, Y.; Jiang, X.; et al. Targeting the brain with PEG-PLGA nanoparticles modified with phage-displayed peptides. Biomaterials 2011, 32, 4943–4950. [Google Scholar] [CrossRef]
- Galdeano, C.; Coquelle, N.; Cieslikiewicz-Bouet, M.; Bartolini, M.; Pérez, B.; Clos, M.; Silman, I.; Jean, L.; Colletier, J.P.; Renard, P.Y.; et al. Increasing Polarity in Tacrine and Huprine Derivatives: Potent Anticholinesterase Agents for the Treatment of Myasthenia Gravis. Molecules 2018, 23, 634. [Google Scholar] [CrossRef] [PubMed]
- Kaproń, B.; Łuszczki, J.; Paneth, A.; Wujec, M.; Siwek, A.; Karcz, T.; Mordyl, B.; Głuch-Lutwin, M.; Gryboś, A.; Nowak, G.; et al. Molecular mechanism of action and safety of 5-(3-chlorophenyl)-4-hexyl-2,4-dihydro-3H-1,2,4-triazole-3-thione—A novel anticonvulsant drug candidate. Int. J. Med. Sci. 2017, 14, 741–749. [Google Scholar] [CrossRef][Green Version]
- Patsalos, P.N.; Berry, D.J.; Bourgeois, B.F.; Cloyd, J.C.; Glauser, T.A.; Johannessen, S.I.; Leppik, I.E.; Tomson, T.; Perucca, E. Antiepileptic drugs-best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008, 49, 1239–1276. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Suzuki, Y.; Fujisaki, K.; Sato, Y.; Takeyama, M. Correlation between plasma ammonia level and serum trough concentration of free valproic acid in patients with epilepsy. Biol. Pharm. Bull. 2012, 35, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, E.; Olsson, R. Suspected drug-induced liver fatalities reported to the WHO database. Dig. Liver Dis. 2006, 38, 33–38. [Google Scholar] [CrossRef]
- Gerstner, T.; Bell, N.; König, S. Oral valproic acid for epilepsy-long-term experience in therapy and side effects. Expert Opin. Pharmacother. 2008, 9, 285–292. [Google Scholar] [CrossRef]
- Hadzagic-Catibusic, F.; Hasanbegovic, E.; Melunovic, M.; Zubcevic, S.; Uzicanin, S. Effects of Carbamazepine and Valproate on Serum Aspartate Aminotransferase, Alanine Aminotransferase and Gamma. Glutamyltransferase Child. Med. Arch. 2017, 17, 239–242. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; LLerena, A.; Jung-Cook, H.; López-López, M. Carbamazepine adverse drug reactions. Expert Rev. Clin. Pharmacol. 2008, 11, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Sachdeva, T.; Bafna, P. Drug-induced hepatotoxicity: A review. J. Appl. Pharm. Sci. 2012, 2, 233–243. [Google Scholar] [CrossRef]
- Li, L.F.; Ma, C. Epidemiological study of severe cutaneous adverse drug reactions in a city district of China. Clin. Exp. Dermatol. 2006, 31, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E. Overtreatment in epilepsy: Adverse consequences and mechanisms. Epilepsy Res. 2002, 52, 25–33. [Google Scholar] [CrossRef]
- Craig, S. Phenytoin poisoning. Neurocrit Care 2005, 3, 161–170. [Google Scholar] [CrossRef]
- Perucca, P.; Gilliam, F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012, 11, 792–802. [Google Scholar] [CrossRef]
- Tanaka, E. Clinically significant pharmacokinetic drug interactions between antiepileptic drugs. J. Clin. Pharm. Ther. 1999, 24, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E. Clinically relevant drug interactions with antiepileptic drug. Br. J. Clin. Pharmacol. 2005, 61, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.; Bramley, T.J.; Kozma, C.; Doshi, D.; Rupnow, M.E.T. Potential drug-drug interactions with antiepileptic drugs in medical recepients. Am. J. Health Syst. Pharm. 2008, 65, 1720–1726. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Froscher, W.; Pisani, F.; Rijan, C.M. The importance of drug interactions in epilepsy therapy. Epilepsia 2002, 43, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Bialer, M. The pharmacokinetics and interactions of new antiepileptic drugs. Drug Monit. 2005, 27, 722–726. [Google Scholar] [CrossRef]
- Turnheim, K. Arzneimittelwechselwirkungen mit antiepileptica. Wien Klin. Wochenschr. 2004, 116, 112–118. [Google Scholar] [CrossRef]
- Wang, J.; Cheung, W.; Grant, D. Determination of pesticides in apple-based infant foods using liquid chromatography electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Tsukada, K. Matrix effect and correction by standard addition in quantitative liquid chromatographic-mass spectrometric analysis of diarrhetic shellfish poisoning toxins. J. Chromatogr. A 2002, 943, 39–46. [Google Scholar] [CrossRef]
- Rybak, M.E.; Calvey, E.M.; Harnly, J.M. Quantitative determination of allicin in garlic: Supercritical fluid extraction and standard addition of alliin. J. Agric. Food Chem. 2004, 52, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.; Liu, C.; Lin, Y.; Chen, H.; Su, S.; Chou, H.; Su, S.C.; Chou, H.K.; Chou, S.S.; Shih, D.Y.C. Analysis of 81 pesticides and metabolite residues in fruits and vegetables by diatomaceous earth column extraction and LC/MS/MS determination. J. Food Drug Anal. 2009, 17, 319–332. [Google Scholar] [CrossRef]
- Andersen, J.E. The standard addition method revisited. Trends Anal. Chem. 2017, 89, 21–33. [Google Scholar] [CrossRef]
- Flieger, J.; Pizoń, M.; Plech, T.; Łuszczki, J.J. Analysis of new potential anticonvulsant compounds in mice brain tissue by SPE/HPLC/DAD. J. Chromatogr. B 2012, 909, 26–33. [Google Scholar] [CrossRef]
- Flieger, J.; Pizoń, M.; Plech, T.; Łuszczki, J.J. Determination of 5-(3-Chlorophenyl)-4-hexyl-2,4-dihydro-3H-1,2,4-triazole-3-thione in Mouse Brain Tissue by Microwave-Assisted Extraction and High-Performance Liquid Chromatography with Fluorescence Detection. Anal. Lett. 2015, 48, 318–327. [Google Scholar] [CrossRef]
- Koukouritaki, S.B.; Manro, J.R.; Marsh, S.A.; Stevens, J.C.; Rettie, A.E.; McCarver, D.G.; Hines, R.N. Developmental expression of human hepatic CYP2C9 and CYP2C19. J. Pharmacol. Exp. Ther. 2004, 308, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Li-Wan-Po, A.; Girard, T.; Farndon, P.; Cooley, C.; Lithgow, J. Pharmacogenetics of CYP2C19: Functional and clinical implications of a new variant CYP2C19*17. Br. J. Clin. Pharm. 2010, 69, 222–230. [Google Scholar] [CrossRef]
- ICH, Q2(R1). Validation of Analytical Procedures: Text and Methodology; International Conference on Harmonization: Geneva, Switzerland, 2005. [Google Scholar]
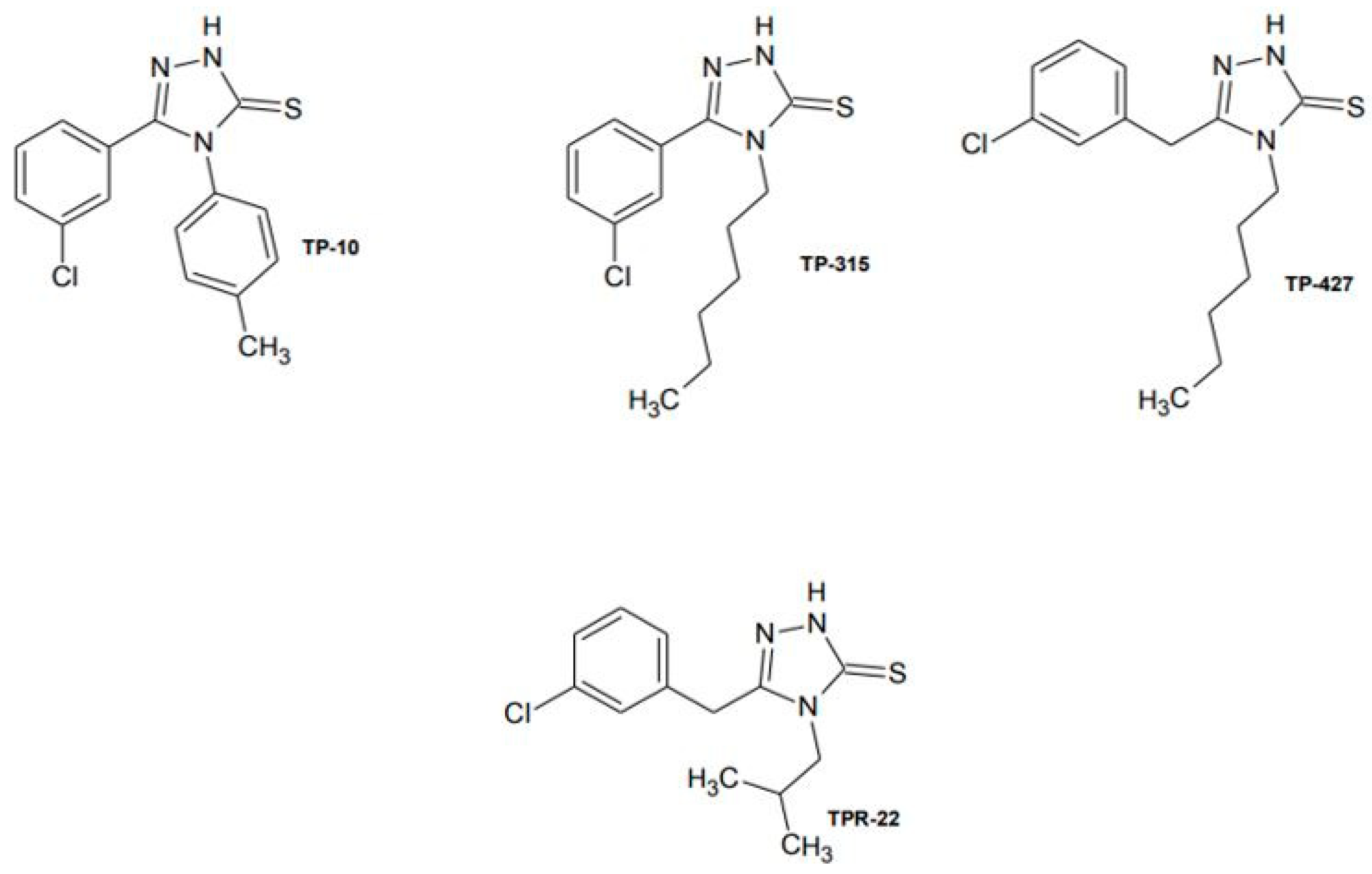


| Compound | Permeability Coefficients (Pe × 10−6) ± SD [cm/s] |
|---|---|
| Valproic acid | 15.37 ± 4.71 |
| TP-10 | 40.2 ± 5.1 |
| TP-315 | 2983.50 ± 211.02 |
| TP-427 | 476.63 ± 39.59 |
| TPR-22 | 34.43 ± 8.30 |
| Compound | Pretreatment Time (min) | Anticonvulsant Activity in MES Test [10,11,13] | Anticonvulsant Activity in 6 Hz Test [12] | Neurotoxicity in Chimney or Rotarod (†) Tests in Mice [10,11,13] | Affinity towards Batrachotoxin-Binding Site on Sodium Channels [11,18,22] | ||
|---|---|---|---|---|---|---|---|
| ED50 ± S.E. [mg/kg] | PI (TD50/ED50) | ED50 ± SEM [mg/kg] | PI (TD50/ED50) | IC50 (μM) ± SEM | |||
| TP-10 | 15 | 57.0 ± 9.4 | 5.9 | 62.6 ± 13.2 | 5.4 | 338.1 ± 12.0 | no data |
| 30 | 74.5 ± 8.1 | 4.5 | 61.1 ± 9.7 | 5.5 | 338.1 ± 14.7 | ||
| 60 | 187.1 ± 18.8 | 1.8 | 169.7 ± 18.5 | 2.0 | 333.4 ± 18.6 | ||
| 120 | 281.4 ± 13.6 | 1.4 | 167.6 ± 17.4 | 2.4 | 395.1 ± 25.2 | ||
| TP-315 | 15 | 47.6 ± 3.8 | 9.7 | 61.3 ± 10.1 | 7.6 | 462.9 ± 20.0 | 6.21 ± 0.80 |
| 30 | 68.3 ± 10.3 | 6.8 | 59.7 ± 6.8 | 7.8 | 462.9 ± 20.0 | ||
| 60 | 98.1 ± 16.4 | 4.7 | 68.1 ± 11.0 | 6.7 | 456.9 ± 19.7 | ||
| 120 | 159.7 ± 21.7 | 2.8 | 136.2 ± 18.3 | 3.3 | 448.1 ± 21.7 | ||
| TP-427 | 15 | 72.1 ± 7.0 | >13 | 40.9 ± 6.4 | >24.4 | >1000 | 6.17 ± 1.43 |
| 30 | 74.5 ± 8.1 | >13 | 46.6 ± 8.2 | >21.5 | >1000 | ||
| 60 | 83.6 ± 3.8 | 6.5 | 51.6 ± 6.9 | 10.5 | 540.7 ± 20.9 | ||
| 120 | 97.9 ± 10.9 | 5.6 | 64.9 ± 5.6 | 8.5 | 548.5 ± 21.4 | ||
| TPR-22 | 15 | 130.4 ±17.6 | 2.3 | no data | no data | 306.0 ± 19.8 (†) | 18.9 ± 1.2 |
| 30 | 130.4 ± 17.6 | 2.4 | no data | no data | 314.5 ± 22.0 (†) | ||
| 60 | 159.9 ± 21.9 | 2.0 | no data | no data | 325.9 ± 23.1 (†) | ||
| 120 | 195.7 ± 21.5 | 1.7 | no data | no data | 329.9 ± 24.2 (†) | ||
| Parameter | Unit | Control Group | Examined Group | p Value |
|---|---|---|---|---|
| RBC | 10*6/μL | 8.24 ± 0.44 | 8.37 ± 0.84 | 0.8183 |
| HGB | g/dL | 14.03 ± 0.35 | 13.88 ± 1.28 | 0.8499 |
| MCV | µm3 | 49.53 ± 2.04 | 49.1 ± 20.06 | 0.0504 |
| MCH | pg | 17.0 ± 0.72 | 16.62 ± 0.38 | 0.3536 |
| MCHC) | g/dL | 34.33 ± 0.06 | 33.84 ± 0.46 | 0.1244 |
| RDW-CV | % | 13.27 ± 1.49 | 13.46 ± 0.56 | 0.9881 |
| RDW-SD | µm3 | 28.3 ± 2.61 | 28.46 ± 1.36 | 0.4434 |
| HCT | % | 40.77 ± 0.95 | 41.08 ± 3.65 | 0.6810 |
| PLT | 10*3/µL | 879.0 ± 64.21 | 1034.8 ± 268.44 | 0.3285 |
| MPV | µm3 | 5.2 ± 0.2 | 5.08 ± 0.25 | 0.3336 |
| PDW | 15.5 ± 0.36 | 15.24 ± 0.17 | 0.0740 | |
| PCT | % | 0.35 ± 0.17 | 0.53 ± 0.16 | 0.1874 |
| WBC | 10*3/µL | 4.46 ± 1.01 | 4.25 ± 1.2 | 0.8096 |
| BAS | % | 0.43 ± 0.22 | 0.44 ± 0.19 | 0.5824 |
| NEU | % | 22.53 ± 2.4 | 23.28 ± 7.19 | 0.8709 |
| LYM | % | 69.0 ± 2.61 | 65.7 ± 6.96 | 0.4714 |
| MON | % | 3.93 ± 0.48 | 4.04 ± 1.17 | 0.8881 |
| EOS | % | 4.1 ± 0.53 | 6.54 ± 2.05 | 0.0972 |
| Analyte Concentration in the Injected Sample [ppm] | Extraction Yield [%±SD] | Repeatability [RSD%] | LOD [ppm] | LOQ [ppm] |
|---|---|---|---|---|
| 0.06 | 96.66 ± 1.77 | 1.83 | 0.000025 | 0.000084 |
| 0.12 | 99.39 ± 0.44 | 0.44 | ||
| 0.18 | 97.46 ± 0.94 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makuch-Kocka, A.; Andres-Mach, M.; Zagaja, M.; Śmiech, A.; Pizoń, M.; Flieger, J.; Cielecka-Piontek, J.; Plech, T. Effect of Chronic Administration of 5-(3-chlorophenyl)-4-Hexyl-2,4 -Dihydro-3H-1,2,4-Triazole-3-Thione (TP-315)—A New Anticonvulsant Drug Candidate—On Living Organisms. Int. J. Mol. Sci. 2021, 22, 3358. https://doi.org/10.3390/ijms22073358
Makuch-Kocka A, Andres-Mach M, Zagaja M, Śmiech A, Pizoń M, Flieger J, Cielecka-Piontek J, Plech T. Effect of Chronic Administration of 5-(3-chlorophenyl)-4-Hexyl-2,4 -Dihydro-3H-1,2,4-Triazole-3-Thione (TP-315)—A New Anticonvulsant Drug Candidate—On Living Organisms. International Journal of Molecular Sciences. 2021; 22(7):3358. https://doi.org/10.3390/ijms22073358
Chicago/Turabian StyleMakuch-Kocka, Anna, Marta Andres-Mach, Mirosław Zagaja, Anna Śmiech, Magdalena Pizoń, Jolanta Flieger, Judyta Cielecka-Piontek, and Tomasz Plech. 2021. "Effect of Chronic Administration of 5-(3-chlorophenyl)-4-Hexyl-2,4 -Dihydro-3H-1,2,4-Triazole-3-Thione (TP-315)—A New Anticonvulsant Drug Candidate—On Living Organisms" International Journal of Molecular Sciences 22, no. 7: 3358. https://doi.org/10.3390/ijms22073358
APA StyleMakuch-Kocka, A., Andres-Mach, M., Zagaja, M., Śmiech, A., Pizoń, M., Flieger, J., Cielecka-Piontek, J., & Plech, T. (2021). Effect of Chronic Administration of 5-(3-chlorophenyl)-4-Hexyl-2,4 -Dihydro-3H-1,2,4-Triazole-3-Thione (TP-315)—A New Anticonvulsant Drug Candidate—On Living Organisms. International Journal of Molecular Sciences, 22(7), 3358. https://doi.org/10.3390/ijms22073358







