Cyclopentanone Derivative Attenuates Memory Impairment by Inhibiting Amyloid Plaques Formation in the 5xFAD Mice
Abstract
:1. Introduction
2. Results
2.1. Computational Study of 3NCP with β-Secretase
2.1.1. Molecular Docking
2.1.2. Molecular Dynamics (MD) Simulations
2.1.3. Molecular Mechanics-Generalized Born Surface Area (MMGBSA) Binding Free Energy Analysis
2.2. Behavioral Experiments
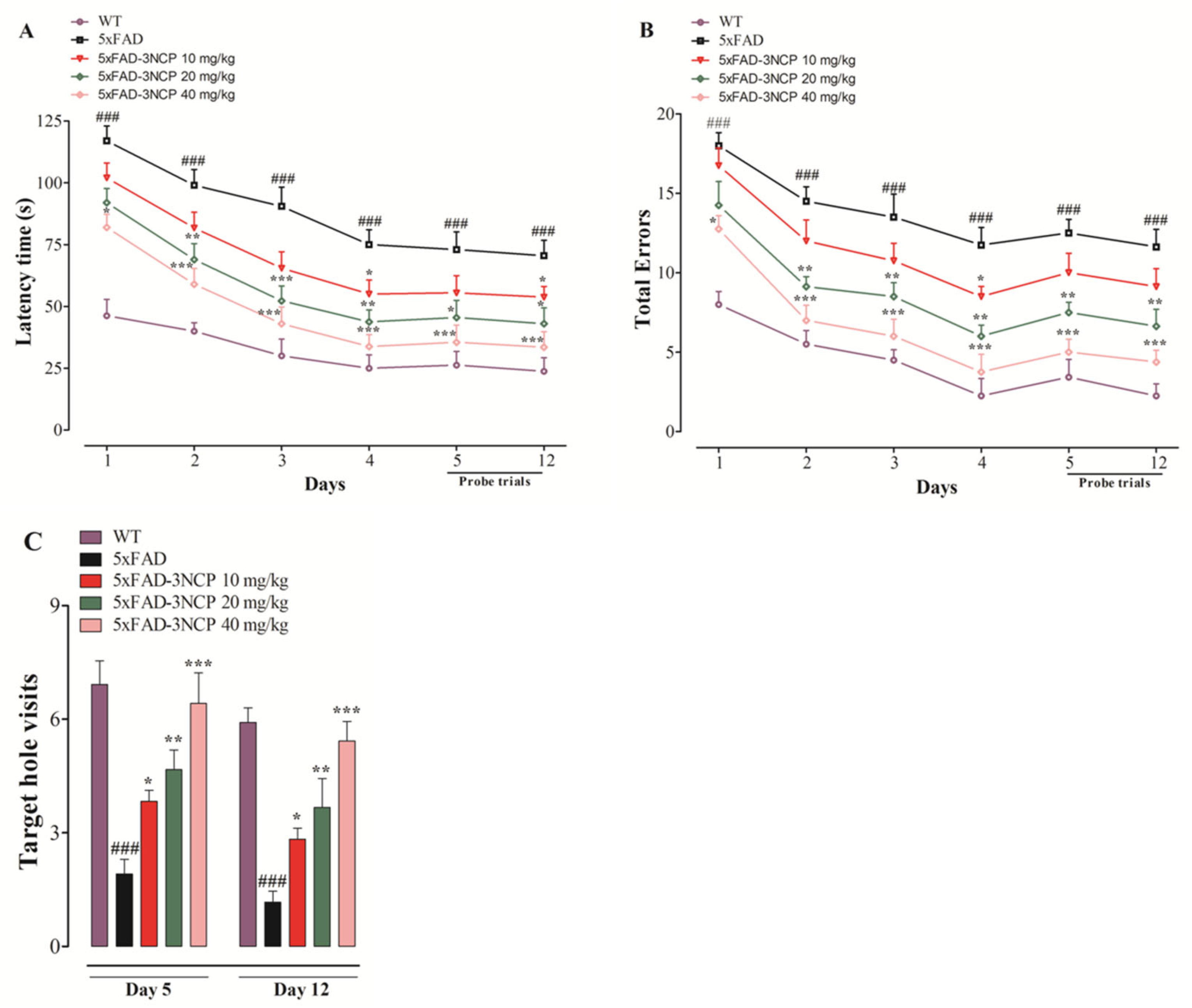
2.2.1. Effect of 3NCP in the LD Test
2.2.2. Effect of 3NCP in the Barnes Maze Test
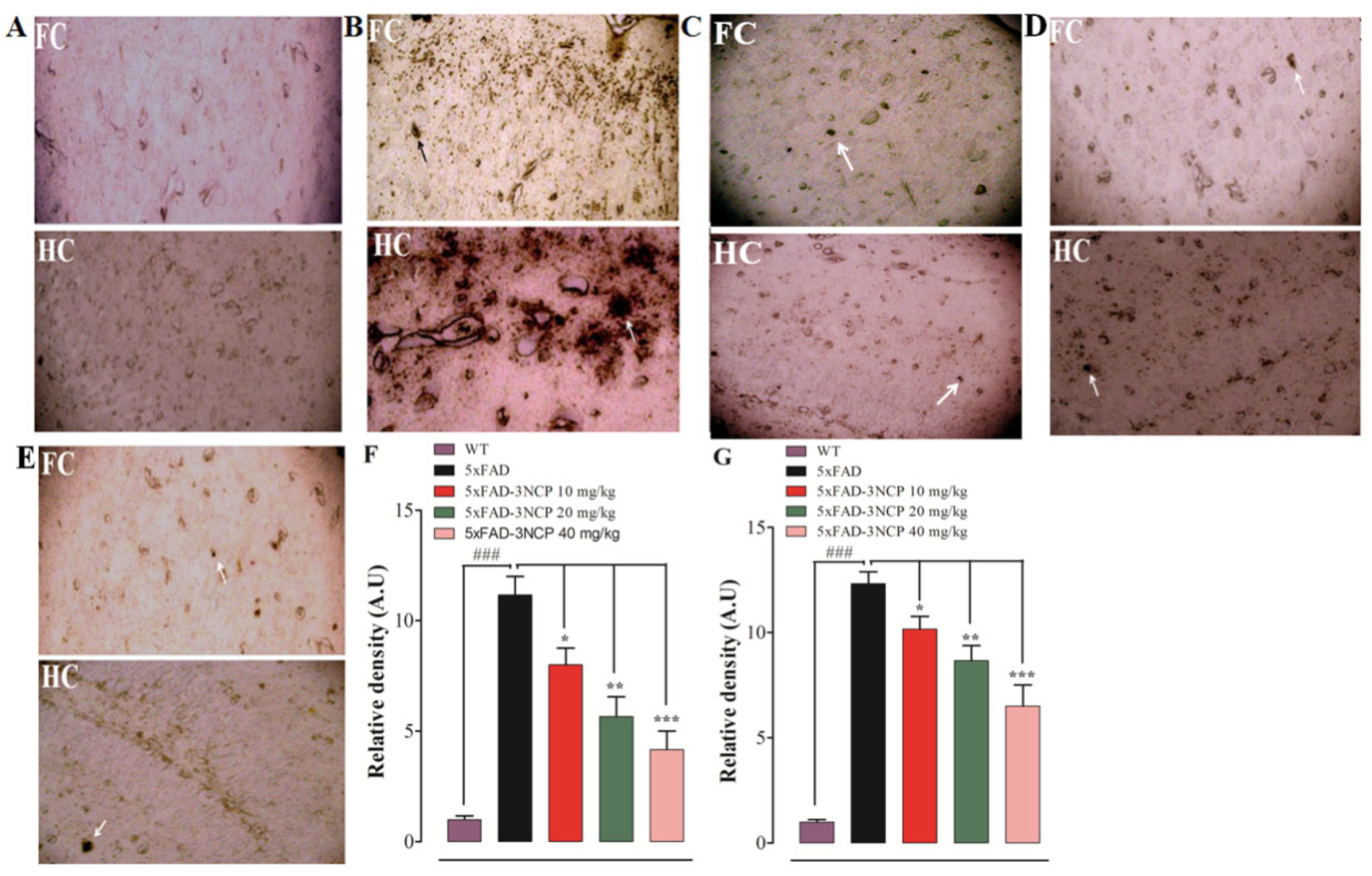
2.3. Effects of 3NCP on Aβ Plaques in Frontal Cortex and Hippocampus
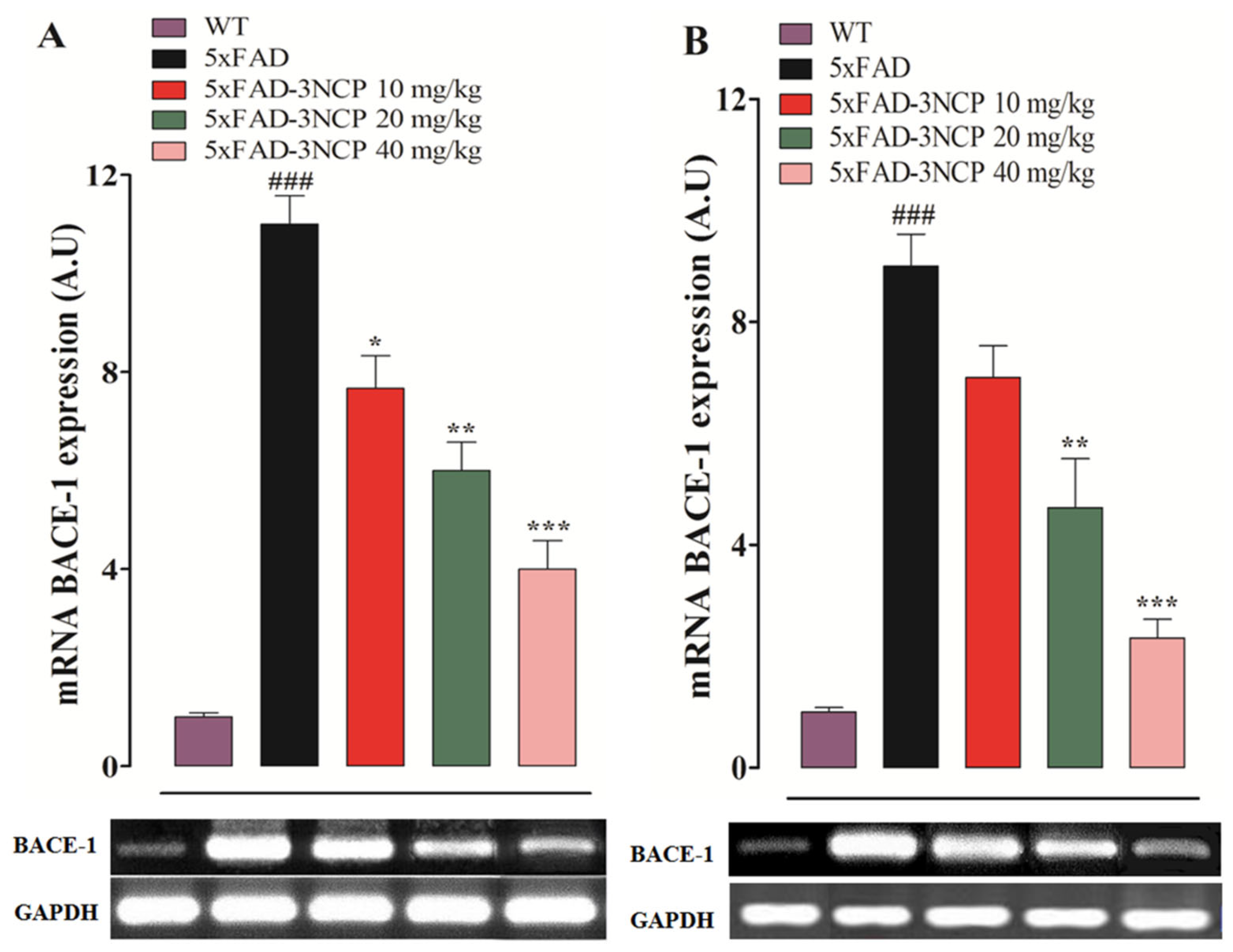
2.4. Effect of 3NCP on BACE-1 Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Computational Study of 3NCP with β-Secretase
4.3.1. Molecular Docking with AutoDock 4.2
4.3.2. Molecular Dynamics Simulation
4.3.3. Binding Free Energy Calculations
4.4. Genotyping of 5xFAD Mice
4.5. Behavioral Experiments
4.5.1. Light/Dark Box (LD) Test
4.5.2. Elevated Plus Maze (EPM) Test
4.5.3. Barnes Maze (BM) Test
4.6. Detection of Neuritic Plaques in Mouse Brain
4.6.1. Thioflavin S Staining Procedure for Detection of Neuritic Plaques
4.6.2. Immunohistochemistry (IHC)
4.7. RT-PCR
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shal, B.; Khan, A.; Khan, A.U.; Ullah, R.; Ali, G.; Islam, S.U.; Haq, I.U.; Ali, H.; Seo, E.-K.; Khan, S. Alleviation of Memory Deficit by Bergenin via the Regulation of Reelin and Nrf-2/NF-κB Pathway in Transgenic Mouse Model. Int. J. Mol. Sci. 2021, 22, 6603. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Piplani, P. Current pharmacotherapy and putative disease-modifying therapy for Alzheimer’s disease. Neurol. Sci. 2016, 37, 1403–1435. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; Volk, S.; Gerbaldo, H. Auguste D and Alzheimer’s disease. Lancet 1997, 349, 1546–1549. [Google Scholar] [CrossRef]
- Lage, J.M.M. 100 Years of Alzheimer’s disease (1906–2006). J. Alzheimer’s Dis. 2006, 9, 15–26. [Google Scholar] [CrossRef]
- Alzheimer, A. Uber eine eigenartige Erkrankung der Hirnrinde. Zentralbl. Nervenh. Psych. 1907, 18, 177–179. [Google Scholar]
- D’andrea, M.; Nagele, R.; Wang, H.Y.; Peterson, P.; Lee, D. Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer’s disease. Histopathology 2001, 38, 120–134. [Google Scholar] [CrossRef]
- Suemoto, T.; Okamura, N.; Shiomitsu, T.; Suzuki, M.; Shimadzu, H.; Akatsu, H.; Yamamoto, T.; Kudo, Y.; Sawada, T. In vivo labeling of amyloid with BF-108. Neurosci. Res. 2004, 48, 65–74. [Google Scholar] [CrossRef]
- Askarova, S.; Yang, X.; Lee, J.C.-M. Impacts of membrane biophysics in Alzheimer’s disease: From amyloid precursor protein processing to Aβ peptide-induced membrane changes. Int. J. Alzheimer’s Dis. 2011, 2011, 134971. [Google Scholar] [CrossRef] [Green Version]
- Accardo, A.; Shalabaeva, V.; Cotte, M.; Burghammer, M.; Krahne, R.; Riekel, C.; Dante, S. Amyloid β peptide conformational changes in the presence of a lipid membrane system. Langmuir 2014, 30, 3191–3198. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Haertel, C.; Maelicke, A.; Montag, D. Galantamine slows down plaque formation and behavioral decline in the 5XFAD mouse model of Alzheimer’s disease. PLoS ONE 2014, 9, e89454. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.L.; Vassar, R. The Alzheimer’s disease β-secretase enzyme, BACE1. Mol. Neurodegener. 2007, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Sastre, M. Troubleshooting methods for APP processing in vitro. J. Pharmacol. Toxicol. Methods 2010, 61, 86–91. [Google Scholar] [CrossRef]
- Vassar, R. Bace 1. J. Mol. Neurosci. 2004, 23, 105–113. [Google Scholar] [CrossRef]
- Lahiri, D.; Kotwal, G.; Farlow, M.; Sima, A.; Kupsky, W.; Sarkar, F.; Sambamurti, K. The Role of the Carboxyl-Terminal Fragments of Amyloid Precursor Protein in Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 2002, 973, 334–339. [Google Scholar] [CrossRef]
- Lauritzen, I.; Pardossi-Piquard, R.; Bauer, C.; Brigham, E.; Abraham, J.-D.; Ranaldi, S.; Fraser, P.; St-George-Hyslop, P.; Le Thuc, O.; Espin, V. The β-secretase-derived C-terminal fragment of βAPP, C99, but not Aβ, is a key contributor to early intraneuronal lesions in triple-transgenic mouse hippocampus. J. Neurosci. 2012, 32, 16243–16255. [Google Scholar] [CrossRef]
- Cai, H.; Wang, Y.; McCarthy, D.; Wen, H.; Borchelt, D.R.; Price, D.L.; Wong, P.C. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat. Neurosci. 2001, 4, 233–234. [Google Scholar] [CrossRef]
- Kitazawa, M.; Medeiros, R.; Laferla, F.M. Transgenic mouse models of Alzheimer disease: Developing a better model as a tool for therapeutic interventions. Curr. Pharm. Des. 2012, 18, 1131–1147. [Google Scholar] [CrossRef] [Green Version]
- Sadleir, K.R.; Popovic, J.; Vassar, R. ER stress is not elevated in the 5XFAD mouse model of Alzheimer’s disease. J. Biol. Chem. 2018, 293, 18434–18443. [Google Scholar] [CrossRef] [Green Version]
- Maarouf, C.L.; Kokjohn, T.A.; Whiteside, C.M.; Macias, M.P.; Kalback, W.M.; Sabbagh, M.N.; Beach, T.G.; Vassar, R.; Roher, A.E. Molecular Differences and Similarities Between Alzheimer’s Disease and the 5XFAD Transgenic Mouse Model of Amyloidosis. Biochem. Insights. 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Casey, D.A.; Antimisiaris, D.; O’Brien, J. Drugs for Alzheimer’s disease: Are they effective? Pharm. Ther. 2010, 35, 208. [Google Scholar]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharmacal. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Ullah, R.; Ali, G.; Ahmad, N.; Akram, M.; Kumari, G.; Amin, M.U.; Umar, M.N. Attenuation of Spatial Memory in 5xFAD Mice by Halting Cholinesterases, Oxidative Stress and Neuroinflammation Using a Cyclopentanone Derivative. Pharmaceuticals 2020, 13, 318. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Ali, G.; Muhammad, T.; Ullah, R.; Umar, M.N.; Hashmi, A.N. Synthetic β-hydroxy ketone derivative inhibits cholinesterases, rescues oxidative stress and ameliorates cognitive deficits in 5XFAD mice model of AD. Mol. Biol. Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- Secci, D.; Carradori, S.; Petzer, A.; Guglielmi, P.; D’Ascenzio, M.; Chimenti, P.; Bagetta, D.; Alcaro, S.; Zengin, G.; Petzer, J.P. 4-(3-Nitrophenyl) thiazol-2-ylhydrazone derivatives as antioxidants and selective hMAO-B inhibitors: Synthesis, biological activity and computational analysis. J. Enzym. Inhib. Med. Chem. 2019, 34, 597–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkerhoff, R.C.; Santa-Helena, E.; do Amaral, P.C.; Cabrera, D.d.C.; Ongaratto, R.F.; de Oliveira, P.M.; D’Oca, C.D.R.M.; Gonçalves, C.A.N.; Nery, L.E.M.; D’Oca, M.G.M. Evaluation of the antioxidant activities of fatty polyhydroquinolines synthesized by Hantzsch multicomponent reactions. RSC Adv. 2019, 9, 24688–24698. [Google Scholar] [CrossRef] [Green Version]
- Resende, M.F.; Lino, C.I.; Souza-Fagundes, E.M.D.; Rettore, J.V.P.; Oliveira, R.B.D.; Labanca, R.A. Assessment of anti-diabetic activity of a novel hydrazine-thiazole derivative: In vitro and in vivo method. Braz. J. Pharm. Sci. 2019, 55. [Google Scholar] [CrossRef] [Green Version]
- Deng, D.S.; Cai, J. Stereoselective aldol reactions catalyzed by acyclic amino acids in aqueous micelles. Helv. Chim. Acta 2007, 90, 114–120. [Google Scholar] [CrossRef]
- Tian, H.; Gao, J.-L.; Xu, H.; Zheng, L.-Y.; Huang, W.-B.; Liu, Q.-W.; Zhang, S.-Q. Proline-based dipeptides as efficient organocatalysts for asymmetric aldol reactions in brine. Tetrahedron Asymmetry 2011, 22, 1074–1080. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef]
- Wagner, J.M.; Sichler, M.E.; Schleicher, E.M.; Franke, T.N.; Irwin, C.; Löw, M.J.; Beindorff, N.; Bouter, C.; Bayer, T.A.; Bouter, Y. Analysis of motor function in the Tg4–42 mouse model of Alzheimer’s disease. Front. Behav. Neurosci. 2019, 13, 107. [Google Scholar] [CrossRef] [Green Version]
- Hui, J.; Feng, G.; Zheng, C.; Jin, H.; Jia, N. Maternal separation exacerbates Alzheimer’s disease-like behavioral and pathological changes in adult APPswe/PS1dE9 mice. Behav. Brain Res. 2017, 318, 18–23. [Google Scholar] [CrossRef]
- Brooks, S.P.; Dunnett, S.B. Tests to assess motor phenotype in mice: A user’s guide. Nat. Rev. Neurosci. 2009, 10, 519–529. [Google Scholar] [CrossRef]
- Bhanumathy, M.; Harish, M.; Shivaprasad, H.; Sushma, G. Nootropic activity of Celastrus paniculatus seed. Pharm. Biol. 2010, 48, 324–327. [Google Scholar] [CrossRef]
- Biala, G.; Kruk, M. Cannabinoid receptor ligands suppress memory-related effects of nicotine in the elevated plus maze test in mice. Behav. Brain Res. 2008, 192, 198–202. [Google Scholar] [CrossRef]
- Manasa, A.; Karimulla, S.; Gobinath, M. Ameliorative effect of Cleome gynandra Linn against scopolamine induced amnesia in mice. Int. J. Res. Pharm. Sci. 2017, 8, 642–649. [Google Scholar]
- Attar, A.; Liu, T.; Chan, W.-T.C.; Hayes, J.; Nejad, M.; Lei, K.; Bitan, G. A shortened Barnes maze protocol reveals memory deficits at 4-months of age in the triple-transgenic mouse model of Alzheimer’s disease. PLoS ONE 2013, 8, e80355. [Google Scholar] [CrossRef]
- Yankner, B.A.; Lu, T. Amyloid β-protein toxicity and the pathogenesis of Alzheimer disease. J. Biol. Chem. 2009, 284, 4755–4759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Echeverria, V.; Ducatenzeiler, A.; Dowd, E.; Jänne, J.; Grant, S.; Szyf, M.; Wandosell, F.; Avila, J.; Grimm, H.; Dunnett, S. Altered mitogen-activated protein kinase signaling, tau hyperphosphorylation and mild spatial learning dysfunction in transgenic rats expressing the β-amyloid peptide intracellularly in hippocampal and cortical neurons. Neuroscience 2004, 129, 583–592. [Google Scholar] [CrossRef]
- Lee, Y.K.; Yuk, D.Y.; Lee, J.W.; Lee, S.Y.; Ha, T.Y.; Oh, K.W.; Yun, Y.P.; Hong, J.T. (−)-Epigallocatechin-3-gallate prevents lipopolysaccharide-induced elevation of beta-amyloid generation and memory deficiency. Brain Res. 2009, 1250, 164–174. [Google Scholar] [CrossRef]
- Bhattarai, A.; Devkota, S.; Bhattarai, S.; Wolfe, M.S.; Miao, Y. Mechanisms of γ-secretase activation and substrate processing. ACS Cent. Sci. 2020, 6, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, S.; Wang, W.; Sun, H.; Zhang, Q.; Liu, X. Binding of Inhibitors to BACE1 Affected by pH-Dependent Protonation: An Exploration from Multiple Replica Gaussian Accelerated Molecular Dynamics and MM-GBSA Calculations. ACS Chem. Neurosci. 2021, 12, 2591–2607. [Google Scholar] [CrossRef]
- Zhu, K.; Peters, F.; Filser, S.; Herms, J. Consequences of pharmacological BACE inhibition on synaptic structure and function. Biol. Psychiatry 2018, 84, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, D.; Tournoy, J.; Hartmann, D.; Huth, T.; Cryns, K.; Deforce, S.; Serneels, L.; Camacho, I.E.; Marjaux, E.; Craessaerts, K. Phenotypic and biochemical analyses of BACE1-and BACE2-deficient mice. J. Biol. Chem. 2005, 280, 30797–30806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The β-Secretase BACE1 in Alzheimer’s Disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef]
- Mukda, S.; Panmanee, J.; Boontem, P.; Govitrapong, P. Melatonin administration reverses the alteration of amyloid precursor protein-cleaving secretases expression in aged mouse hippocampus. Neurosci. Lett. 2016, 621, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, B.S. The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aliaga, K.; Bermejo-Bescós, P.; Benedí, J.; Martín-Aragón, S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011, 89, 939–945. [Google Scholar] [CrossRef]
- Jawhar, S.; Trawicka, A.; Jenneckens, C.; Bayer, T.A.; Wirths, O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Aβ aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 196.e29–196.e40. [Google Scholar] [CrossRef]
- Girard, S.D.; Baranger, K.; Gauthier, C.; Jacquet, M.; Bernard, A.; Escoffier, G.; Marchetti, E.; Khrestchatisky, M.; Rivera, S.; Roman, F.S. Evidence for early cognitive impairment related to frontal cortex in the 5XFAD mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 33, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Oehlrich, D.; Peschiulli, A.; Tresadern, G.; Van Gool, M.; Vega, J.A.; De Lucas, A.I.; Alonso de Diego, S.A.; Prokopcova, H.; Austin, N.; Van Brandt, S. Evaluation of a series of β-secretase 1 inhibitors containing novel heteroaryl-fused-piperazine amidine warheads. ACS Med. Chem. Lett. 2019, 10, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Huey, R.; Morris, G.M.; Forli, S. Using AutoDock 4 and AutoDock vina with AutoDockTools: A tutorial. Scripps Res. Inst. Mol. Graph. Lab. 2012, 10550, 91000–92037. [Google Scholar]
- Ullah, R.; Ali, G.; Subhan, F.; Naveed, M.; Khan, A.; Khan, J.; Halim, S.A.; Ahmad, N.; Zakiullah; Al-Harrasi, A. Attenuation of nociceptive and paclitaxel-induced neuropathic pain by targeting inflammatory, CGRP and Substance P signaling using 3-Hydroxyflavone. Neurochem. Int. 2021, 144, 104981. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Parihar, D.; Shah, M.; Yadav, S.; Managori, H.; Bhowmick, S.; Patil, P.C.; Alissa, S.A.; Wabaidur, S.M.; Islam, M.A. Computational screening of promising beta-secretase 1 inhibitors through multi-step molecular docking and molecular dynamics simulations-Pharmacoinformatics approach. J. Mol. Struct. 2020, 1205, 127660. [Google Scholar] [CrossRef]
- Taha, M.O.; Habash, M.; Al-Hadidi, Z.; Al-Bakri, A.; Younis, K.; Sisan, S. Docking-based comparative intermolecular contacts analysis as new 3-D QSAR concept for validating docking studies and in silico screening: NMT and GP inhibitors as case studies. J. Chem. Inf. Modeling 2011, 51, 647–669. [Google Scholar] [CrossRef]
- Lee, T.-S.; Allen, B.K.; Giese, T.J.; Guo, Z.; Li, P.; Lin, C.; McGee, T.D., Jr.; Pearlman, D.A.; Radak, B.K.; Tao, Y. Alchemical binding free energy calculations in AMBER20: Advances and best practices for drug discovery. J. Chem. Inf. Modeling 2020, 60, 5595–5623. [Google Scholar] [CrossRef]
- Abro, A.; Azam, S.S. Binding free energy based analysis of arsenic (+3 oxidation state) methyltransferase with S-adenosylmethionine. J. Mol. Liq. 2016, 220, 375–382. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An overview of the Amber biomolecular simulation package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, G.; Rio, A.D.; Degliesposti, G.; Sgobba, M. Fast and accurate predictions of binding free energies using MM-PBSA and MM-GBSA. J. Comput. Chem. 2010, 31, 797–810. [Google Scholar] [CrossRef]
- Bathini, R.; Sivan, S.K.; Fatima, S.; Manga, V. Molecular docking, MM/GBSA and 3D-QSAR studies on EGFR inhibitors. J. Chem. Sci. 2016, 128, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Khan, J.; Ali, G.; Rashid, U.; Khan, R.; Jan, M.S.; Ullah, R.; Ahmad, S.; Abbasi, S.W.; Khan Khalil, A.A.; Sewell, R.D.E. Mechanistic evaluation of a novel cyclohexanone derivative’s functionality against nociception and inflammation: An in-vitro, in-vivo and in-silico approach. Eur. J. Pharmacol. 2021, 174091. [Google Scholar] [CrossRef]
- Reiserer, R.; Harrison, F.; Syverud, D.; McDonald, M. Impaired spatial learning in the APPSwe+ PSEN1ΔE9 bigenic mouse model of Alzheimer’s disease. Genes Brain Behav. 2007, 6, 54–65. [Google Scholar] [CrossRef]
- Xu, W.; Xu, F.; Anderson, M.E.; Kotarba, A.E.; Davis, J.; Robinson, J.K.; Van Nostrand, W.E. Cerebral microvascular rather than parenchymal amyloid-β protein pathology promotes early cognitive impairment in transgenic mice. J. Alzheimer’s Dis. 2014, 38, 621–632. [Google Scholar] [CrossRef] [Green Version]
- Dhingra, D.; Kumar, V. Memory-enhancing activity of palmatine in mice using elevated plus maze and Morris water maze. Adv. Pharmacol. Sci. 2012, 2012, 357368. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Price, T.O.; Banks, W.A.; Ercal, N.; Morley, J.E. Effect of alpha-lipoic acid on memory, oxidation, and lifespan in SAMP8 mice. J. Alzheimer’s Dis. 2012, 32, 447–455. [Google Scholar] [CrossRef]
- Ly, P.T.; Cai, F.; Song, W. Detection of neuritic plaques in Alzheimer’s disease mouse model. J. Vis. Exp. JoVE 2011, 53, e2831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Gong, Y.; Luan, Y.; Li, Y.; Liu, J.; Yue, Z.; Yuan, B.; Sun, J.; Xie, C.; Li, L. BHBA treatment improves cognitive function by targeting pleiotropic mechanisms in transgenic mouse model of Alzheimer’s disease. FASEB J. 2020, 34, 1412–1429. [Google Scholar] [CrossRef] [Green Version]
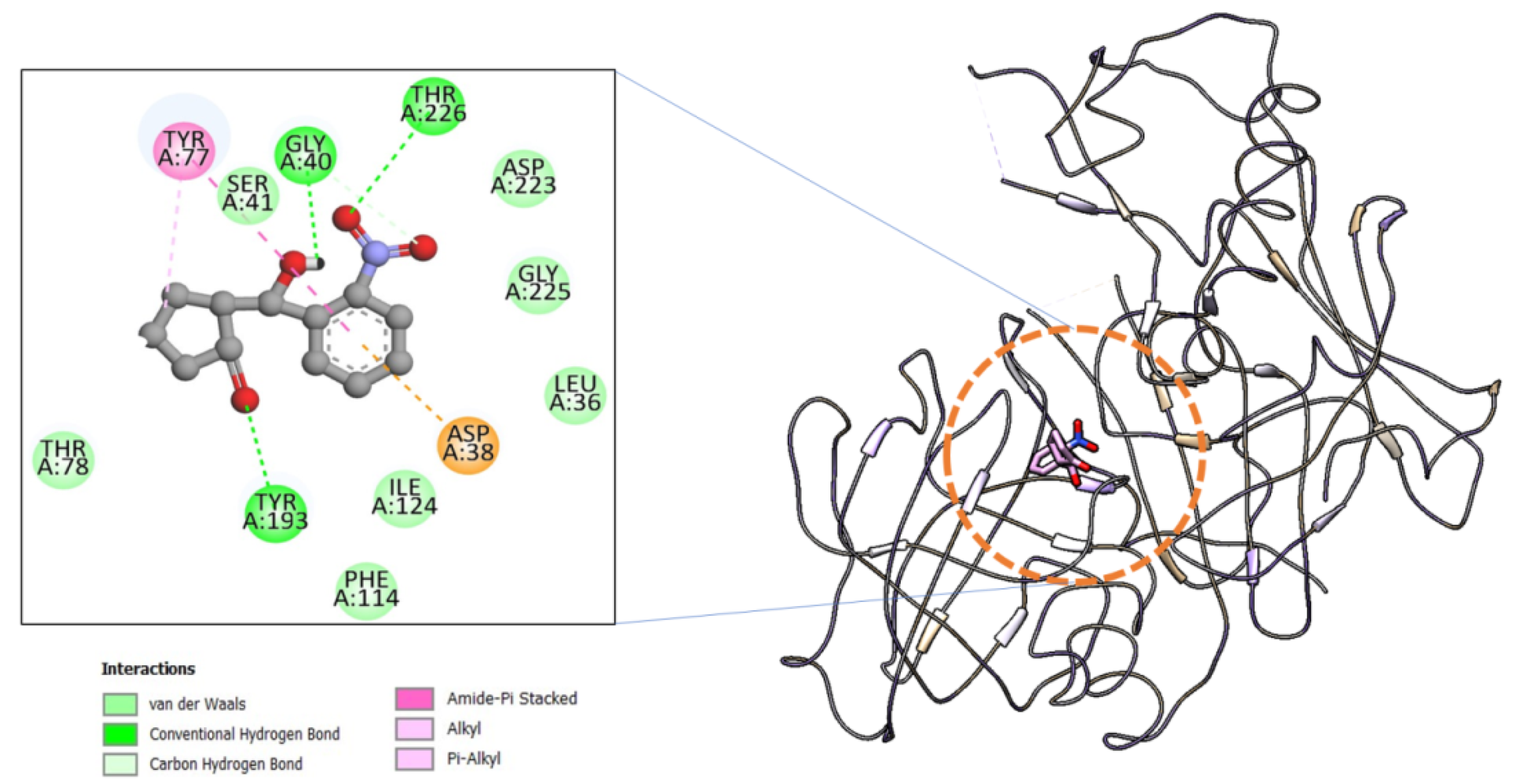
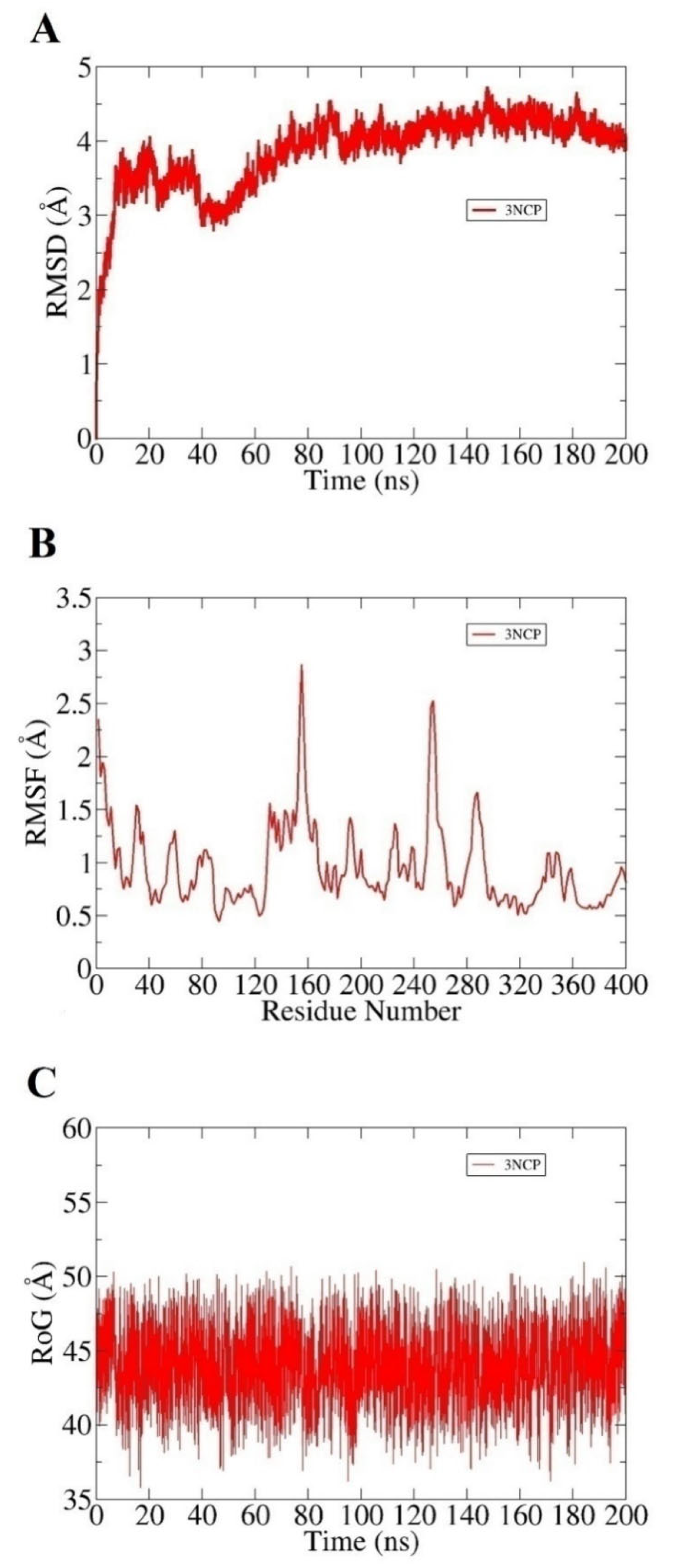
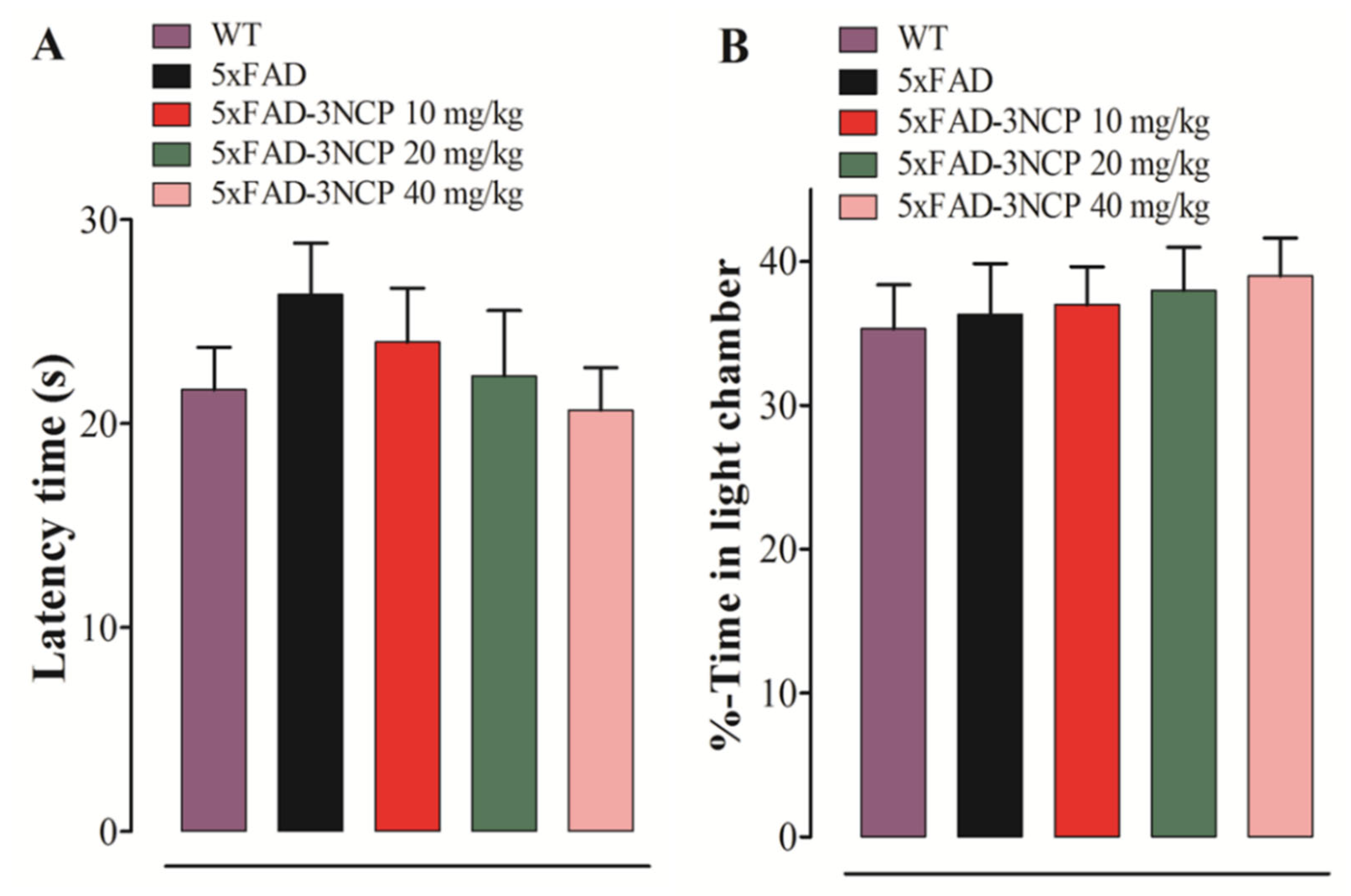
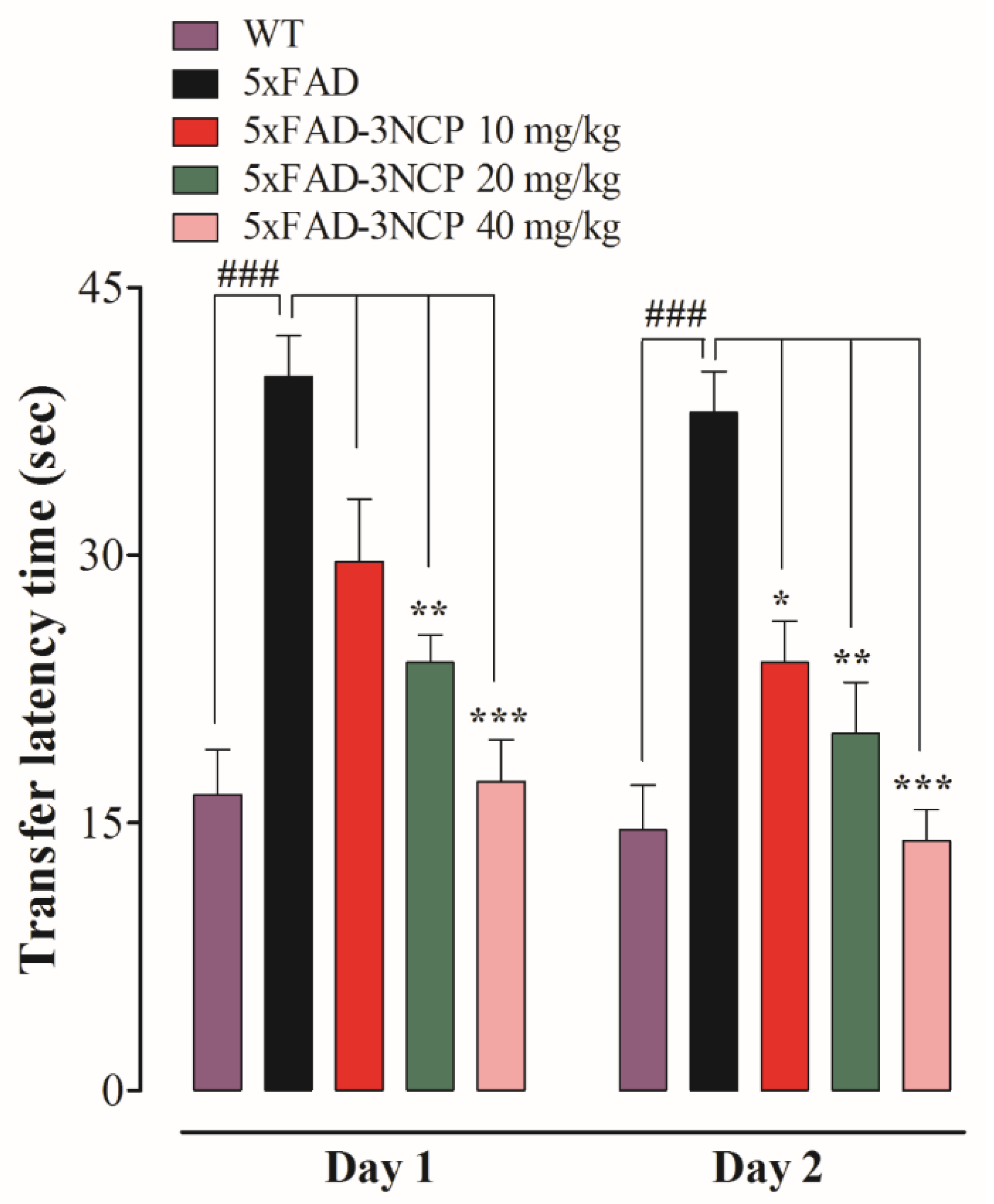

| Table Cont. | 3NCP Complex |
|---|---|
| Van der Waals energy | −27.72 |
| Electrostatic energy | −37.01 |
| Polar solvation energy | 47.36 |
| Nonpolar solvation energy | −3.6 |
| Net gas phase energy | −64.73 |
| Net solvation energy | 43.76 |
| Net total energy | −20.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, R.; Ali, G.; Khan, A.; Ahmad, S.; Al-Harrasi, A. Cyclopentanone Derivative Attenuates Memory Impairment by Inhibiting Amyloid Plaques Formation in the 5xFAD Mice. Int. J. Mol. Sci. 2021, 22, 9559. https://doi.org/10.3390/ijms22179559
Ullah R, Ali G, Khan A, Ahmad S, Al-Harrasi A. Cyclopentanone Derivative Attenuates Memory Impairment by Inhibiting Amyloid Plaques Formation in the 5xFAD Mice. International Journal of Molecular Sciences. 2021; 22(17):9559. https://doi.org/10.3390/ijms22179559
Chicago/Turabian StyleUllah, Rahim, Gowhar Ali, Ajmal Khan, Sajjad Ahmad, and Ahmed Al-Harrasi. 2021. "Cyclopentanone Derivative Attenuates Memory Impairment by Inhibiting Amyloid Plaques Formation in the 5xFAD Mice" International Journal of Molecular Sciences 22, no. 17: 9559. https://doi.org/10.3390/ijms22179559
APA StyleUllah, R., Ali, G., Khan, A., Ahmad, S., & Al-Harrasi, A. (2021). Cyclopentanone Derivative Attenuates Memory Impairment by Inhibiting Amyloid Plaques Formation in the 5xFAD Mice. International Journal of Molecular Sciences, 22(17), 9559. https://doi.org/10.3390/ijms22179559








