Lantibiotics Produced by Oral Inhabitants as a Trigger for Dysbiosis of Human Intestinal Microbiota
Abstract
1. Introduction
2. Results
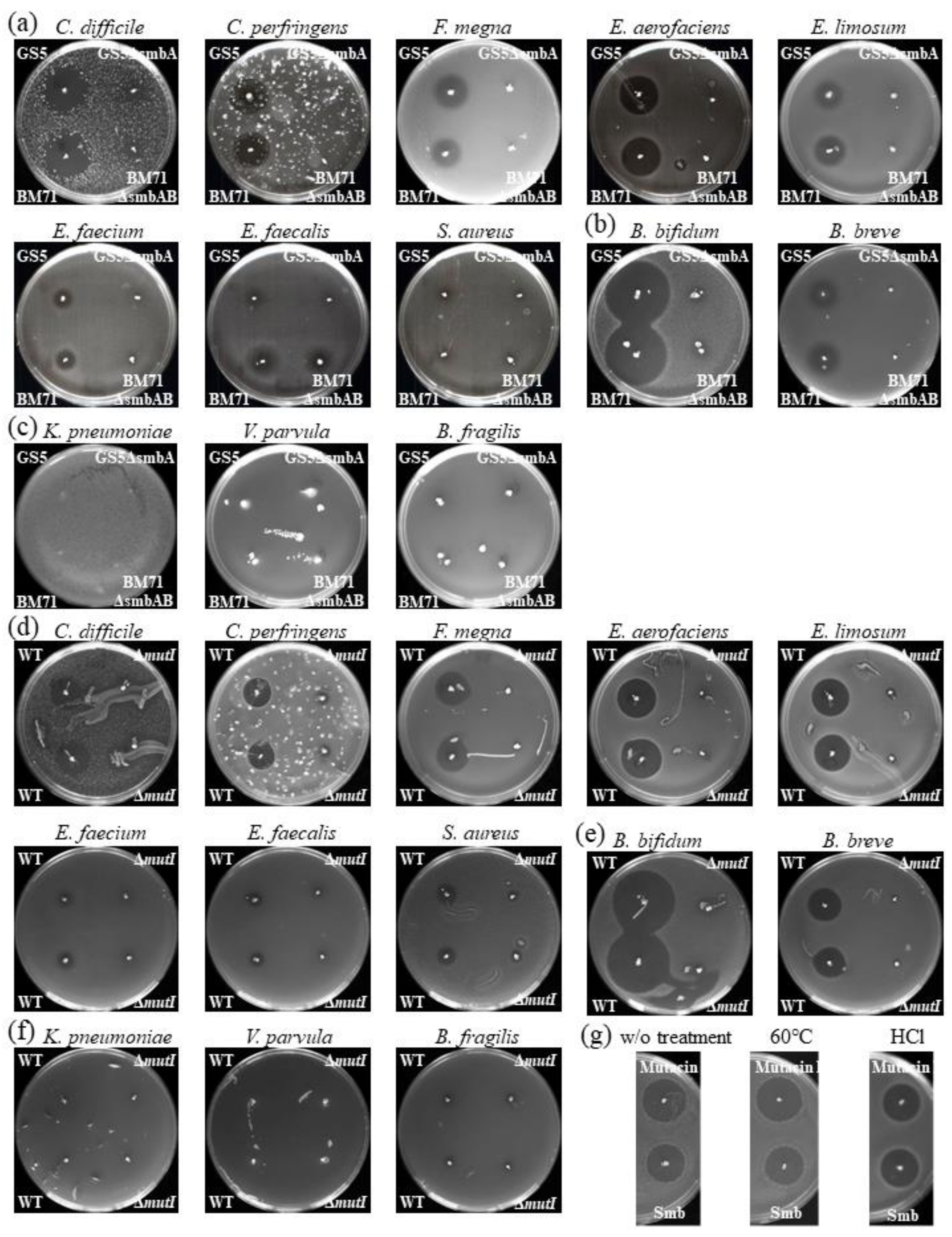
2.1. Overall In Vitro Susceptibility of Intestinal Microbiota Bacteria to Smb
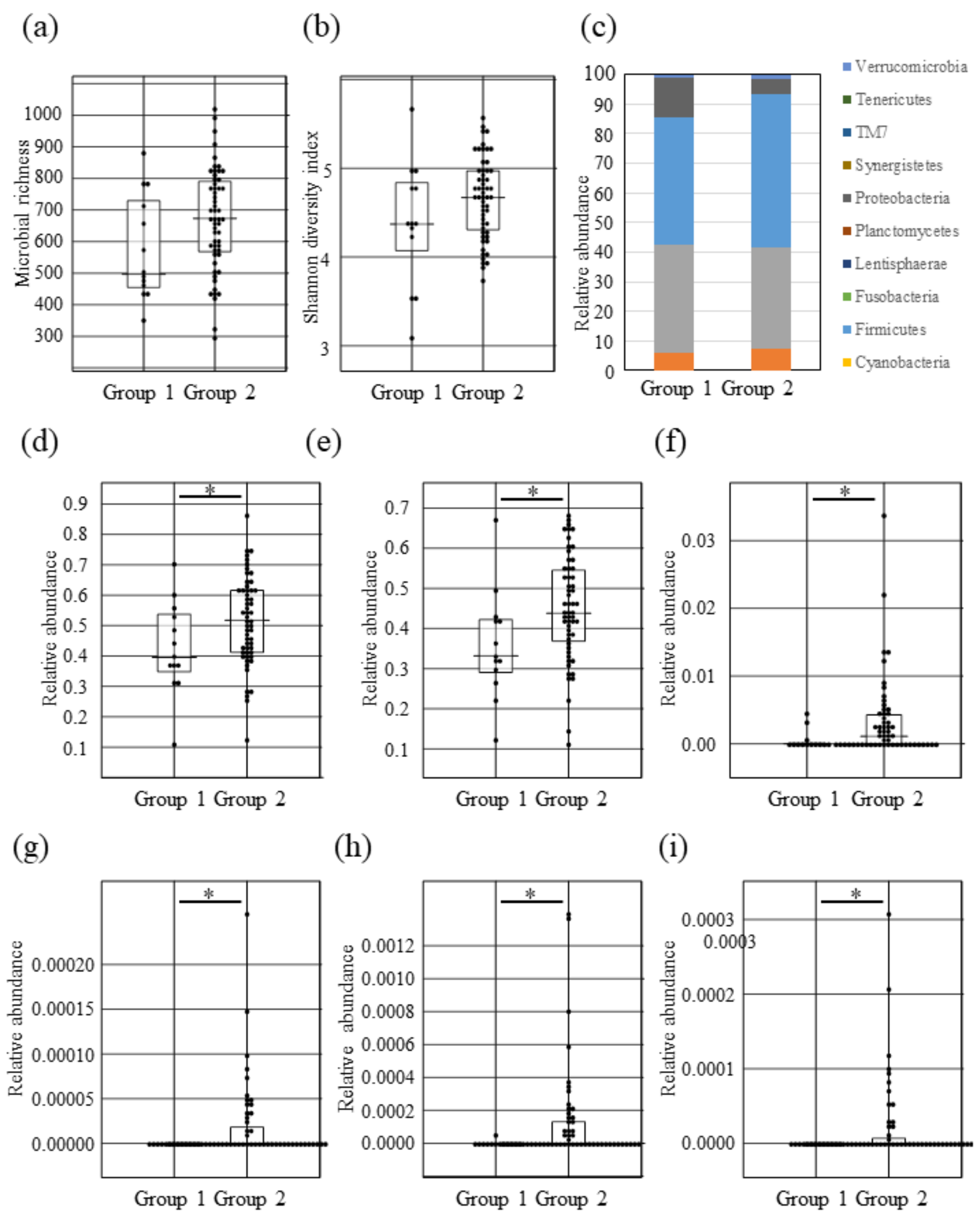
2.2. Possible Impact of Lantibiotics Produced by Oral Bacteria on Intestinal Microbiota
2.3. Intestinal Microbial Composition Changes between Lantibiotic Positive and Negative Subjects
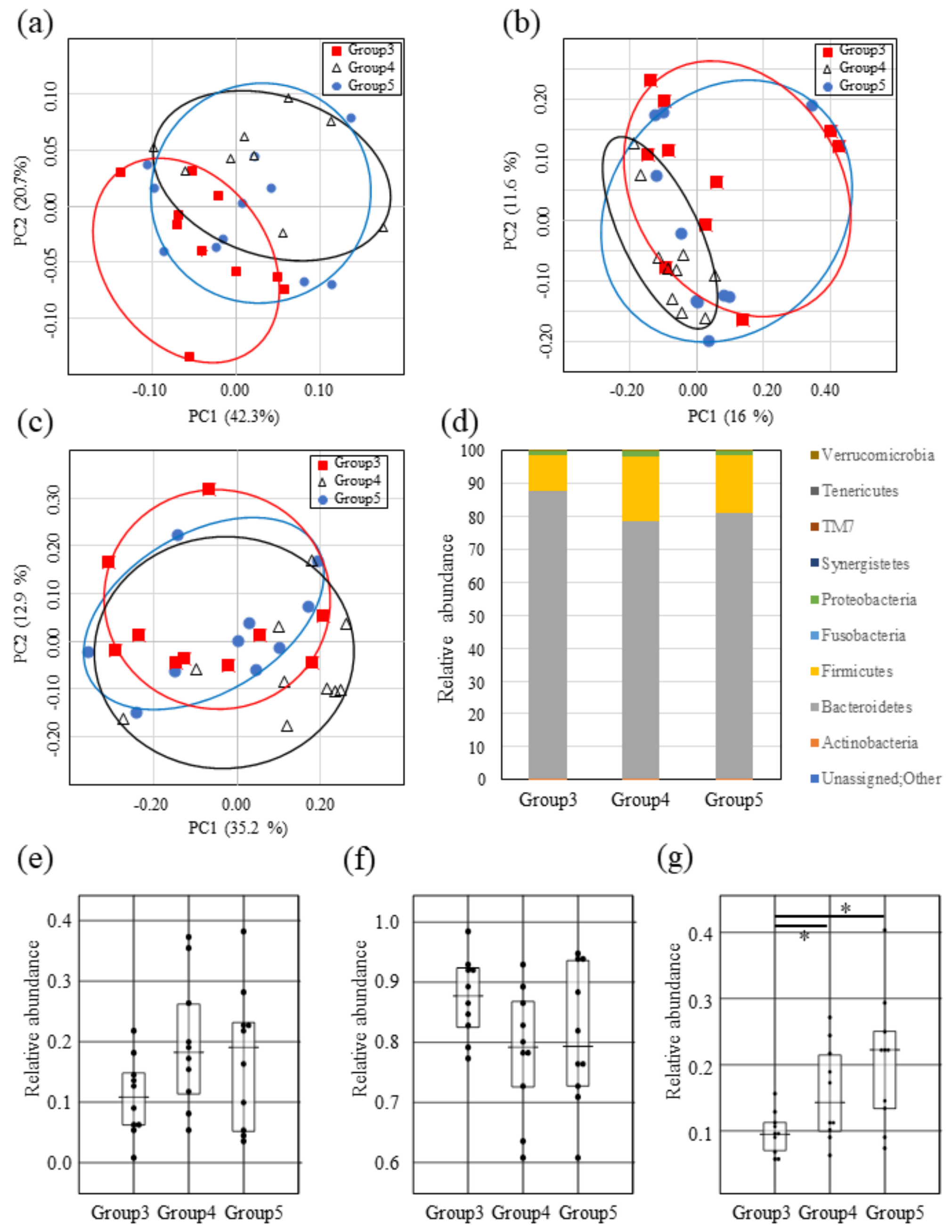
2.4. Impact of Both Mutacin I/III and Smb on Intestinal Microbiota
2.5. Lantibiotics Produced by Oral Bacteria Do Not Affect Salivary Microbiota
2.6. Administration of the Supernatant Containing Mutacin I Reduces Firmicute Bacteria in the Mouse Intestine
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Agar Plate Bacteriocin Assay
4.2. Fecal and Saliva Sample Collection
4.3. Mouse Fecal Sample Collection
4.4. Isolation of S. mutans and Construction of Mutants
4.5. DNA Extraction from Fecal and Saliva Samples and 16S rRNA Sequences
4.6. Quantitative Real-Time PCR
4.7. 16S rRNA Sequence Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the Gut Microbiota in Immunity and Inflammatory Disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Blaser, M.J. Fecal Microbiota Transplantation for Dysbiosis—Predictable Risks. N. Engl. J. Med. 2019, 381, 2064–2066. [Google Scholar] [CrossRef]
- Das, B.; Nair, G.B. Homeostasis and Dysbiosis of the Gut Microbiome in Health and Disease. J. Biosci. 2019, 44, 5. [Google Scholar] [CrossRef]
- Manges, A.R.; Labbe, A.; Loo, V.G.; Atherton, J.K.; Behr, M.A.; Masson, L.; Tellis, P.A.; Brousseau, R. Comparative Meta-Genomic Study of Alterations to the Intestinal Microbiota and Risk of Nosocomial Clostridum Dificile-Associated Disease. J. Infect. Dis. 2010, 202, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nat. Cell Biol. 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Pedersen, H.K. Disentangling Type 2 Diabetes and Metformin Treatment Signatures in the Human Gut Microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Mitev, K.; Taleski, V. Association between the Gut Microbiota and Obesity. Open Access Maced. J. Med. Sci. 2019, 7, 2050–2056. [Google Scholar] [CrossRef]
- Chatterjee, C.; Paul, M.; Xie, L.; van der Donk, W.A. Biosynthesis and Mode of Action of Lantibiotics. Chem. Rev. 2005, 105, 633–684. [Google Scholar] [CrossRef]
- Willey, J.M.; van der Donk, W.A. Lantibiotics: Peptides of Diverse Structure and Function. Annu. Rev. Microbiol. 2007, 61, 477–501. [Google Scholar] [CrossRef]
- Kuramitsu, H.K. Virulence Factors of Mutans Streptococci: Role of Molecular Genetics. Crit. Rev. Oral Biol. Med. 1993, 4, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.; Qi, F. The Mutacins of Streptococcus mutans: Regulation and Ecology. Mol. Oral Microbiol. 2011, 27, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Caufield, P.W.; Miller, E.J. Isolation and Biochemical Characterization of a Novel Lantibiotic Mutacin from Streptococcus mutans. J. Bacteriol. 1994, 176, 4316–4320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chikindas, M.L.; Novák, J.; Driessen, A.J.; Konings, W.; Schilling, K.M.; Caufield, P.W. Mutacin II, a Bactericidal Antibiotic from Streptococcus mutans. Antimicrob. Agents Chemother. 1995, 39, 2656–2660. [Google Scholar] [CrossRef][Green Version]
- Qi, F.; Chen, P.; Caufield, P.W. Purification of Mutacin III from Group III Streptococcus mutans UA787 and Genetic Analyses of Mutacin III Biosynthesis Genes. Appl. Environ. Microbiol. 1999, 65, 3880–3887. [Google Scholar] [CrossRef]
- Yonezawa, H.; Kuramitsu, H.K. Genetic Analysis of a Unique Bacteriocin, Smb, Produced by Streptococcus mutans GS5. Antimicrob. Agents Chemother. 2005, 49, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Nes, I.F.; Diep, D.B.; Håvarstein, L.S.; Brurberg, M.B.; Eijsink, V.; Holo, H. Biosynthesis of Bacteriocins in Lactic Acid Bacteria. Lact. Acid Bact. Genet. Metab. Appl. 1996, 70, 17–32. [Google Scholar] [CrossRef]
- Denoel, T.; Lemaire, C.; Luxen, A. Progress in Lanthionine and Protected Lanthionine Synthesis. Chemistry 2018, 24, 15421–15441. [Google Scholar] [CrossRef]
- Escano, J.; Ravichandran, A.; Salamat, B.; Smith, L. Carboxyl Analogue of Mutacin 1140, a Scaffold for Lead Antibacterial Discovery. Appl. Environ. Microbiol. 2017, 83, 14. [Google Scholar] [CrossRef] [PubMed]
- Linnett, P.E.; Strominger, J.L. Additional Antibiotic Inhibitors of Peptidoglycan Synthesis. Antimicrob. Agents Chemother. 1973, 4, 231–236. [Google Scholar] [CrossRef]
- Chihib, N.-E.; Tholozan, J.-L. Effect of Rapid Cooling and Acidic pH on Cellular Homeostasis of Pectinatus Frisingensis, a Strictly Anaerobic Beer-Spoilage Bacterium. Int. J. Food Microbiol. 1999, 48, 191–202. [Google Scholar] [CrossRef]
- Parrot, M.; Caufield, P.W.; Lavoie, M.C. Preliminary Characterization of Four Bacteriocins from Streptococcus mutans. Can. J. Microbiol. 1990, 36, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the Immune System. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-Induced Extinctions in the Gut Microbiota Compound over Generations. Nat. Cell Biol. 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Virta, L.J.; Kekkonen, R.A.; Forslund, K.; Bork, P.; De Vos, W.M. Intestinal Microbiome Is Related to Lifetime Antibiotic Use in Finnish Pre-School Children. Nat. Commun. 2016, 7, 10410. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The Gut Microbiota Shapes Intestinal Immune Responses during Health and Disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Holmes, E.; Li, J.V.; Marchesi, J.R.; Nicholson, J.K. Gut Microbiota Composition and Activity in Relation to Host Metabolic Phenotype and Disease Risk. Cell Metab. 2012, 16, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, E.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Gut Microbiota as a Source of Novel Antimicrobials. Gut Microbes 2019, 10, 1–21. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and Consequences of Intestinal Dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Diversity, Metabolism and Microbial Ecology of Butyrate-Producing Bacteria from the Human Large Intes-Tine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Doumatey, A.P.; Adeyemo, A.; Zhou, J.; Lei, L.; Adebamowo, S.N.; Adebamowo, C.; Rotimi, C.N. Gut Microbiome Profiles Are Associated with Type 2 Diabetes in Urban Africans. Front. Cell. Infect. Microbiol. 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Hokamura, K.; Taniguchi, N.; Wada, K.; Kudo, C.; Nomura, R.; Kojima, A.; Naka, S.; Muranaka, Y.; Thura, M.; et al. The Collagen-Binding Protein of Streptococcus mutans Is Involved in Haemorrhagic Stroke. Nat. Commun. 2011, 2, 485. [Google Scholar] [CrossRef]
- Liu, L.; Hao, T.; Xie, Z.; Horsman, G.P.; Chen, Y. Genome Mining Unveils Widespread Natural Product Biosynthetic Capacity in Human Oral Microbe Streptococcus mutans. Sci. Rep. 2016, 6, 37479. [Google Scholar] [CrossRef]
- Robson, C.L.; Wescombe, P.A.; Klesse, N.A.; Tagg, J.R. Isolation and Partial Characterization of the Streptococcus mutans Type AII Lantibiotic Mutacin K8. Microbiology 2007, 153, 1631–1641. [Google Scholar] [CrossRef][Green Version]
- Yonezawa, H.; Kuramitsu, H.K.; Nakayama, S.-I.; Mitobe, J.; Motegi, M.; Nakao, R.; Watanabe, H.; Senpuku, H. Differential Expression of the Smb Bacteriocin in Streptococcus mutans Isolates. Antimicrob. Agents Chemother. 2008, 52, 2742–2749. [Google Scholar] [CrossRef] [PubMed]
- Wescombe, P.A.; Heng, N.C.K.; Burton, J.P.; Tagg, J.R. Something Old and Something New: An Update on the Amazing Repertoire of Bacteriocins Produced by Streptococcus salivarius. Probiotics Antimicrob. Proteins 2009, 2, 37–45. [Google Scholar] [CrossRef]
- Van Tyne, D.; Martin, M.J.; Gilmore, M.S. Structure, Function, and Biology of the Enterococcus faecalis Cytolysin. Toxins 2013, 5, 895–911. [Google Scholar] [CrossRef]
- Mishiro, T.; Oka, K.; Kuroki, Y.; Takahashi, M.; Tatsumi, K.; Saitoh, T.; Tobita, H.; Ishimura, N.; Sato, S.; Ishihara, S.; et al. Oral Microbiome Alterations of Healthy Volunteers with Proton Pump Inhibitor. J. Gastroenterol. Hepatol. 2018, 33, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Motegi, M.; Takagi, Y.; Yonezawa, H.; Hanada, N.; Terajima, J.; Watanabe, H.; Senpuku, H. Assessment of Genes Associated with Streptococcus mutans Biofilm Morphology. Appl. Environ. Microbiol. 2006, 72, 6277–6287. [Google Scholar] [CrossRef]
- Shiroza, T.; Kuramitsu, H.K. Construction of a Model Secretion System for Oral Streptococci. Infect. Immun. 1993, 61, 3745–3755. [Google Scholar] [CrossRef]
- Okuda, M.; Osaki, T.; Kikuchi, S.; Ueda, J.; Lin, Y.; Yonezawa, H.; Maekawa, K.; Hojo, F.; Kamiya, S.; Fukuda, Y. Evaluation of a Stool Antigen Test Using a mAb for Native Catalase for Diagnosis of Helicobacter pylori Infection in Children and Adults. J. Med. Microbiol. 2014, 63, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xia, X.; Tang, R.; Zhou, J.; Zhao, H.; Wang, K. Development of a Real-Time PCR Method for Firmicutes and Bac-teroidetes in Faeces and Its Application to Quantify Intestinal Population of Obese and Lean Pigs. Lett. Appl. Microbiol. 2008, 47, 367–373. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16s Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2012, 41, e1. [Google Scholar] [CrossRef]
- Kuczynski, J.; Stombaugh, J.; Walters, W.A.; Gonzalez, A.; Caporaso, J.G.; Knight, R. Using QIIME to Analyze 16S rRNA Gene Sequences from Microbial Communities. Curr. Protoc. Bioinform. 2011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Zapala, M.A.; Schork, N.J. Multivariate Regression Analysis of Distance Matrices for Testing Associations between Gene Expression Patterns and Related Variables. Proc. Natl. Acad. Sci. USA 2006, 103, 19430–19435. [Google Scholar] [CrossRef] [PubMed]
| Primer. | Nucleotide Sequence (5′-3′) | Source of Reference |
|---|---|---|
| MutI/III F1st | GAGGCTAATGGTGGTATTAT | This study |
| MutI/III R1st | CCCACTTTACTATGAGTATC | This study |
| MutI/III F2nd | GTTTTCTGATATGCTTCTACTG | This study |
| MutI/III R2nd | CTAATATCAAAAGATTGTGCCG | This study |
| MutII F1st | GTGGTAAAAAAGATGGTAAACTG | This study |
| MutII R1st | TTAACAAGGTCCTGGTGGT | This study |
| MutII F2nd | ATGAACAAGTTAAACAGTAACGC | This study |
| MutII R2nd | CCGGTAAGTACATAGTGC | This study |
| Smb F1st | GCAATAACTTTTGGGTGGC | This study |
| Smb R1st | CCTTTATTTCCCAATACAATG | This study |
| Smb F2nd | GGAGCATTATGATGATAGGT | This study |
| Smb R2nd | TTCTTGCAAGCCTGCTTT | This study |
| Firm934F | GGAGYATGTGGTTTAATTCGAAGCA | 49 |
| Firm1060R | AGCTGACGACAACCATGCAC | 49 |
| Eub338F | AGCTGACGACAACCATGCAC | 49 |
| Eub518R | CGCTACTTGGCTGGTTCAG | 49 |
| MutI UF | GAAGAGTGGACTGAGTATG | This study |
| MutI URBam | CGGGATCCAGTATCTGTCCTCCTCGAA | This study |
| MutI DFBam | CGGGATCCCAAGGACTTCTAATAATTGTGTG | This study |
| MutI DR | GTTTAGAAACTTCTGTTTGACTATAC | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yonezawa, H.; Motegi, M.; Oishi, A.; Hojo, F.; Higashi, S.; Nozaki, E.; Oka, K.; Takahashi, M.; Osaki, T.; Kamiya, S. Lantibiotics Produced by Oral Inhabitants as a Trigger for Dysbiosis of Human Intestinal Microbiota. Int. J. Mol. Sci. 2021, 22, 3343. https://doi.org/10.3390/ijms22073343
Yonezawa H, Motegi M, Oishi A, Hojo F, Higashi S, Nozaki E, Oka K, Takahashi M, Osaki T, Kamiya S. Lantibiotics Produced by Oral Inhabitants as a Trigger for Dysbiosis of Human Intestinal Microbiota. International Journal of Molecular Sciences. 2021; 22(7):3343. https://doi.org/10.3390/ijms22073343
Chicago/Turabian StyleYonezawa, Hideo, Mizuho Motegi, Atsushi Oishi, Fuhito Hojo, Seiya Higashi, Eriko Nozaki, Kentaro Oka, Motomichi Takahashi, Takako Osaki, and Shigeru Kamiya. 2021. "Lantibiotics Produced by Oral Inhabitants as a Trigger for Dysbiosis of Human Intestinal Microbiota" International Journal of Molecular Sciences 22, no. 7: 3343. https://doi.org/10.3390/ijms22073343
APA StyleYonezawa, H., Motegi, M., Oishi, A., Hojo, F., Higashi, S., Nozaki, E., Oka, K., Takahashi, M., Osaki, T., & Kamiya, S. (2021). Lantibiotics Produced by Oral Inhabitants as a Trigger for Dysbiosis of Human Intestinal Microbiota. International Journal of Molecular Sciences, 22(7), 3343. https://doi.org/10.3390/ijms22073343






