Abstract
Skeletal muscle regeneration is highly dependent on the inflammatory response. A wide variety of innate and adaptive immune cells orchestrate the complex process of muscle repair. This review provides information about the various types of immune cells and biomolecules that have been shown to mediate muscle regeneration following injury and degenerative diseases. Recently developed cell and drug-based immunomodulatory strategies are highlighted. An improved understanding of the immune response to injured and diseased skeletal muscle will be essential for the development of therapeutic strategies.
1. Introduction
Skeletal muscle injury or disease can result in an inflammatory response in which phagocytes and lymphocytes rapidly invade the tissue to influence satellite cell activation, proliferation, and terminal differentiation (Figure 1) [1,2]. Immediately after injury, the muscle cells begin to undergo necrosis. A compromised sarcolemma causes the cellular contents to be released into the extracellular space. Infiltrating immune cells, such as neutrophils and macrophages, start clearing out the necrotic cell debris and also secrete various growth factors and cytokines to recruit more immune cells to the site of injury [3]. Neutrophils appear to play a transient role and are quickly replaced by macrophages, which persist for weeks to months (Figure 2). Macrophages exhibit diverse phenotypes and can promote either muscle injury or repair depending upon the severity of the injury and the timing of phenotypic (M1/M2) transition [4,5,6].
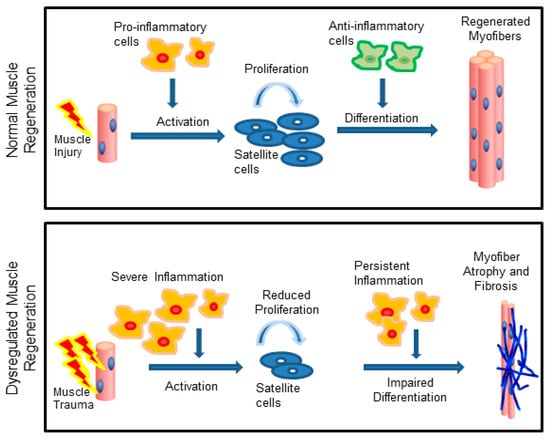
Figure 1.
Skeletal muscle regeneration is dependent on the inflammatory response. Following acute injuries, the pro-inflammatory cells support satellite cell proliferation, while anti-inflammatory cells support differentiation (top). In chronic injuries, persistent inflammation impairs satellite cell activity resulting in muscle wasting and fibrosis (bottom). Adapted from [7].
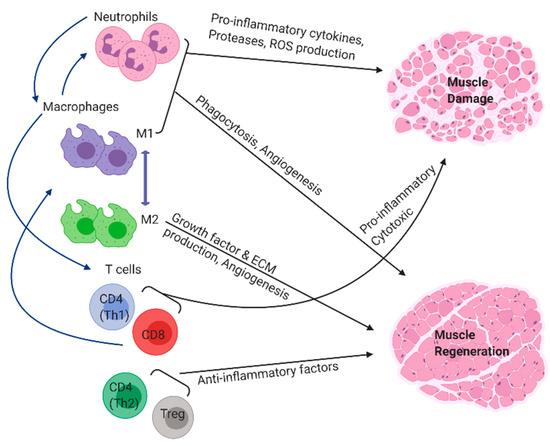
Figure 2.
Inflammatory cells of the innate and adaptive immune system participate in the process of muscle regeneration and repair. Blue arrows indicate immune cells recruiting each other through the secretion of various soluble mediators.
Macrophages are also believed to be responsible for recruiting T cells to the site of muscle injury [8]. The two main T cell types—helper (CD4) and cytotoxic (CD8) can be detected in the wound site as early as three days post-injury [9,10,11]. Similar to macrophages, T cells have been shown to persist at the site of injury for several weeks after trauma. The sustained presence of these cell types at the site of injury suggests that they are critically involved in the regenerative process.
In this work, we review various experimental studies that have attempted to distinguish between the features of the inflammatory response that promote muscle damage, fibrosis, or repair. Several cell types and biomolecules have emerged as targets for therapy design and the development of immunomodulatory strategies is an active area of research for skeletal muscle trauma. While it has been suggested that a heightened and prolonged inflammatory response is detrimental for muscle regeneration, it has also been shown that ablating inflammation is not an effective approach for muscle repair. The precise conditions in which inflammation results in a functionally beneficial regenerative response are unclear. Nevertheless, the activity of invading immune cell types and their actions on resident muscle stem cells largely dictate the regenerative outcome, and the purpose of this review is to highlight and describe the activity of key cellular players in the immune response to injured and diseased muscle tissue.
2. Innate Immune Cells Influence Muscle Regeneration
2.1. The Role of Neutrophils in Muscle Regeneration
Muscle damage is common and can result from sports-related injuries or diseases, such as muscular dystrophy. Mechanical changes that cause trauma or stress to muscle membranes, resulting from injury or overload, initiate a cascade of events that ultimately lead to granulocyte, primarily phagocytic neutrophil infiltration into the site of injury [12,13,14]. Perturbations of skeletal muscle propagates the release of pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin (IL)-1β [12]. These cytokines act on the endothelium of adjacent vasculature causing the release of IL-6 and IL-8 (also known as CXCL8 or neutrophil chemotactic factor) [3,12]. These cytokines, especially IL-8, serve as chemoattractants for neutrophils and cause their migration from circulation into areas of damage [3]. Neutrophils can appear as early as one hour after muscle injury [15,16] and persist for up to a week [17,18].
Their primary role in injured tissue is to remove cellular debris through phagocytosis and recruit macrophages to the wound environment [12,14]. Once activated, neutrophils generate a robust inflammatory response through the release of cytokines (e.g., TNF-α, IL-1, and more IL-8) [19], proteases, and reactive oxygen species (ROS; ex: O₂ˉ, H₂O₂, OHˉ, HClO) [20]. The removal of cellular and fiber debris facilitates muscle regeneration and connective tissue deposition [12]. Additionally, neutrophils can secrete chemoattractants to recruit macrophages to the site of damage. Macrophage infiltration can influence various steps in the process of muscle regeneration, which are described in detail below. Interestingly, macrophage mediated clearance of apoptotic neutrophils stimulates the secretion of TGF-β1 and IL-10, and polarizes the macrophages to an anti-inflammatory phenotype [21]. Besides muscle fiber regeneration, neutrophils can also impact nerve healing and removal of neutrophils may be detrimental to reinnervation [22]. The role of neutrophils is not fully understood, but there is some evidence from a variety of systems to suggest that they can also promote tissue regeneration through regulating angiogenesis and lymphangiogenesis [23,24].
Further evidence for the critical role of neutrophils in muscle regeneration is provided by studies where neutrophils were depleted. For instance, mice injected with a snake venom toxin had significant necrosis and an impaired regenerative response in the absence of neutrophils [25]. Other studies have suggested that neutrophils do not significantly impact muscle recovery. In one research study, animals were subjected to ten days of hind limb unloading, followed by depletion of neutrophils (using the Ly6G/Ly6C (Gr-1) antibody) and then subsequent reloading [26]. The results showed that neutrophil depletion had no impact on the loss of force or recovery of the atrophied fibers [26]. However, it should be noted that incubating soleus muscle after unloading/loading with LPS, a neutrophil activator, led to a 20% greater deficit in force, as well as increased muscle damage, when compared to muscle with depleted neutrophils. The potential for neutrophils to worsen muscle damage and exacerbate injury has also been studied extensively. For example, neutrophil-derived mediators can cause myofiber lesions, membrane lysis, and the oxidative degradation of lipids [2,12,27,28]. While muscle cells will release protective factors and antioxidants such as superoxide dismutase 2 (SOD-2), glutathione peroxidase (GPX), catalase (CAT), and thioredoxin (TRX) which will shield them from the detrimental effects of neutrophil oxidants, overproduction of cytolytic and cytotoxic compounds by neutrophils can further injure the already damaged surrounding tissue [2,29,30].
Substantial research focused on the impact of neutrophils in muscle damage began in the 1980s, and strong evidence suggests that they hinder rather than help muscle regeneration. One of the earliest studies conducted on the topic demonstrates that blocking neutrophil adhesion activity with the anti-CD11b antibody M1/70 reduces oxidant production and myofiber damage after stretch injury at early time points [31]. Blocking CD11b or CD11a has had beneficial effects in a traumatic skeletal injury model induced by a needle puncture as well, with a general reduction in myofiber damage [32]. Other methods of inhibiting neutrophil activity after exercise also mitigate overall muscle cell damage. For example, inhibiting neutrophil activity by mutating the gp91-phox gene, which ultimately leads to superoxide production, protects fibers from damage after hind limb unloading, with a significant reduction in membrane lysis [33]. In a similar study of loading and unloading exercise, mice deficient in neutrophil-derived myeloperoxidase enzyme (MPO) enzyme had significantly less myofiber lysis [34].
Further support for the detrimental role of neutrophils came from a study that reportedly depleted them through with a Ly6G antibody to block activity after exhaustive exercise, and this resulted in reduced myofiber lesions [35]. These observations were correlated with a reduction in TNF-α, IL-6, and macrophage infiltration providing evidence that these are some of the mediators by which neutrophils can cause tissue damage [35]. Neutrophils also mediate destruction through the β2 integrin receptor CD18 and inhibiting this receptor led to a decrease in force deficit and overall oxidative damage with a concomitant increase in the proportion and cross-sectional area of regenerating fibers [15].
The role of neutrophils in exacerbating ischemia reperfusion (I/R) injuries has been well documented [36]. At the site of I/R injury, neutrophils release ROS, proteinases, proinflammatory cytokines and chemokines [2]. Together, these mediators can increase the immune response and lead to tissue necrosis. Once again, the depletion of neutrophils reduced the extent of injury [37,38] and functional deficits [39] following I/R injury.
Besides injuries, neutrophil-mediated muscle damage is also heavily implicated in myopathies, such as muscular dystrophy that show chronic and persistent inflammation [29]. Neutrophil elastase and ROS were found elevated in an animal model of muscular dystrophy (mdx) and contribute to a reduction in myoblast survival and differentiation [40]. Depleting neutrophils in young mdx mice reduced muscle breakdown and blocking TNF-α activity with etanercept decreased exercise-induced muscle damage in adult mdx mice [41]. Utilizing other TNF-α blockers, such as infliximab, also proved beneficial and reduced muscle damage, as evidenced by reduced inflammation and necrosis, along with increased myotube formation [42]. Collectively, these results suggest that either removing neutrophils or blocking mediators of inflammatory activity, such as TNF-α, has beneficial effects on muscle regeneration and supports the hypothesis that neutrophils harm muscle.
Neutrophils have also been implicated in the age-associated decline in muscle regeneration. Neutrophils and macrophages often work in concert to promote the immune response following injury [43]. Similar correlation has been found in aged animals following contusion injury, where an increase in macrophage and neutrophil populations was found [44,45]. However, some studies have highlighted a decline in cellular activity with aging. For instance, a lower phagocytic capacity of macrophages was implicated in the slowing down of the regenerative process [46].
Based on the review of published literature, it appears that neutrophils play dual roles in the muscle regeneration process where they can promote muscle damage and contribute to muscle repair through different mechanisms.
2.2. Macrophages
Macrophages are highly versatile innate effector cells that play a critical role in the mounting and resolution of inflammatory responses [47,48]. Monocytes, originating in the bone marrow, differentiate into macrophages once they reach the tissue and are influenced by the inflammatory milieu and pathogen-associated pattern recognition receptors (PRR) [49,50]. Both tissue-resident and recruited macrophages play important roles in skeletal muscle tissue repair following injury [1,51,52]. While resident macrophages act as initial sensors of pathological events, recruited macrophages amplify the inflammatory response and form a link between innate and adaptive immunological responses [53]. Recent studies further highlight that resident and recruited macrophages are developmentally and functionally distinct [54,55]. Macrophages have several functions in muscle repair and remodeling, including phagocytosis, enzyme secretion, cytokine and growth factor production, antigen presentation, and immune cell recruitment.
Macrophages exhibit complex and hybrid phenotypes as a result of the wide range of activation states they experience [56]. According to a nomenclature introduced in 2000 [57], macrophage activation states may include “classically activated” (M1) macrophages that are pro-inflammatory and “alternatively activated” (M2) macrophages that are anti-inflammatory [52]. Under in vitro conditions, the activation state of macrophages was observed to be analogous to the helper T cell type 1 (Th1) and type 2 (Th2) observed for T lymphocytes (T cells); however, in vivo macrophages demonstrate a wide variety of phenotypes based on the complexity of signals present in the tissue [52,56,58]. In response to LPS treatment or interferon γ (IFN-γ), macrophages become classically activated. M1 macrophages are characterized by the expression of inducible nitrogen oxide synthase (iNOS), pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6, IL-12), CD68, and Toll-like receptor (TLR) ligands. In response to IL-4 and IL-13 acting on common receptor chain, IL-4Rα, macrophages become alternatively activated [59]. M2 macrophages are characterized by the expression of arginase-1 (ARG1), scavenger receptors (CD163), mannose receptors (CD206), and/or anti-inflammatory cytokines (e.g., IL-10) [5,47,48,60,61,62]. Mantovani et al. further subdivided M2 macrophages into M2a, M2b, M2c, and M2d subtypes based on their function and how they are induced by different stimulatory factors. The M2a phenotype is obtained when macrophages are stimulated with IL-4 and IL-13 and are also named wound-healing macrophages for their anti-inflammatory function. M2b macrophages, named regulatory macrophages, play a role in immunoregulation by monitoring the extent of the immune response and the inflammatory reaction. M2b macrophages are induced when exposed to immune complexes (IC) and TLR agonists or IL-1 receptor (IL-1R) agonists. Stimulating macrophages with IL-10 and glucocorticoids induces the M2c phenotype aiding in immunosuppression, phagocytosis, and tissue remodeling [4,62,63,64,65,66]. M2d macrophages, a novel subset of macrophages considered tumor-associated macrophages (TAMs) and are induced by the co-stimulation of TLR ligands and A2 adenosine receptor (A2R) agonists or IL-6. TAMs have been found to contribute to angiogenesis and cancer metastasis while also playing a critical role in the inflammatory response of neoplastic tissue [63,67,68]. While these macrophage phenotypes have been extensively characterized in vitro, their role in regulating tissue regeneration in vivo is unclear. It should also be noted that the M1 and M2 polarization states are hypothetical ends of a spectrum of activation states in which macrophages can exist. The in vivo polarization state of macrophages can be highly complex and heterogeneous with a high degree of plasticity.
Skeletal muscle has remarkable regenerative capabilities following injury. After an injury, a plethora of events occurs, including the activation of satellite cells (MuSC) (Pax7+) and the infiltration of immune cells, such as neutrophils and macrophages [47,69]. Several studies have demonstrated the importance of macrophages in the regeneration of skeletal muscle using various injury models (e.g., hind limb ischemia, freeze-injury, unloading/loading sequences, myotoxic agent injection) [1,48,51,59,69,70,71,72]. After the initial neutrophil influx at the site of injury, macrophages become the dominant inflammatory cell type with peak levels at day 3 post injury [1,47,71].
The recovery of skeletal muscle requires macrophages to phagocytize and remove cellular debris from necrotic muscle tissue, while also producing a vast array of chemokines and cytokines for recruitment of stem cells and coordination of the repair process. The activation state of macrophages influences the myogenic process by acting on myogenic precursor cells. In co-cultures, pro-inflammatory M1 macrophages were more influential in enhancing myogenic precursor cells proliferation, whereas anti-inflammatory M2 macrophages enhanced differentiation, indicated by myogenin expression and myotube formation [59]. Another study demonstrated the supportive role pro-inflammatory M1 macrophages can play in vivo when injected together with myoblasts. The study found that the co-delivery of these cells increased the efficiency of myoblast engraftment and played a role in muscle regeneration by increasing migration and proliferation events but delaying differentiation [73].
Besides paracrine influence on cellular function, macrophages can also have direct cell-to-cell interactions. One study demonstrated that myogenic precursor cells were rescued from apoptosis through direct contact with macrophages in vitro and in vivo [74] via adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1; CD54) and platelet endothelial cell adhesion molecule-1 (PECAM-1; CD31) [75].
2.3. Role of Macrophages in Muscle Injury
Muscle repair and regeneration following an acute muscle injury rely on an inflammatory response which activates a series of interactions between immune cells and muscle cells. The initial response mimics a Th1-like inflammatory response, with an influx of neutrophils, followed by CD68+ macrophages [1]. The presence of IFN-γ and TNF-α promotes classical activation of Ly6C+ monocytes into the M1 phenotype macrophages [1,60]. Monocytes are recruited to the site of injury by cells of both the innate and adaptive immune system, such as neutrophils and CD8+ T cells, and are classified by the expression level of Ly6C [76]. The interaction of CCR2/CCL2 and CX3CR1/CX3CL1 aids in the recruitment and differentiation of monocytes into macrophages [59,77,78,79].
Cytotoxic (CD8+) T cells facilitate the expression of CCL2 by resident macrophages in injured muscle, aiding in the recruitment of inflammatory monocytes [80]. Murine models deficient in CCR2 experienced decreased monocyte infiltration impairing angiogenesis and muscle regeneration whilst promoting adipocyte accumulation following a cardiotoxin-induced injury [81]. Another study showcased that the presence of CCR2 is essential to muscle regeneration, independent of TLR signaling, age, and sex in murine models as the decreased infiltration of monocytes/macrophages promoted a pro-inflammatory microenvironment [82]. Another study using CD8 knockout mice showed decreased expression of CCL2 resulting in impaired muscle regeneration and increased fibrosis [80]. Studies in mice lacking CCR2 or CCL2 have shown that monocyte/macrophage infiltration is impaired, altering the regenerative process directing myogenic precursor cells to promote an adipogenic phenotype [83,84,85,86,87]. The role of CX3CR1/CX3CL1 in skeletal muscle regeneration is still not well studied; however, one study found that a CX3CR1 deficiency rescues impaired muscle regeneration in CCL2 deficient mice following a notexin-induced muscle injury [77]. Another study also found that a CX3CR1 deficiency affected macrophage phagocytic functions, decreased insulin-like growth factor 1 (IGF-1) and IL-6 expression by macrophages, and may have impaired myogenic precursor cell differentiation [70].
The differentiation of monocytes to macrophages is critical to an appropriate healing response. In one study, the monocyte/macrophage population was depleted at various stages before and after cardiotoxin-induced muscle injury using the CD11b-diphtheria toxin receptor (DTR) transgenic mice. The results suggested that early ablations (days 0–2) resulted in greater impairment in regeneration, whereas later ablation (day 4) had a minimal effect [48]. Early ablation resulted in deficient muscle regeneration due to a decreased number of macrophages present at days 2 to 4, highlighting a role of pro-inflammatory macrophages in tissue repair [48]. M1 macrophages are typically associated with exacerbating tissue damage, with the potential of provoking a fibrotic healing response [88,89]. However, a study found that treatment of lacerated mouse muscles with exogenous M1 macrophages reduced fibrosis and enhanced muscle regeneration, highlighting a beneficial role for M1 macrophages in tissue repair [90]. In support, another study also showed that in vitro polarized M1 (LPS/IFN-γ) macrophages may positively influence muscle regeneration when injected into the gastrocnemius muscles after a tourniquet-induced ischemia-reperfusion (TK-I/R) injury. It was shown that the early delivery of M1 macrophages improved functional recovery, accelerated myofiber repair, decreased fibrotic tissue deposition, and increased whole muscle expression of IGF-1 following TK-I/R injury [91].
As mentioned earlier, macrophage phenotypes (M1/M2) play distinct but complementary roles in skeletal muscle healing. In vitro and in vivo, M1 macrophages promote muscle damage through the release of ROS and production of nitric oxide (NO) [2,12,92]. The concentration of NO present at the site of injury plays a role in its function; at high concentrations, apoptosis may be induced, while at low concentrations it can help protect cells against oxidative damage [93,94,95]. One study showed that NO activation was critical in the early phases of skeletal muscle repair in a murine muscle crush injury, as it increased MuSC proliferation and quantity [96,97]. The study demonstrated that the released NO may play a role as a regulator between skeletal muscle regeneration and fibrosis [97].
Once pro-inflammatory (or M1-like) macrophages reach their peak concentration, the pro-inflammatory microenvironment begins to convert into an anti-inflammatory microenvironment with a higher concentration of M2-like macrophages. Recently, studies have found that Forkhead box p3 (Foxp3+) CD4+ regulatory T cells (Treg) play a role in inducing the M2 phenotype and aid in the shift from M1 macrophages to M2 macrophages by producing IL-10 [98,99]. The study also showed that Tregs are recruited to the site of injury by CCL2, similar to monocytes [99]. Studies show that the switch from M1 macrophages to M2 macrophages is a crucial step in skeletal muscle regeneration. The role of IL-10 in mediating the transition between M1 to M2 macrophages was investigated in mice experiencing muscle reloading after the induction of disuse atrophy due to hindlimb unloading. IL-10 ablation significantly reduced the expression of M2 macrophage markers (i.e., CD163 and Arg 1) following 4 days of reloading, indicating a key role in inducing M2 polarization. The study also found that, without the induction of M2 macrophages, muscle regeneration was reduced, and muscle fiber growth was significantly slowed [100]. The beneficial effect of M2 macrophages has also been demonstrated following endurance exercise training in humans [101]. A significant increase in M2 macrophage quantity after 12 weeks of endurance exercise training was associated with myofiber hypertrophy and satellite cell accumulation.
Therefore, the transition to an M2 macrophage phenotype is believed to be essential for effective and complete tissue regeneration. A great deal of effort is being made to tissue engineer therapies that will rapidly elicit an M2 macrophage phenotype upon transplantation. For instance, scaffolds that release IL-4 [102,103], IL-10 [104,105] or present large enough pore-sizes [106], are being designed with the goal to promote an M2 macrophage phenotype in vivo.
At least one study has highlighted the importance of timing at which the polarization of M1 and M2 macrophages takes place. It was shown that introducing M1 macrophages in a temporally coordinated manner can improve angiogenesis and skeletal muscle regeneration following hind limb ischemia [107]. The study further concluded that the persistent activation of M1 macrophages may exacerbate muscle tissue damage; however, the premature introduction of M2 macrophages in the acute inflammatory phase can interfere with muscle regeneration, impeding muscle repair while promoting fibrosis [107].
Regeneration of skeletal muscle following chronic muscle injuries varies greatly from the healing response of an acute muscle injury. The inflammatory response following a chronic muscle injury differs from the cellular response observed in muscle injuries such as crush, freeze, or toxin-induced injuries. Genetic dystrophies are associated with an inflammatory component, evoked by DAMP release due to muscle fiber damage sustained during muscle contraction, and this ultimately results in fiber degeneration and loss of motor unit function [11]. In one study, IL-10 expression was ablated in mdx mice resulting in increased muscle damage and decreased strength [108]. The results of this study suggest that IL-10 plays a role in deactivating M1 macrophages, attenuating the dystrophic pathology at early, acute stages of the disease [108]. One study demonstrated that the depletion of macrophages in an mdx murine model at the early, acute peak of muscle pathology resulted in large reductions of lesions in the sarcolemma of muscle fibers [109]. Taken together, these studies demonstrate that M1 macrophages play a detrimental role in muscular dystrophy due to their highly cytolytic nature, but a reduction in muscle damage may be observed through M2 phenotype switch [110,111,112].
A defective MPC response, infiltration of macrophages, and heightened proliferation of matrix-producing cells are characteristics of chronic inflammation. Similar to muscular dystrophy, VML also exhibits chronic inflammation with repeated degeneration–regeneration cycles [113]. Studies working with VML defects in both rats and pigs found that macrophages persisted in the defects for weeks to months, unlike the immune response associated with an acute injury [113,114]. The role of extracellular matrix (ECM) scaffolds in modulating macrophage phenotypes (M1/M2) has been extensively studied in the context of VML. While some studies have shown positive regenerative outcomes with the induction of an M2 macrophage phenotype, others have highlighted that synergistic roles played by both M1 and M2 phenotypes that are critical for regeneration [115,116]. Following autograft treatment of VML injury, mixed upregulation of pro- and anti-inflammatory macrophage phenotypes is observed for several weeks post-injury. This mixed macrophage phenotype was associated with improved regenerative outcomes and muscle function.
2.4. Role of Macrophages in Aged Muscle
Skeletal muscle undergoes several cellular and metabolic changes with aging, but the underlying mechanisms are poorly characterized. Skeletal muscle aging is strongly influenced by the imbalance of the damage and regeneration processes, at the molecular and cellular levels [45,117,118,119]. The immune system is known to experience changes in function with aging, and one study showed that factors released by young but not old bone marrow cells supported myoblast proliferation and differentiation in vitro [120].
The predominant phenotypes of cells could also change with aging. In a study comparing subpopulations of macrophages in young (average 31.9 years) and elderly individuals (average 71.4 years), the results showed that the quantity of pro-inflammatory M1 macrophages (CD11b+) was lower in elderly muscle at baseline and also in response to resistance exercise when compared to younger muscle, while M2 macrophages (CD163+) were more prevalent [121]. M2 macrophages are the major type of macrophage in healthy skeletal muscle, while M1 macrophages are typically lower in abundance. Due to the low-grade chronic state of inflammation observed in aged muscle, one would expect to see an increase in M1 macrophages and decrease in M2 macrophages; however, a study found that M2 macrophages (CD206+) increased with age while M1 macrophages (CD80+) declined with age [122]. Another study comparing young (21–33 years) and elderly individuals (70–81 years) showed similar results. Higher CD206 gene expression was observed in the muscles of elderly individuals, while no difference in total macrophage content (CD68+) was detected. CD206, a M2 macrophage marker, is suggestive of an increase in the proportion of anti-inflammatory macrophages in senescent muscle [123,124]. One study showed an increase in IL-4 driven M2a macrophages (CD163+) in aged muscles, which were implicated in muscle fibrosis. Loss of NO production in aged muscle enabled the rise of M2a macrophages, resembling the mechanism driving fibrosis in mdx mice [109,125,126]. Collectively, these studies highlight a shift in M1/M2 phenotype balance with age, and a potential detrimental role of increased M2 macrophage accumulation.
Interestingly, it has also been shown that ECM scaffolds derived from animals show different remodeling outcomes and macrophage polarization depending on the age of the source animal. It was found that ECM scaffolds from younger pigs (3–12 weeks old) stimulated a predominantly M2 macrophage response compared to older pigs (26–52 weeks old). An M2 macrophage response was associated with greater myogenesis and lower fibrosis [127]. Therefore, age as a biological variable should be accounted for when studying macrophage polarization.
3. Adaptive Immune Cells Mediate Muscle Regeneration
3.1. Helper and Cytotoxic T Cells
T and B lymphocytes are two of the main cell families that comprise the adaptive immune system, which generates hyper-specific memory responses to immunological threats. This review focuses on the T cells, which consist of CD4+ (helper) and CD8+ (cytotoxic) groups. The CD8+ group is important for clearance of intracellular pathogens (e.g., viruses, mycobacteria, etc.) and emergent neoplasms by direct cell killing. The CD4+ population is the most abundant, well-characterized, and impactful group on overall immune function as their name, helper, indicates their coordination of all immune responses. This help to all the other cells of the immune system is proffered by the cytokine milieu produced by different effector phenotypes of CD4+ T cells, which includes Th1, Th2, Th17, Tfh, and Treg subsets. The help these cells offer is tuned to the type of threat or stage/progress of immune response. These phenotypes are well-characterized and detailed elsewhere [128]. Many of the cytokine signals that potentiate or come from CD8+, Th1 and Th17 cells polarize macrophages toward a pro-inflammatory M1 phenotype, while Th2 and Treg-inducing cytokines promote an anti-inflammatory M2 phenotype. The timing and sources of cytokines eliciting macrophage and Treg responses may be tissue-dependent and is an important line of inquiry requiring further research [129,130,131].
CD8+ T cells develop into cytotoxic T cells which can directly destroy infected cells by inducing apoptosis via release of contents from cytolytic granules and/or interaction with death receptor Fas on target cells by Fas Ligand on the CD8+ [128]. The T cell granules contain perforins, cytolysins, proteases, and granulolysins that are all designed to degrade a cell [128]. The main focus of the review is pro-inflammatory CD8+ and CD4+ (Th1, Th2, and Th17) and immunosuppressive regulatory CD4+ (Treg) cells as they relate to muscle physiology; however, B cells and Natural Killer cells have been found in myopathies and post-exercise, respectively [132,133,134].
3.2. T Cell Response to Muscle Injury
Growing evidence suggests that T cells can mediate tissue repair by influencing cell types such as macrophages, stem cells, and myoblasts which act in concert to promote muscle fiber regeneration. They also secrete a wide variety of factors that can skew the healing environment towards either a pro-inflammatory or pro-regenerative response.
In a cardiotoxin model of muscle injury, T cells facilitated regeneration and their absence dramatically hindered recovery. Mice lacking CD8+ T cells (CD8-/-) after cardiotoxin injury showed markedly less regeneration as evidenced by smaller cross-sectional area (CSA) and heightened matrix deposition [80]. When T cells were then transplanted into CD8-/- animals, regeneration was enhanced [80]. It is likely that the improvement is due to the presence of Gr1High macrophages, which are recruited to the muscle by monocyte chemoattractant protein 1 (MCP-1), released by CD8+ T cells [80]. As Gr1High macrophages promote MuSC proliferation, the stem cell pool decreases in the absence of T cells leading to diminished regeneration [80].
In another model of cardiotoxin injury, Rag1-/- mice, which lack T and B-cells, were subjected to injury and displayed significantly delayed regeneration marked by a reduction in fiber organization, size, and myofibers with centrally located nuclei (CLN) [135]. These detrimental effects were recovered after transplanting activated CD3+ T and B-cells into injured Rag1-/- mice [135]. In addition to mediating macrophage activity, the negative side effects of removing T cells may be partly due to reduced stem cell and myogenic activity in their absence. Activated T cells or T cell conditioned media cultured with muscle stem cells caused an increase in the proliferation of stem cells that were Pax7high and MyoDlow [135]. Likewise, conditioned media collected from IL-2 and CD3 activated lymphocytes was cultured with myoblasts and they exhibited reduced differentiation, with downregulation of myoblast determination protein 1 (MyoD) and myogenin [136].
Similar effects have been seen in multi-muscle injury models, in which the extensor digitorum longus (EDL) and tibialis anterior (TA) are injured with a cardiotoxin injection. In these injuries, blocking IFN-γ receptor signaling with an antibody negatively impacted muscle regeneration [137]. CD4+ T cells, natural killer cells, macrophages, and myoblasts express IFN-γ during muscle injury, and blocking the IFN-γ receptors on these cell types resulted in decreased fiber regeneration, cellular proliferation, and MyoD+ cells [137]. In vitro, blocking the IFN-γ receptor on C2C12 cells caused a decrease in proliferation and myoblast fusion providing further evidence of the positive impact that T cells contribute to muscle regeneration through the release of their cytokines [137].
In composite injuries consisting of VML defects and open tibia fractures, the extent of damage can prolong T cell presence past the 3-day mark where they are initially found significantly elevated in VML injuries [138]. VML injury impaired bone regeneration and maintained elevated levels of both CD4+ and CD8+ T cells at 3, 14, and 28 days post-composite trauma [9]. Minced muscle graft (MMG) treatment attenuated the inflammatory response generated by composite injuries and reduced CD4+ T cell but not CD8+ T cell infiltration into the defect site 3 days post-injury [138]. MMG treatment had an overall depressive effect on the inflammatory response and encouraged regeneration through factor release (e.g., MCP-1, IL-10, and IGF-1) and satellite cell delivery [138].
It has also been shown that in treating muscle with bioengineered scaffolds, T cells are necessary for proper healing. In a traumatic muscle injury model, in which a portion of the quadriceps was excised and subsequently treated with either a bone or cardiac derived ECM scaffold, T cells were significantly elevated in response to the scaffold [139]. The ECM scaffolds reportedly modified the T cell composition towards a pro-regenerative CD4+ Th2 cell response [139]. Th2 cytokine IL-4 was also present in scaffold-treated wounds, while Th1 cytokine genes Ifng, Fasl, Cd28, and Tbx21 were downregulated [139]. Associated with the Th2 response was an inhibition of M1 macrophages and activation of M2 macrophages, which are essential for muscle regeneration [139]. CD4+ T cells were also found to be important in the healing of ischemia-induced muscle injuries. In a model of ischemia, the gastrocnemius, plantaris, and soleus muscles were treated with alginate gels containing conditioned media from different subsets of CD4+ T cells (Th1, Th2, Th17, and Treg) [140]. By mixing cytokines from the different subsets of cells, it was found that conditioned media from differing combinations of CD4+ T cell subsets could regulate angiogenesis and myogenesis [140]. For instance, Th2 and Th17 cytokines augmented angiogenesis while Th1 cytokines induced vascular regression. A combination of Th1, Th2, and Th17 cytokines supported MPC proliferation but not differentiation. Treg cytokines had little to no effect on angiogenesis or myogenesis in vitro or in vivo in this model.
In support, other studies have also shown that T cells may play a supportive role in muscle regeneration through interaction with MPCs. Activated T cells release several growth factors that can influence MuSC activity, including but not limited to fibroblast growth factor-2 (FGF-2), IFN-γ, TGF-β, TNF-α, IL-1, IL-4, and IL-13 [135,141,142]. One study identified that the combination of IL-1, IL-13, TNF-α, and IFN-γ pro-inflammatory cytokines, played a role in maintaining MPC potency and stimulating MPCs to proliferate in vivo [135].
An over-abundance of T cell infiltrate can be detrimental to tissue regeneration. The effects of this were illustrated in a study that induced TA injury via a cardiotoxin in Cbl-b (ubiquitin ligase) deficient mice, which increased the activation and infiltration of macrophages [8]. Cbl-b-/- mice displayed poor healing, as indicated by increased fibrosis and inflammation [8]. As the ubiquitin ligase also helps regulate CD8+ T cells, the presence of the T cells was increased in the injured muscle [8]. The authors also identified that RANTES (CCL5), a chemokine secreted by macrophages, delayed T cell clearance and allowed them to persist for up to two weeks after injury [8]. Blocking RANTES activity with a neutralizing antibody rescued regeneration, leading to reduced CD8+ T cell infiltrate and fibroblast aggregation [8].
It should be noted that most of the models presented here are acute injuries, and in more chronic injuries, as seen with composite bone and muscle damage [10], T cell presence can be prolonged and potentially contribute to further damage. Therefore, when selecting therapies for muscle, T cells may present a good target to reduce inflammation and aberrant regeneration in chronic injuries.
3.3. T Cell Response to Exercise
Exercise-induced muscle damage can increase T cell presence in skeletal muscle. However, the extent of T cell infiltration and activity appears to be highly dependent on the type, intensity, and duration of exercise. For instance, strenuous eccentric cycling for 30 min did not increase T or B cell detection in skeletal muscle [143]. In another study, human subjects undergoing 45 min uphill or downhill running exercises did not experience significant differences in inflammation compared to non-exercised controls. Only the downhill running group reported delayed-onset muscle soreness (DOMS) with elevated T cells, neutrophils, and macrophages compared to the non-DOMS experiencing group. However, neither group was significantly different from the control group [144]. In contrast, other studies have reported heightened T cell presence following exercise. For example, T cell levels were found elevated in experienced athletes participating in an ultra-endurance exercise bout for 24 h. The numbers of total T cells, CD8+ cells, and macrophages (CD68+) were found to be 2–3 fold higher after 28 h of exercise [145]. Along with an increase in T cell populations, major histocompatibility class 1 (MHC I) expression was increased in muscle fibers. As healthy skeletal muscle normally shows very low MHC class I expression, it is possible that damaged muscle increases MHC class I expression to communicate with CD8+ T cells [145]. In another study investigating lengthening contractions, human participants underwent two sessions of exercise. It was found that CD8+ T cells and CD68+ macrophages infiltrated skeletal muscle only after the second session, with a concomitant increase in cytokines such as MCP-1 and IFN-γ-induced protein 10 (IP-10) [146]. The increased presence of CD8+ T cells was implicated in muscle adaptation to repeated eccentric contractions [146,147]. The study used DOMS as an indirect indicator of muscle damage, which was found significantly reduced after the second bout. As CD8+ T cells were increased significantly only after a second bout of exercise when evidence of muscle damage was reduced, it was suggested that CD8+ T cells do not exacerbate injury but facilitate repair [147,148]. Interestingly, this study reported no significant increase in MHC I following muscle damage.
Similar results were reported in another study with elite athletes that performed two bouts of high-intensity endurance exercise [149]. Higher concentrations of neutrophils, lymphocytes, T cells (CD4+ and CD8+) as well as natural killer (NK) cells (CD56+) were reported after the second bout of exercise. The study also reported a reduction in the percentage of CD56+CD69+ cells suggesting reduced state of NK cell activation and cytolytic activity.
In support, other studies have also indicated that NK cell cytolytic activity decreases after prolonged, intense, and stressful exercise [150]. These effects are possibly due to a temporary depression of the immune system post-exercise due to the presence of IL-6 in combination with anti-inflammatory cytokines such as IL-10, and interleukin 1 receptor antagonist (IL-1ra), which lead to decreased Th1 mediated responses [150]. However, there is controversy over the conclusions from these results and an extensive review challenged the role of exercise in immunosuppression [151]. Furthermore, in mouse models of skin and lung cancer there was an enhanced NK cell response with exercise. While this model has its challenges because the mice could wheel run ad libitum, there was a beneficial effect of exercise, and it was dependent on IL-6 (presumably muscle-derived) and epinephrine [152]. A similar study using a breast cancer mouse model supports this immune-potentiating effect by showing that exercise reduced myeloid-derived suppressor development [153].
Taken together, these studies highlight the need to investigate the role of T cells and their subsets not only in muscle repair following contraction induced injury but also in mediating the repeated bout effect. As described in the previous section, T cell derived factors can stimulate muscle stem cell proliferation. Therefore, future studies should explore the regulation of muscle stem cell activity via specific T cell subsets as a potential mechanism for muscle repair and adaptation following exercise.
3.4. Cytotoxic T Cells Mediate Continued Pathology Following Disease and Aging
Research confirming the deleterious role of T cells in dystrophy models is extensive and studies focused on depleting T cells demonstrate that their removal can dramatically improve muscle fiber regeneration. T cells account for roughly 3% of infiltrating cells in mdx models of dystrophy, with CD4+ and CD8+ T cells making up almost half of that proportion [154,155]. CD4+/CD8+ T cells have been found in dystrophic muscle at disease onset, with activated (CD44high) T cells present in both muscle and blood [154,156]. Muscle fibers invaded by CD8+ T cells typically express MHC-I, although the expression does not seem to be necessary for T cell-mediated cytotoxicity [157]. CD4+ T cells tend to dominate in dysferlinopathy, which is accompanied by the presence of membrane attack complex on the sarcolemma of fibers [158].
In an A/J limb/girdle dysferlin dystrophy- severe combined immunodeficiency (SCID) mouse model, the absence of T and B lymphocytes resulted in significantly improved muscle regeneration along with a concomitant increase in force production [159]. In the mdx/scid mouse model, depleting T and B lymphocytes led to a significant decrease in the expression of transforming growth factor β (TGF-β), a growth factor heavily implicated in fibrosis, accompanied by a decrease in fibrosis [160]. While these effects did not lead to any differences in myofibers with CLN, necrosis, degeneration, or muscle force compared to mdx controls, the research highlights the impact of T cells on fibrosis development [160].
Research targeting specific T cell populations for depletion has shown that CD8+ T cells are the main culprits of continued muscle degeneration. Depleting CD8+ T cells in mdx mice (by crossing them with perforin knockout mice) resulted in a marked reduction in apoptotic myonuclei and necrosis [154]. Interestingly, mdx mice that also lacked perforin experienced minimal apoptosis and macrophage invasion into the connective tissue, providing support that T cells cause damage through perforin [154]. Depleting CD8+ T cells can also lead to a reduction in muscle pathology as measured by areas of inflammation as well as necrotic fibers [156]. CD4+ T cells are also found in mdx muscle, and antibody depletion resulted in decreased muscle pathology similar to that seen after depleting CD8+ T cells [156]. In another study, CD45RChigh T cell depletion through a monoclonal antibody resulted in increased strength as measured through a grip test [161].
PKC theta, a regulator of T cell activation and proliferation, was knocked out in mdx mice (mdx/θ-/-) and the deficiency attenuated muscle wasting in the diaphragm [162]. Specifically, there was an increase in myogenin/eMHC positive cells and functional activity as seen by improved running ability [162]. Treating mdx mice subject to cardiotoxin injury in the TA and quadriceps femoris with sphingosine-1-phosphate, a T cell inhibitor, increased force production in the EDL. The increase in force is likely due to the reduced fibrosis and fat accumulation seen in the EDL, with a simultaneous increase in myogenic cells and regenerating fibers [163,164].
Lastly, treating mdx mice with rapamycin (an immunosuppressant) for six weeks decreased effector CD4+/CD8+ T cells in the muscle while maintaining Foxp3+ Treg cells, which were beneficial to muscle healing [165]. In contrast, one research study did report that depleting CD4+ and CD8+ T cells at four weeks of age in an mdx mouse model had no impact on fibrotic tissue development in the diaphragm, despite a decrease in TGF-β after double CD4+/CD8+ depletion [166]. The authors suggested that the early time point chosen may have impacted the results as the cells/environment that induce fibrosis could have already been present.
Perforin-expressing CD4+/CD8+ T cells are one of the main immune cell types found in polymyositis (PM) and dermatomyositis (DM) [134,167,168]. In PM, the inflammatory cells primarily reside in the endomysial space, whereas in DM they reside in the perivascular/perimysial space, but similar levels of CD8+ cells are found in both conditions [134]. Both cell types express Fas Ligand in PM, which is associated with high levels of muscle apoptosis [167]. Increased levels of effector T cells, with CD8+ T cells being predominant, have also been found infiltrating muscle tissues in patients with juvenile DM syndrome where they localize around blood vessels [168]. In adult DM, while CD8+/CD4+ T cells are present, it has been suggested that humoral effector mechanisms are the main driver of muscle degeneration, since activated B cells are found in the peripheral blood of patients [134,169].
3.5. Role of Treg in Acute and Chronic Muscle Injury
Regulatory T (Treg) lymphocytes (Foxp3+CD4+) have been recognized for their ability to suppress and attenuate inflammation; phenotypically and functionally distinct populations of Treg cells have been identified, such as muscle specific Treg [99,170,171,172,173,174]. Skeletal muscle Treg (mTreg) are considered distinct from their lymphoid-organ counterparts by the following criteria: their prevalence, transcriptome, and T cell receptor (TCR) repertoire [99]. mTreg were first documented in 2013 [99] and have a critical role in muscle regeneration following both acute and chronic muscle injuries [99,175]. Expression of Helios and neuropilin-1 (Nrp-1) by mTreg cells showcases that this is a thymic derived Treg cell [99,176,177]. However, mTreg cells have a distinct gene-expression profile, notably upregulating genes encoding Treg mediated suppression (e.g., IL-10, Gzmb, Ctla-4, Tim-3, Klrg1), tissue repair (e.g., Il1rl1, Areg, Pdgf), and chemokine receptors (e.g., Ccr1, Ccr2, Ccr3), while downregulating genes encoding proteins implicated in the Wnt signaling pathway (e.g., Tcf7, Lef1, Satb1), and certain chemokine receptors (e.g., Cxcr5, Ccr7) [78,99,170,178,179]. Of the transcripts expressed or repressed by mTreg, amphiregulin (Areg), special AT-rich sequence-binding protein-1 (SATB1), and suppression of tumorigenicity 2 (ST2) are of interest due to their role in regenerative activities of the muscle. Areg, a member of the epithelial growth factor family, can directly impact muscle regeneration by promoting MuSC myogenicity and enhancing Treg ability to suppress immune responses [178,180]. SATB1, a chromatin organizer is capable of affecting Treg functionality [99,181,182]. ST2, encoded by Interleukin 1 Receptor Like 1 (Il1rl1), binds interleukin-33 (IL-33) and affects mTreg accumulation and function, promoting effective repair. IL-33 is also known as an alarmin and its levels spike within hours of injury, drive accumulation of Treg in muscle [183]. Ablation of the Il1rl1 gene is known to prevent IL-33 from interacting with ST2, resulting in impaired Treg recruitment and delayed regeneration [183,184].
Muscle regeneration following an acute muscle injury is influenced by the accumulation of Treg cells at the site of insult. Treg influence macrophage phenotype, modulating muscle regeneration. mTreg gather at the site of injury by day 4, just as the myeloid cell infiltrate switches from a pro- to anti-inflammatory phenotype following acute injuries (e.g., cardiotoxin-induced injury and cryo-injury) [99]. In vitro, macrophages co-cultured with Foxp3+CD4+CD25+ Treg exhibited the ability of Treg to steer monocyte differentiation towards the anti-inflammatory phenotype. Treg produce IL-10, IL-4, and IL-13, cytokines critical in this context for resolving an inflammatory response. Macrophages upregulated the expression of CD206 and CD163, increased the production of CCL18, and enhanced phagocytic capacity when co-cultured with Tregs, these events, with the exception of CD206 upregulation, were partly dependent on IL-10 [98]. It is perhaps paradoxical that IL-4 and IL-13 are critical for an effective Th2 response, but the timing, anatomic location and form of stimulus, and concomitant cytokines (namely well-established immunosuppressive IL-10) are important for this division of effector functions. Co-culture of monocytes with Treg also inhibited the ability of monocytes to produce pro-inflammatory cytokines such as TNF-α and IL-6 [185].
In an acute injury model induced by cardiotoxin injection, ablation of Treg resulted in increased production of pro-inflammatory cytokine IFN-γ by NK cells and effector T cells, likely CD8+ and Th1. The production of IFN-γ led to increased accumulation of the pro-inflammatory macrophage (MHCII+) subset and fibrosis. When Treg were present at the injury site, IFN-γ production was limited, promoting the accrual of macrophages and regulating their phenotypic switch [186]. Ablation of Treg in Foxp3-DTR transgenic mice following cardiotoxin injury supported this: the cellular infiltrate was increased, and myeloid cells failed to undergo the phenotypic switch from pro- to anti-inflammatory macrophages. Histologically, Treg ablation led to a disorganized tissue structure with several foci of inflammation, greater collagen deposition, and a decreased number of centrally nucleated fibers [99].
In vitro exposure to activated Treg cells induced MuSC expansion and concomitantly inhibited myogenic differentiation. In vivo studies revealed that the recruitment of Treg cells to acutely injured muscle was limited to the time frame of MuSC expansion [187]. Local expansion of Treg cells is required for skeletal muscle repair, as observed with the results aforementioned, and evident when treatment with an anti-CD25 mAb targeting Foxp3+CD4+CD25hi Treg cells increased muscle damage in dystrophic mice. Similarly, treatment with complexes of recombinant IL-2 with an anti-IL-2 mAb prevented muscle damage in dystrophic mice while enhancing Treg activity and increasing IL-10 production [99,175,180]. Osteopontin (OPN) is an immunomodulator in mdx muscle which promotes fibrosis and expression of TGF-β. Studies ablating OPN concluded that myeloid cells shifted to a pro-regenerative phenotype and led to an increase in intramuscular Treg with reduction in fibrosis [188,189]. Protozoan parasite chronic muscle infections, such as Toxoplasma gondii, led to impaired Treg-mediated immunomodulation which directly contributes to macrophage-mediated muscle damage. As mentioned above, the restoration of Treg activity rescues muscle regeneration; however, in the case of chronic infection, the suppression of the Treg population promoted regeneration and increased the proportion of M2 macrophages [180,190]. These findings show the complexity of Treg and their function in muscle injury, infection, and disease.
3.6. Role of Treg in Aged Muscle
The aging of skeletal muscle is associated with a steady decline in bulk, function, and regenerative capacity due to both intrinsic and environmental factors [191,192]. Poor regeneration is associated with the age-associated decrease in MuSC frequency and function, which can influence Treg recruitment to the muscle [187].
Treg cells increase in lymphoid organs with age; however, they are sparse in aged muscle following injury [183,193]. Following a cardiotoxin-induced injury, Treg accumulation was diminished in the muscle of aged mice, reflecting the poor recruitment, proliferation, and retention of Treg in aged muscle. The exogenous administration of IL-33 restored the Treg population in injured aged muscle, promoting regeneration. IL-33 did not affect the recruitment of Treg to the site of injury; however, it enhanced Treg cell proliferation, these results were observed in both a cardiotoxin-induced injury and cryo-injury [183]. These findings highlight the importance of the IL-33:ST2 axis and its role in mTreg cell function and expansion.
Overall, studies show that a lack of mTreg is partly responsible for the poor muscle regeneration seen in older individuals. Future studies should investigate therapeutic strategies to improve Treg quantity and function in aged muscle.
4. Immunomodulators in Skeletal Muscle Regeneration
The inflammatory response should be mitigated under certain conditions to encourage regeneration. Factors that can modulate the immune response have been explored specifically in the context of muscle healing and they include, but are not limited to, mesenchymal stem cell (MSC) therapy with or without a scaffold delivery system and immune suppressant drugs such as FK506 and FTY720 [194].
4.1. Immunomodulatory Properties of MSCs
The immunomodulatory properties of MSC have been well documented in application to many different tissues, including but not limited to skeletal muscle, bone, skin, peripheral nerve, blood vessels, cartilage, and tendon [195,196]. Their role in regulating the immune system comes from their ability to secrete a variety of growth factors (e.g., TGF-β1, HGF, prostaglandin 2, SDF-1, NO) and cytokines (e.g., IL-4, IL-6, and IL-10) that suppress pro-inflammatory responses to damage [195]. Specifically, these factors are released upon stem cell activation from pro-inflammatory cytokines and they inhibit T cell proliferation, encourage the shift from a Th1 to a Th2 response, and impair the maturation of dendritic cells. The shift from a Th1 to a Th2 response may also cause a shift in macrophage phenotype because the Th2 response skews macrophages to a pro-regenerative M2 phenotype [195,196]. They can also impact a significant number of other pro-inflammatory cells including B-cells, and natural killer cells [195]. Collectively, inhibiting these cell types directly impacts the level of inflammation found in the defect area. In a recent clinical study, allogeneic placenta-derived, mesenchymal-like adherent cells were found to increase muscle strength and volume in patients who underwent hip arthroplasty. The authors concluded that the cell therapy was safe and the beneficial effects to injured skeletal muscle were attributed to MSC induced immunomodulation [197].
The impact of MSCs on immune cells has been studied in several ways, including in vitro, in vivo, and in disease models such as muscular dystrophy. When culturing MSCs with CD8+ T cells, these cells decreased the expression of CD8, CD28, and CD44, indicating a potential shift in cellular phenotype and function [198]. Additionally, MSCs reduced the T cell release of IFN-γ and granzyme B, which required stimulation of CD8+ T cells directly from monocytes, while increasing the release of IL-4 [198,199]. Phenotypic changes were also seen in CD4+ T cells that were cultured with bone marrow derived MSCs, and CD4+/CD8+ T cells downregulated CD25, CD38, and CD69, preventing their activation [200].
In vitro studies involving the co-culture of M0, M1, and M2 macrophages with MSCs demonstrated promising results. Naive macrophages cultured with MSCs were shifted toward an M1 state and had an increased ability to kill pathogens via a boost in the respiratory burst response. In contrast, M1s cultured with MSCs shifted towards an M2 phenotype, as evidenced by increased M2 gene expression. Therefore, MSCs exhibit diverse effects on of macrophages that are largely attributed to a prostaglandin E2 (PGE₂) dependent mechanism [201].
Animals with substantial skeletal muscle damage that were implanted with MSCs showed a significant decrease in pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, TGF-β, and IFN-γ) and an increase in an important anti-inflammatory cytokine IL-10 [202]. The changes seen at the molecular level corresponded to histological differences in muscle regeneration, with less collagen content and fibrosis in MSC treated animals coinciding with improvements in microvasculature formation within the muscle [202]. In another model of VML injury, MSCs were seeded onto an ECM scaffold, which upon implantation led to a shift in macrophage polarization from an M1 to an M2 phenotype, resulting in improved muscle regeneration and reduced fibrotic tissue deposition [203]. In contrast, another study showed that the delivery of MSCs on an ECM scaffold supported functional recovery following VML with little to no muscle fiber regeneration. The authors attributed the improvement in function to a fibrotic scar mediated bridging effect that allowed for increased force transmission vs. production [204].
δ-sarcoglycan null dystrophic hamsters that received an intramuscular injection of MSCs showed a reduction in the upregulation of pro-inflammatory markers in the circulatory system, including immunoglobulin A, vascular cell adhesion molecule-1, and myeloperoxidase [205]. Other beneficial results were seen within the muscle itself, which demonstrated that leukocyte antigens, oxidative stress, and NF-kB levels did not increase in comparison to animals not receiving the MSC treatment [205]. MSC derived nuclei were observed in both center and periphery of myofibers, suggesting their role in fiber growth via donation of nucleus or cell fusion. In another study, MSC administration into dystrophic mice reduced inflammatory cytokines (such as TNF-α, IL-6) and oxidative stress. Simultaneously, the MSC treatment increased VEGF, IL-10, and IL-4 release [206].
Immunomodulatory benefits can also be exhibited by MSC derived exosomes, which are nanovesicles secreted by mammalian cells for intercellular communication [207]. These vesicles contain bioactive molecules such as lipids, proteins, mRNAs and microRNAs (miRNAs) unique to the cell of origin [208,209]. To evaluate the therapeutic potential of MSC-derived exosomes, several clinical trials (http://clinicaltrials.gov, accessed on 10 February 2021) have been completed or are currently ongoing. Exosomes recapitulate the broad therapeutic effects attributed to MSCs [210,211]. For instance, MSC exosomes contain numerous anti-inflammatory and anti-fibrotic miRNAs [211] as well as several cytokines and growth factors including IL-6, IL-10, TGF-β1, VEGF and HGF [212]. These molecules can promote both immunomodulation and tissue regeneration. As an acellular MSC byproduct, exosomes can readily circulate through organs, elicit a minimal immune response, avoid phagocytosis and elicit cellular responses by binding to specific receptors on the target cell [213]. In mice with cardiotoxin injury, MSC exosomes support muscle regeneration, enhanced angiogenesis, and reduced fibrosis [214]. In another study, MSC-derived exosome treatment following cardiotoxin injury increased the expression of markers associated with an M2 macrophage phenotype (Arg1+ and Ym1+), which coincided with improved muscle regeneration evidenced by increased quantity of myoblasts and fibers with centrally located nuclei [215]. In a murine VML model, co-delivery of muscle ECM and MSC extracellular vesicles (EV) enhanced angiogenesis and myogenesis but reduced fibrosis. A decrease in M1-like markers (iNOS) with an increase in M2-like markers (CD163 and Arg1) was also reported [216]. The same study created a co-culture model with C2C12 cells with macrophages and introduced a cardiotoxin to mimic an injured microenvironment in vitro. Treatment of the in vitro cardiotoxin injury with MSC EVs elicited a protective action against cell apoptosis and increased cell survival as shown by new laminin deposition and increased number of proliferating cells [216].
In conclusion, MSCs display a versatile ability to enhance the pro-regenerative environment by reducing inflammation and encouraging myofiber formation. The molecular impact of MSCs on the cellular niche of regenerating muscle has also improved functional deficits seen with muscle damage.
4.2. Immunosuppressants as Therapies for Muscle Regeneration
Immunosuppressant drugs, such as FK506 and FTY720, have been used for modulation of the immune response in skeletal muscle. FTY720 is a sphingosine-1-phosphate receptor modulator that can regulate chronic inflammation and fibrosis deposition [217]. FK506, also known as tacrolimus, is a macrolide antibiotic that acts on T cells and can suppress their immune response [218]. In a model of limb-girdle type 2C muscular dystrophy, FTY720 was administered for 3 weeks to mice aged 3 weeks and greatly reduced muscle membrane permeability and fibrosis [219]. Additionally, it also increased the upregulation of sarcoglycan which could lead to the protection of the sarcolemma from shear forces. Therefore, the drug could partly protect the muscle from the damaging effects of inflammation commonly associated with the disease. In another study conducted on FTY720, mice were subjected to hind limb ischemia-reperfusion muscle injuries and then subsequently treated with the drug [220]. Although local effects of muscle regeneration (i.e., cross sectional area of muscles, myofibers with CLN, etc.) were not measured, the study revealed that FTY720 will reduce systemic inflammation by causing a decrease in peripheral blood T cell levels. In conjunction with the T cell decrease, a significant reduction in serum creatinine and important cytokines, such as TNF-α, IL-6, IL-10, and IL-18, were also observed.
In complex models of VML that include bone fractures, or osteotomies, the use of FK506 induced better healing in bone fractures that correlated with changes in cell types in the overlying, injured musculature [10]. Bone defects compounded by VML of the TA are difficult to treat. Introducing the FK506 drug reduced the numbers of T-lymphocytes and macrophages found in the TA, with reduced T-lymphocytes in the callus of the bone [10]. Administering the drug did not improve TA force production, but it did restore mechanical properties of the bone in the presence of the muscle defect [10]. These findings suggest that the drug’s impact on the injured muscle, such as the reduction of T cell infiltration into all areas of damage, strongly and positively impact the healing ability of the bone. Regulating the immune response with FK506 delivered in conjunction with a MMG to a VML injury in swine muscle led to a marginal improvement of force deficit with a higher fraction of muscle fibers vs. fibrotic tissue [221].
5. Conclusions
The physiological repair mechanisms following skeletal muscle injury or disease are currently under investigation. Cells of the innate and adaptive immune system play diverse and complex roles in muscle healing, and the interplay between endogenous stem cell populations and infiltrating immune cells likely determines the regenerative outcome. Greater insight into these cellular interactions will pave the way for new therapeutic strategies. Recent substantial progress in just identifying the cells present in healthy muscle and regenerating skeletal muscle should help directing impactful hypotheses in this area [222,223,224,225]. Mounting evidence indicates that immune cell subsets can have both pro- and anti-reparative functions. The environmental cues and molecular triggers which control the switch between pro- and anti-reparative functions need further investigation. Some studies suggest that a transient and controlled immune response is critical to muscle healing, but an overactive and persistent immune response can be detrimental to tissue repair. Since the clinical treatment options for muscle injuries are scarce, modulation of the immune response might present an effective approach to boost the innate regenerative capacity of the skeletal muscle niche.
Author Contributions
N.Z., G.H., N.A.P., and K.G. drafted the manuscript. K.G. and N.Z. made the figures. K.G., N.A.P., and N.Z. revised and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by United States Department of Defense Applied Research Award OR170286 and National Institute of General Medical Sciences (NIGMS)-1R15GM129731-01 awarded to KG. The APC was funded by NIGMS-1R15GM129731-01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to thank Jeffrey Au, Hannah Chauvin, Charles West, and Allison Paoli, for their assistance with this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef]
- Tidball, J.G. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R345–R353. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hu, P. Skeletal muscle regeneration is modulated by inflammation. J. Orthop. Transl. 2018, 13, 25–32. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, E.; Zordan, P.; Sciorati, C.; Rovere-Querini, P.; Brunelli, S. Macrophage plasticity in skeletal muscle repair. Biomed. Res. Int. 2014, 2014, 560629. [Google Scholar] [CrossRef]
- Umansky, K.B.; Gruenbaum-Cohen, Y.; Tsoory, M.; Feldmesser, E.; Goldenberg, D.; Brenner, O.; Groner, Y. Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration. PLoS Genet. 2015, 11, e1005457. [Google Scholar] [CrossRef]
- Kohno, S.; Ueji, T.; Abe, T.; Nakao, R.; Hirasaka, K.; Oarada, M.; Harada-Sukeno, A.; Ohno, A.; Higashibata, A.; Mukai, R.; et al. Rantes secreted from macrophages disturbs skeletal muscle regeneration after cardiotoxin injection in Cbl-b-deficient mice. Muscle Nerve 2011, 43, 223–229. [Google Scholar] [CrossRef]
- Hurtgen, B.J.; Ward, C.L.; Garg, K.; Pollot, B.E.; Goldman, S.M.; McKinley, T.O.; Wenke, J.C.; Corona, B.T. Severe muscle trauma triggers heightened and prolonged local musculoskeletal inflammation and impairs adjacent tibia fracture healing. J. Musculoskelet. Neuronal Interact. 2016, 16, 122–134. [Google Scholar]
- Hurtgen, B.J.; Henderson, B.E.P.; Ward, C.L.; Goldman, S.M.; Garg, K.; McKinley, T.O.; Greising, S.M.; Wenke, J.C.; Corona, B.T. Impairment of early fracture healing by skeletal muscle trauma is restored by FK506. BMC Musculoskelet. Disord. 2017, 18, 253. [Google Scholar] [CrossRef]
- Brunelli, S.; Rovere-Querini, P. The immune system and the repair of skeletal muscle. Pharmacol. Res. 2008, 58, 117–121. [Google Scholar] [CrossRef]
- Butterfield, T.A.; Best, T.M.; Merrick, M.A. The dual roles of neutrophils and macrophages in inflammation: A critical balance between tissue damage and repair. J. Athl. Train 2006, 41, 457–465. [Google Scholar]
- Toumi, H.; F’Guyer, S.; Best, T.M. The role of neutrophils in injury and repair following muscle stretch. J. Anat. 2006, 208, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Pizza, F.X.; Peterson, J.M.; Baas, J.H.; Koh, T.J. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J. Physiol. 2005, 562, 899–913. [Google Scholar] [CrossRef]
- Fielding, R.A.; Manfredi, T.J.; Ding, W.; Fiatarone, M.A.; Evans, W.J.; Cannon, J.G. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am. J. Physiol. 1993, 265, R166–R172. [Google Scholar] [CrossRef] [PubMed]
- QUINDRY, J.C.; STONE, W.L.; KING, J.; BROEDER, C.E. The effects of acute exercise on neutrophils and plasma oxidative stress. Med. Sci. Sports Exerc. 2003, 35, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Brickson, S.; Hollander, J.; Corr, D.T.; Ji, L.L.; Best, T.M. Oxidant production and immune response after stretch injury in skeletal muscle. Med. Sci. Sports Exerc. 2001, 33, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Cassatella, M.A. Neutrophil-derived proteins: Selling cytokines by the pound. Adv. Immunol. 1999, 73, 369–509. [Google Scholar] [CrossRef]
- Toumi, H.; Best, T. The inflammatory response: Friend or enemy for muscle injury? Br. J. Sports Med. 2003, 37, 284–286. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Lindborg, J.A.; Mack, M.; Zigmond, R.E. Neutrophils Are Critical for Myelin Removal in a Peripheral Nerve Injury Model of Wallerian Degeneration. J. Neurosci. 2017, 37, 10258–10277. [Google Scholar] [CrossRef] [PubMed]
- Herath, T.D.K.; Larbi, A.; Teoh, S.H.; Kirkpatrick, C.J.; Goh, B.T. Neutrophil-mediated enhancement of angiogenesis and osteogenesis in a novel triple cell co-culture model with endothelial cells and osteoblasts. J. Tissue Eng. Regen. Med. 2018, 12, e1221–e1236. [Google Scholar] [CrossRef]
- McCourt, M.; Wang, J.H.; Sookhai, S.; Redmond, H.P. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch. Surg. 1999, 134, 1325–1331, discussion 1331–1322. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.F.; Zamunér, S.R.; Zuliani, J.P.; Fernandes, C.M.; Cruz-Hofling, M.A.; Fernandes, I.; Chaves, F.; Gutiérrez, J.M. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle Nerve 2003, 28, 449–459. [Google Scholar] [CrossRef]
- Dumont, N.; Bouchard, P.; Frenette, J. Neutrophil-induced skeletal muscle damage: A calculated and controlled response following hindlimb unloading and reloading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1831–R1838. [Google Scholar] [CrossRef]
- Pizza, F.X.; Koh, T.J.; McGregor, S.J.; Brooks, S.V. Muscle inflammatory cells after passive stretches, isometric contractions, and lengthening contractions. J. Appl. Physiol. 2002, 92, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Frenette, J.; St-Pierre, M.; Cote, C.H.; Mylona, E.; Pizza, F.X. Muscle impairment occurs rapidly and precedes inflammatory cell accumulation after mechanical loading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R351–R357. [Google Scholar] [CrossRef]
- Brickson, S.; Ji, L.L.; Schell, K.; Olabisi, R.; St Pierre Schneider, B.; Best, T.M. M1/70 attenuates blood-borne neutrophil oxidants, activation, and myofiber damage following stretch injury. J. Appl. Physiol. 2003, 95, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Zerria, K.; Jerbi, E.; Hammami, S.; Maaroufi, A.; Boubaker, S.; Xiong, J.P.; Arnaout, M.A.; Fathallah, D.M. Recombinant integrin CD11b A-domain blocks polymorphonuclear cells recruitment and protects against skeletal muscle inflammatory injury in the rat. Immunology 2006, 119, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Tidball, J.G. Null mutation of gp91phox reduces muscle membrane lysis during muscle inflammation in mice. J. Physiol. 2003, 553, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Lusis, A.J.; Tidball, J.G. Null mutation of myeloperoxidase in mice prevents mechanical activation of neutrophil lysis of muscle cell membranes in vitro and in vivo. J. Physiol. 2005, 565, 403–413. [Google Scholar] [CrossRef]
- Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Neutrophil Depletion Attenuates Muscle Injury after Exhaustive Exercise. Med. Sci. Sports Exerc. 2016, 48, 1917–1924. [Google Scholar] [CrossRef]
- Wehling-Henricks, M.; Sokolow, S.; Lee, J.J.; Myung, K.H.; Villalta, S.A.; Tidball, J.G. Major basic protein-1 promotes fibrosis of dystrophic muscle and attenuates the cellular immune response in muscular dystrophy. Hum. Mol. Genet. 2008, 17, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Crinnion, J.N.; Homer-Vanniasinkam, S.; Hatton, R.; Parkin, S.M.; Gough, M.J. Role of neutrophil depletion and elastase inhibition in modifying skeletal muscle reperfusion injury. Cardiovasc. Surg. 1994, 2, 749–753. [Google Scholar] [PubMed]
- Kyriakides, C.; Austen, W., Jr.; Wang, Y.; Favuzza, J.; Kobzik, L.; Moore, F.D., Jr.; Hechtman, H.B. Skeletal muscle reperfusion injury is mediated by neutrophils and the complement membrane attack complex. Am. J. Physiol. 1999, 277, C1263–C1268. [Google Scholar] [CrossRef]
- Walden, D.L.; McCutchan, H.J.; Enquist, E.G.; Schwappach, J.R.; Shanley, P.F.; Reiss, O.K.; Terada, L.S.; Leff, J.A.; Repine, J.E. Neutrophils accumulate and contribute to skeletal muscle dysfunction after ischemia-reperfusion. Am. J. Physiol. 1990, 259, H1809–H1812. [Google Scholar] [CrossRef]
- Arecco, N.; Clarke, C.J.; Jones, F.K.; Simpson, D.M.; Mason, D.; Beynon, R.J.; Pisconti, A. Elastase levels and activity are increased in dystrophic muscle and impair myoblast cell survival, proliferation and differentiation. Sci. Rep. 2016, 6, 24708. [Google Scholar] [CrossRef]
- Hodgetts, S.; Radley, H.; Davies, M.; Grounds, M.D. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx mice. Neuromuscul. Disord. 2006, 16, 591–602. [Google Scholar] [CrossRef]
- Grounds, M.D.; Torrisi, J. Anti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 676–682. [Google Scholar] [CrossRef]
- Prame Kumar, K.; Nicholls, A.J.; Wong, C.H.Y. Partners in crime: Neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018, 371, 551–565. [Google Scholar] [CrossRef]
- Ghaly, A.; Marsh, D.R. Aging-associated oxidative stress modulates the acute inflammatory response in skeletal muscle after contusion injury. Exp. Gerontol. 2010, 45, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.; Della Gatta, P.; Cameron-Smith, D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1485–R1495. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D. Phagocytosis of necrotic muscle in muscle isografts is influenced by the strain, age, and sex of host mice. J. Pathol. 1987, 153, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Juban, G.; Chazaud, B. Metabolic regulation of macrophages during tissue repair: Insights from skeletal muscle regeneration. FEBS Lett. 2017, 591, 3007–3021. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Melton, D.W.; Porter, L.; Sarwar, Z.U.; McManus, L.M.; Shireman, P.K. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am. J. Pathol. 2014, 184, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Serbina, N.V.; Jia, T.; Hohl, T.M.; Pamer, E.G. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008, 26, 421–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Zhao, L.; Zeng, Z.; Xiao, W.; Chen, P. Macrophage depletion impairs skeletal muscle regeneration: The roles of regulatory factors for muscle regeneration. Cell Biol. Int. 2017, 41, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Brigitte, M.; Schilte, C.; Plonquet, A.; Baba-Amer, Y.; Henri, A.; Charlier, C.; Tajbakhsh, S.; Albert, M.; Gherardi, R.K.; Chrétien, F. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheumatol. 2010, 62, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Mass, E. Delineating the origins, developmental programs and homeostatic functions of tissue-resident macrophages. Int. Immunol. 2018, 30, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Ginhoux, F. Fetal monocytes and the origins of tissue-resident macrophages. Cell Immunol. 2018, 330, 5–15. [Google Scholar] [CrossRef]
- Tonkin, J.; Temmerman, L.; Sampson, R.D.; Gallego-Colon, E.; Barberi, L.; Bilbao, D.; Schneider, M.D.; Musarò, A.; Rosenthal, N. Monocyte/Macrophage-derived IGF-1 Orchestrates Murine Skeletal Muscle Regeneration and Modulates Autocrine Polarization. Mol. Ther. 2015, 23, 1189–1200. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Pillon, N.J.; Bilan, P.J.; Fink, L.N.; Klip, A. Cross-talk between skeletal muscle and immune cells: Muscle-derived mediators and metabolic implications. Am. J. Physiol. -Endocrinol. Metab. 2012, 304, E453–E465. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Wang, L.-X.; Zhang, S.-X.; Wu, H.-J.; Rong, X.-L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ni, H.; Lan, L.; Wei, X.; Xiang, R.; Wang, Y. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res. 2010, 20, 701. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, J.-B.; He, Y.-L.; Peng, J.-J.; Zhang, X.-H.; Chen, C.-Q.; Li, W.; Cai, S.-R. Tumor-associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J. Surg. Oncol. 2012, 106, 462–468. [Google Scholar] [CrossRef]
- Bosurgi, L.; Manfredi, A.A.; Rovere-Querini, P. Macrophages in injured skeletal muscle: A perpetuum mobile causing and limiting fibrosis, prompting or restricting resolution and regeneration. Front. Immunol. 2011, 2, 62. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, H.; Wang, X.; Ransohoff, R.M.; Zhou, L. CX3CR1 deficiency delays acute skeletal muscle injury repair by impairing macrophage functions. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 380–393. [Google Scholar] [CrossRef]
- Summan, M.; Warren, G.L.; Mercer, R.R.; Chapman, R.; Hulderman, T.; Van Rooijen, N.; Simeonova, P.P. Macrophages and skeletal muscle regeneration: A clodronate-containing liposome depletion study. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2006, 290, R1488–R1495. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Wehling-Henricks, M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J. Physiol. 2007, 578, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Bencze, M.; Negroni, E.; Vallese, D.; Yacoub-Youssef, H.; Chaouch, S.; Wolff, A.; Aamiri, A.; Di Santo, J.P.; Chazaud, B.; Butler-Browne, G.; et al. Proinflammatory Macrophages Enhance the Regenerative Capacity of Human Myoblasts by Modifying Their Kinetics of Proliferation and Differentiation. Mol. Ther. 2012, 20, 2168–2179. [Google Scholar] [CrossRef]
- Sonnet, C.; Lafuste, P.; Arnold, L.; Brigitte, M.; Poron, F.; Authier, F.; Chrétien, F.; Gherardi, R.K.; Chazaud, B. Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. J. Cell Sci. 2006, 119, 2497–2507. [Google Scholar] [CrossRef]
- Chazaud, B.; Sonnet, C.; Lafuste, P.; Bassez, G.; Rimaniol, A.-C.; Poron, F.; Authier, F.-J.; Dreyfus, P.A.; Gherardi, R.K. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell Biol. 2003, 163, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Saclier, M.; Cuvellier, S.; Magnan, M.; Mounier, R.; Chazaud, B. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 2013, 280, 4118–4130. [Google Scholar] [CrossRef]
- Arnold, L.; Perrin, H.; de Chanville, C.B.; Saclier, M.; Hermand, P.; Poupel, L.; Guyon, E.; Licata, F.; Carpentier, W.; Vilar, J.; et al. CX3CR1 deficiency promotes muscle repair and regeneration by enhancing macrophage ApoE production. Nat. Commun. 2015, 6, 8972. [Google Scholar] [CrossRef]
- Warren, G.L.; Hulderman, T.; Mishra, D.; Gao, X.; Millecchia, L.; O’Farrell, L.; Kuziel, W.A.; Simeonova, P.P. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J. 2005, 19, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Combadière, C.; Potteaux, S.; Rodero, M.; Simon, T.; Pezard, A.; Esposito, B.; Merval, R.; Proudfoot, A.; Tedgui, A.; Mallat, Z. Combined inhibition of Ccl2, Cx3cr1 and Ccr5 abrogates Ly6chi and Ly6clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 2008, 117, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, Z.; Qu, C.; Cui, W.; Wang, X.; Du, J. CD8 T cells are involved in skeletal muscle regeneration through facilitating MCP-1 secretion and Gr1high macrophage infiltration. J. Immunol. 2014, 193, 5149–5160. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.O.; McHale, M.J.; Wells, J.T.; Ochoa, O.; Michalek, J.E.; McManus, L.M.; Shireman, P.K. Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R832–R842. [Google Scholar] [CrossRef] [PubMed]
- Melton, D.W.; Roberts, A.C.; Wang, H.; Sarwar, Z.; Wetzel, M.D.; Wells, J.T.; Porter, L.; Berton, M.T.; McManus, L.M.; Shireman, P.K. Absence of CCR2 results in an inflammaging environment in young mice with age-independent impairments in muscle regeneration. J. Leukoc. Biol. 2016, 100, 1011–1025. [Google Scholar] [CrossRef]
- Ochoa, O.; Sun, D.; Reyes-Reyna, S.M.; Waite, L.L.; Michalek, J.E.; McManus, L.M.; Shireman, P.K. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2007, 293, R651–R661. [Google Scholar] [CrossRef]
- Contreras-Shannon, V.; Ochoa, O.; Reyes-Reyna, S.M.; Sun, D.; Michalek, J.E.; Kuziel, W.A.; McManus, L.M.; Shireman, P.K. Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2−/− mice following ischemic injury. Am. J. Physiol.-Cell. Physiol. 2007, 292, C953–C967. [Google Scholar] [CrossRef]
- Shireman, P.K.; Contreras-Shannon, V.; Ochoa, O.; Karia, B.P.; Michalek, J.E.; McManus, L.M. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J. Leukoc. Biol. 2007, 81, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Huang, D.; Saederup, N.; Charo, I.F.; Ransohoff, R.M.; Zhou, L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Huang, D.; Ransohoff, R.M.; Zhou, L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 3344–3355. [Google Scholar] [CrossRef]
- Duffield, J.S.; Lupher, M.; Thannickal, V.J.; Wynn, T.A. Host responses in tissue repair and fibrosis. Annu. Rev. Pathol. 2013, 8, 241–276. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Novak, M.L.; Weinheimer-Haus, E.M.; Koh, T.J. Macrophage activation and skeletal muscle healing following traumatic injury. J. Pathol. 2014, 232, 344–355. [Google Scholar] [CrossRef]
- Rybalko, V.; Hsieh, P.-L.; Merscham-Banda, M.; Suggs, L.J.; Farrar, R.P. The Development of Macrophage-Mediated Cell Therapy to Improve Skeletal Muscle Function after Injury. PLoS ONE 2015, 10, e0145550. [Google Scholar] [CrossRef] [PubMed]
- Filippin, L.I.; Moreira, A.J.; Marroni, N.P.; Xavier, R.M. Nitric oxide and repair of skeletal muscle injury. Nitric Oxide 2009, 21, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Yamamuro, A.; Maeda, S. Nitric oxide at a low concentration protects murine macrophage RAW264 cells against nitric oxide-induced death via cGMP signaling pathway. Br. J. Pharm. 2003, 139, 28–34. [Google Scholar] [CrossRef]
- Wink, D.A.; Hanbauer, I.; Laval, F.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. Nitric Oxide Protects against the Cytotoxic Effects of Reactive Oxygen Species. Ann. N. Y. Acad. Sci. 1994, 738, 265–278. [Google Scholar] [CrossRef]
- Fujii, Y.; Guo, Y.; Hussain, S.N.A. Regulation of nitric oxide production in response to skeletal muscle activation. J. Appl. Physiol. 1998, 85, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Ley, K. M1 Means Kill; M2 Means Heal. J. Immunol. 2017, 199, 2191. [Google Scholar] [CrossRef]
- Filippin, L.I.; Cuevas, M.J.; Lima, E.; Marroni, N.P.; Gonzalez-Gallego, J.; Xavier, R.M. Nitric oxide regulates the repair of injured skeletal muscle. Nitric Oxide 2011, 24, 43–49. [Google Scholar] [CrossRef]
- Tiemessen, M.M.; Jagger, A.L.; Evans, H.G.; van Herwijnen, M.J.C.; John, S.; Taams, L.S. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. USA 2007, 104, 19446–19451. [Google Scholar] [CrossRef]
- Burzyn, D.; Kuswanto, W.; Kolodin, D.; Shadrach, J.L.; Cerletti, M.; Jang, Y.; Sefik, E.; Tan, T.G.; Wagers, A.J.; Benoist, C.; et al. A special population of regulatory T cells potentiates muscle repair. Cell 2013, 155, 1282–1295. [Google Scholar] [CrossRef]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef]
- Walton, R.G.; Kosmac, K.; Mula, J.; Fry, C.S.; Peck, B.D.; Groshong, J.S.; Finlin, B.S.; Zhu, B.; Kern, P.A.; Peterson, C.A. Human skeletal muscle macrophages increase following cycle training and are associated with adaptations that may facilitate growth. Sci. Rep. 2019, 9, 969. [Google Scholar] [CrossRef]
- Hachim, D.; LoPresti, S.T.; Yates, C.C.; Brown, B.N. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials 2017, 112, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.E.; Kim, D.S.; Chung, H.C.; Shing, B.; Moon, K.H.; George, S.K.; Kim, M.W.; Atala, Z.; Kim, J.H.; Ko, I.K.; et al. Controlled Delivery of Stem Cell-Derived Trophic Factors Accelerates Kidney Repair After Renal Ischemia-Reperfusion Injury in Rats. Stem. Cells Transl. Med. 2019, 8, 959–970. [Google Scholar] [CrossRef]
- Sok, M.C.P.; Baker, N.; McClain, C.; Lim, H.S.; Turner, T.; Hymel, L.; Ogle, M.; Olingy, C.; Palacios, J.I.; Garcia, J.R.; et al. Dual delivery of IL-10 and AT-RvD1 from PEG hydrogels polarize immune cells towards pro-regenerative phenotypes. Biomaterials 2021, 268, 120475. [Google Scholar] [CrossRef]
- Potas, J.R.; Haque, F.; Maclean, F.L.; Nisbet, D.R. Interleukin-10 conjugated electrospun polycaprolactone (PCL) nanofibre scaffolds for promoting alternatively activated (M2) macrophages around the peripheral nerve in vivo. J. Immunol. Methods 2015, 420, 38–49. [Google Scholar] [CrossRef]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef]
- Hsieh, P.-L.; Rybalko, V.; Baker, A.B.; Suggs, L.J.; Farrar, R.P. Recruitment and therapeutic application of macrophages in skeletal muscles after hind limb ischemia. J. Vasc. Surg. 2018, 67, 1908–1920.e1901. [Google Scholar] [CrossRef]
- Villalta, S.A.; Rinaldi, C.; Deng, B.; Liu, G.; Fedor, B.; Tidball, J.G. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum. Mol. Genet. 2011, 20, 790–805. [Google Scholar] [CrossRef]
- Wehling, M.; Spencer, M.J.; Tidball, J.G. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J. Cell Biol. 2001, 155, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Villalta, S.A.; Deng, B.; Rinaldi, C.; Wehling-Henricks, M.; Tidball, J.G. IFN-γ promotes muscle damage in the mdx mouse model of Duchenne muscular dystrophy by suppressing M2 macrophage activation and inhibiting muscle cell proliferation. J. Immunol. 2011, 187, 5419–5428. [Google Scholar] [CrossRef] [PubMed]
- Mojumdar, K.; Liang, F.; Giordano, C.; Lemaire, C.; Danialou, G.; Okazaki, T.; Bourdon, J.; Rafei, M.; Galipeau, J.; Divangahi, M.; et al. Inflammatory monocytes promote progression of Duchenne muscular dystrophy and can be therapeutically targeted via CCR2. EMBO Mol. Med. 2014, 6, 1476–1492. [Google Scholar] [CrossRef] [PubMed]
- Segawa, M.; Fukada, S.-I.; Yamamoto, Y.; Yahagi, H.; Kanematsu, M.; Sato, M.; Ito, T.; Uezumi, A.; Hayashi, S.i.; Miyagoe-Suzuki, Y.; et al. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp. Cell Res. 2008, 314, 3232–3244. [Google Scholar] [CrossRef] [PubMed]
- Greising, S.M.; Rivera, J.C.; Goldman, S.M.; Watts, A.; Aguilar, C.A.; Corona, B.T. Unwavering Pathobiology of Volumetric Muscle Loss Injury. Sci. Rep. 2017, 7, 13179. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.A.; Greising, S.M.; Watts, A.; Goldman, S.M.; Peragallo, C.; Zook, C.; Larouche, J.; Corona, B.T. Multiscale analysis of a regenerative therapy for treatment of volumetric muscle loss injury. Cell Death. Discov. 2018, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Ward, C.L.; Rathbone, C.R.; Corona, B.T. Transplantation of devitalized muscle scaffolds is insufficient for appreciable de novo muscle fiber regeneration after volumetric muscle loss injury. Cell Tissue. Res. 2014, 358, 857–873. [Google Scholar] [CrossRef]
- Garg, K.; Ward, C.L.; Corona, B.T. Asynchronous inflammation and myogenic cell migration limit muscle tissue regeneration mediated by a cellular scaffolds. Inflamm Cel. Signal. 2014, 1. [Google Scholar] [CrossRef]
- Roth, S.M.; Metter, E.J.; Ling, S.; Ferrucci, L. Inflammatory factors in age-related muscle wasting. Curr. Opin. Rheumatol. 2006, 18, 625–630. [Google Scholar] [CrossRef]
- Hepple, R.T.; Rice, C.L. Innervation and neuromuscular control in ageing skeletal muscle. J. Physiol. 2016, 594, 1965–1978. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef]
- Wang, Y.; Wehling-Henricks, M.; Welc, S.S.; Fisher, A.L.; Zuo, Q.; Tidball, J.G. Aging of the immune system causes reductions in muscle stem cell populations, promotes their shift to a fibrogenic phenotype, and modulates sarcopenia. FASEB J. 2019, 33, 1415–1427. [Google Scholar] [CrossRef]
- Przybyla, B.; Gurley, C.; Harvey, J.F.; Bearden, E.; Kortebein, P.; Evans, W.J.; Sullivan, D.H.; Peterson, C.A.; Dennis, R.A. Aging alters macrophage properties in human skeletal muscle both at rest and in response to acute resistance exercise. Exp. Gerontol. 2006, 41, 320–327. [Google Scholar] [CrossRef]
- Cui, C.-Y.; Driscoll, R.K.; Piao, Y.; Chia, C.W.; Gorospe, M.; Ferrucci, L. Skewed macrophage polarization in aging skeletal muscle. Aging Cell 2019, 18, e13032. [Google Scholar] [CrossRef]
- Tam, C.S.; Sparks, L.M.; Johannsen, D.L.; Covington, J.D.; Church, T.S.; Ravussin, E. Low Macrophage Accumulation in Skeletal Muscle of Obese Type 2 Diabetics and Elderly Subjects. Obesity 2012, 20, 1530–1533. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wehling-Henricks, M.; Samengo, G.; Tidball, J.G. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell 2015, 14, 678–688. [Google Scholar] [CrossRef]
- Wehling-Henricks, M.; Jordan, M.C.; Gotoh, T.; Grody, W.W.; Roos, K.P.; Tidball, J.G. Arginine Metabolism by Macrophages Promotes Cardiac and Muscle Fibrosis in mdx Muscular Dystrophy. PLoS ONE 2010, 5, e10763. [Google Scholar] [CrossRef]
- Sicari, B.M.; Johnson, S.A.; Siu, B.F.; Crapo, P.M.; Daly, K.A.; Jiang, H.; Medberry, C.J.; Tottey, S.; Turner, N.J.; Badylak, S.F. The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials 2012, 33, 5524–5533. [Google Scholar] [CrossRef] [PubMed]
- Cano, R.L.E.; Lopera, H.D.E. Introduction to T and B lymphocytes. In Autoimmunity from Bench to Bedside [Internet]; Chapter 5; Anaya, J.M., Shoenfeld, Y., Rojas-Villarraga, A., Levy, R.A., Cervera, R., Eds.; El Rosario University Press: Bogota, Colombia, 18 July 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459471/ (accessed on 21 March 2021).
- Sun, W.; Wei, F.Q.; Li, W.J.; Wei, J.W.; Zhong, H.; Wen, Y.H.; Lei, W.B.; Chen, L.; Li, H.; Lin, H.Q.; et al. A positive-feedback loop between tumour infiltrating activated Treg cells and type 2-skewed macrophages is essential for progression of laryngeal squamous cell carcinoma. Br. J. Cancer 2017, 117, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Fanelli, G.; Tan, N.; Nova-Lamperti, E.; McGregor, R.; Lechler, R.I.; Lombardi, G.; Scottà, C. Expanded Regulatory T Cells Induce Alternatively Activated Monocytes with a Reduced Capacity to Expand T Helper-17 Cells. Front. Immunol. 2018, 9, 1625. [Google Scholar] [CrossRef]
- Schmidt, A.; Zhang, X.M.; Joshi, R.N.; Iqbal, S.; Wahlund, C.; Gabrielsson, S.; Harris, R.A.; Tegnér, J. Human macrophages induce CD4(+)Foxp3(+) regulatory T cells via binding and re-release of TGF-β. Immunol. Cell Biol. 2016, 94, 747–762. [Google Scholar] [CrossRef]
- Shephard, R.J.; Rhind, S.; Shek, P.N. Exercise and the immune system. Natural killer cells, interleukins and related responses. Sports Med. 1994, 18, 340–369. [Google Scholar] [CrossRef]
- Marshall-Gradisnik, S.; Weatherby, R.; Deakin, G.; Coutts, R.; Meir, R.; Connellan, P.; Stevenson, L.; Rogerson, S. Natural killer cell activity following 6 weeks of strength training in healthy young males with/without testosterone enanthate administration. J. Exerc. Sci. Fit. 2009, 6, 106–114. [Google Scholar]
- Goebels, N.; Michaelis, D.; Engelhardt, M.; Huber, S.; Bender, A.; Pongratz, D.; Johnson, M.A.; Wekerle, H.; Tschopp, J.; Jenne, D.; et al. Differential expression of perforin in muscle-infiltrating T cells in polymyositis and dermatomyositis. J. Clin. Investig. 1996, 97, 2905–2910. [Google Scholar] [CrossRef]
- Fu, X.; Xiao, J.; Wei, Y.; Li, S.; Liu, Y.; Yin, J.; Sun, K.; Sun, H.; Wang, H.; Zhang, Z.; et al. Combination of inflammation-related cytokines promotes long-term muscle stem cell expansion. Cell Res. 2015, 25, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Al-Shanti, N.; Durcan, P.; Al-Dabbagh, S.; Dimchev, G.A.; Stewart, C.E. Activated lymphocytes secretome inhibits differentiation and induces proliferation of C2C12 myoblasts. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 33, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Nguyen, M.H.; Fantuzzi, G.; Koh, T.J. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am. J. Physiol. Cell. Physiol. 2008, 294, C1183–C1191. [Google Scholar] [CrossRef]
- Hurtgen, B.J.; Ward, C.L.; Leopold Wager, C.M.; Garg, K.; Goldman, S.M.; Henderson, B.E.P.; McKinley, T.O.; Greising, S.M.; Wenke, J.C.; Corona, B.T. Autologous minced muscle grafts improve endogenous fracture healing and muscle strength after musculoskeletal trauma. Physiol. Rep. 2017, 5, e13362. [Google Scholar] [CrossRef] [PubMed]
- Sadtler, K.; Estrellas, K.; Allen, B.W.; Wolf, M.T.; Fan, H.; Tam, A.J.; Patel, C.H.; Luber, B.S.; Wang, H.; Wagner, K.R.; et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 2016, 352, 366–370. [Google Scholar] [CrossRef]
- Kwee, B.J.; Budina, E.; Najibi, A.J.; Mooney, D.J. CD4 T-cells regulate angiogenesis and myogenesis. Biomaterials 2018, 178, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Levings, M.K.; Sangregorio, R.; Sartirana, C.; Moschin, A.L.; Battaglia, M.; Orban, P.C.; Roncarolo, M.-G. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 2002, 196, 1335–1346. [Google Scholar] [CrossRef]
- Dumke, B.R.; Lees, S.J. Age-related impairment of T cell-induced skeletal muscle precursor cell function. Am. J. Physiol. Cell Physiol. 2011, 300, C1226–C1233. [Google Scholar] [CrossRef] [PubMed]
- Malm, C.; Nyberg, P.; Engstrom, M.; Sjodin, B.; Lenkei, R.; Ekblom, B.; Lundberg, I. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J. Physiol. 2000, 529 Pt 1, 243–262. [Google Scholar] [CrossRef]
- Malm, C.; Sjodin, T.L.; Sjoberg, B.; Lenkei, R.; Renstrom, P.; Lundberg, I.E.; Ekblom, B. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J. Physiol. 2004, 556, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Marklund, P.; Mattsson, C.M.; Wahlin-Larsson, B.; Ponsot, E.; Lindvall, B.; Lindvall, L.; Ekblom, B.; Kadi, F. Extensive inflammatory cell infiltration in human skeletal muscle in response to an ultraendurance exercise bout in experienced athletes. J. Appl. Physiol. 2013, 114, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Deyhle, M.R.; Gier, A.M.; Evans, K.C.; Eggett, D.L.; Nelson, W.B.; Parcell, A.C.; Hyldahl, R.D. Skeletal Muscle Inflammation Following Repeated Bouts of Lengthening Contractions in Humans. Front. Physiol. 2015, 6, 424. [Google Scholar] [CrossRef]
- Hyldahl, R.D.; Chen, T.C.; Nosaka, K. Mechanisms and Mediators of the Skeletal Muscle Repeated Bout Effect. Exerc. Sport Sci. Rev. 2017, 45, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Deyhle, M.R.; Hyldahl, R.D. The Role of T Lymphocytes in Skeletal Muscle Repair from Traumatic and Contraction-Induced Injury. Front. Physiol. 2018, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Ronsen, O.; Pedersen, B.K.; Oritsland, T.R.; Bahr, R.; Kjeldsen-Kragh, J. Leukocyte counts and lymphocyte responsiveness associated with repeated bouts of strenuous endurance exercise. J. Appl. Physiol. 2001, 91, 425–434. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C. The T cell and NK cell immune response to exercise. Ann. Transpl. 2005, 10, 43–48. [Google Scholar]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef]
- Garritson, J.; Krynski, L.; Haverbeck, L.; Haughian, J.M.; Pullen, N.A.; Hayward, R. Physical activity delays accumulation of immunosuppressive myeloid-derived suppressor cells. PLoS ONE 2020, 15, e0234548. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.J.; Walsh, C.M.; Dorshkind, K.A.; Rodriguez, E.M.; Tidball, J.G. Myonuclear apoptosis in dystrophic mdx muscle occurs by perforin-mediated cytotoxicity. J. Clin. Investig. 1997, 99, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Madaro, L.; Bouche, M. From innate to adaptive immune response in muscular dystrophies and skeletal muscle regeneration: The role of lymphocytes. Biomed. Res. Int. 2014, 2014, 438675. [Google Scholar] [CrossRef]
- Spencer, M.J.; Montecino-Rodriguez, E.; Dorshkind, K.; Tidball, J.G. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin. Immunol. 2001, 98, 235–243. [Google Scholar] [CrossRef]
- Emslie-Smith, A.M.; Arahata, K.; Engel, A.G. Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum. Pathol. 1989, 20, 224–231. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, Y.E.; Kim, S.I.; Kim, J.I.; Lee, C.H.; Park, K.H.; Kim, D.S. Differential immunohistological features of inflammatory myopathies and dysferlinopathy. J. Korean Med. Sci. 2009, 24, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Farini, A.; Sitzia, C.; Navarro, C.; D’Antona, G.; Belicchi, M.; Parolini, D.; Del Fraro, G.; Razini, P.; Bottinelli, R.; Meregalli, M.; et al. Absence of T and B lymphocytes modulates dystrophic features in dysferlin deficient animal model. Exp. Cell Res. 2012, 318, 1160–1174. [Google Scholar] [CrossRef]
- Farini, A.; Meregalli, M.; Belicchi, M.; Battistelli, M.; Parolini, D.; D’Antona, G.; Gavina, M.; Ottoboni, L.; Constantin, G.; Bottinelli, R.; et al. T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J. Pathol. 2007, 213, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ouisse, L.-H.; Remy, S.; Lafoux, A.; Larcher, T.; Tesson, L.; Chenouard, V.; Guillonneau, C.; Brusselle, L.; Vimond, N.; Rouger, K.; et al. Immunological characterization of a rat model of Duchenne’s disease and demonstration of improved muscle strength after anti-CD45RC antibody treatment. Front. Immunol. 2019, 10, 2131. [Google Scholar] [CrossRef]
- Madaro, L.; Pelle, A.; Nicoletti, C.; Crupi, A.; Marrocco, V.; Bossi, G.; Soddu, S.; Bouche, M. PKC theta ablation improves healing in a mouse model of muscular dystrophy. PLoS ONE 2012, 7, e31515. [Google Scholar] [CrossRef]
- Ieronimakis, N.; Pantoja, M.; Hays, A.L.; Dosey, T.L.; Qi, J.; Fischer, K.A.; Hoofnagle, A.N.; Sadilek, M.; Chamberlain, J.S.; Ruohola-Baker, H.; et al. Increased sphingosine-1-phosphate improves muscle regeneration in acutely injured mdx mice. Skelet Muscle 2013, 3, 20. [Google Scholar] [CrossRef]
- Jin, Y.; Knudsen, E.; Wang, L.; Bryceson, Y.; Damaj, B.; Gessani, S.; Maghazachi, A.A. Sphingosine 1-phosphate is a novel inhibitor of T-cell proliferation. Blood 2003, 101, 4909–4915. [Google Scholar] [CrossRef]
- Eghtesad, S.; Jhunjhunwala, S.; Little, S.R.; Clemens, P.R. Rapamycin ameliorates dystrophic phenotype in mdx mouse skeletal muscle. Mol. Med. 2011, 17, 917–924. [Google Scholar] [CrossRef]
- Morrison, J.; Palmer, D.B.; Cobbold, S.; Partridge, T.; Bou-Gharios, G. Effects of T-lymphocyte depletion on muscle fibrosis in the mdx mouse. Am. J. Pathol. 2005, 166, 1701–1710. [Google Scholar] [CrossRef]
- Sugiura, T.; Murakawa, Y.; Nagai, A.; Kondo, M.; Kobayashi, S. Fas and Fas ligand interaction induces apoptosis in inflammatory myopathies: CD4+ T cells cause muscle cell injury directly in polymyositis. Arthritis Rheumatol. 1999, 42, 291–298. [Google Scholar] [CrossRef]
- Mizuno, K.; Yachie, A.; Nagaoki, S.; Wada, H.; Okada, K.; Kawachi, M.; Toma, T.; Konno, A.; Ohta, K.; Kasahara, Y.; et al. Oligoclonal expansion of circulating and tissue-infiltrating CD8+ T cells with killer/effector phenotypes in juvenile dermatomyositis syndrome. Clin. Exp. Immunol. 2004, 137, 187–194. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Koarada, S.; Tada, Y.; Ushiyama, O.; Morito, F.; Suzuki, N.; Ohta, A.; Horiuchi, T.; Miyake, K.; Nagasawa, K. Difference in B cell activation between dermatomyositis and polymyositis: Analysis of the expression of RP105 on peripheral blood B cells. Ann. Rheum. Dis. 2001, 60, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Josefowicz, S.Z.; Lu, L.-F.; Rudensky, A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Green, J.A.; Moltedo, B.; Arvey, A.; Hemmers, S.; Yuan, S.; Treuting, P.M.; Rudensky, A.Y. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 2015, 162, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, Y.; O’Hagan, T.; Dittmer, M.; Penalva, R.; Mayoral, S.R.; Bankhead, P.; Fleville, S.; Eleftheriadis, G.; Zhao, C.; Naughton, M.; et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 2017, 20, 674. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.P.; Sheng, D.Z.; Sugimoto, K.; Gonzalez-Rajal, A.; Nakagawa, S.; Hesselson, D.; Kikuchi, K. Zebrafish Regulatory T Cells Mediate Organ-Specific Regenerative Programs. Dev. Cell 2017, 43, 659–672.e655. [Google Scholar] [CrossRef]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930. [Google Scholar] [CrossRef] [PubMed]
- Villalta, S.A.; Rosenthal, W.; Martinez, L.; Kaur, A.; Sparwasser, T.; Tidball, J.G.; Margeta, M.; Spencer, M.J.; Bluestone, J.A. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci. Transl. Med. 2014, 6, 258ra142. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.M.; Korty, P.E.; Kim, Y.C.; Martens, C.; Shevach, E.M. Helios expression defines a phenotypically distinct population of Treg cells. J. Immunol. 2018, 200, 116–119. [Google Scholar]
- Yadav, M.; Louvet, C.; Davini, D.; Gardner, J.M.; Martinez-Llordella, M.; Bailey-Bucktrout, S.; Anthony, B.A.; Sverdrup, F.M.; Head, R.; Kuster, D.J.; et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 2012, 209, 1713–S1719. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, D.M.W.; van Loosdregt, J.; Gorlani, A.; Bekker, C.P.J.; Gröne, A.; Sibilia, M.; van Bergen en Henegouwen, P.M.P.; Roovers, R.C.; Coffer, P.J.; Sijts, A.J.A.M. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 2013, 38, 275–284. [Google Scholar] [CrossRef] [PubMed]
- DiSpirito, J.R.; Zemmour, D.; Ramanan, D.; Cho, J.; Zilionis, R.; Klein, A.M.; Benoist, C.; Mathis, D. Molecular diversification of regulatory T cells in nonlymphoid tissues. Sci. Immunol. 2018, 3, eaat5861. [Google Scholar] [CrossRef]
- Jin, R.M.; Warunek, J.; Wohlfert, E.A. Therapeutic administration of IL-10 and amphiregulin alleviates chronic skeletal muscle inflammation and damage induced by infection. Immunohorizons 2018, 2, 142–154. [Google Scholar] [CrossRef]
- Beyer, M.; Thabet, Y.; Müller, R.-U.; Sadlon, T.; Classen, S.; Lahl, K.; Basu, S.; Zhou, X.; Bailey-Bucktrout, S.L.; Krebs, W.; et al. Repression of the genome organizer SATB1 in regulatory T cells is required for suppressive function and inhibition of effector differentiation. Nat. Immunol. 2011, 12, 898. [Google Scholar] [CrossRef]
- Alvarez, J.D.; Yasui, D.H.; Niida, H.; Joh, T.; Loh, D.Y.; Kohwi-Shigematsu, T. The MAR-binding protein SATB1 orchestrates temporal and spatial expression of multiple genes during T-cell development. Genes Dev. 2000, 14, 521–535. [Google Scholar] [PubMed]
- Kuswanto, W.; Burzyn, D.; Panduro, M.; Wang, K.K.; Jang, Y.C.; Wagers, A.J.; Benoist, C.; Mathis, D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 2016, 44, 355–367. [Google Scholar] [CrossRef]
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat. Rev. Drug. Discov. 2008, 7, 827. [Google Scholar] [CrossRef]
- Taams, L.S.; van Amelsfort, J.M.R.; Tiemessen, M.M.; Jacobs, K.M.G.; de Jong, E.C.; Akbar, A.N.; Bijlsma, J.W.J.; Lafeber, F.P.J.G. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum. Immunol. 2005, 66, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Panduro, M.; Benoist, C.; Mathis, D. Treg cells limit IFN-γ production to control macrophage accrual and phenotype during skeletal muscle regeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E2585–E2593. [Google Scholar] [CrossRef]
- Castiglioni, A.; Corna, G.; Rigamonti, E.; Basso, V.; Vezzoli, M.; Monno, A.; Almada, A.E.; Mondino, A.; Wagers, A.J.; Manfredi, A.A.; et al. FOXP3+ T Cells Recruited to Sites of Sterile Skeletal Muscle Injury Regulate the Fate of Satellite Cells and Guide Effective Tissue Regeneration. PLoS ONE 2015, 10, e0128094. [Google Scholar] [CrossRef] [PubMed]
- Capote, J.; Kramerova, I.; Martinez, L.; Vetrone, S.; Barton, E.R.; Sweeney, H.L.; Miceli, M.C.; Spencer, M.J. Osteopontin ablation ameliorates muscular dystrophy by shifting macrophages to a pro-regenerative phenotype. J. Cell Biol. 2016, 213, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Vetrone, S.A.; Montecino-Rodriguez, E.; Kudryashova, E.; Kramerova, I.; Hoffman, E.P.; Liu, S.D.; Miceli, M.C.; Spencer, M.J. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-β. J. Clin. Investig. 2009, 119, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.M.; Blair, S.J.; Warunek, J.; Heffner, R.R.; Blader, I.J.; Wohlfert, E.A. Regulatory T Cells Promote Myositis and Muscle Damage in Toxoplasma gondii Infection. J. Immunol. 2017, 198, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Blau, H.M.; Cosgrove, B.D.; Ho, A.T.V. The central role of muscle stem cells in regenerative failure with aging. Nat. Med. 2015, 21, 854. [Google Scholar] [CrossRef] [PubMed]
- Brack, A.S.; Muñoz-Cánoves, P. The ins and outs of muscle stem cell aging. Skelet Muscle 2016, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Lages, C.S.; Suffia, I.; Velilla, P.A.; Huang, B.; Warshaw, G.; Hildeman, D.A.; Belkaid, Y.; Chougnet, C. Functional Regulatory T Cells Accumulate in Aged Hosts and Promote Chronic Infectious Disease Reactivation. J. Immunol. 2008, 181, 1835–1848. [Google Scholar] [CrossRef]
- Qazi, T.H.; Duda, G.N.; Ort, M.J.; Perka, C.; Geissler, S.; Winkler, T. Cell therapy to improve regeneration of skeletal muscle injuries. J. Cachexia Sarcopenia Muscle 2019, 10, 501–516. [Google Scholar] [CrossRef]
- Klimczak, A.; Kozlowska, U.; Kurpisz, M. Muscle Stem/Progenitor Cells and Mesenchymal Stem Cells of Bone Marrow for Skeletal Muscle Regeneration in Muscular Dystrophies. Arch. Immunol. Ther. Exp. 2018, 66, 13. [Google Scholar]
- Caseiro, A.; Pereira, T.; Bártolo, P.; Santos, J.; Luís, A.; Maurício, A.L.A.A.C. Trends in Mesenchymal Stem Cells’ Applications for Skeletal Muscle Repair and Regeneration; Demirer, T., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- Winkler, T.; Perka, C.; von Roth, P.; Agres, A.N.; Plage, H.; Preininger, B.; Pumberger, M.; Geissler, S.; Hagai, E.L.; Ofir, R.; et al. Immunomodulatory placental-expanded, mesenchymal stromal cells improve muscle function following hip arthroplasty. J. Cachexia Sarcopenia Muscle 2018, 9, 880–897. [Google Scholar] [CrossRef]
- Hof-Nahor, I.; Leshansky, L.; Shivtiel, S.; Eldor, L.; Aberdam, D.; Itskovitz-Eldor, J.; Berrih-Aknin, S. Human mesenchymal stem cells shift CD8+ T cells towards a suppressive phenotype by inducing tolerogenic monocytes. J. Cell Sci. 2012, 125, 4640–4650. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Groh, M.E.; Maitra, B.; Szekely, E.; Koc, O.N. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp. Hematol. 2005, 33, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 2016, 6, 38308. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.A.M.; Shaheen, N.E.M.; Abu Zahra, F.A. Immunomodulatory capacity of the local mesenchymal stem cells transplantation after severe skeletal muscle injury in female rats. Immunopharmacol. Immunotoxicol. 2016, 38, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Liu, S.; Zhang, H.; Zhu, B.; Su, Y.; Zheng, C.; Tian, R.; Wang, M.; Kuang, H.; Zhao, X.; et al. Mesenchymal stem cells and extracellular matrix scaffold promote muscle regeneration by synergistically regulating macrophage polarization toward the M2 phenotype. Stem. Cell Res. Ther. 2018, 9, 88. [Google Scholar] [CrossRef]
- Corona, B.T.; Wu, X.; Ward, C.L.; McDaniel, J.S.; Rathbone, C.R.; Walters, T.J. The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM. Biomaterials 2013, 34, 3324–3335. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Zisa, D.; Leiker, M.; Johnston, C.; Lin, H.; Lee, T. Muscular dystrophy therapy by nonautologous mesenchymal stem cells: Muscle regeneration without immunosuppression and inflammation. Transplantation 2009, 87, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.H.; Dunn, A.J.; Talovic, M.; Haas, G.J.; Marcinczyk, M.; Elmashhady, H.; Kalaf, E.G.; Sell, S.A.; Garg, K. Aligned nanofibers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro. Biomed. Mater. 2019, 14, 035010. [Google Scholar] [CrossRef]
- Li, Y.; Jin, D.; Xie, W.; Wen, L.; Chen, W.; Xu, J.; Ding, J.; Ren, D.; Xiao, Z. Mesenchymal Stem Cells-Derived Exosomes: A Possible Therapeutic Strategy for Osteoporosis. Curr. Stem. Cell Res. Ther. 2018, 13, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transpl. 2019, 54, 789–792. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem. Cell Res. Ther. 2018, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.W.; Wang, J.; Lee, C.J.; Liu, M.; Neelamegham, S.; Canty, J.M.; Nguyen, J. The microRNA regulatory landscape of MSC-derived exosomes: A systems view. Sci. Rep. 2018, 8, 1419. [Google Scholar] [CrossRef]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front. Cell Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef]
- Riau, A.K.; Ong, H.S.; Yam, G.H.F.; Mehta, J.S. Sustained Delivery System for Stem Cell-Derived Exosomes. Front. Pharm. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem. Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Magarotto, F.; Sgrò, A.; Dorigo Hochuli, A.H.; Andreetta, M.; Grassi, M.; Saggioro, M.; Nogara, L.; Tolomeo, A.M.; Francescato, R.; Collino, F.; et al. Muscle functional recovery is driven by extracellular vesicles combined with muscle extracellular matrix in a volumetric muscle loss murine model. Biomaterials 2021, 269, 120653. [Google Scholar] [CrossRef]
- Baer, A.; Colon-Moran, W.; Bhattarai, N. Characterization of the effects of immunomodulatory drug fingolimod (FTY720) on human T cell receptor signaling pathways. Sci. Rep. 2018, 8, 10910. [Google Scholar] [CrossRef]
- Thomson, A.W.; Bonham, C.A.; Zeevi, A. Mode of action of tacrolimus (FK506): Molecular and cellular mechanisms. Ther. Drug. Monit. 1995, 17, 584–591. [Google Scholar] [CrossRef]
- Heydemann, A. Severe murine limb-girdle muscular dystrophy type 2C pathology is diminished by FTY720 treatment. Muscle Nerve 2017, 56, 486–494. [Google Scholar] [CrossRef]
- Foster, A.D.; Vicente, D.; Sexton, J.J.; Johnston, L.; Clark, N.; Leonhardt, C.; Elster, E.A.; Davis, T.A.; Bradley, M.J. Administration of FTY720 during Tourniquet-Induced Limb Ischemia Reperfusion Injury Attenuates Systemic Inflammation. Mediat. Inflamm. 2017, 2017, 4594035. [Google Scholar] [CrossRef]
- Corona, B.T.; Rivera, J.C.; Wenke, J.C.; Greising, S.M. Tacrolimus as an adjunct to autologous minced muscle grafts for the repair of a volumetric muscle loss injury. J. Exp. Orthop. 2017, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Dell’Orso, S.; Juan, A.H.; Ko, K.D.; Naz, F.; Perovanovic, J.; Gutierrez-Cruz, G.; Feng, X.; Sartorelli, V. Single cell analysis of adult mouse skeletal muscle stem cells in homeostatic and regenerative conditions. Development 2019, 146. [Google Scholar] [CrossRef]
- De Micheli, A.J.; Laurilliard, E.J.; Heinke, C.L.; Ravichandran, H.; Fraczek, P.; Soueid-Baumgarten, S.; De Vlaminck, I.; Elemento, O.; Cosgrove, B.D. Single-Cell Analysis of the Muscle Stem Cell Hierarchy Identifies Heterotypic Communication Signals Involved in Skeletal Muscle Regeneration. Cell Rep. 2020, 30, 3583–3595.e3585. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, A.B.; Smith, G.R.; Raue, U.; Begue, G.; Minchev, K.; Ruf-Zamojski, F.; Nair, V.D.; Wang, X.; Zhou, L.; Zaslavsky, E.; et al. Single-cell transcriptional profiles in human skeletal muscle. Sci. Rep. 2020, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- De Micheli, A.J.; Spector, J.A.; Elemento, O.; Cosgrove, B.D. A reference single-cell transcriptomic atlas of human skeletal muscle tissue reveals bifurcated muscle stem cell populations. Skelet. Muscle 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).