The Role of Endoglin in Hepatocellular Carcinoma
Abstract
1. Introduction
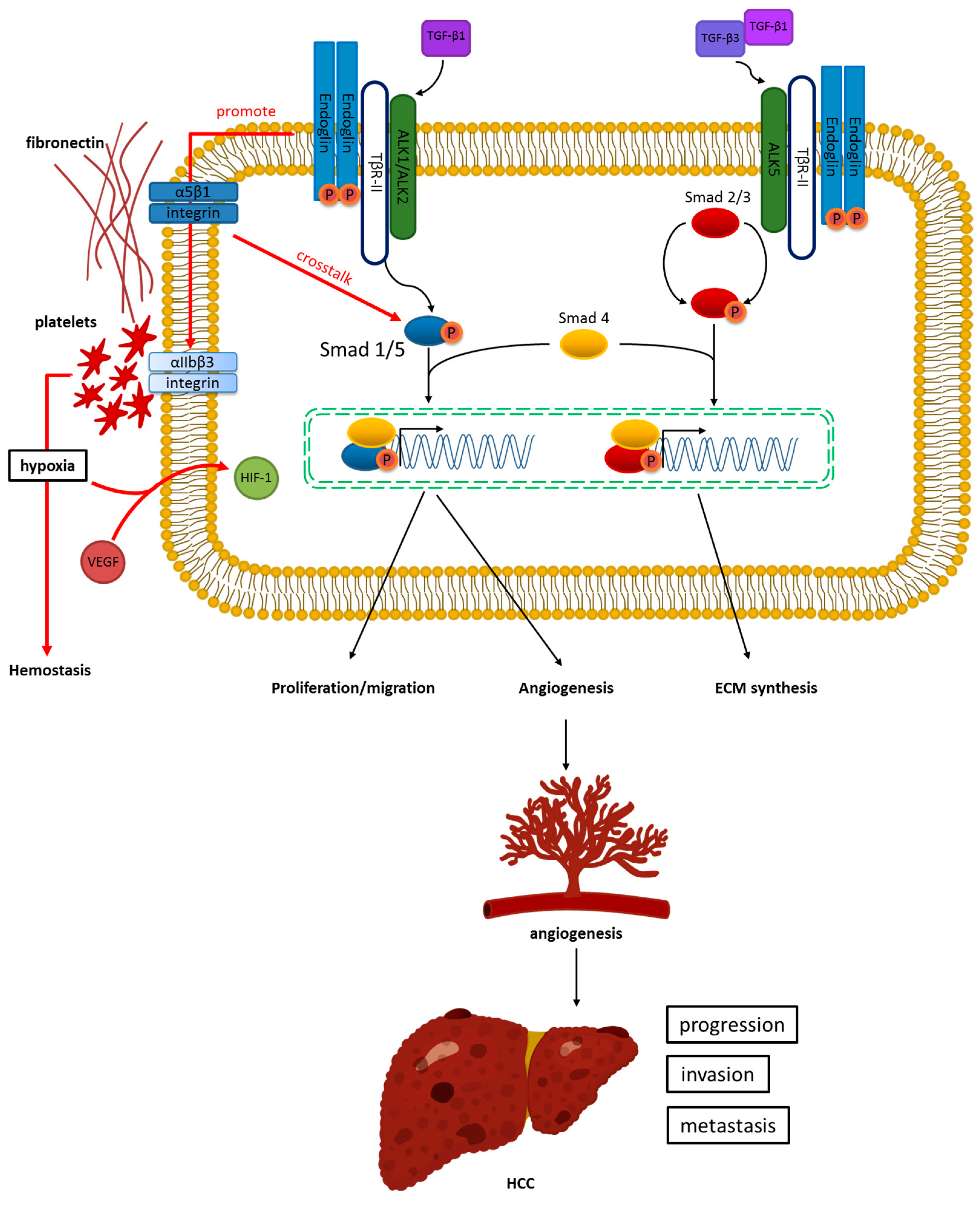
2. Structure, Function, and Signaling Pathway of Endoglin
3. Angiogenesis of HCC and Its Relation to Endoglin
4. Circulating Endoglin in HCC Patients
5. Hepatitis C Virus Core Protein Modulates the Endoglin Signaling Pathway and the Role of Endoglin in Cancer Stem Cells, Hepatic Stellate Cells and Cancer Associated Fibroblasts
6. Endoglin in Tumor-Derived Endothelial Cells and HCC
7. Endoglin Detection and the Inhibition of Endoglin in HCC Therapy
8. Endoglin as a Biomarker and Controversial Issues in Endoglin-Targeted Therapy
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayiner, M.; Golabi, P.; Younossi, Z.M. Disease Burden of Hepatocellular Carcinoma: A Global Perspective. Dig. Dis. Sci. 2019, 64, 910–917. [Google Scholar] [CrossRef]
- Mossenta, M.; Busato, D.; Baboci, L.; Di Cintio, F.; Toffoli, G.; Bo, M.D. New Insight into Therapies Targeting Angiogenesis in Hepatocellular Carcinoma. Cancers 2019, 11, 1086. [Google Scholar] [CrossRef]
- Jamshidi-Parsian, A.; Griffin, R.J.; Kore, R.A.; Todorova, V.K.; Makhoul, I. Tumor-Endothelial Cell Interaction in an Experimental Model of Human Hepatocellular Carcinoma. Exp. Cell Res. 2018, 372, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, J.P.; Bernardo, V.; Canedo, N.H.S.; Ribeiro, O.D.; Caroli-Bottino, A.; Pannain, V.L. Microvascular Density of Regenerative Nodule to Small Hepatocellular Carcinoma by Automated Analysis Using CD105 and CD34 Immunoexpression. BMC Cancer 2014, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the Angiogenic Switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Kerbel, R.S. Tumor Angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Shao, S.; Yan, Y.; Yang, S.; Bai, S.; Wu, Y.; Zhang, J.; Liu, R.; Ma, H.; Chai, L.; et al. Association between G-Protein Coupled Receptor 4 Expression and Microvessel Density, Clinicopathological Characteristics and Survival in Hepatocellular Carcinoma. Oncol. Lett. 2020, 19, 2609–2620. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Lu, W.-Q.; Huang, G.-W.; Wang, W. Correlation between CD105 Expression and Postoperative Recurrence and Metastasis of Hepatocellular Carcinoma. BMC Cancer 2006, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Gougos, A.; Letarte, M. Identification of a Human Endothelial Cell Antigen with Monoclonal Antibody 44G4 Produced against a Pre-B Leukemic Cell Line. J. Immunol. 1988, 141, 1925–1933. [Google Scholar]
- Fernández-Ruiz, E.; St-Jacques, S.; Bellón, T.; Letarte, M.; Bernabéu, C. Assignment of the Human Endoglin Gene (END) to 9q34→qter. Cytogenet. Genome Res. 1993, 64, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Gougos, A.; Letarte, M. Primary Structure of Endoglin, an RGD-Containing Glycoprotein of Human Endothelial Cells. J. Biol. Chem. 1990, 265, 8361–8364. [Google Scholar] [CrossRef]
- López-Novoa, J.M.; Bernabeu, C. The Physiological Role of Endoglin in the Cardiovascular System. Am. J. Physiol. Circ. Physiol. 2010, 299, H959–H974. [Google Scholar] [CrossRef]
- Maring, J.A.; Trojanowska, M.; Dijke, P.T. Role of Endoglin in Fibrosis and Scleroderma. Int. Rev. Cell Mol. Biol. 2012, 297, 295–308. [Google Scholar] [CrossRef]
- Kwon, Y.-C.; Sasaki, R.; Meyer, K.; Ray, R. Hepatitis C Virus Core Protein Modulates Endoglin (CD105) Signaling Pathway for Liver Pathogenesis. J. Virol. 2017, 91, e01235-17. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A.; Adamek, A. Role of Endoglin (CD105) in the Progression of Hepatocellular Carcinoma and Anti-Angiogenic Therapy. Int. J. Mol. Sci. 2018, 19, 3887. [Google Scholar] [CrossRef]
- Ray, B.N.; Lee, N.Y.; How, T.; Blobe, G.C. ALK5 Phosphorylation of the Endoglin Cytoplasmic Domain Regulates Smad1/5/8 Signaling and Endothelial Cell Migration. Carcinogenesis 2009, 31, 435–441. [Google Scholar] [CrossRef]
- Bernabeu, C.; Lopez-Novoa, J.M.; Quintanilla, M. The Emerging Role of TGF-β Superfamily Coreceptors in Cancer. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2009, 1792, 954–973. [Google Scholar] [CrossRef] [PubMed]
- Finnson, K.W.; Philip, A. Endoglin in Liver Fibrosis. J. Cell Commun. Signal. 2011, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bellón, T.; Corbi, A.; Lastres, P.; Calés, C.; Cebrián, M.; Vera, S.; Cheifetz, S.; Massagué, J.; Letarte, M.; Bernabeu, C. Identification and Expression of Two Forms of the Human Transforming Growth Factor-β-Binding Protein Endoglin with Distinct Cytoplasmic Regions. Eur. J. Immunol. 1993, 23, 2340–2345. [Google Scholar] [CrossRef]
- Blanco, F.J.; Bernabeu, C. Alternative Splicing Factor or Splicing Factor-2 Plays a Key Role in Intron Retention of the Endoglin Gene during Endothelial Senescence. Aging Cell 2011, 10, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Alsamman, M.; Sterzer, V.; Meurer, S.K.; Sahin, H.; Schaeper, U.; Kuscuoglu, D.; Strnad, P.; Weiskirchen, R.; Trautwein, C.; Scholten, D. Endoglin in Human Liver Disease and Murine Models of Liver Fibrosis—A Protective Factor against Liver Fibrosis. Liver Int. 2017, 38, 858–867. [Google Scholar] [CrossRef]
- Pérez-Gómez, E.; Eleno, N.; López-Novoa, J.M.; Ramirez, J.R.; Velasco, B.; Letarte, M.; Bernabeu, C.; Quintanilla, M. Characterization of Murine S-endoglin Isoform and Its Effects on Tumor Development. Oncogene 2005, 24, 4450–4461. [Google Scholar] [CrossRef] [PubMed]
- Meurer, S.K.; Tihaa, L.; Borkham-Kamphorst, E.; Weiskirchen, R. Expression and Functional Analysis of Endoglin in Isolated Liver Cells and Its Involvement in Fibrogenic Smad Signalling. Cell. Signal. 2011, 23, 683–699. [Google Scholar] [CrossRef]
- Velasco, S.; Pericacho, M.; Alvarez-Muñoz, P.; Rodríguez-Barbero, A.; Dijke, P.T.; Bernabeu, C.; López-Novoa, J.M. L-and S-endoglin Differentially Modulate TGF 1 Signaling Mediated by ALK1 and ALK5 in L6E9 Myoblasts. J. Cell Sci. 2008, 121, 913–919. [Google Scholar] [CrossRef]
- Lebrin, F.; Deckers, M.; Bertolino, P.; Dijke, P.T. TGF-β Receptor Function in the Endothelium. Cardiovasc. Res. 2005, 65, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Dallas, N.A.; Samuel, S.; Xia, L.; Fan, F.; Gray, M.J.; Lim, S.J.; Ellis, L.M. Endoglin (CD105): A Marker of Tumor Vasculature and Potential Target for Therapy. Clin. Cancer Res. 2008, 14, 1931–1937. [Google Scholar] [CrossRef]
- Cheifetz, S.; Bellon, T.; Cales, C.; Vera, S.; Bernabeu, C.; Massague, J.; Letarte, M. Endoglin Is a Component of the Trans-Forming Growth Factor-Beta Receptor System in Human Endothelial Cells. J. Biol. Chem. 1992, 267, 19027–19030. [Google Scholar] [CrossRef]
- Fonsatti, E.; Del Vecchio, L.; Altomonte, M.; Sigalotti, L.; Nicotra, M.R.; Coral, S.; Natali, P.G.; Maio, M. Endoglin: An Accessory Component of the TGF-β-Binding Receptor-Complex with Diagnostic, Prognostic, and Bioimmunotherapeutic Potential in Human Malignancies. J. Cell. Physiol. 2001, 188, 1–7. [Google Scholar] [CrossRef]
- Guerrero-Esteo, M.; Sánchez-Elsner, T.; Letamendia, A.; Bernabéu, C. Extracellular and Cytoplasmic Domains of Endoglin Interact with the Transforming Growth Factor-β Receptors I and II. J. Biol. Chem. 2002, 277, 29197–29209. [Google Scholar] [CrossRef]
- Blanco, F.J.; Santibanez, J.F.; Guerrero-Esteo, M.; Langa, C.; Vary, C.P.H.; Bernabeu, C. Interaction and Functional Interplay between Endoglin and ALK-1, Two Components of the Endothelial Transforming Growth Factor-β Receptor Complex. J. Cell. Physiol. 2005, 204, 574–584. [Google Scholar] [CrossRef]
- Pomeraniec, L.; Hector-Greene, M.; Ehrlich, M.; Blobe, G.C.; Henis, Y.I. Regulation of TGF-β Receptor Hetero-Oligomerization and Signaling by Endoglin. Mol. Biol. Cell 2015, 26, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Rius, C.; Smith, J.D.; Almendro, N.; Langa, C.; Botella, L.M.; Marchuk, D.A.; Vary, C.P.; Bernabeu, C. Cloning of the Promoter Region of Human Endoglin, the Target Gene for Hereditary Hemorrhagic Telangiectasia Type 1. Blood 1998, 92, 4677–4690. [Google Scholar] [CrossRef]
- Nassiri, F.; Cusimano, M.D.; Scheithauer, B.W.; Rotondo, F.; Fazio, A.; Yousef, G.M.; Syro, L.V.; Kovacs, K.; Lloyd, R.V. Endoglin (CD105): A Review of Its Role in Angiogenesis and Tumor Diagnosis, Progression and Therapy. Anticancer. Res. 2011, 31, 2283–2290. [Google Scholar]
- Lebrin, F.; Goumans, M.-J.; Jonker, L.; Carvalho, R.L.C.; Valdimarsdottir, G.; Thorikay, M.; Mummery, C.; Arthur, H.M.; Dijke, P.T. Endoglin Promotes Endothelial Cell Proliferation and TGF-β/ALK1 Signal Transduction. EMBO J. 2004, 23, 4018–4028. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Morine, K.J.; Letarte, M. Endoglin: A Critical Mediator of Cardiovascular Health. Vasc. Health Risk Manag. 2013, 9, 195–206. [Google Scholar] [CrossRef]
- Rossi, E.; Sanz-Rodriguez, F.; Eleno, N.; Düwell, A.; Blanco, F.J.; Langa, C.; Botella, L.M.; Cabañas, C.; Lopez-Novoa, J.M.; Bernabeu, C. Endothelial Endoglin Is Involved in Inflammation: Role in Leukocyte Adhesion and Transmigration. Blood 2013, 121, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Barbero, A.; Obreo, J.; Álvarez-Muñoz, P.; Pandiella, A.; Bernabéu, C.; López-Novoa, J.M. Endoglin Modulation of TGF-ß1-Induced Collagen Synthesis is Dependent on ERK1/2 MAPK Activation. Cell. Physiol. Biochem. 2006, 18, 135–142. [Google Scholar] [CrossRef]
- Lee, N.Y.; Blobe, G.C. The Interaction of Endoglin with β-Arrestin2 Regulates Transforming Growth Factor-β-mediated ERK Activation and Migration in Endothelial Cells. J. Biol. Chem. 2007, 282, 21507–21517. [Google Scholar] [CrossRef]
- Scherner, O.; Meurer, S.K.; Tihaa, L.; Gressner, A.M.; Weiskirchen, R. Endoglin Differentially Modulates Antagonistic Transforming Growth Factor-β1 and BMP-7 Signaling*. J. Biol. Chem. 2007, 282, 13934–13943. [Google Scholar] [CrossRef]
- Tian, H.; Mythreye, K.; Golzio, C.; Katsanis, N.; Blobe, G.C. Endoglin Mediates Fibronectin/α5β1 Integrin and TGF-β Pathway Crosstalk in Endothelial Cells. EMBO J. 2012, 31, 3885–3900. [Google Scholar] [CrossRef]
- Rossi, E.; Pericacho, M.; Bachelot-Loza, C.; Pidard, D.; Gaussem, P.; Poirault-Chassac, S.; Blanco, F.J.; Langa, C.; González-Manchón, C.; Novoa, J.M.L.; et al. Human Endoglin as a Potential New Partner Involved in Platelet–Endothelium Interactions. Cell. Mol. Life Sci. 2018, 75, 1269–1284. [Google Scholar] [CrossRef] [PubMed]
- Onoe, T.; Ohdan, H.; Tokita, D.; Hara, H.; Tanaka, Y.; Ishiyama, K.; Asahara, T. Liver Sinusoidal Endothelial Cells Have a Capacity for Inducing Nonresponsiveness of T Cells across Major Histocompatibility Complex Barriers. Transpl. Int. 2005, 18, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Duda, D.G.; Sahani, D.V.; Jain, R.K. HCC and Angiogenesis: Possible Targets and Future Directions. Nat. Rev. Clin. Oncol. 2011, 8, 292–301. [Google Scholar] [CrossRef]
- Honda, H.; Tajima, T.; Kajiyama, K.; Kuroiwa, T.; Yoshimitsu, K.; Irie, H.; Aibe, H.; Shimada, M.; Masuda, K. Vascular Changes in Hepatocellular Carcinoma: Correlation of Radiologic and Pathologic Findings. Am. J. Roentgenol. 1999, 173, 1213–1217. [Google Scholar] [CrossRef]
- OsamuMatsui, O.; Kobayashi, S.; Sanada, J.; Kouda, W.; Ryu, Y.; Kozaka, K.; Kitao, A.; Nakamura, K.; Gabata, T. Hepatocelluar Nodules in Liver Cirrhosis: Hemodynamic Evaluation (Angiography-Assisted CT) with Special Reference to Multi-Step Hepatocarcinogenesis. Abdom. Imaging 2011, 36, 264–272. [Google Scholar] [CrossRef]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.-E. Liver Sinusoidal Endothelial Cells: Physiology and Role in Liver Diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef]
- Muto, J.; Shirabe, K.; Sugimachi, K.; Maehara, Y. Review of Angiogenesis in Hepatocellular Carcinoma. Hepatol. Res. 2014, 45, 1–9. [Google Scholar] [CrossRef]
- Sanz-Cameno, P.; Trapero-Marugán, M.; Chaparro, M.; Jones, E.A.; Moreno-Otero, R. Angiogenesis: From Chronic Liver Inflammation to Hepatocellular Carcinoma. J. Oncol. 2010, 2010, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Poon, R.T. Vascular Changes in Hepatocellular Carcinoma. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2008, 291, 721–734. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, S.; Zhang, D.; Du, J.; Guo, H.; Zhao, X.; Zhang, W.; Hao, X. Vasculogenic Mimicry Is Associated with High Tumor Grade, Invasion and Metastasis, and Short Survival in Patients with Hepatocellular Carcinoma. Oncol. Rep. 2006, 16, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, X.; Zhu, D.; Liu, T.; Liang, X.; Liu, F.; Zhang, Y.; Dong, X.; Sun, B. HIF-1α Promoted Vasculogenic Mimicry Formation in Hepatocellular Carcinoma through LOXL2 up-Regulation in Hypoxic Tumor Microenvironment. J. Exp. Clin. Cancer Res. 2017, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Segatelli, V.; De Oliveira, E.C.; Boin, I.F.S.F.; Ataide, E.C.; Escanhoela, C.A.F. Evaluation and Comparison of Microvessel Density Using the Markers CD34 and CD105 in Regenerative Nodules, Dysplastic Nodules and Hepatocellular Carcinoma. Hepatol. Int. 2014, 8, 260–265. [Google Scholar] [CrossRef]
- Yu, D.; Sun, X.; Qiu, Y.; Zhou, J.; Wu, Y.; Zhuang, L.; Chen, J.; Ding, Y. Identification and Clinical Significance of Mobilized Endothelial Progenitor Cells in Tumor Vasculogenesis of Hepatocellular Carcinoma. Clin. Cancer Res. 2007, 13, 3814–3824. [Google Scholar] [CrossRef]
- Yu, D.-C.; Chen, J.; Ding, Y.-T. Hypoxic and Highly Angiogenic Non-Tumor Tissues Surrounding Hepatocellular Carcinoma: The ‘Niche’ of Endothelial Progenitor Cells. Int. J. Mol. Sci. 2010, 11, 2901–2909. [Google Scholar] [CrossRef]
- Park, Y.N.; Kim, Y.B.; Yang, K.M.; Park, C. Increased Expression of Vascular Endothelial Growth Factor and Angiogenesis in the Early Stage of Multistep Hepatocarcinogenesis. Arch. Pathol. Lab. Med. 2000, 124, 1061–1065. [Google Scholar] [CrossRef]
- Teixeira, A.C.; Brasil, I.R.; Torres, A.F.; Tavora, F. The Evaluation of Angiogenesis Markers in Hepatocellular Carcinoma and Precursor Lesions in Liver Explants from a Single Institution. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Kin, M.; Torimura, T.; Ueno, T.; Inuzuka, S.; Tanikawa, K. Sinusoidal Capillarization in Small Hepatocellular Carcinoma. Pathol. Int. 1994, 44, 771–778. [Google Scholar] [CrossRef]
- Poon, R.T.-P.; Ng, I.O.-L.; Lau, C.; Yu, W.-C.; Yang, Z.-F.; Fan, S.-T.; Wong, J. Tumor Microvessel Density as a Predictor of Recurrence After Resection of Hepatocellular Carcinoma: A Prospective Study. J. Clin. Oncol. 2002, 20, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ghellal, A.; Li, C.; Byrne, G.; Haboubi, N.; Wang, J.M.; Bundred, N. Breast Carcinoma: Vascular Density Determined Using CD105 Antibody Correlates with Tumor Prognosis. Cancer Res. 1999, 59, 856–861. [Google Scholar] [PubMed]
- Fonsatti, E.; Altomonte, M.; Nicotra, M.R.; Natali, P.G.; Maio, M. Endoglin (CD105): A Powerful Therapeutic Target on Tumor-Associated Angiogenetic Blood Vessels. Oncogene 2003, 22, 6557–6563. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Muro, H.; Suzuki, S.; Sakaguchi, T.; Konno, H.; Baba, S.; Syed, A.S. Immunohistochemical Studies on Endothelial Cell Phenotype in Hepatocellular Carcinoma. Hepatology 1997, 26, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Pan, Y.; Chen, J.; Sun, X.; Qiu, Y.; Ding, Y. Endoglin (CD105) Expression in Angiogenesis of Primary Hepatocellular Carcinomas: Analysis Using Tissue Microarrays and Comparisons with CD34 and VEGF. Ann. Clin. Lab. Sci. 2007, 37, 39–48. [Google Scholar]
- Ho, J.W.; Poon, R.T.; Sun, C.K.; Xue, W.-C.; Fan, S.-T. Clinicopathological and Prognostic Implications of Endoglin (CD105) Expression in Hepatocellular Carcinoma and Its Adjacent Non-tumorous Liver. World J. Gastroenterol. 2005, 11, 176–181. [Google Scholar] [CrossRef]
- Benetti, A.; Berenzi, A.; Gambarotti, M.; Garrafa, E.; Gelati, M.; Dessy, E.; Portolani, N.; Piardi, T.; Giulini, S.M.; Caruso, A.; et al. Transforming Growth Factor-β1 and CD105 Promote the Migration of Hepatocellular Carcinoma–Derived Endothelium. Cancer Res. 2008, 68, 8626–8634. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, L.; Chen, X.; Qian, H.; Zhang, S.; Chen, Y.; Luo, R.; Shao, J.; Liu, H.; Chen, J. Phenotypic and Functional Characterization of Tumor-Derived Endothelial Cells Isolated from Primary Human Hepatocellular Carcinoma. Hepatol. Res. 2018, 48, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.-H.; Guo, P.; Yan, L.-N.; He, D. Tumor Microvascular Density Detected by Anti-CD105 and Anti-CD34 in Hepatocellular Carcinoma Patients and Its Predictive Value of Tumor Recurrence after Liver Transplantation. Sichuan Da Xue Xue Bao. Yi Xue Ban/J. Sichuan Univ. Med Sci. Ed. 2010, 41, 818–821. [Google Scholar]
- Xiong, Y.-Q.; Sun, H.-C.; Zhang, W.; Zhu, X.-D.; Zhuang, P.-Y.; Zhang, J.-B.; Wang, L.; Wu, W.-Z.; Qin, L.-X.; Tang, Z.-Y. Human Hepatocellular Carcinoma Tumor–derived Endothelial Cells Manifest Increased Angiogenesis Capability and Drug Resistance Compared with Normal Endothelial Cells. Clin. Cancer Res. 2009, 15, 4838–4846. [Google Scholar] [CrossRef]
- Sánchez-Elsner, T.; Botella, L.M.; Velasco, B.; Langa, C.; Bernabéu, C. Endoglin Expression Is Regulated by Transcriptional Cooperation between the Hypoxia and Transforming Growth Factor-β Pathways. J. Biol. Chem. 2002, 277, 43799–43808. [Google Scholar] [CrossRef]
- Li, C.; Guo, B.; Ding, S.; Rius, C.; Langa, C.; Kumar, P.; Bernabeu, C.; Kumar, S. TNF Alpha Down-Regulates CD105 Expression in Vascular Endothelial Cells: A Comparative Study with TGF beta 1. Anticancer Res. 2003, 23, 1189–1196. [Google Scholar]
- Tian, F.; Zhou, A.-X.; Smits, A.M.; Larsson, E.; Goumans, M.-J.; Heldin, C.-H.; Borén, J.; Akyürek, L.M. Endothelial Cells Are Activated during Hypoxia via Endoglin/ALK-1/SMAD1/5 Signaling in Vivo and in Vitro. Biochem. Biophys. Res. Commun. 2010, 392, 283–288. [Google Scholar] [CrossRef]
- Wikström, P.; Lissbrant, I.F.; Stattin, P.; Egevad, L.; Bergh, A. Endoglin (CD105) is Expressed on Immature Blood Vessels and Is a Marker for Survival in Prostate Cancer. Prostate 2002, 51, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Darcy, K.M.; Birrer, M.J. Translational Research in the Gynecologic Oncology Group: Evaluation of Ovarian Cancer Markers, Profiles, and Novel Therapies. Gynecol. Oncol. 2010, 117, 429–439. [Google Scholar] [CrossRef]
- Tanaka, F.; Otake, Y.; Yanagihara, K.; Kawano, Y.; Miyahara, R.; Li, M.; Yamada, T.; Hanaoka, N.; Inui, K.; Wada, H. Evaluation of Angiogenesis in Non-small Cell Lung Cancer: Comparison between Anti-CD34 Antibody and Anti-CD105 Antibody. Clin. Cancer Res. 2001, 7, 3410–3415. [Google Scholar]
- Margaritescu, C.; Simionescu, C.; Mogoanta, L.; Badea, P.; Pirici, D.; Stepan, A.; Ciurea, R. Endoglin (CD105) and Microvessel Density in Oral Squamous Cell Carcinoma. Rom. J. Morphol. Embryol. 2008, 49, 321–326. [Google Scholar]
- Henry-Berger, J.; Mouzat, K.; Baron, S.; Bernabeu, C.; Marceau, G.; Saru, J.-P.; Sapin, V.; Lobaccaro, J.-M.A.; Caira, F. Endoglin (CD105) Expression Is Regulated by the Liver X Receptor Alpha (NR1H3) in Human Trophoblast Cell Line JAR1. Biol. Reprod. 2008, 78, 968–975. [Google Scholar] [CrossRef]
- Yagmur, E.; Rizk, M.; Stanzel, S.; Hellerbrand, C.; Lammert, F.; Trautwein, C.; Wasmuth, H.E.; Gressner, A.M. Elevation of Endoglin (CD105) Concentrations in Serum of Patients with Liver Cirrhosis and Carcinoma. Eur. J. Gastroenterol. Hepatol. 2007, 19, 755–761. [Google Scholar] [CrossRef]
- Hawinkels, L.J.; Kuiper, P.; Wiercinska, E.; Verspaget, H.W.; Liu, Z.; Pardali, E.; Sier, C.F.; Dijke, P.T. Matrix Metalloproteinase-14 (MT1-MMP)–Mediated Endoglin Shedding Inhibits Tumor Angiogenesis. Cancer Res. 2010, 70, 4141–4150. [Google Scholar] [CrossRef]
- Gallardo-Vara, E.; Blanco, F.J.; Roqué, M.; Friedman, S.L.; Suzuki, T.; Botella, L.M.; Bernabeu, C. Transcription Factor KLF6 Upregulates Expression of Metalloprotease MMP14 and Subsequent Release of Soluble Endoglin during Vascular Injury. Angiogenesis 2016, 19, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gómez, E.; Del Castillo, G.; Santibáñez, J.F.; Lêpez-Novoa, J.M.; Bernabéu, C.; Quintanilla, M. The Role of the TGF-β Coreceptor Endoglin in Cancer. Sci. World J. 2010, 10, 2367–2384. [Google Scholar] [CrossRef]
- Duff, S.E.; Li, C.; Garland, J.M.; Kumar, S. CD105 is Important for Angiogenesis: Evidence and Potential Applications. FASEB J. 2003, 17, 984–992. [Google Scholar] [CrossRef]
- Elnemr, D.M.; Abdel-Azeez, H.A.; Labib, H.A.; Abo-Taleb, F.M. Clinical Relevance of Serum Endoglin Level in Egyptian Hepatocellular Carcinoma Patients. Clin. Lab. 2012, 58, 1023–1028. [Google Scholar] [PubMed]
- Teama, S.; Fawzy, A.; Teama, S.; Helal, A.; Drwish, A.D.; Elbaz, T.; Desouky, E. Increased Serum Endoglin and Transforming Growth Factor β1 mRNA Expression and Risk of Hepatocellular Carcinoma in Cirrhotic Egyptian Patients. Asian Pac. J. Cancer Prev. 2016, 17, 2429–2434. [Google Scholar]
- Li, Y.; Zhai, Z.; Liu, D.; Zhong, X.; Meng, X.; Yang, Q.; Liu, J.; Li, H.-Y. CD105 Promotes Hepatocarcinoma Cell Invasion and Metastasis through VEGF. Tumor Biol. 2014, 36, 737–745. [Google Scholar] [CrossRef]
- Kwon, Y.-C.; Bose, S.K.; Steele, R.; Meyer, K.; Di Bisceglie, A.M.; Ray, R.B.; Ray, R. Promotion of Cancer Stem-Like Cell Properties in Hepatitis C Virus-Infected Hepatocytes. J. Virol. 2015, 89, 11549–11556. [Google Scholar] [CrossRef]
- Mardomi, A.; Sabzichi, M.; Somi, M.H.; Shanehbandi, D.; Rahbarghazi, R.; Sanjarani, O.T.; Samadi, N. Trafficking Mechanism of Bone Marrow-Derived Mesenchymal Stem Cells toward Hepatocellular Carcinoma HepG2 Cells by Modulating Endoglin, CXCR4 and TGF-β. Cell. Mol. Boil. 2016, 62, 81–86. [Google Scholar]
- Yu, D.; Zhuang, L.; Sun, X.; Chen, J.; Yao, Y.; Meng, K.; Ding, Y. Particular Distribution and Expression Pattern of Endoglin (CD105) in the Liver of Patients with Hepatocellular Carcinoma. BMC Cancer 2007, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- About, F.; Bibert, S.; Jouanguy, E.; Nalpas, B.; Lorenzo, L.; Rattina, V.; Zarhrate, M.; Hanein, S.; Munteanu, M.; Müllhaupt, B.; et al. Identification of an Endoglin Variant Associated With HCV-Related Liver Fibrosis Progression by Next-Generation Sequencing. Front. Genet. 2019, 10, 1024. [Google Scholar] [CrossRef]
- Meurer, S.; Wimmer, A.E.; Van De Leur, E.; Weiskirchen, R. Leur Endoglin Trafficking/Exosomal Targeting in Liver Cells Depends on N-Glycosylation. Cells 2019, 8, 997. [Google Scholar] [CrossRef]
- Gerrits, T.; Zandbergen, M.; Wolterbeek, R.; Bruijn, J.A.; Baelde, H.J.; Scharpfenecker, M. Endoglin Promotes Myofibroblast Differentiation and Extracellular Matrix Production in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 7713. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, C.; Song, Q.; Zhu, M.; Xu, Y.; Xiao, M.; Zheng, W. Identifying Cancer-Associated Fibroblasts as Emerging Targets for Hepatocellular Carcinoma. Cell Biosci. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, M.; Zhao, R.; Huang, Y.; Liu, F.; Li, B.; Qin, Y. CAF-Induced Placental Growth Factor Facilitates Neoangiogenesis in Hepatocellular Carcinoma. Acta Biochim. Biophys. Sin. 2019, 52, 18–25. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, Q.; Guo, L.; Han, C. The Prognostic Correlation between CD105 Expression Level in Tumor Tissue and Peripheral Blood and Sunitinib Administration in Advanced Hepatocellular Carcinoma. Cancer Biol. Ther. 2018, 19, 1006–1014. [Google Scholar] [CrossRef]
- Ribeiro, O.D.; Canedo, N.H.S.; Pannain, V.L. Immunohistochemical Angiogenic Biomarkers in Hepatocellular Carcinoma and Cirrhosis: Correlation with Pathological Features. Clinical 2016, 71, 639–643. [Google Scholar] [CrossRef]
- Zhong, L.; Zou, H.; Huang, Y.; Gong, W.; He, J.; Tan, J.; Lai, Z.; Li, Y.; Zhou, C.; Zhang, G.; et al. Magnetic Endoglin Aptamer Nanoprobe for Targeted Diagnosis of Solid Tumor. J. Biomed. Nanotechnol. 2019, 15, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.; Ulahannan, S.V.; Cao, L.; Rahma, O.E.; Makarova-Rusher, O.V.; Kleiner, D.E.; Fioravanti, S.; Walker, M.D.; Carey, S.; Yu, Y.; et al. A Phase II Study of TRC105 in Patients with Hepatocellular Carcinoma Who Have Progressed on Sorafenib. United Eur. Gastroenterol. J. 2015, 3, 453–461. [Google Scholar] [CrossRef]
- Karmani, L.; Bouchat, V.; Bouzin, C.; Leveque, P.; LaBar, D.; Bol, A.; Deumer, G.; Marega, R.; Bonifazi, D.; Haufroid, V.; et al. 89Zr-Labeled Anti-endoglin Antibody-Targeted Gold Nanoparticles for Imaging Cancer: Implications for Future Cancer Therapy. Nanomedicine 2014, 9, 1923–1937. [Google Scholar] [CrossRef]
- Duan, C.-L.; Hou, G.-H.; Liu, Y.-P.; Liang, T.; Song, J.; Han, J.-K.; Zhang, C. Tumor Vascular Homing Endgolin-Targeted Radioimmunotherapy in Hepatocellular Carcinoma. Tumor Biol. 2014, 35, 12205–12215. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Paauwe, M.; Nixon, A.B.; Hawinkels, L.J. Endoglin Targeting: Lessons Learned and Questions That Remain. Int. J. Mol. Sci. 2020, 22, 147. [Google Scholar] [CrossRef]
- Schoonderwoerd, M.J.; Koops, M.F.; Angela, R.A.; Koolmoes, B.; Toitou, M.; Paauwe, M.; Barnhoorn, M.C.; Liu, Y.; Sier, C.F.; Hardwick, J.C.; et al. Targeting Endoglin-Expressing Regulatory T Cells in the Tumor Microenvironment Enhances the Effect of PD1 Checkpoint Inhibitor Immunotherapy. Clin. Cancer Res. 2020, 26, 3831–3842. [Google Scholar] [CrossRef]

| Trial Description | Condition or Disease | FDA Approval Status | NCT# |
|---|---|---|---|
| Sorafenib and | Hepatoma | Phase 1/2 | NCT01306058 |
| TRC105 in | Liver neoplasms | ||
| hepatocellular | Adenoma, liver | ||
| cancer | hepatocellular carcinoma | ||
| Liver neoplasms, experimental | |||
| TRC105 for | Hepatocellular carcinoma | Phase 2 | NCT01375569 |
| liver cancer | Hepatocellular cancer | ||
| that has not responded to sorafenib | Carcinoma, hepatocellular | ||
| Trial of TRC105 and sorafenib in patients with HCC | Hepatocellular carcinoma | Phase 1/2 | NCT02560779 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeng, K.-S.; Sheen, I.-S.; Lin, S.-S.; Leu, C.-M.; Chang, C.-F. The Role of Endoglin in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 3208. https://doi.org/10.3390/ijms22063208
Jeng K-S, Sheen I-S, Lin S-S, Leu C-M, Chang C-F. The Role of Endoglin in Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2021; 22(6):3208. https://doi.org/10.3390/ijms22063208
Chicago/Turabian StyleJeng, Kuo-Shyang, I-Shyan Sheen, Shu-Sheng Lin, Chuen-Miin Leu, and Chiung-Fang Chang. 2021. "The Role of Endoglin in Hepatocellular Carcinoma" International Journal of Molecular Sciences 22, no. 6: 3208. https://doi.org/10.3390/ijms22063208
APA StyleJeng, K.-S., Sheen, I.-S., Lin, S.-S., Leu, C.-M., & Chang, C.-F. (2021). The Role of Endoglin in Hepatocellular Carcinoma. International Journal of Molecular Sciences, 22(6), 3208. https://doi.org/10.3390/ijms22063208






