Functional Expression of TRPV1 Ion Channel in the Canine Peripheral Blood Mononuclear Cells
Abstract
1. Introduction
2. Results
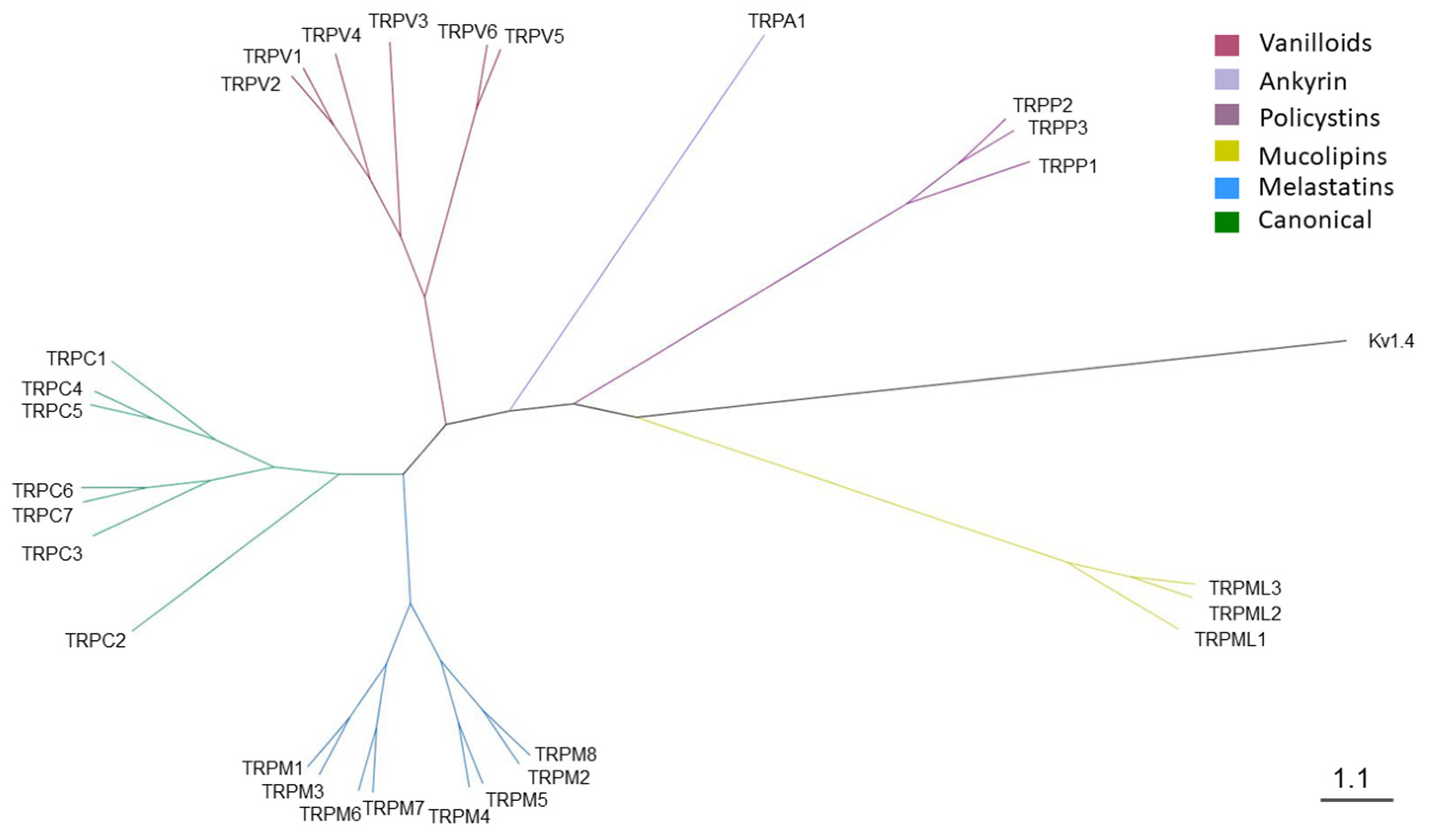
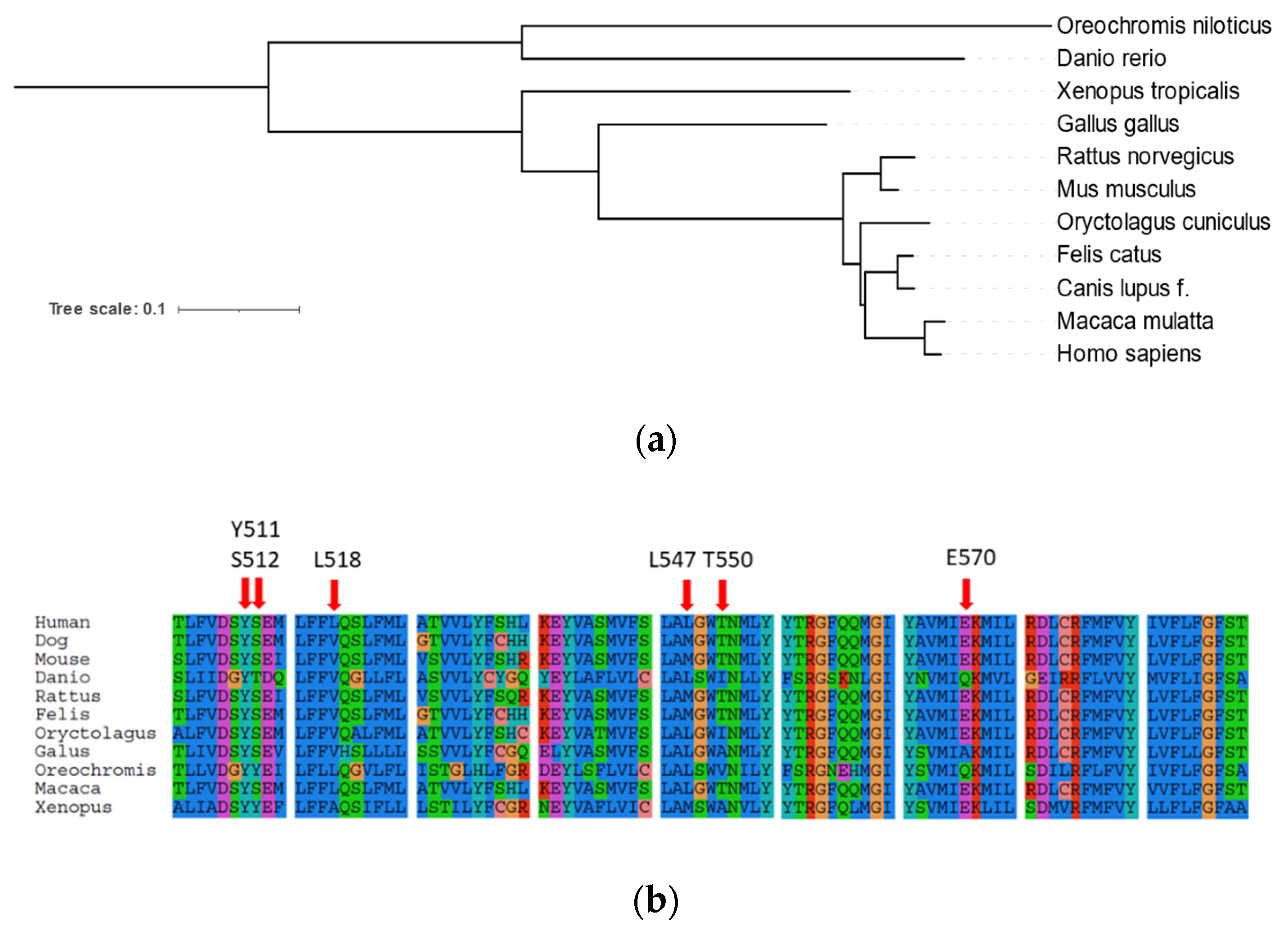
2.1. Phylogenetic Analysis of Canine TRP Ion Channels Family and TRPV1 Orthologs
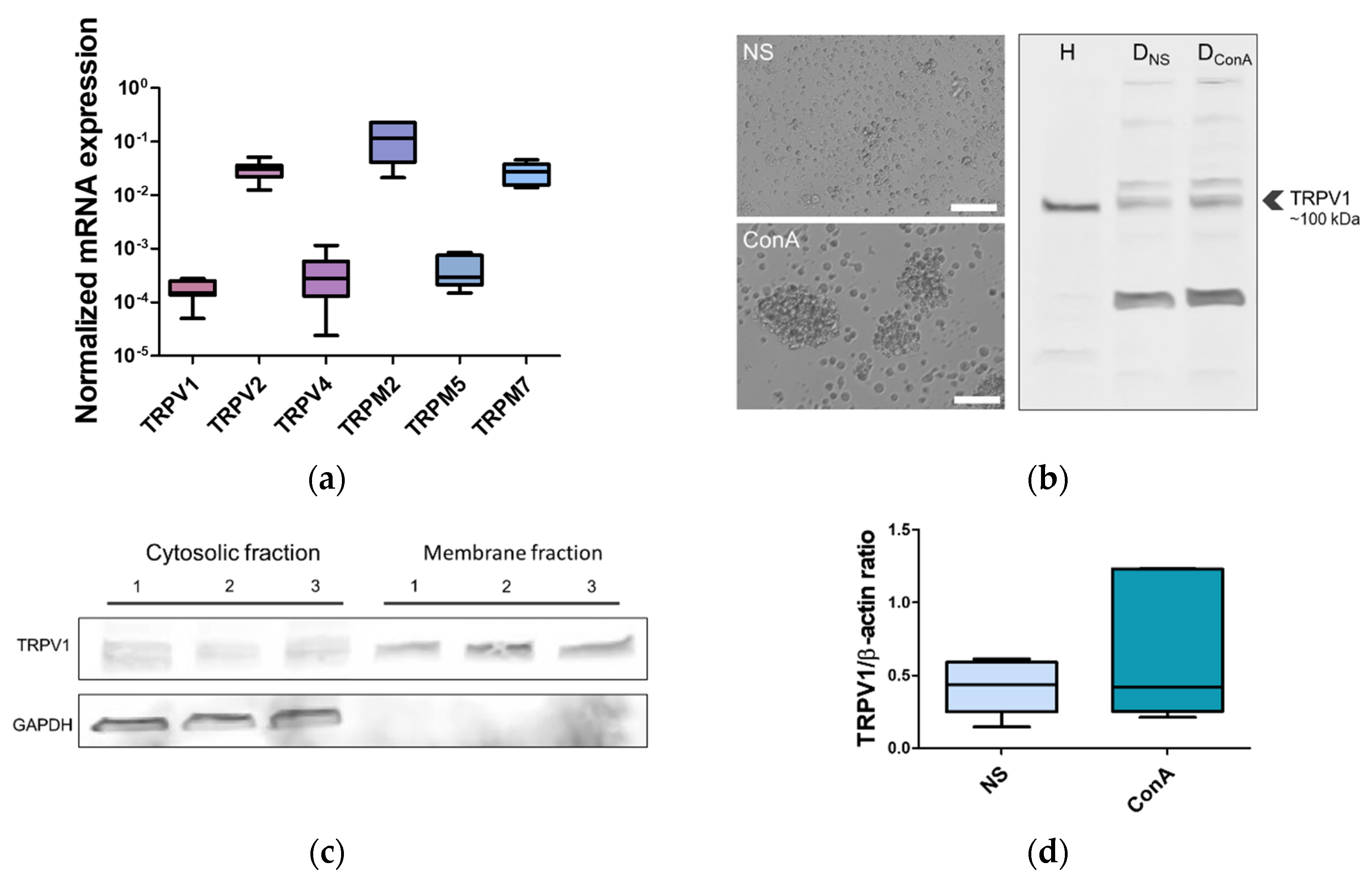
2.2. TRPV1 Expression in the Canine PBMC
2.3. Patch-Clamp Recording of Canine PBMC in Response to Capsaicin Treatment
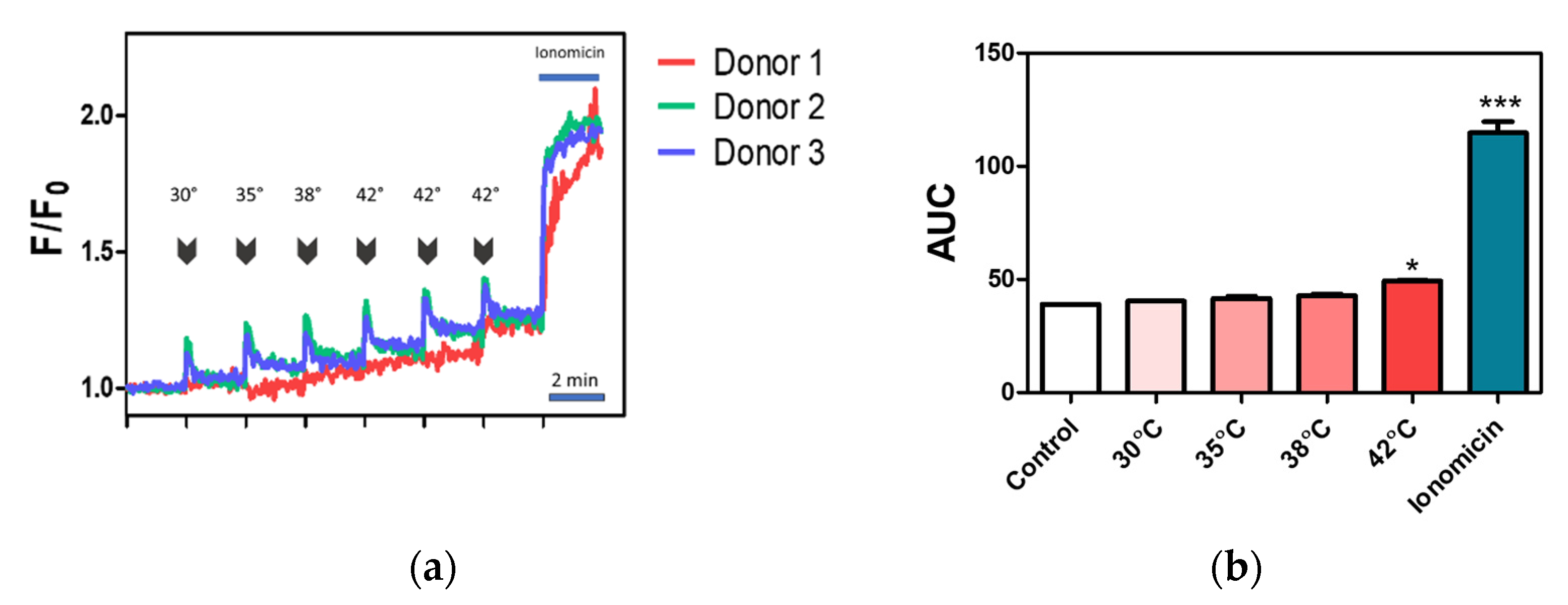
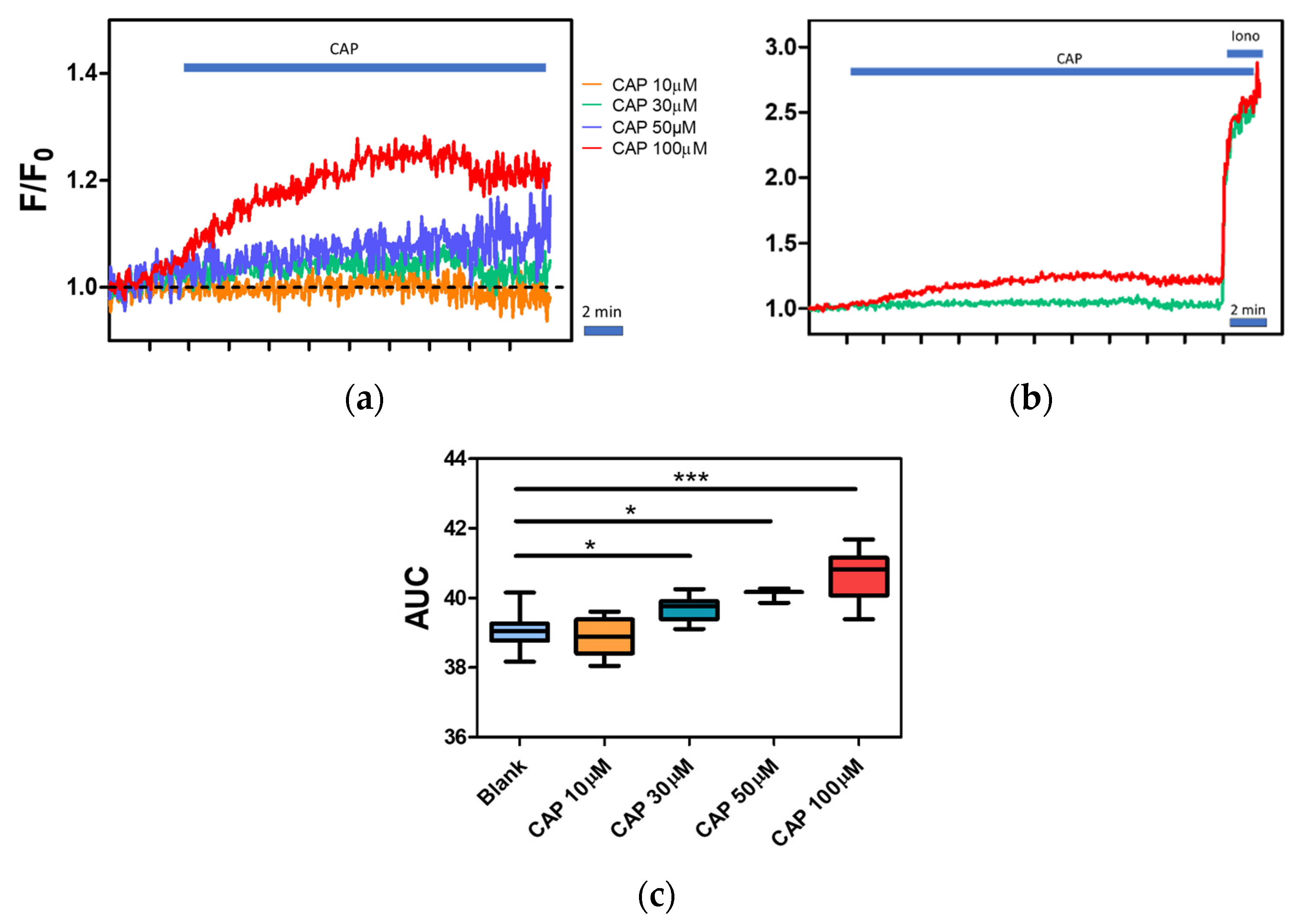
2.4. Effect of Temperature on Calcium Influx in Canine PBMC
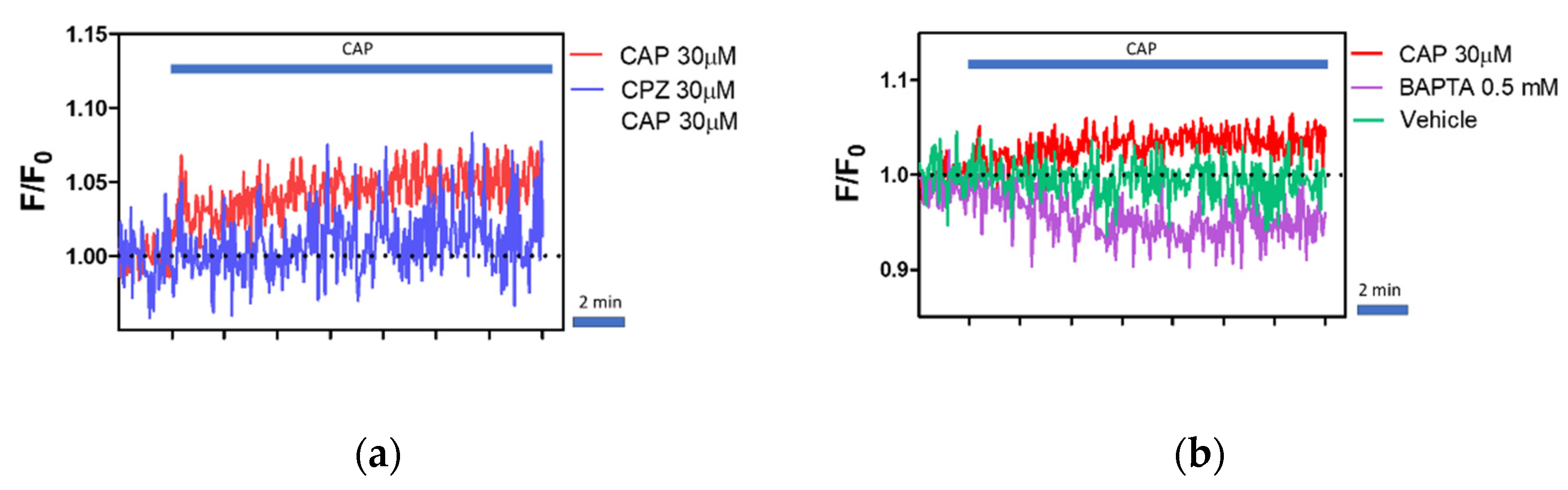
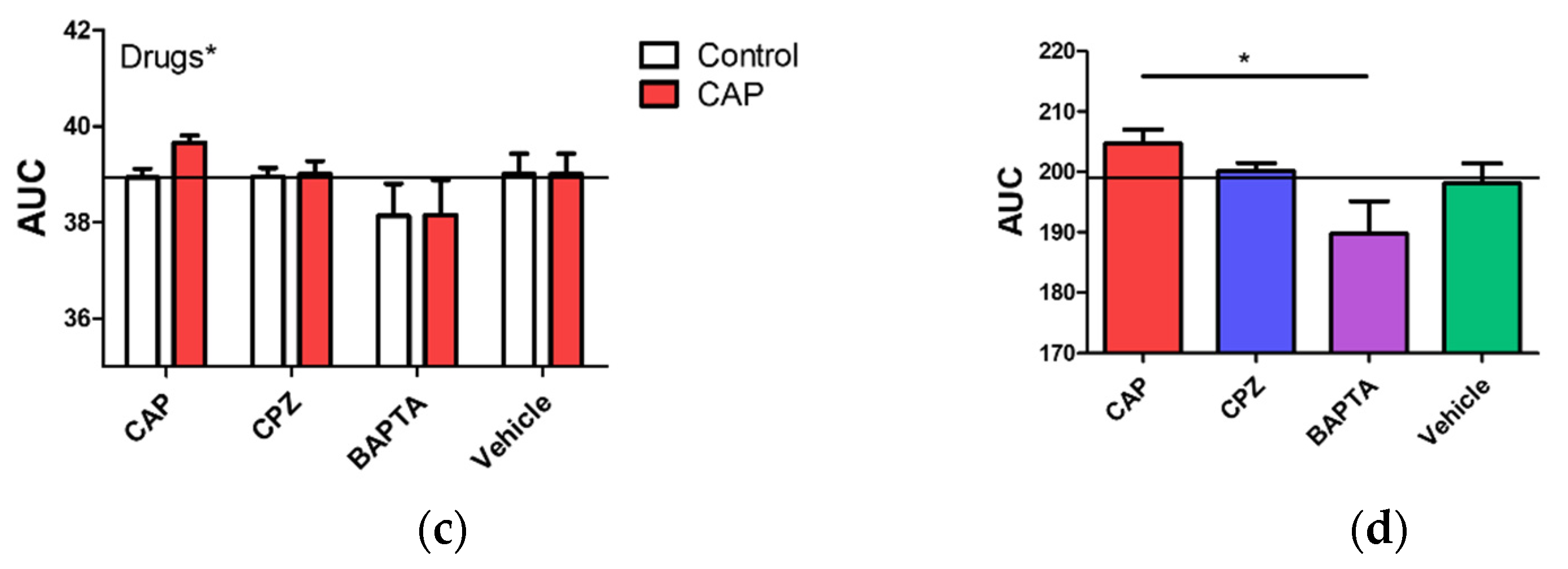
2.5. Regulation of Intracellular Ca2+ Level by Capsaicin, Capsazepine and BAPTA
3. Discussion
4. Materials and Methods
4.1. Phylogenetic Analysis
4.2. PBMC Isolation and Cell Culture
4.3. RT-qPCR
4.4. Western Blot
4.5. Patch-Clamp
4.6. Calcium Assay
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becchetti, A.; Munaron, L.; Arcangeli, A. The Role of Ion Channels and Transporters in Cell Proliferation and Cancer. Front. Physiol. 2013, 4, 312. [Google Scholar] [CrossRef]
- Kim, J.B. Channelopathies. Korean J. Pediatr 2014, 57, 1–18. [Google Scholar] [CrossRef]
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A Comprehensive Map of Molecular Drug Targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef]
- Hutchings, C.J.; Colussi, P.; Clark, T.G. Ion Channels as Therapeutic Antibody Targets. MAbs 2019, 11, 265–296. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, K.; Xue, S.; Buchmann, P.; Charpin-El-Hamri, G.; Saxena, P.; Hussherr, M.D.; Shao, J.; Ye, H.; Xie, M.; Fussenegger, M. Electrogenetic Cellular Insulin Release for Real-time Glycemic Control in Type 1 Diabetic Mice. Science 2020, 368, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Lee, S.Y.; Ramakrishnan, C.; Deisseroth, K. Structure-guided Transformation of Channelrhodopsin into a Light-activated Chloride Channel. Science 2014, 344, 420–424. [Google Scholar] [CrossRef]
- Stanley, S.A.; Sauer, J.; Kane, R.S.; Dordick, J.S.; Friedman, J.M. Remote Regulation of Glucose Homeostasis in Mice Using Genetically Encoded Nanoparticles. Nat. Med. 2015, 21, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.A.; Kelly, L.; Latcha, K.N.; Schmidt, S.F.; Yu, X.; Nectow, A.R.; Sauer, J.; Dyke, J.P.; Dordick, J.S.; Friedman, J.M. Bidirectional Electromagnetic Control of the Hypothalamus Regulates Feeding and Metabolism. Nature 2016, 531, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yoon, S.; Sun, J.; Huang, Z.; Lee, C.; Allen, M.; Wu, Y.; Chang, Y.J.; Sadelain, M.; Shung, K.K.; et al. Mechanogenetics for the Remote and Noninvasive Control of Cancer Immunotherapy. Proc. Natl. Acad. Sci. USA 2018, 115, 992–997. [Google Scholar] [CrossRef]
- Tam, M.; Gómez, S.; González-Gross, M.; Marcos, A. Possible Roles of Magnesium on the Immune System. Eur. J. Clin. Nutr. 2003, 57, 1193–1197. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc in Human Health: Effect of Zinc on Immune Cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef]
- Feske, S.; Wulff, H.; Skolnik, E.Y. Ion Channels in Innate and Adaptive Immunity. Annu. Rev. Immunol. 2015, 33, 291–353. [Google Scholar] [CrossRef] [PubMed]
- Winslow, M.M.; Crabtree, G.R. Immunology. Decoding Calcium Signaling. Science 2005, 307, 56–57. [Google Scholar] [CrossRef]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A Mutation in Orai1 Causes Immune Deficiency by Abrogating CRAC Channel Function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Fung-Leung, W.P.; Edwards, W.; Liu, Y.; Ngo, K.; Angsana, J.; Castro, G.; Wu, N.; Liu, X.; Swanson, R.V.; Wickenden, A.D. T Cell Subset and Stimulation Strength-Dependent Modulation of T Cell Activation by Kv1.3 Blockers. PLoS ONE 2017, 12, e0170102. [Google Scholar] [CrossRef]
- Cahalan, M.D.; Chandy, K.G. The Functional Network of Ion Channels in T Lymphocytes. Immunol. Rev. 2009, 231, 59–87. [Google Scholar] [CrossRef] [PubMed]
- Bose, T.; Cieślar-Pobuda, A.; Wiechec, E. Role of Ion Channels in Regulating Ca²⁺ Homeostasis during the Interplay between Immune and Cancer Cells. Cell Death Dis. 2015, 6, e1648. [Google Scholar] [CrossRef] [PubMed]
- Hardie, R.C. Photosensitive TRPs. Handb. Exp. Pharmacol. 2014, 223, 795–826. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Voets, T. TRP Channels: A TR(I)P through a World of Multifunctional Cation Channels. Pflugers Arch. 2005, 451, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The Transient Receptor Potential Family of Ion Channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef]
- Chávez, A.E.; Chiu, C.Q.; Castillo, P.E. TRPV1 Activation by Endogenous Anandamide Triggers Postsynaptic Long-term Depression in Dentate gyrus. Nat. Neurosci. 2010, 13, 1511–1518. [Google Scholar] [CrossRef]
- Boonen, B.; Alpizar, Y.A.; Sanchez, A.; López-Requena, A.; Voets, T.; Talavera, K. Differential Effects of Lipopolysaccharide on Mouse Sensory TRP Channels. Cell Calcium 2018, 73, 72–81. [Google Scholar] [CrossRef]
- Premkumar, L.S. Transient Receptor Potential Channels as Targets for Phytochemicals. ACS Chem. Neurosci. 2014, 5, 1117–1130. [Google Scholar] [CrossRef]
- Nakamura, T.; Miyoshi, N.; Ishii, T.; Nishikawa, M.; Ikushiro, S.; Watanabe, T. Activation of Transient Receptor Potential Ankyrin 1 by Quercetin and Its Analogs. Biosci. Biotechnol. Biochem. 2016, 80, 949–954. [Google Scholar] [CrossRef]
- Parenti, A.; De Logu, F.; Geppetti, P.; Benemei, S. What is the Evidence for the Role of TRP Channels in Inflammatory and Immune Cells? Br. J. Pharmacol. 2016, 173, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Alliger, K.; Weidinger, C.; Yerinde, C.; Wirtz, S.; Becker, C.; Engel, M.A. Functional Role of Transient Receptor Potential Channels in Immune Cells and Epithelia. Front. Immunol. 2018, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Farfariello, V.; Liberati, S.; Morelli, M.B.; Nabissi, M.; Santoni, M.; Amantini, C. The Role of Transient Receptor Potential Vanilloid Type-2 Ion Channels in Innate and Adaptive Immune Responses. Front. Immunol. 2013, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Scheraga, R.G.; Southern, B.D.; Grove, L.M.; Olman, M.A. The Role of TRPV4 in Regulating Innate Immune Cell Function in Lung Inflammation. Front. Immunol. 2020, 11, 1211. [Google Scholar] [CrossRef]
- Michalick, L.; Kuebler, W.M. TRPV4-A Missing Link Between Mechanosensation and Immunity. Front. Immunol. 2020, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Majhi, R.K.; Sahoo, S.S.; Yadav, M.; Pratheek, B.M.; Chattopadhyay, S.; Goswami, C. Functional Expression of TRPV Channels in T Cells and Their Implications in Immune Regulation. FEBS J. 2015, 282, 2661–2681. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.H.; Gimzewski, J.K.; Ghoneum, A.; Katano, H.; Paw U, C.N.; Agrawal, A. Inhibition of TRPV1 Channel Activity in Human CD4⁺ T Cells by Nanodiamond and Nanoplatinum Liquid, DPV576. Nanomaterials 2018, 8, 770. [Google Scholar] [CrossRef]
- Melzer, N.; Hicking, G.; Göbel, K.; Wiendl, H. TRPM2 Cation Channels Modulate T Cell Effector Functions and Contribute to Autoimmune CNS Inflammation. PLoS ONE 2012, 7, e47617. [Google Scholar] [CrossRef]
- Beceiro, S.; Radin, J.N.; Chatuvedi, R.; Piazuelo, M.B.; Horvarth, D.J.; Cortado, H.; Gu, Y.; Dixon, B.; Gu, C.; Lange, I.; et al. TRPM2 Ion Channels Regulate Macrophage Polarization and Gastric Inflammation during Helicobacter pylori Infection. Mucosal. Immunol. 2017, 10, 493–507. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Okumura, R.; Ono, C.; Okuzaki, D.; Kawai, T.; Okochi, Y.; Tanimura, N.; Murakami, M.; Kayama, H.; Umemoto, E.; et al. TRPM5 Negatively Regulates Calcium-Dependent Responses in Lipopolysaccharide-Stimulated B Lymphocytes. Cell Rep. 2020, 31, 107755. [Google Scholar] [CrossRef]
- Romagnani, A.; Vettore, V.; Rezzonico-Jost, T.; Hampe, S.; Rottoli, E.; Nadolni, W.; Perotti, M.; Meier, M.A.; Hermanns, C.; Geiger, S.; et al. TRPM7 Kinase Activity is Essential for T Cell Colonization and Alloreactivity in the Gut. Nat. Commun. 2017, 8, 1917. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; David, J. The Capsaicin Receptor: A Heat-activated Ion Channel in the Pain Pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, E.A.; Potatieva, N.N.; Bolshakov, K.V. Voltage-Dependent Interaction of Capsaicine and Protons on TRPV1-Receptors. Acta Nat. 2017, 9, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jara-Oseguera, A.; Chang, T.H.; Bae, C.; Hanson, S.M.; Swartz, K.J. Heat Activation is Intrinsic to the Pore Domain of TRPV1. Proc. Natl. Acad. Sci. USA 2018, 115, E317–E324. [Google Scholar] [CrossRef] [PubMed]
- Bertin, S.; Aoki-Nonaka, Y.; de Jong, P.R.; Nohara, L.L.; Xu, H.; Stanwood, S.R.; Srikanth, S.; Lee, J.; To, K.; Abramson, L.; et al. The Ion Channel TRPV1 Regulates the Activation and Proinflammatory Properties of CD4⁺ T Cells. Nat. Immunol. 2014, 15, 1055–1063. [Google Scholar] [CrossRef]
- Saunders, C.I.; Kunde, D.A.; Crawford, A.; Geraghty, D.P. Expression of Transient Receptor Potential Vanilloid 1 (TRPV1) and 2 (TRPV2) in Human Peripheral Blood. Mol. Immunol. 2007, 44, 1429–1435. [Google Scholar] [CrossRef]
- Inada, H.; Iida, T.; Tominaga, M. Different Expression Patterns of TRP Genes in Murine B and T Lymphocytes. Biochem. Biophys. Res. Commun. 2006, 350, 762–767. [Google Scholar] [CrossRef]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity-Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef]
- Roger, S.; Gillet, L.; Le Guennec, J.Y.; Besson, P. Voltage-gated Sodium Channels and Cancer: Is Excitability Their Primary Role? Front. Pharmacol. 2015, 6, 152. [Google Scholar] [CrossRef]
- Phelps, P.T.; Anthes, J.C.; Correll, C.C. Cloning and Functional Characterization of Dog Transient Receptor Potential Vanilloid Receptor-1 (TRPV1). Eur. J. Pharmacol. 2005, 513, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lucroy, M.D.; Suckow, M.A. Predictive Modeling for Cancer Drug Discovery Using Canine Models. Expert Opin. Drug Discov. 2020, 15, 731–738. [Google Scholar] [CrossRef]
- Bujak, J.K.; Pingwara, R.; Nelson, M.H.; Majchrzak, K. Adoptive Cell Transfer: New Perspective Treatment in Veterinary Oncology. Acta Vet. Scand. 2018, 60, 60. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, N.H.; Fan, T.M.; Schachtschneider, K.M.; Principe, D.R.; Schook, L.B.; Jungersen, G. Of Mice, Dogs, Pigs, and Men: Choosing the Appropriate Model for Immuno-Oncology Research. ILAR J. 2018, 59, 247–262. [Google Scholar] [CrossRef]
- Cha, S.K.; Kim, J.H.; Huang, C.L. Flow-induced Activation of TRPV5 and TRPV6 Channels Stimulates Ca(2+)-activated K(+) Channel Causing Membrane Hyperpolarization. Biochim. Biophys. Acta 2013, 1833, 3046–3053. [Google Scholar] [CrossRef]
- Voets, T. TRP Channels and Thermosensation. Handb. Exp. Pharmacol. 2014, 223, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Cohen, B.E.; Chuang, H.H. A Single TRPV1 Amino Acid Controls Species Sensitivity to Capsaicin. Sci. Rep. 2020, 10, 8038. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, J. Understand Spiciness: Mechanism of TRPV1 Channel Activation by Capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef]
- Gavva, N.R.; Klionsky, L.; Qu, Y.; Shi, L.; Tamir, R.; Edenson, S.; Zhang, T.J.; Viswanadhan, V.N.; Toth, A.; Pearce, L.V.; et al. Molecular Determinants of Vanilloid Sensitivity in TRPV1. J. Biol. Chem. 2004, 279, 20283–20295. [Google Scholar] [CrossRef]
- Montell, C. Physiology, Phylogeny, and Functions of the TRP Superfamily of Cation Channels. Sci. STKE 2001, 2001, re1. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Zaelzer, C.; Brauchi, S. Structure–functional Intimacies of Transient Receptor Potential Channels. Q. Rev. Biophys. 2009, 42, 201–246. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A Capsaicin-receptor Homologue with a High Threshold for Noxious Heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Perálvarez-Marín, A.; Doñate-Macian, P.; Gaudet, R. What Do We Know about the Transient Receptor Potential Vanilloid 2 (TRPV2) Ion Channel? FEBS J. 2013, 280, 5471–5487. [Google Scholar] [CrossRef]
- Zhang, F.; Hanson, S.M.; Jara-Oseguera, A.; Krepkiy, D.; Bae, C.; Pearce, L.V.; Blumberg, P.M.; Newstead, S.; Swartz, K.J. Engineering Vanilloid-sensitivity into the Rat TRPV2 Channel. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.E.; Julius, D. Molecular Basis for Species-specific Sensitivity to “Hot” Chili Peppers. Cell 2002, 108, 421–430. [Google Scholar] [CrossRef]
- Gau, P.; Poon, J.; Ufret-Vincenty, C.; Snelson, C.D.; Gordon, S.E.; Raible, D.W.; Dhaka, A. The Zebrafish Ortholog of TRPV1 is Required for Heat-induced Locomotion. J. Neurosci. 2013, 33, 5249–5260. [Google Scholar] [CrossRef]
- Spinsanti, G.; Zannolli, R.; Panti, C.; Ceccarelli, I.; Marsili, L.; Bachiocco, V.; Frati, F.; Aloisi, A.M. Quantitative Real-Time PCR Detection of TRPV1-4 Gene Expression in Human Leukocytes from Healthy and Hyposensitive Subjects. Mol. Pain 2008, 4, 51. [Google Scholar] [CrossRef]
- Veldhuis, N.A.; Lew, M.J.; Abogadie, F.C.; Poole, D.P.; Jennings, E.A.; Ivanusic, J.J.; Eilers, H.; Bunnett, N.W.; McIntyre, P. N-glycosylation Determines Ionic Permeability and Desensitization of the TRPV1 Capsaicin Receptor. J. Biol. Chem. 2012, 287, 21765–21772. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Eilers, H. TRPV1 Splice Variants: Structure and Function. Front. Biosci. 2010, 15, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.H.; Neelands, T.R.; McDonald, H.A.; Choi, W.; Kroeger, P.E.; Puttfarcken, P.S.; Faltynek, C.R.; Moreland, R.B.; Han, P. TRPV1b Overexpression Negatively Regulates TRPV1 Responsiveness to Capsaicin, Heat and Low pH in HEK293 Cells. J. Neurochem. 2006, 99, 1088–1102. [Google Scholar] [CrossRef] [PubMed]
- Storti, B.; Di Rienzo, C.; Cardarelli, F.; Bizzarri, R.; Beltram, F. Unveiling TRPV1 Spatio-temporal Organization in Live Cell Membranes. PLoS ONE 2015, 10, e0116900. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, R.; Tsang, S.Y. Versatile Roles of Intracellularly Located TRPV1 Channel. J. Cell. Physiol. 2017, 232, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; Córdova, C.; Marchant, I.; Zúñiga, R.; Ochova, P.; Ramírez-Barrantes, R.; González-Arriagada, W.A.; Rodriguez, B.; Olivero, P. Intracellular Aggregated TRPV1 is Associated with Lower Survival in Breast Cancer Patients. Breast Cancer 2018, 10, 161–168. [Google Scholar] [CrossRef]
- Kochukov, M.Y.; McNearney, T.A.; Yin, H.; Zhang, L.; Ma, F.; Ponomareva, L.; Abshire, S.; Westlund, K.N. Tumor Necrosis Factor-alpha (TNF-alpha) Enhances Functional Thermal and Chemical Responses of TRP Cation Channels in Human Synoviocytes. Mol. Pain 2009, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Donaldson, P.J.; Petrova, R.S. Verification and Spatial Mapping of TRPV1 and TRPV4 Expression in the Embryonic and Adult Mouse Lens. Exp. Eye Res. 2019, 186, 107707. [Google Scholar] [CrossRef]
- Liu, Y.J.; Lin, X.X.; Fang, J.Q.; Fang, F. Involvement of MrgprC in Electroacupuncture Analgesia for Attenuating CFA-Induced Thermal Hyperalgesia by Suppressing the TRPV1 Pathway. Evid. Based Complement. Altern. Med. 2018, 2018, 9102107. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Hou, P.; Yan, Z.; Kong, W.; Liu, B.; Li, X.; Yao, J.; Zhang, Y.; Qin, F.; et al. TRPV1 Channels are Functionally Coupled with BK(mSlo1) Channels in Rat Dorsal Root Ganglion (DRG) Neurons. PLoS ONE 2013, 8, e78203. [Google Scholar] [CrossRef]
- Fischer, M.J.; Balasuriya, D.; Jeggle, P.; Goetze, T.A.; McNaughton, P.A.; Reeh, P.W.; Edwardson, J.M. Direct Evidence for Functional TRPV1/TRPA1 Heteromers. Pflugers Arch. 2014, 466, 2229–2241. [Google Scholar] [CrossRef]
- Geron, M.; Kumar, R.; Zhou, W.; Faraldo-Gómez, J.D.; Vásquez, V.; Priel, A. TRPV1 Pore Turret Dictates Distinct DkTx and Capsaicin Gating. Proc. Natl. Acad. Sci. USA 2018, 115, E11837–E11846. [Google Scholar] [CrossRef] [PubMed]
- Velisetty, P.; Stein, R.A.; Sierra-Valdez, F.J.; Vásquez, V.; Cordero-Morales, J.F. Expression and Purification of the Pain Receptor TRPV1 for Spectroscopic Analysis. Sci. Rep. 2017, 7, 9861. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.S.; Qin, D.; Kim, K.; McDonald, T.V. Capsaicin Inhibits Jurkat T-cell Activation by Blocking Calcium Entry Current I(CRAC). J. Pharmacol. Exp. Ther. 2001, 299, 238–246. [Google Scholar] [PubMed]
- Lu, G.; Henderson, D.; Liu, L.; Reinhart, P.H.; Simon, S.A. TRPV1b, a Functional Human Vanilloid Receptor Splice Variant. Mol. Pharmacol. 2005, 67, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Wang, M.H.; Hwang, S.W.; Cho, H.; Cho, S.Y.; Kwon, M.J.; Lee, S.Y.; Oh, U. Differences in Sensitivity of Vanilloid Receptor 1 Transfected to Human Embryonic Kidney Cells and Capsaicin-activated Channels in Cultured Rat Dorsal Root Ganglion Neurons to Capsaicin Receptor Agonists. Neurosci. Lett. 2001, 299, 135–139. [Google Scholar] [CrossRef]
- Voolstra, O.; Huber, A. Post-Translational Modifications of TRP Channels. Cells 2014, 3, 258–287. [Google Scholar] [CrossRef] [PubMed]
- Prescott, E.D.; Julius, D. A Modular PIP2 Binding Site as a Determinant of Capsaicin Receptor Sensitivity. Science 2003, 300, 1284–1288. [Google Scholar] [CrossRef]
- Staruschenko, A.; Jeske, N.A.; Akopian, A.N. Contribution of TRPV1-TRPA1 Interaction to the Single Channel Properties of the TRPA1 Channel. J. Biol. Chem. 2010, 285, 15167–15177. [Google Scholar] [CrossRef]
- Shibasaki, K.; Sugio, S.; Takao, K.; Yamanaka, A.; Miyakawa, T.; Tominaga, M.; Ishizaki, Y. TRPV4 Activation at the Physiological Temperature is a Critical Determinant of Neuronal Excitability and Behavior. Pflugers Arch. 2015, 467, 2495–2507. [Google Scholar] [CrossRef]
- Cao, E.; Cordero-Morales, J.F.; Liu, B.; Qin, F.; Julius, D. TRPV1 Channels are Intrinsically Heat Sensitive and Negatively Regulated by Phosphoinositide Lipids. Neuron 2013, 77, 667–679. [Google Scholar] [CrossRef]
- Kashio, M.; Tominaga, M. The TRPM2 Channel: A Thermo-sensitive Metabolic Sensor. Channels 2017, 11, 426–433. [Google Scholar] [CrossRef]
- Xiao, B.; Coste, B.; Mathur, J.; Patapoutian, A. Temperature-dependent STIM1 Activation Induces Ca2+ influx and Modulates Gene Expression. Nat. Chem. Biol. 2011, 7, 351–358. [Google Scholar] [CrossRef]
- Mamatova, K.N.; Kang, T.M. Activation of Rat Transient Receptor Potential Cation Channel Subfamily V Member 1 Channels by 2-aminoethoxydiphenyl Borate. Integr. Med. Res. 2013, 2, 112–123. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Hu, H.Z.; Colton, C.K.; Wood, J.D.; Zhu, M.X. An Alternative Splicing Product of the Murine trpv1 Gene Dominant Negatively Modulates the Activity of TRPV1 Channels. J. Biol. Chem. 2004, 279, 37423–37430. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-P.; Tseng, C.-S.; Sun, S.-P.; Chen, Y.-S.; Tsai, C.-R.; Hsu, M.-F. Capsaicin Stimulates the Non-store-operated Ca2+ Entry but Inhibits the Store-operated Ca2+ Entry in Neutrophils. Toxicol. Appl. Pharmacol. 2005, 209, 134–144. [Google Scholar] [CrossRef]
- Feske, S.; Concepcion, A.R.; Coetzee, W.A. Eye on Ion Channels in Immune Cells. Sci. Signal. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Pecze, L.; Jósvay, K.; Blum, W.; Petrovics, G.; Vizler, C.; Oláh, Z.; Schwaller, B. Activation of Endogenous TRPV1 Fails to Induce Overstimulation-based Cytotoxicity in Breast and Prostate Cancer Cells but not in Pain-sensing Neurons. Biochim. Biophys. Acta 2016, 1863, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yang, F.; Liu, S.; Colton, C.K.; Wang, C.; Cui, Y.; Cao, X.; Zhu, M.X.; Sun, C.; Wang, K.; et al. Heteromeric Heat-sensitive Transient Receptor Potential Channels Exhibit Distinct Temperature and Chemical Response. J. Biol. Chem. 2012, 287, 7279–7288. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.V.; Ann, J.; Blumberg, P.M.; Lee, J. Combination of a Rapidly Penetrating Agonist and a Slowly Penetrating Antagonist Affords Agonist Action of Limited Duration at the Cellular Level. Biomol. Ther. 2019, 27, 435–441. [Google Scholar] [CrossRef]
- Kistner, K.; Siklosi, N.; Babes, A.; Khalil, M.; Selescu, T.; Zimmermann, K.; Wirtz, S.; Becker, C.; Neurath, M.F.; Reeh, P.W.; et al. Systemic Desensitization through TRPA1 Channels by Capsazepine and Mustard Oil—A Novel Strategy against Inflammation and Pain. Sci. Rep. 2016, 6, 28621. [Google Scholar] [CrossRef]
- Lin, Y.J.; Lin, R.L.; Ruan, T.; Khosravi, M.; Lee, L.Y. A Synergistic Effect of Simultaneous TRPA1 and TRPV1 Activations on Vagal Pulmonary C-fiber Afferents. J. Appl. Physiol. 2015, 118, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.S.; Majhi, R.K.; Tiwari, A.; Acharya, T.; Kumar, P.S.; Saha, S.; Kumar, A.; Goswami, C.; Chattopadhyay, S. Transient Receptor Potential Ankyrin1 Channel is Endogenously Expressed in T Cells and is Involved in Immune Functions. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Bertin, S.; Aoki-Nonaka, Y.; Lee, J.; de Jong, P.R.; Kim, P.; Han, T.; Yu, T.; To, K.; Takahashi, N.; Boland, B.S.; et al. The TRPA1 Ion Channel is Expressed in CD4+ T Cells and Restrains T-cell-mediated Colitis through Inhibition of TRPV1. Gut 2017, 66, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wang, Y.; Qu, H. Loading Calcium Fluorescent Probes into Protoplasts to Detect Calcium in the Flesh Tissue Cells of Malus domestica. Hortic Res. 2020, 7, 91. [Google Scholar] [CrossRef]
- Palmer, A.E.; Tsien, R.Y. Measuring Calcium Signaling Using Genetically Targetable Fluorescent Indicators. Nat. Protoc. 2006, 1, 1057–1065. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, Scalable Generation of High-quality Protein Multiple Sequence Alignments Using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian Inference of Phylogenetic Trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Junier, T.; Zdobnov, E.M. The Newick Utilities: High-throughput Phylogenetic Tree Processing in the UNIX Shell. Bioinformatics 2010, 26, 1669–1670. [Google Scholar] [CrossRef]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.fr: New Generation Phylogenetic Services for Non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP-phylogeny inference package (Version 3.2). Cladistics 2002, 5, 164–166. [Google Scholar]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New Capabilities and Interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Brinkhof, B.; Spee, B.; Rothuizen, J.; Penning, L.C. Development and Evaluation of Canine Reference Genes for Accurate Quantification of Gene Expression. Anal. Biochem. 2006, 356, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Target Name | Accession Number | Forward Primer | Reverse Primer | Size (bp) |
|---|---|---|---|---|
| TRPV1 | NM_001003970.1 | AACATGCTCATTGCCCTCAT | GACCTCATCCACCCTGAAAC | 216 |
| TRPV2 | XM_546641.6 | TGAACTGCTCTTCCTGGTCC | GCAAGCCGCGTGTGTAATAG | 143 |
| TRPV4 | NM_001127315.1 | CCTGTATGAGTCCTCCGTGG | CTCTGTGGCTGCTTCTCGAT | 135 |
| TRPM2 | XM_022413206.1 | GGACAACGCCTGGATTGAGA | GTGGTTGGCATACAGTGGGA | 160 |
| TRPM5 | XM_022405530.1 | ATGGCAAGTTTGTGAAGGTGC | GACTTCATGGCAAACGACCG | 136 |
| TRPM7 | XM_535475.6 | TCAGCAACTCGTCAGGTGTTT | AAGCATCCGTTGGACTCTGT | 167 |
| RPS-19 | Xm_533657 | CCTTCCTCAAAAA/GTCTGGG | GTTCTCATCGTAGGGAGCAAG | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujak, J.K.; Kosmala, D.; Majchrzak-Kuligowska, K.; Bednarczyk, P. Functional Expression of TRPV1 Ion Channel in the Canine Peripheral Blood Mononuclear Cells. Int. J. Mol. Sci. 2021, 22, 3177. https://doi.org/10.3390/ijms22063177
Bujak JK, Kosmala D, Majchrzak-Kuligowska K, Bednarczyk P. Functional Expression of TRPV1 Ion Channel in the Canine Peripheral Blood Mononuclear Cells. International Journal of Molecular Sciences. 2021; 22(6):3177. https://doi.org/10.3390/ijms22063177
Chicago/Turabian StyleBujak, Joanna K., Daria Kosmala, Kinga Majchrzak-Kuligowska, and Piotr Bednarczyk. 2021. "Functional Expression of TRPV1 Ion Channel in the Canine Peripheral Blood Mononuclear Cells" International Journal of Molecular Sciences 22, no. 6: 3177. https://doi.org/10.3390/ijms22063177
APA StyleBujak, J. K., Kosmala, D., Majchrzak-Kuligowska, K., & Bednarczyk, P. (2021). Functional Expression of TRPV1 Ion Channel in the Canine Peripheral Blood Mononuclear Cells. International Journal of Molecular Sciences, 22(6), 3177. https://doi.org/10.3390/ijms22063177






