Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection
Abstract
1. Introduction
2. Results
2.1. Extraction of EGCG
2.1.1. EGCG Extract
2.1.2. Various Formulations of EGCG
2.2. In Vitro Cell Analysis
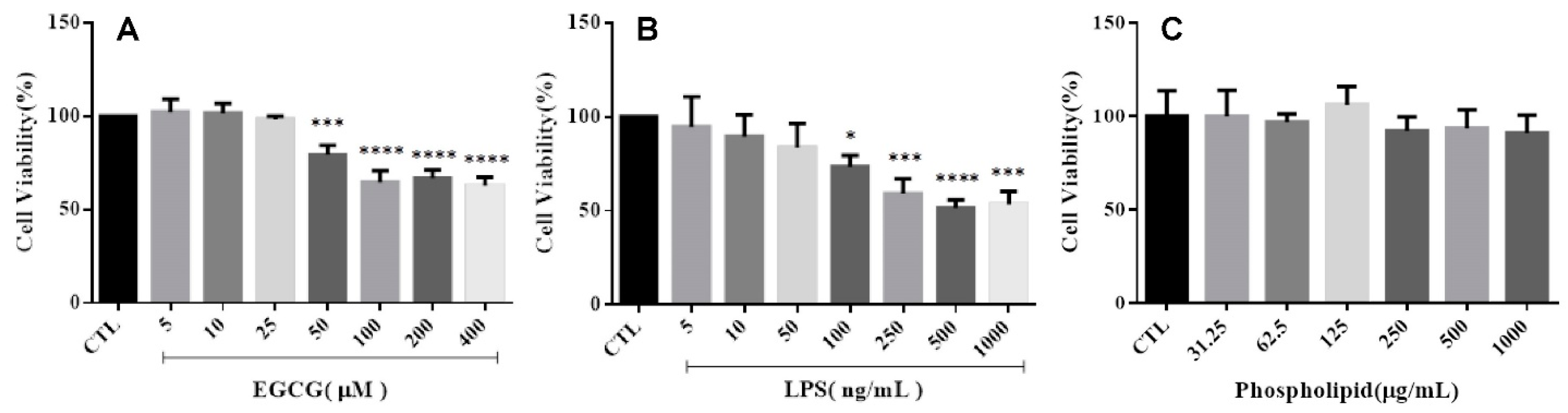
2.2.1. Cell Viability
2.2.2. Cell Morphology
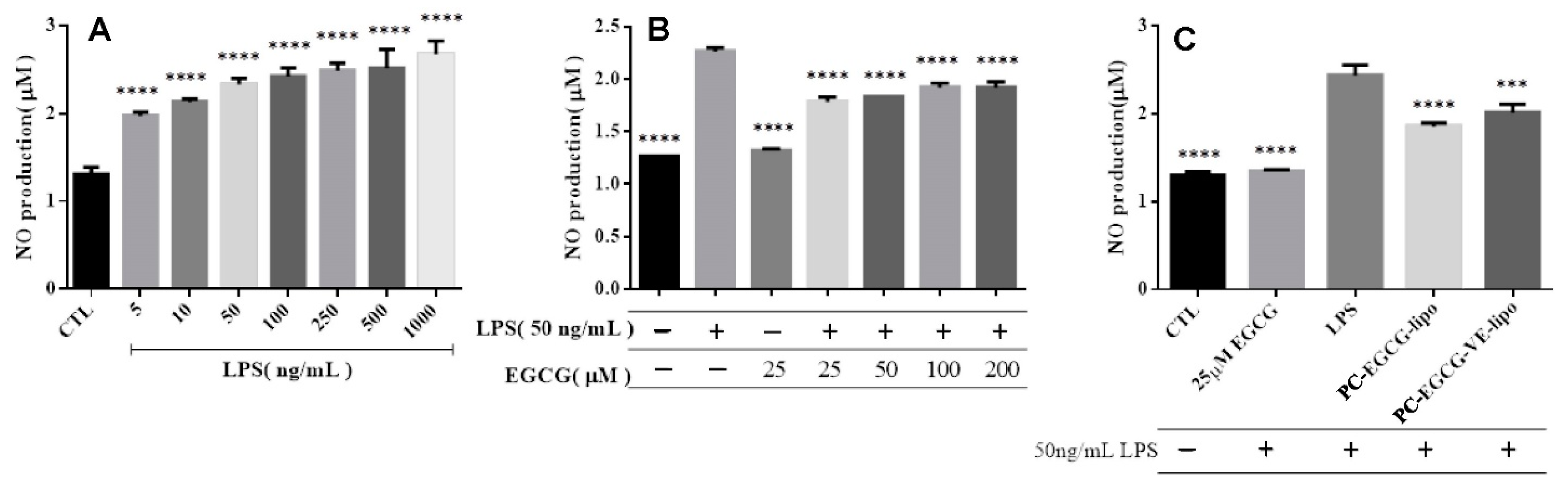
2.2.3. NO Release
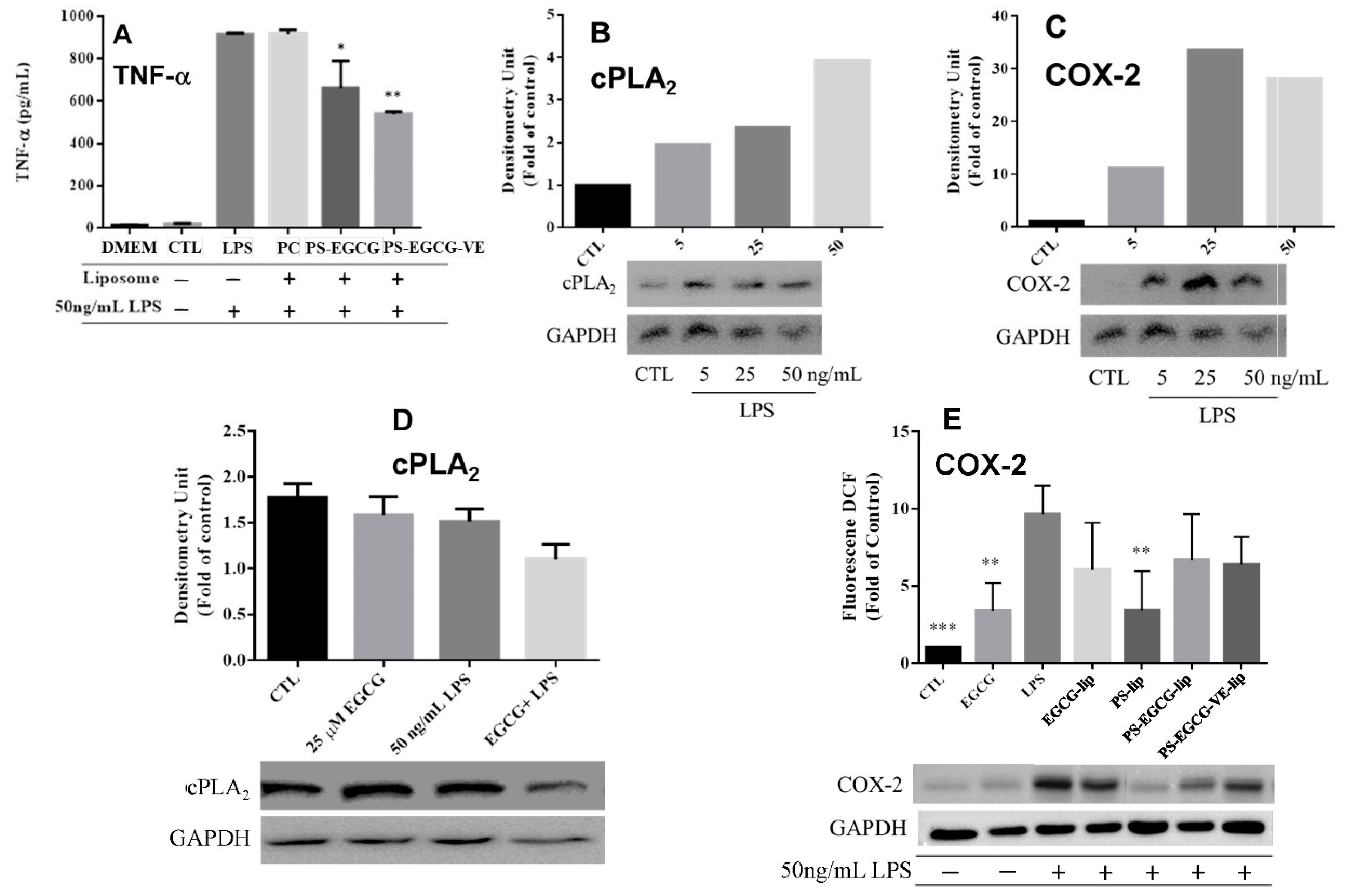
2.2.4. Cytokine Analysis
2.3. In Vivo Animal Test
2.3.1. Animal Behavioral Test
2.3.2. Inflammatory Markers Analysis
3. Discussion
4. Material and Methods
4.1. The Source of Epigallocatechin-3-Gallate
4.1.1. Epigallocatechin-3-Gallate Extraction
4.1.2. Free Radical Scavenging Activity Analysis
4.2. Liposome Preparation
Analysis of Fabricated Liposome
4.3. In Vitro Cell Analysis
4.3.1. Cell Culture
4.3.2. Cell Viability
4.3.3. Cell Morphology
4.3.4. Nitric Oxide Release
4.3.5. Cytokine Analysis
TNF-α Assessment
COX-2 and cPLA2 Analysis by Western Blotting
4.4. Animal Study
4.4.1. Ethics Statement
4.4.2. Rat management
4.4.3. Parkinsonian Syndrome Rat Model
4.4.4. Animal Behavioral Test
4.4.5. Animal Inflammatory Factor Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef]
- Ekdahl, C.; Kokaia, Z.; Lindvall, O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience 2009, 158, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Papageorgiou, I.E.; Fetani, A.F.; Lewen, A.; Heinemann, U.; Kann, O. Widespread activation of microglial cells in the hippocampus of chronic epileptic rats correlates only partially with neurodegeneration. Brain Struct. Funct. 2015, 220, 2423–2439. [Google Scholar] [CrossRef] [PubMed]
- Dutta, G.; Zhang, P.; Liu, B. The lipopolysaccharide Parkinson’s disease animal model: Mechanistic studies and drug discovery. Fundam. Clin. Pharmacol. 2008, 22, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Tsuchiya, S.; Aramaki, Y. Involvement of ERK, a MAP kinase, in the production of TGF-β by macrophages treated with liposomes composed of phosphatidylserine. Biochem. Biophys. Res. Commun. 2004, 324, 1400–1405. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.-G.; Fang, D.; Le, W.-D. (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J. Neurosci. Res. 2004, 78, 723–731. [Google Scholar] [CrossRef]
- Liu, J.; Hong, Z.; Ding, J.; Liu, J.; Zhang, J.; Chen, S. Predominant release of lysosomal enzymes by newborn rat microglia after LPS treatment revealed by proteomic studies. J. Proteome Res. 2008, 7, 2033–2049. [Google Scholar] [CrossRef]
- Cho, H.-S.; Kim, S.; Lee, S.-Y.; Park, J.A.; Kim, S.-J.; Chun, H.S. Protective effect of the green tea component, L-theanine on environmental toxins-induced neuronal cell death. Neurotoxicology 2008, 29, 656–662. [Google Scholar] [CrossRef]
- Mariani, M.M.; Kielian, T. Microglia in infectious diseases of the central nervous system. J. Neuroimmune Pharmacol. 2009, 4, 448–461. [Google Scholar] [CrossRef]
- Santiago, R.M.; Barbieiro, J.; Lima, M.M.; Dombrowski, P.A.; Andreatini, R.; Vital, M.A. Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Bing, G. Lipopolysaccharide animal models for Parkinson’s disease. Parkinson’s Dis. 2011, 2011, 327089. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-C.; Teismann, P.; Tieu, K.; Vila, M.; Jackson-Lewis, V.; Ischiropoulos, H.; Przedborski, S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 6145–6150. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Surh, Y.-J.; Chun, K.-S.; Cha, H.-H.; Han, S.S.; Keum, Y.-S.; Park, K.-K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2001, 480, 243–268. [Google Scholar] [CrossRef]
- Renaud, J.; Nabavi, S.F.; Daglia, M.; Nabavi, S.M.; Martinoli, M.-G. Epigallocatechin-3-gallate, a promising molecule for Parkinson’s disease? Rejuvenation Res. 2015, 18, 257–269. [Google Scholar] [CrossRef]
- Yang, J.E.; Rhoo, K.Y.; Lee, S.; Lee, J.T.; Park, J.H.; Bhak, G.; Paik, S.R. EGCG-mediated Protection of the Membrane Disruption and Cytotoxicity Caused by the ‘Active Oligomer’of α-Synuclein. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Bieschke, J.; Russ, J.; Friedrich, R.P.; Ehrnhoefer, D.E.; Wobst, H.; Neugebauer, K.; Wanker, E.E. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci.USA 2010, 107, 7710–7715. [Google Scholar] [CrossRef]
- Yoshida, W.; Kobayashi, N.; Sasaki, Y.; Ikebukuro, K.; Sode, K. Partial peptide of α-synuclein modified with small-molecule inhibitors specifically inhibits amyloid fibrillation of α-synuclein. Int. J. Mol. Sci. 2013, 14, 2590–2600. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Quan, Z.; Wong, W.; Guo, J.; Zhang, R.; Yang, Q.; Dai, R.; McGeer, P.L.; Qing, H. Epigallocatechin gallate (EGCG) inhibits alpha-synuclein aggregation: A potential agent for Parkinson’s disease. Neurochem. Res. 2016, 41, 2788–2796. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Lu, Z.; Yang, Q.; Liu, L.; Jiang, Z.; Zhang, L.; Zhang, X.; Qing, H. “Cell-addictive” dual-target traceable nanodrug for Parkinson’s disease treatment via flotillins pathway. Theranostics 2018, 8, 5469. [Google Scholar] [CrossRef]
- Xu, Q.; Langley, M.; Kanthasamy, A.G.; Reddy, M.B. Epigallocatechin gallate has a neurorescue effect in a mouse model of Parkinson disease. J. Nutr. 2017, 147, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhu, M.; Liang, Z. (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2018, 17, 4883–4888. [Google Scholar] [CrossRef]
- Park, E.; Chun, H.S. Green tea polyphenol Epigallocatechine gallate (EGCG) prevented LPS-induced BV-2 micoglial cell activation. J. Life Sci. 2016, 26, 640–645. [Google Scholar] [CrossRef][Green Version]
- Liu, J.-B.; Zhou, L.; Wang, Y.-Z.; Wang, X.; Zhou, Y.; Ho, W.-Z.; Li, J.-L. Neuroprotective Activity of ()-Epigallocatechin Gallate against Lipopolysaccharide-Mediated Cytotoxicity. J. Immunol. Res. 2016, 2016, 4962351. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.-J.; Lee, H.-G.; Kook, M.S.; Ko, H.-M.; Jung, J.-Y.; Kim, W.-J. Epigallocatechin-3-gallate rescues LPS-impaired adult hippocampal neurogenesis through suppressing the TLR4-NF-κB signaling pathway in mice. Korean J. Physiol. Pharmacol. 2016, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Pho, Q.-H.; Wu, X.-Y.; Chin, T.-Y.; Chen, C.-M.; Fang, P.-H.; Lin, Y.-C.; Hsieh, M.-F. PLGA microspheres loaded with β-cyclodextrin complexes of epigallocatechin-3-gallate for the anti-inflammatory properties in activated microglial cells. Polymers 2018, 10, 519. [Google Scholar] [CrossRef]
- Buszello, K.; Harnisch, S.; Müller, R.; Müller, B. The influence of alkali fatty acids on the properties and the stability of parenteral O/W emulsions modified with Solutol HS 15®. Eur. J. Pharm. Biopharm. 2000, 49, 143–149. [Google Scholar] [CrossRef]
- Shashi, K.; Satinder, K.; Bharat, P. A complete review on: Liposomes. Int. Res. J. Pharm. 2012, 3, 10–16. [Google Scholar]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, Z.; Zhu, X.; Lin, R.; Chen, L. Salidroside reduces cell mobility via NF-κB and MAPK signaling in LPS-induced BV2 microglial cells. Evid. Based Complementary Altern. Med. 2014, 2014, 383821. [Google Scholar] [CrossRef]
- Tambuyzer, B.R.; Ponsaerts, P.; Nouwen, E.J. Microglia: Gatekeepers of central nervous system immunology. J. Leukoc. Biol. 2009, 85, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Jeong, G.-S. Butein provides neuroprotective and anti-neuroinflammatory effects through Nrf2/ARE-dependent haem oxygenase 1 expression by activating the PI3K/Akt pathway. Br. J. Pharmacol. 2016, 173, 2894–2909. [Google Scholar] [CrossRef]
- Matsuno, R.; Aramaki, Y.; Tsuchiya, S. Involvement of TGF-β in inhibitory effects of negatively charged liposomes on nitric oxide production by macrophages stimulated with LPS. Biochem. Biophys. Res. Commun. 2001, 281, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fujii, S.; Wu, Z.; Hashioka, S.; Tanaka, Y.; Shiratsuchi, A.; Nakanishi, Y.; Nakanishi, H. Involvement of COX-1 and up-regulated prostaglandin E synthases in phosphatidylserine liposome-induced prostaglandin E2 production by microglia. J. Neuroimmunol. 2006, 172, 112–120. [Google Scholar] [CrossRef]
- Ramos, G.C.; Fernandes, D.; Charao, C.T.; Souza, D.G.; Teixeira, M.M.; Assreuy, J. Apoptotic mimicry: Phosphatidylserine liposomes reduce inflammation through activation of peroxisome proliferator-activated receptors (PPARs) in vivo. Br. J. Pharmacol. 2007, 151, 844–850. [Google Scholar] [CrossRef]
- Abe, N.; Nishihara, T.; Yorozuya, T.; Tanaka, J. Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems. Cells 2020, 9, 2132. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Hsieh, M.-F.; Ho, Y.-N.; Huang, C.-M.; Lee, J.-S.; Yang, C.-Y.; Chang, Y. Enhancement of catechin skin permeation via a newly fabricated mPEG-PCL-graft-2-hydroxycellulose membrane. J. Membr. Sci. 2011, 371, 134–140. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Bin Azizi, J.; Ramanathan, S.; Ismail, S.; Sasidharan, S.; Said, M.I.M.; Mansor, S.M. Evaluation of antioxidant and antibacterial activities of aqueous, methanolic and alkaloid extracts from Mitragyna speciosa (Rubiaceae family) leaves. Molecules 2009, 14, 3964–3974. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Molina, A.; Buhlin, K.; Klinge, B. Periodontal diseases and association with atherosclerotic disease. Periodontology 2000 2020, 83, 66–89. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Creese, I.; Burt, D.R.; Snyder, S.H. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science 1977, 197, 596–598. [Google Scholar] [CrossRef] [PubMed]



| Samples | Molar Ratio | Diameter (nm) | Encapsulation Efficiency(%) | PDI | |||
|---|---|---|---|---|---|---|---|
| PC | PS | CH | VE | ||||
| PC-liposome | 0.73 | - | 0.27 | - | 184.4 ± 1.59 | - | 0.218 |
| PS-liposome | 0.24 | 0.49 | 0.27 | - | 117.47 ± 1.12 | - | 0.092 |
| PC-EGCG-liposome | 0.73 | - | 0.27 | - | 155.2 ± 1.23 | 55.4% | 0.121 |
| PS-EGCG-liposome | 0.24 | 0.49 | 0.27 | - | 132.86 ± 2.05 | 70.4% | 0.094 |
| PC-EGCG-VE-liposome | 0.73 | - | 0.27 | 0.07 | 161.5 ± 0.56 | 60.2% | 0.058 |
| PS-EGCG-VE-liposome | 0.24 | 0.49 | 0.27 | 0.07 | 142.9 ± 0.36 | 76.8% | 0.101 |
| Experimental Groups of Rats | LPS Injected 3 μL (5 μg/μL) | EGCG-Loaded Liposomes 2 μL (12.5 μM) | PBS 5 μL | Amount |
|---|---|---|---|---|
| Rats with Parkinson Disease syndrome | Yes | No | Yes | 4 |
| Rats with Parkinson Disease syndrome treated | Yes | Yes | No | 5 |
| Control group | No | No | Yes | 5 |
| Gene | Forward Primers (5′→3′) | Reverse Primers (5′→3′) |
|---|---|---|
| TNF-α | TGA CTC GTG GGA TGA TGA CG | CTG GAG ACT GCC CAT TCT CG |
| IL-1β | CTC ACA CTC AGA TCA TCT TCT C | GGT ATG AAA TGG CAA ATC GG |
| BDNF | GGT CAC AGC GGC AGA TAA AAA G | TTC GGC ATT GCG AGT TCC AG |
| GAPDH | GA AGA GAG AGG CCC TCA G | TGT GAG GGA GAT GCT CAG TG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-Y.; Barro, L.; Tsai, S.-T.; Feng, T.-W.; Wu, X.-Y.; Chao, C.-W.; Yu, R.-S.; Chin, T.-Y.; Hsieh, M.F. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. Int. J. Mol. Sci. 2021, 22, 3037. https://doi.org/10.3390/ijms22063037
Cheng C-Y, Barro L, Tsai S-T, Feng T-W, Wu X-Y, Chao C-W, Yu R-S, Chin T-Y, Hsieh MF. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. International Journal of Molecular Sciences. 2021; 22(6):3037. https://doi.org/10.3390/ijms22063037
Chicago/Turabian StyleCheng, Chun-Yuan, Lassina Barro, Shang-Ting Tsai, Tai-Wei Feng, Xiao-Yu Wu, Che-Wei Chao, Ruei-Siang Yu, Ting-Yu Chin, and Ming Fa Hsieh. 2021. "Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection" International Journal of Molecular Sciences 22, no. 6: 3037. https://doi.org/10.3390/ijms22063037
APA StyleCheng, C.-Y., Barro, L., Tsai, S.-T., Feng, T.-W., Wu, X.-Y., Chao, C.-W., Yu, R.-S., Chin, T.-Y., & Hsieh, M. F. (2021). Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. International Journal of Molecular Sciences, 22(6), 3037. https://doi.org/10.3390/ijms22063037






