Prognostic and Theranostic Applications of Positron Emission Tomography for a Personalized Approach to Metastatic Castration-Resistant Prostate Cancer
Abstract
1. Introduction
2. Potential Role of PET Tracers as Predictive Biomarkers of mCRPC Response to Therapy
2.1. Radiolabeled Choline (11C-Choline, 18F-Methyl-Choline, 18F-Ethyl-Choline)
2.2. 18F-fluorodeoxyglucose (18F-FDG)
2.3. Androgen Receptor (AR)
3. Theranostic PET Tracers
3.1. Bone-Targeting Theranostic Radiopharmaceuticals
3.1.1. 18F-Sodium Fluoride (18F-NaF)
3.1.2. Radiolabeled Zolendronic Acid
3.2. Molecular Theranostic Radiopharmaceuticals
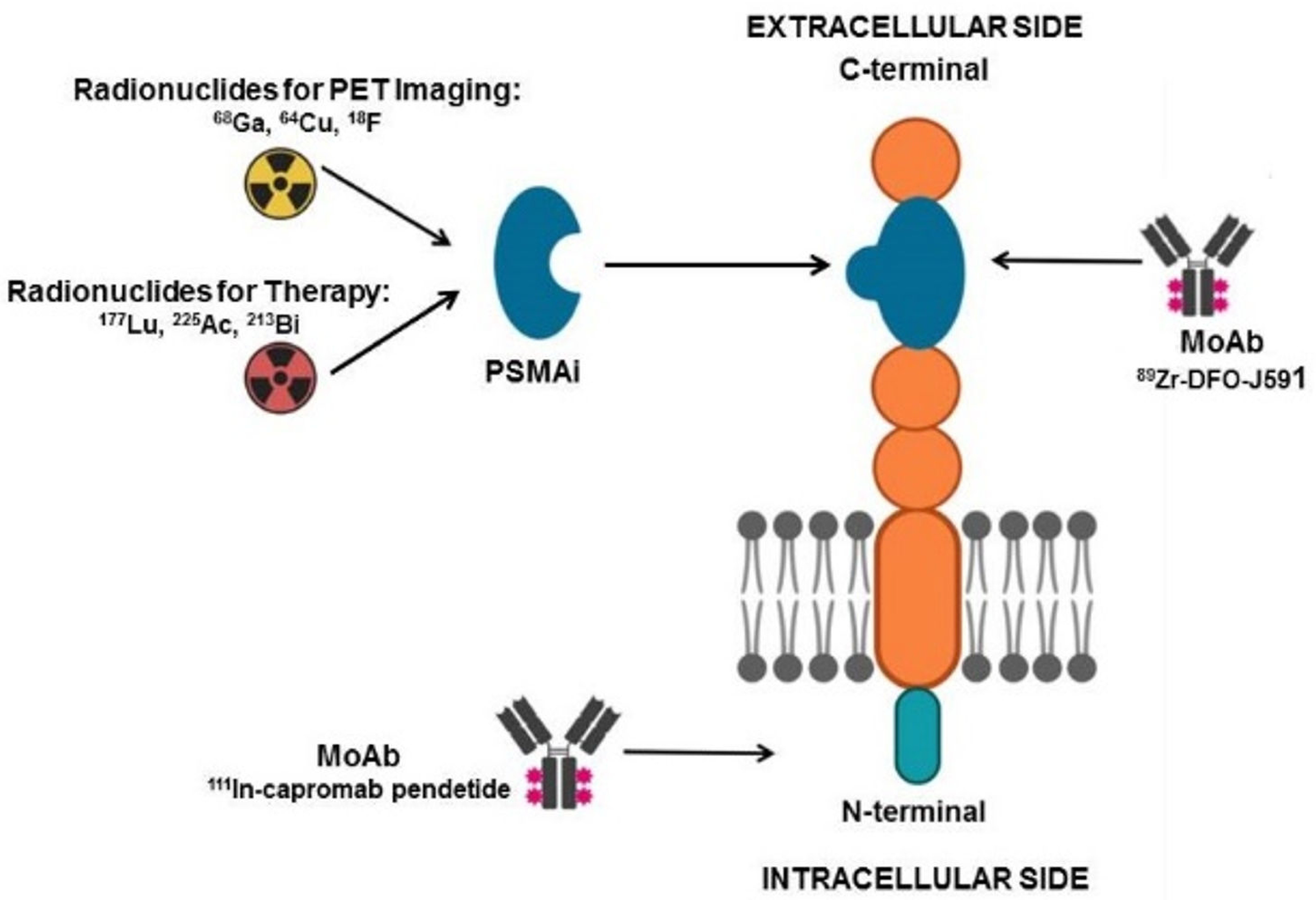
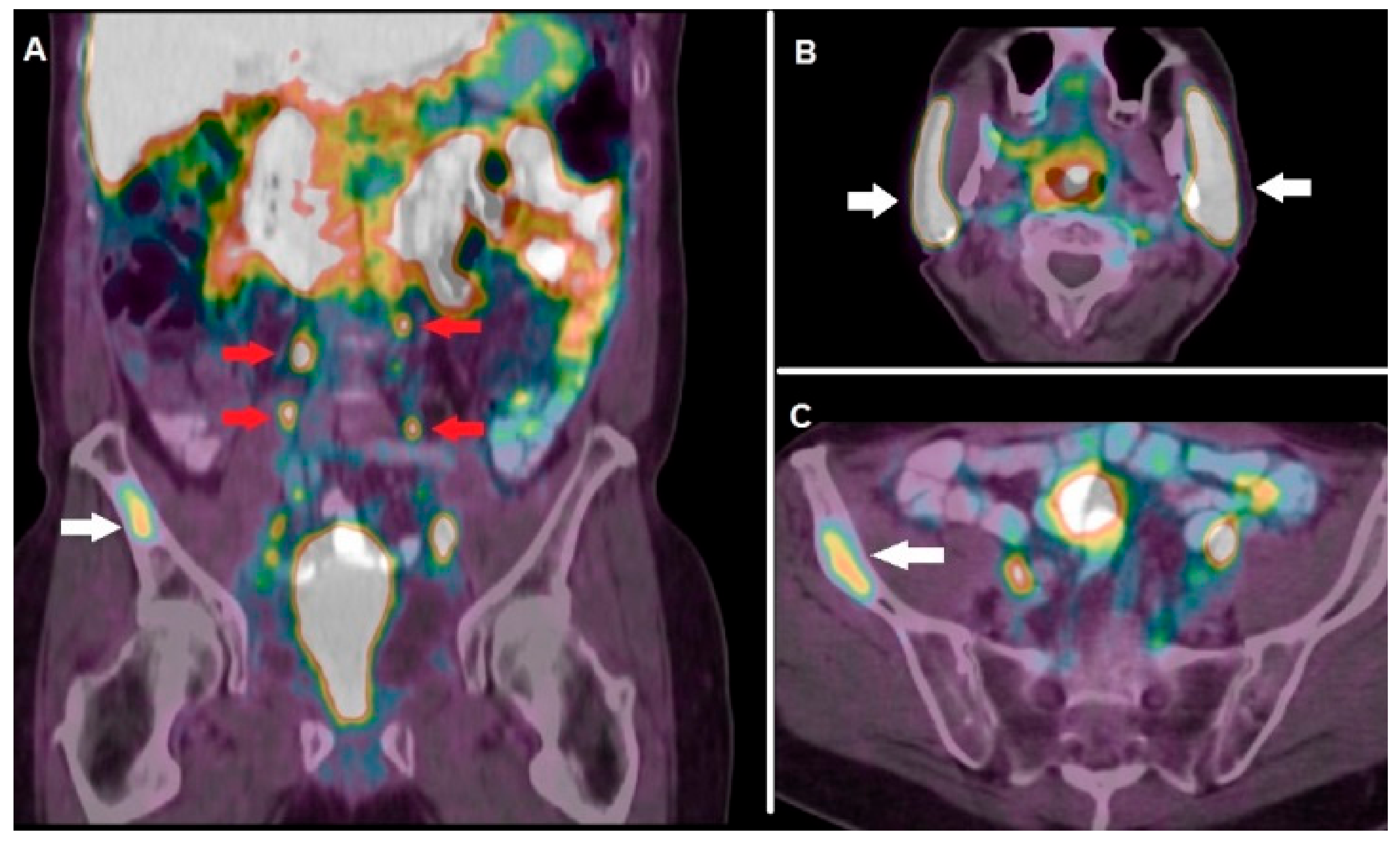
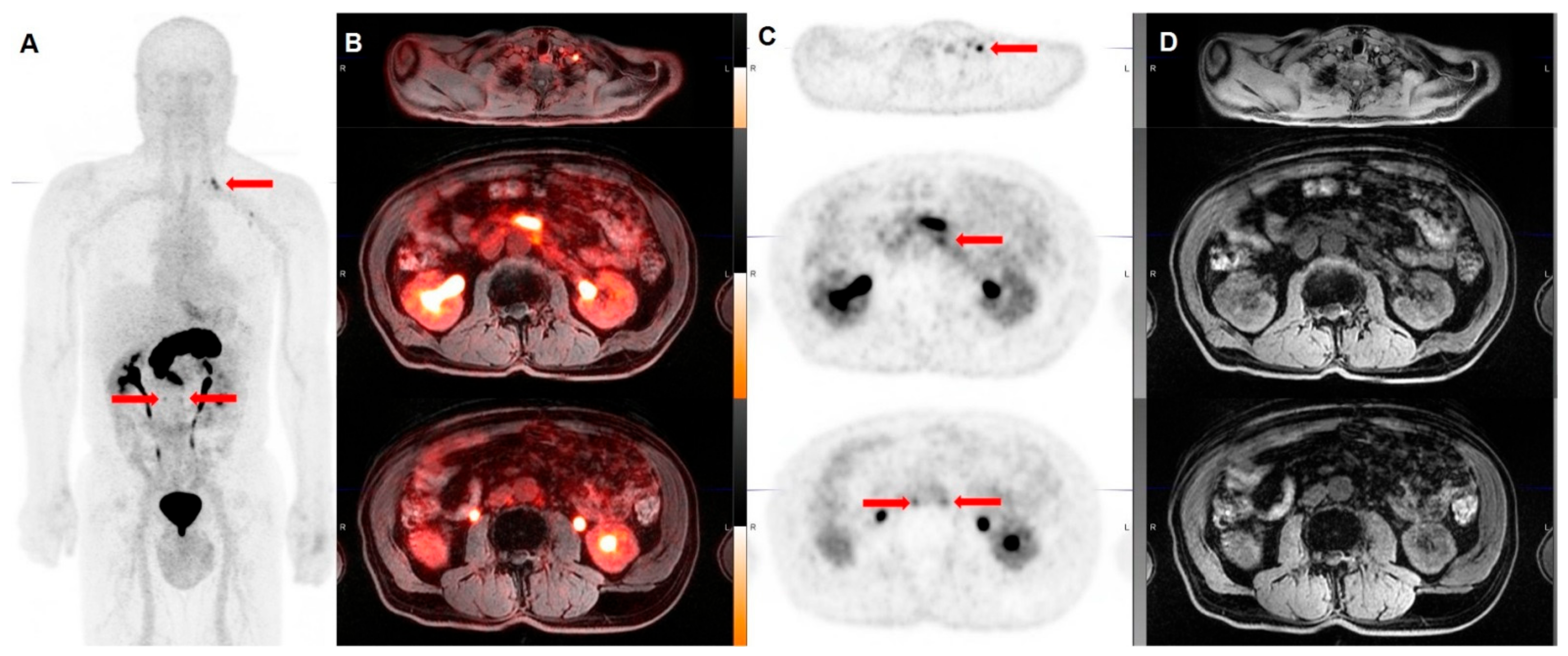
3.2.1. Prostate Specific Membrane Antigen (PSMA)
3.2.2. Poly (ADP-Ribose) Polymerase-1 (PARP-1)
3.2.3. Gastrin Releasing Peptide Receptor (GRPR)
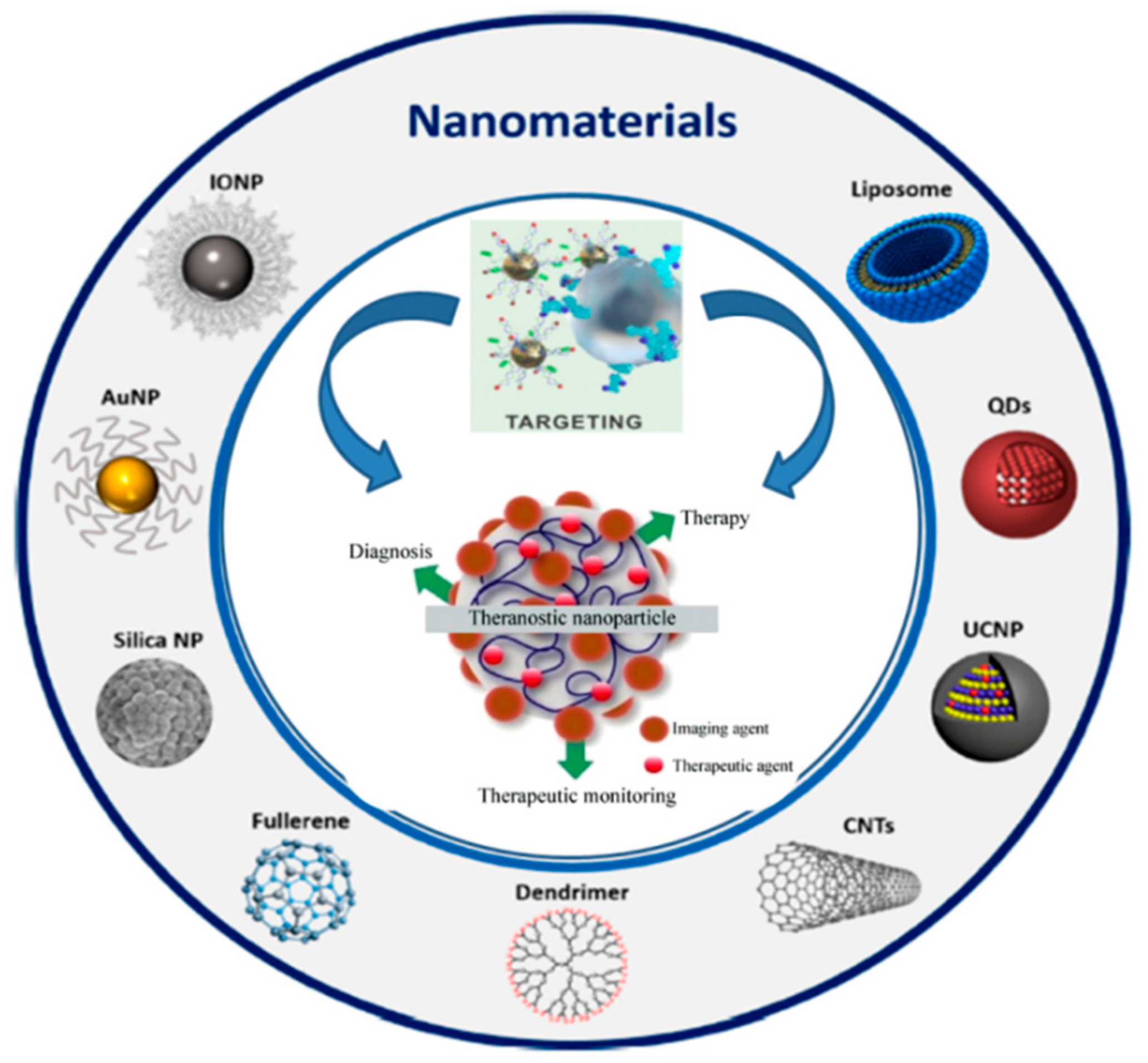
4. Theranostic Applications of Radioconjugated Nanomaterials in Prostate Cancer
5. Future Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N. Androgen deprivation therapy for prostate cancer. JAMA 2005, 294, 238. [Google Scholar] [CrossRef] [PubMed]
- Swami, U.; McFarland, T.R.; Nussenzveig, R.; Agarwal, N. Advanced prostate cancer: Treatment advances and future directions. Trends Cancer 2020, 6, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Corn, P.G.; Agarwal, N.; Araujo, J.C.; Sonpavde, G. Taxane-based combination therapies for metastatic prostate cancer. Eur. Urol. Focus 2019, 5, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-generation antiandrogens: From discovery to standard of care in castration resistant prostate cancer. Front. Oncol. 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Merseburger, A.S.; Haas, G.P.; von Klot, C.-A. An update on enzalutamide in the treatment of prostate cancer. Ther. Adv. Urol. 2015, 7, 9–21. [Google Scholar] [CrossRef]
- Chen, A. PARP inhibitors: Its role in treatment of cancer. Chin. J. Cancer 2011, 30, 463–471. [Google Scholar] [CrossRef]
- Kawalec, P.; Paszulewicz, A.; Holko, P.; Pilc, A. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. A systematic review and meta-analysis. Arch. Med. Sci. 2012, 8, 767–775. [Google Scholar] [CrossRef]
- Sartor, O.; Coleman, R.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014, 15, 738–746. [Google Scholar] [CrossRef]
- Jones, W.; Griffiths, K.; Barata, P.C.; Paller, C.J. PSMA theranostics: Review of the current status of PSMA-targeted imaging and radioligand therapy. Cancers 2020, 12, 1367. [Google Scholar] [CrossRef]
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Cianni, R.; Bagni, O. Theranostic approaches in nuclear medicine: Current status and future prospects. Expert Rev. Med. Devices 2020, 17, 331–343. [Google Scholar] [CrossRef]
- Dhiantravan, N.; Violet, J.; Eapen, R.; Alghazo, O.; Scalzo, M.; Jackson, P.; Keam, S.P.; Mitchell, C.; Neeson, P.J.; Sandhu, S.; et al. Clinical trial protocol for LuTectomy: A Single-arm study of the dosimetry, safety, and potential benefit of 177Lu-PSMA-617 prior to prostatectomy. Eur. Urol. Focus 2020. [Google Scholar] [CrossRef]
- Marcu, L.; Bezak, E.; Allen, B.J. Global comparison of targeted alpha vs targeted beta therapy for cancer: In vitro, in vivo and clinical trials. Crit. Rev. Oncol. Hematol. 2018, 123, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Pavel, M.E.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Scopinaro, F.; Pelle, G.; Cianni, R.; Salvatori, R.; Schillaci, O.; Bagni, O. Molecular response assessed by (68)Ga-DOTANOC and survival after (90)Y microsphere therapy in patients with liver metastases from neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 432–440. [Google Scholar] [CrossRef]
- Murray, I.; Chittenden, S.J.; Denis-Bacelar, A.M.; Hindorf, C.; Parker, C.C.; Chua, S.; Flux, G.D. The potential of 223Ra and 18F-Fluoride imaging to predict bone lesion response to treatment with 223Ra-Dichloride in castration resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1832–1844. [Google Scholar] [CrossRef]
- Kairemo, K.; Joensuu, T. Radium-223-dichloride in castration resistant metastatic prostate cancer-preliminary results of the response evaluation using F-18-fluoride PET/CT. Diagnostics 2015, 5, 413–427. [Google Scholar] [CrossRef]
- Khawar, A.; Eppard, E.; Roesch, F.; Ahmadzadehfar, H.; Kürpig, S.; Meisenheimer, M.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. Preliminary results of biodistribution and dosimetric analysis of [68Ga]Ga-DOTAZOL: A new zoledronate-based bisphosphonate for PET/CT diagnosis of bone diseases. Ann. Nucl. Med. 2019, 33, 404–413. [Google Scholar] [CrossRef]
- Khawar, A.; Eppard, E.; Roesch, F.; Ahmadzadehfar, H.; Kürpig, S.; Meisenheimer, M.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. Biodistribution and post-therapy dosimetric analysis of [177Lu]Lu-DOTAZOL in patients with osteoblastic metastases: First results. EJNMMI Res. 2019, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Eppard, E.; Lehnert, W.; Jimenez-Franco, L.D.; Soza-Ried, C.; Ceballos, M.; Ribbeck, J.; Kluge, A.; Roesch, F.; Meckel, M.; et al. Evaluation of safety and dosimetry of 177 Lu DOTA-ZOL for therapy of bone metastases. J. Nucl. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kwee, S.A.; Lim, J.; Watanabe, A.; Kromer-Baker, K.; Coel, M.N. Prognosis Related to Metastatic Burden Measured by 18F-Fluorocholine PET/CT in Castration-Resistant Prostate Cancer. J. Nucl. Med. 2014, 55, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sato, M.M.; Coel, M.N.; Lee, K.-H.; Kwee, S.A. Prediction of PSA Progression in Castration-Resistant Prostate Cancer Based on Treatment-Associated Change in Tumor Burden Quantified by 18F-Fluorocholine PET/CT. J. Nucl. Med. 2016, 57, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Caroli, P.; De Giorgi, U.; Scarpi, E.; Fantini, L.; Moretti, A.; Galassi, R.; Celli, M.; Conteduca, V.; Rossi, L.; Bianchi, E.; et al. Prognostic value of 18F-choline PET/CT metabolic parameters in patients with metastatic castration-resistant prostate cancer treated with abiraterone or enzalutamide. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 348–354. [Google Scholar] [CrossRef]
- Filippi, L.; Spinelli, G.P.; Chiaravalloti, A.; Schillaci, O.; Equitani, F.; Bagni, O. Prognostic Value of 18F-Choline PET/CT in Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Radium-223. Biomedicines 2020, 8, 555. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.J.; Gavane, S.C.; Blanc-Autran, E.; Nehmeh, S.; Gönen, M.; Beattie, B.; Vargas, H.A.; Schöder, H.; Humm, J.L.; Fine, S.W.; et al. Positron emission tomography/computed tomography—Based assessments of androgen receptor expression and glycolytic activity as a prognostic biomarker for metastatic castration-resistant prostate cancer. JAMA Oncol. 2018, 4, 217–224. [Google Scholar] [CrossRef]
- Bauckneht, M.; Capitanio, S.; Donegani, M.I.; Zanardi, E.; Miceli, A.; Murialdo, R.; Raffa, S.; Tomasello, L.; Vitti, M.; Cavo, A.; et al. Role of Baseline and Post-Therapy 18F-FDG PET in the Prognostic Stratification of Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients Treated with Radium-223. Cancers 2019, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for ImmunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 2010, 51, 1293–1300. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Seifert, R.; Seitzer, K.; Herrmann, K.; Kessel, K.; Schäfers, M.; Kleesiek, J.; Weckesser, M.; Boegemann, M.; Rahbar, K. Analysis of PSMA expression and outcome in patients with advanced Prostate Cancer receiving 177Lu-PSMA-617 radioligand therapy. Theranostics 2020, 10, 7812–7820. [Google Scholar] [CrossRef]
- Bouvet, V.; Wuest, M.; Jans, H.-S.; Janzen, N.; Genady, A.R.; Valliant, J.F.; Benard, F.; Wuest, F. Automated synthesis of [18F]DCFPyL via direct radiofluorination and validation in preclinical prostate cancer models. EJNMMI Res. 2016, 6, 40. [Google Scholar] [CrossRef]
- Müller, C.; Singh, A.; Umbricht, C.A.; Kulkarni, H.R.; Johnston, K.; Benešová, M.; Senftleben, S.; Müller, D.; Vermeulen, C.; Schibli, R.; et al. Preclinical investigations and first-in-human application of 152Tb-PSMA-617 for PET/CT imaging of prostate cancer. EJNMMI Res. 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Umbricht, C.A.; Gracheva, N.; Tschan, V.J.; Pellegrini, G.; Bernhardt, P.; Zeevaart, J.R.; Köster, U.; Schibli, R.; van der Meulen, N.P. Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1919–1930. [Google Scholar] [CrossRef]
- Dehdashti, F.; Picus, J.; Michalski, J.M.; Dence, C.S.; Siegel, B.A.; Katzenellenbogen, J.A.; Welch, M.J. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Beer, T.M.; Higano, C.S.; Anand, A.; Taplin, M.-E.; Efstathiou, E.; Rathkopf, D.; Shelkey, J.; Yu, E.Y.; Alumkal, J.; et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1–2 study. Lancet 2010, 375, 1437–1446. [Google Scholar] [CrossRef]
- Vargas, H.A.; Kramer, G.M.; Scott, A.M.; Weickhardt, A.; Meier, A.A.; Parada, N.; Beattie, B.J.; Humm, J.L.; Staton, K.D.; Zanzonico, P.B.; et al. Reproducibility and repeatability of semiquantitative 18 F-Fluorodihydrotestosterone uptake metrics in castration-resistant prostate cancer metastases: A prospective multicenter study. J. Nucl. Med. 2018, 59, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Xu, J.; Mpoy, C.; Chu, W.; Kim, S.H.; Li, H.; Rogers, B.E.; Katzenellenbogen, J.A. Preliminary evaluation of a novel 18F-labeled PARP-1 ligand for PET imaging of PARP-1 expression in prostate cancer. Nucl. Med. Biol. 2018, 66, 26–31. [Google Scholar] [CrossRef]
- Zhang-Yin, J.; Provost, C.; Cancel-Tassin, G.; Rusu, T.; Penent, M.; Radulescu, C.; Comperat, E.; Cussenot, O.; Montravers, F.; Renard-Penna, R.; et al. A comparative study of peptide-based imaging agents [68Ga]Ga-PSMA-11, [68Ga]Ga-AMBA, [68Ga]Ga-NODAGA-RGD and [68Ga]Ga-DOTA-NT-20.3 in preclinical prostate tumour models. Nucl. Med. Biol. 2020, 84–85, 88–95. [Google Scholar] [CrossRef]
- Dam, J.H.; Olsen, B.B.; Baun, C.; Høilund-Carlsen, P.-F.; Thisgaard, H. In Vivo Evaluation of a Bombesin Analogue Labeled with Ga-68 and Co-55/57. Mol. Imaging Biol. 2016, 18, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Maddalena, M.E.; Fox, J.; Chen, J.; Feng, W.; Cagnolini, A.; Linder, K.E.; Tweedle, M.F.; Nunn, A.D.; Lantry, L.E. 177Lu-AMBA Biodistribution, Radiotherapeutic Efficacy, Imaging, and Autoradiography in Prostate Cancer Models with Low GRP-R Expression. J. Nucl. Med. 2009, 50, 2017–2024. [Google Scholar] [CrossRef]
- Zhang, H.; Schuhmacher, J.; Waser, B.; Wild, D.; Eisenhut, M.; Reubi, J.C.; Maecke, H.R. DOTA-PESIN, a DOTA-conjugated bombesin derivative designed for the imaging and targeted radionuclide treatment of bombesin receptor-positive tumours. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1198–1208. [Google Scholar] [CrossRef]
- Wieser, G.; Mansi, R.; Grosu, A.L.; Schultze-Seemann, W.; Dumont-Walter, R.A.; Meyer, P.T.; Maecke, H.R.; Reubi, J.C.; Weber, W.A. Positron Emission Tomography (PET) imaging of prostate cancer with a gastrin releasing peptide receptor antagonist—From mice to men. Theranostics 2014, 4, 412–419. [Google Scholar] [CrossRef]
- Maina, T.; Bergsma, H.; Kulkarni, H.R.; Mueller, D.; Charalambidis, D.; Krenning, E.P.; Nock, B.A.; de Jong, M.; Baum, R.P. Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [68Ga]SB3 and PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 964–973. [Google Scholar] [CrossRef]
- Lymperis, E.; Kaloudi, A.; Sallegger, W.; Bakker, I.L.; Krenning, E.P.; de Jong, M.; Maina, T.; Nock, B.A. Radiometal-dependent biological profile of the radiolabeled gastrin-releasing peptide receptor antagonist SB3 in cancer theranostics: Metabolic and biodistribution patterns defined by neprilysin. Bioconjug. Chem. 2018, 29, 1774–1784. [Google Scholar] [CrossRef]
- Chatalic, K.L.S.; Konijnenberg, M.; Nonnekens, J.; de Blois, E.; Hoeben, S.; de Ridder, C.; Brunel, L.; Fehrentz, J.-A.; Martinez, J.; van Gent, D.C.; et al. In vivo stabilization of a gastrin-releasing peptide receptor antagonist enhances PET imaging and radionuclide therapy of prostate cancer in preclinical studies. Theranostics 2016, 6, 104–117. [Google Scholar] [CrossRef]
- Dalm, S.U.; Bakker, I.L.; de Blois, E.; Doeswijk, G.N.; Konijnenberg, M.W.; Orlandi, F.; Barbato, D.; Tedesco, M.; Maina, T.; Nock, B.A.; et al. 68Ga/177Lu-NeoBOMB1, a novel radiolabeled GRPR antagonist for theranostic use in oncology. J. Nucl. Med. 2017, 58, 293–299. [Google Scholar] [CrossRef]
- Kurth, J.; Krause, B.J.; Schwarzenböck, S.M.; Bergner, C.; Hakenberg, O.W.; Heuschkel, M. First-in-human dosimetry of gastrin-releasing peptide receptor antagonist [177Lu]Lu-RM2: A radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Yari, H.; Nkepang, G.; Awasthi, V. Surface modification of liposomes by a lipopolymer targeting prostate specific membrane antigen for theranostic delivery in prostate cancer. Materials 2019, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, A.; Boudon, J.; Mirjolet, C.; Créhange, G.; Millot, N. Taxane-grafted metal-oxide nanoparticles as a new theranostic tool against cancer: The promising example of docetaxel-functionalized titanate nanotubes on prostate tumors. Adv. Healthc. Mater. 2017, 6, 1700245. [Google Scholar] [CrossRef]
- Jiménez-Mancilla, N.; Ferro-Flores, G.; Santos-Cuevas, C.; Ocampo-García, B.; Luna-Gutiérrez, M.; Azorín-Vega, E.; Isaac-Olivé, K.; Camacho-López, M.; Torres-García, E. Multifunctional targeted therapy system based on 99m Tc/ 177 Lu-labeled gold nanoparticles-Tat(49-57)-Lys 3 -bombesin internalized in nuclei of prostate cancer cells: Therapy with 99mTc/177Lu-labeled gold nanoparticles-Tat-bombesin. J. Label. Compd. Radiopharm. 2013, 56, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Paulo, A.; Pallier, A.; Même, S.; Tóth, É.; Gano, L.; Marques, F.; Geraldes, C.F.G.C.; Castro, M.M.C.A.; Cardoso, A.M.; et al. Dual imaging gold nanoplatforms for targeted radiotheranostics. Materials 2020, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Moeendarbari, S.; Tekade, R.; Mulgaonkar, A.; Christensen, P.; Ramezani, S.; Hassan, G.; Jiang, R.; Öz, O.K.; Hao, Y.; Sun, X. Theranostic nanoseeds for efficacious internal radiation therapy of unresectable solid tumors. Sci. Rep. 2016, 6, 20614. [Google Scholar] [CrossRef]
- Chen, F.; Ma, K.; Zhang, L.; Madajewski, B.; Turker, M.Z.; Gallazzi, F.; Cruickshank, K.; Zhang, X.; Jenjitranant, P.; Touijer, K.A.; et al. Ultrasmall renally clearable silica nanoparticles target prostate cancer. ACS Appl. Mater. Interfaces 2019, 11, 43879–43887. [Google Scholar] [CrossRef] [PubMed]
- Molecular & Diagnostic Imaging in Prostate Cancer: Clinical Applications and Treatment Strategies; Springer Science+Business Media: New York, NY, USA, 2018; ISBN 978-3-319-99285-3.
- Kuang, Y.; Salem, N.; Corn, D.J.; Erokwu, B.; Tian, H.; Wang, F.; Lee, Z. Transport and metabolism of radiolabeled choline in hepatocellular carcinoma. Mol. Pharm. 2010, 7, 2077–2092. [Google Scholar] [CrossRef] [PubMed]
- Katz-Brull, R.; Degani, H. Kinetics of choline transport and phosphorylation in human breast cancer cells; NMR application of the zero trans method. Anticancer Res. 1996, 16, 1375–1380. [Google Scholar]
- Hara, H.; Takahashi, T.; Serada, S.; Fujimoto, M.; Ohkawara, T.; Nakatsuka, R.; Harada, E.; Nishigaki, T.; Takahashi, Y.; Nojima, S.; et al. Overexpression of glypican-1 implicates poor prognosis and their chemoresistance in oesophageal squamous cell carcinoma. Br. J. Cancer 2016, 115, 66–75. [Google Scholar] [CrossRef]
- Conteduca, V.; Scarpi, E.; Caroli, P.; Salvi, S.; Lolli, C.; Burgio, S.L.; Menna, C.; Schepisi, G.; Testoni, S.; Gurioli, G.; et al. Circulating androgen receptor combined with 18F-fluorocholine PET/CT metabolic activity and outcome to androgen receptor signalling-directed therapies in castration-resistant prostate cancer. Sci. Rep. 2017, 7, 15541. [Google Scholar] [CrossRef] [PubMed]
- Ancey, P.-B.; Contat, C.; Meylan, E. Glucose transporters in cancer—From tumor cells to the tumor microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [CrossRef]
- Gonzalez-Menendez, P.; Hevia, D.; Mayo, J.C.; Sainz, R.M. The dark side of glucose transporters in prostate cancer: Are they a new feature to characterize carcinomas?: The role of GLUT transporters in prostate cancer. Int. J. Cancer 2018, 142, 2414–2424. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Mens Health 2019, 37, 288. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; Veach, D.R.; Fox, J.J.; Scher, H.I.; Morris, M.J.; Larson, S.M. Evaluation of Castration-Resistant Prostate Cancer with Androgen Receptor-Axis Imaging. J. Nucl. Med. 2016, 57, 73S–78S. [Google Scholar] [CrossRef][Green Version]
- Rathkopf, D.E.; Morris, M.J.; Fox, J.J.; Danila, D.C.; Slovin, S.F.; Hager, J.H.; Rix, P.J.; Chow Maneval, E.; Chen, I.; Gönen, M.; et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. JCO 2013, 31, 3525–3530. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.D. Radium-223 dichloride for metastatic castration-resistant prostate cancer: The urologist’s perspective. Urology 2015, 85, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Pacilio, M.; Ventroni, G.; De Vincentis, G.; Cassano, B.; Pellegrini, R.; Di Castro, E.; Frantellizzi, V.; Follacchio, G.A.; Garkavaya, T.; Lorenzon, L.; et al. Dosimetry of bone metastases in targeted radionuclide therapy with alpha-emitting 223Ra-dichloride. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 21–33. [Google Scholar] [CrossRef]
- Jadvar, H.; Colletti, P.M. 18F-NaF/223RaCl2 theranostics in metastatic prostate cancer: Treatment response assessment and prediction of outcome. Br. J. Radiol. 2018, 91. [Google Scholar] [CrossRef] [PubMed]
- Blau, M.; Ganatra, R.; Bender, M.A. 18F-fluoride for bone imaging. Semin. Nucl. Med. 1972, 2, 31–37. [Google Scholar] [CrossRef]
- Araz, M.; Aras, G.; Küçük, Ö.N. The role of 18F–NaF PET/CT in metastatic bone disease. J. Bone Oncol. 2015, 4, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Zheng, M. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. JNCI J. Natl. Cancer Inst. 2004, 96, 879–882. [Google Scholar] [CrossRef]
- Pfannkuchen, N.; Meckel, M.; Bergmann, R.; Bachmann, M.; Bal, C.; Sathekge, M.; Mohnike, W.; Baum, R.; Rösch, F. Novel radiolabeled bisphosphonates for PET diagnosis and endoradiotherapy of bone metastases. Pharmaceuticals 2017, 10, 45. [Google Scholar] [CrossRef]
- Chang, S.S. Overview of prostate-specific membrane antigen. Rev. Urol. 2004, 6, S13–S18. [Google Scholar] [PubMed]
- Wright, G.L.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol. Oncol. Semin. Orig. Investig. 1995, 1, 18–28. [Google Scholar] [CrossRef]
- Minner, S.; Wittmer, C.; Graefen, M.; Salomon, G.; Steuber, T.; Haese, A.; Huland, H.; Bokemeyer, C.; Yekebas, E.; Dierlamm, J.; et al. High level PSMA expression is associated with early psa recurrence in surgically treated prostate cancer. Prostate 2011, 71, 281–288. [Google Scholar] [CrossRef]
- Mhawech-Fauceglia, P.; Zhang, S.; Terracciano, L.; Sauter, G.; Chadhuri, A.; Herrmann, F.R.; Penetrante, R. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: An immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology 2007, 50, 472–483. [Google Scholar] [CrossRef]
- Rizvi, T.; Deng, C.; Rehm, P. Indium-111 capromab pendetide (ProstaScint®) demonstrates renal cell carcinoma and aortocaval nodal metastases from prostate adenocarcinoma. World J. Nucl. Med. 2015, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Hardie, A.D.; Rieter, W.J.; Bradshaw, M.L.; Gordon, L.L.; Young, M.A.; Keane, T.E. Improved performance of SPECT-CT In-111 capromab pendetide by correlation with diffusion-weighted magnetic resonance imaging for identifying metastatic pelvic lymphadenopathy in prostate cancer. World J. Urol. 2013, 31, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Biancone, L.; Petruzziello, C.; Schillaci, O. Tc-99m HMPAO-labeled leukocyte scintigraphy with hybrid SPECT/CT detects perianal fistulas in Crohn disease. Clin. Nucl. Med. 2006, 31, 541–542. [Google Scholar] [CrossRef]
- Cimadamore, A.; Cheng, M.; Santoni, M.; Lopez-Beltran, A.; Battelli, N.; Massari, F.; Galosi, A.B.; Scarpelli, M.; Montironi, R. New prostate cancer targets for diagnosis, imaging, and therapy: Focus on prostate-specific membrane antigen. Front. Oncol. 2018, 8, 653. [Google Scholar] [CrossRef]
- Prasad, V.; Steffen, I.G.; Diederichs, G.; Makowski, M.R.; Wust, P.; Brenner, W. Biodistribution of [68Ga]PSMA-HBED-CC in patients with prostate cancer: Characterization of uptake in normal organs and tumour lesions. Mol. Imaging Biol. 2016, 18, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.D.; Poyet, C.; Kroeze, S.G.C.; Kranzbühler, B.; Schüler, H.I.G.; Guckenberger, M.; Kaufmann, P.A.; Hermanns, T.; Burger, I.A. Current and potential future role of PSMA-PET in patients with castration-resistant prostate cancer. World J. Urol. 2019, 37, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Fourquet, A.; Aveline, C.; Cussenot, O.; Créhange, G.; Montravers, F.; Talbot, J.-N.; Gauthé, M. 68Ga-PSMA-11 PET/CT in restaging castration-resistant nonmetastatic prostate cancer: Detection rate, impact on patients’ disease management and adequacy of impact. Sci. Rep. 2020, 10, 2104. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical evaluation of a tailor-made DOTA-Conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar]
- Fendler, W.P.; Rahbar, K.; Herrmann, K.; Kratochwil, C.; Eiber, M. 177Lu-PSMA radioligand therapy for prostate cancer. J. Nucl. Med. 2017, 58, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Bagni, O. The potential of PSMA-targeted alpha therapy in the management of prostate cancer. Expert Rev. Anticancer Ther. 2020, 20, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Singh, A.; Kulkarni, H.; Baum, R.P. Molecular imaging with 64Cu-PSMA PET/CT in Theranostics of prostate cancer. EJEA 2016, 31, 277–286. [Google Scholar] [CrossRef]
- Wondergem, M.; van der Zant, F.M.; Broos, W.a.M.; Roeleveld, T.A.; Donker, R.; Ten Oever, D.; Geenen, R.W.F.; Knol, R.J.J. 18F-DCFPyL PET/CT for primary staging in 160 high-risk prostate cancer patients; metastasis detection rate, influence on clinical management and preliminary results of treatment efficacy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 521–531. [Google Scholar] [CrossRef]
- Wondergem, M.; van der Zant, F.; Broos, W.; Knol, R. Increased PSMA Expression in Castration-Resistant Prostate Cancer Metastases 3 Months After Initiation of Enzalutamide Indicated by 18F-DCFPyL PET/CT. Clin. Nucl. Med. 2019, 44, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.A.; Kraus, W.L. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat. Rev. Mol. Cell Biol. 2012, 13, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Sartor, O.; Barata, P.C.; Paller, C.J. Therapeutic Potential of PARP Inhibitors in the Treatment of Metastatic Castration-Resistant Prostate Cancer. Cancers 2020, 12, 3467. [Google Scholar] [CrossRef] [PubMed]
- Ambur Sankaranarayanan, R.; Kossatz, S.; Weber, W.; Beheshti, M.; Morgenroth, A.; Mottaghy, F.M. Advancements in PARP1 targeted nuclear imaging and theranostic probes. JCM 2020, 9, 2130. [Google Scholar] [CrossRef]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, G.; Jannetti, S.A.; Carter, L.M.; Sadique, A.; Kossatz, S.; Guru, N.; Demétrio De Souza França, P.; Maeda, M.; Zeglis, B.M.; Lewis, J.S.; et al. Targeted Brain Tumor Radiotherapy Using an Auger Emitter. Clin. Cancer Res. 2020, 26, 2871–2881. [Google Scholar] [CrossRef] [PubMed]
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate cancer theranostics targeting gastrin-releasing peptide receptors. Mol. Imaging Biol. 2018, 20, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Kilcoyne, A.; Harisinghani, M.G.; Mahmood, U. Prostate cancer imaging and therapy: Potential role of nanoparticles. J. Nucl. Med. 2016, 57, 105S–110S. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Astruc, D. Docetaxel nanotechnology in anticancer therapy. ChemMedChem 2012, 7, 952–972. [Google Scholar] [CrossRef]
- Ma, K.; Mendoza, C.; Hanson, M.; Werner-Zwanziger, U.; Zwanziger, J.; Wiesner, U. Control of ultrasmall sub-10 nm ligand-functionalized fluorescent core-shell silica nanoparticle growth in water. Chem. Mater. 2015, 27, 4119–4133. [Google Scholar] [CrossRef]
- Zheng, J.; Klinz, S.G.; De Souza, R.; Fitzgerald, J.; Jaffray, D.A. Longitudinal tumor hypoxia imaging with [18F]FAZA-PET provides early prediction of nanoliposomal irinotecan (nal-IRI) treatment activity. EJNMMI Res. 2015, 5, 57. [Google Scholar] [CrossRef]
- Cryer, A.M.; Thorley, A.J. Nanotechnology in the diagnosis and treatment of lung cancer. Pharmacol. Ther. 2019, 198, 189–205. [Google Scholar] [CrossRef]
- Thang, S.P.; Violet, J.; Sandhu, S.; Iravani, A.; Akhurst, T.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; Williams, S.G.; Hicks, R.J.; et al. Poor outcomes for patients with metastatic castration-resistant prostate cancer with low Prostate-Specific Membrane Antigen (PSMA) expression deemed ineligible for 177Lu-labelled PSMA radioligand therapy. Eur. Urol. Oncol. 2019, 2, 670–676. [Google Scholar] [CrossRef] [PubMed]

| Authors | Year | Type of Study | Radiotracer | Target | Comment |
|---|---|---|---|---|---|
| Murray et al. [16] | 2017 | Phase I open-label clinical trial | 18F-NaF | Newly formed bone | Incorporation of 18F-NaF in bone metastases correlated with that of 223Ra-dichloride, thus PET/CT with 18F-NaF might be utilized for targeted alpha therapy response prediction and dosimetric calculation. |
| Kairemo et al. [17] | 2015 | Retrospective, single-center | 18F-NaF | Newly formed bone | PET/CT with 18F-NaF performed at baseline and after the 6th cycle of 223Ra-dichloride therapy correlated with PSA changes and resulted useful for monitoring response to targeted alpha therapy. |
| Khawar et al. [18] | 2019 | Retrospective, single-center | 68Ga-DOTAZOL | Osteoclastic bone resorption | PET/CT with 68Ga-DOTAZOL enables the visualization of mCRPC and bronchial carcinoma bone metastases and can be utilized for provisional dosimetry before therapy with 177Lu-DOTAZOL |
| Khawar et al. [19] | 2019 | Retrospective, single-center | 177Lu-DOTAZOL | Osteoclastic bone resorption | 177Lu-DOTAZOL, presenting high absorbed dose in bones and low kidney dose, represents a promising therapeutic agent for skeletal metastases from mCRPC. |
| Fernandez et al. [20] | 2019 | Prospective, single-center | 177Lu-DOTAZOL | Osteoclastic bone resorption | 177Lu-DOTAZOL is safe and presents a favorable therapeutic index compared to other radiopharmaceuticals applied for the management of bone metastases. |
| Kwee et al. [21] | 2014 | Prospective, single-center | 18F-choline | Cell membrane biosynthesis | PET-derived volumetric parameters, such as MATV and TLA, correlated with PSA level and represent prognostic factors on overall survival in mCRPC. |
| Lee et al. [22] | 2016 | Prospective clinical study | 18F-choline | Cell membrane biosynthesis | MATV changes on PET/CT, performed at baseline and after three months of therapy, correlated with time to PSA progression in mCRPC subjects submitted to systemic therapy (antiandrogens, sipuleucel-T, chemotherapy, 223Ra-dichloride). |
| Caroli et al. [23] | 2018 | Prospective clinical study | 18F-choline | Cell membrane biosynthesis | Overall burden of metabolically active disease (i.e., MATV and TLA calculated on baseline PET/CT) resulted useful to predict mCRPC patients’ outcome after therapy with 2nd generation antiandrogens. |
| Filippi et al. [24] | 2020 | Retrospective, single-center | 18F-choline | Cell membrane biosynthesis | Baseline PSA levels and PET-derived parameters (i.e., TLA and number of lesions) correlated with overall survival of mCRPC patients treated with 223Ra-dichloride, TLA resulted independent predictor in multivariate analysis. |
| Fox et al. [25] | 2018 | Prospective, single-center | 18F-FDG | Glycolytic pathway | Patients affected by mCRPC were submitted to dual tracer PET/CT with 18F-FDG and 18F-FDHT for evaluating androgen receptor (AR) and glycolytic (Gly) status before therapy with 2nd generation antiandrogens. Imaging phenotypes characterized by no-AR expression and positive Gly had the worse prognosis. |
| 18F-FDHT | Androgen receptor | ||||
| Bauckneht et al. [26] | 2019 | Retrospective, single-center | 18F-FDG | Glycolytic pathway | PET with 18F-FDG performed before and after 223Ra-dichloride therapy helped identify patients with less favorable prognostic factors (high MTV) and for monitoring response to treatment. |
| Holland et al. [27] | 2010 | Pre-clinical | 89Zr-DFO-J591 | PSMA | Immuno-PET with 89Zr-DFO-J591 resulted capable of detecting PSMA-expressing tumor xenograft in mice. |
| Hofman et al. [28] | 2018 | Single-arm, single-center, phase 2 trial | 68Ga-PSMA-11 | PSMA | Pre-treatment PET with 68Ga-PSMA-11 showing at least 1 site of metastatic disease with PSMA uptake was utilized as enrollment criterion for 177Lu-PSMA-617 RLT. |
| Seifert et al. [29] | 2020 | Retrospective studies | 68Ga-PSMA-11 | PSMA | Quantitative parameters (i.e., PSMAaverage) calculated on PET/CT with 68Ga-PSMA-11 resulted useful for selecting mCRPC patients before RLT with 177Lu-PSMA-617. |
| Bouvet et al. [30] | 2016 | Pre-clinical | 18F-DCFPyL | PSMA | The radiocompound, obtained via direct radiofluorination, was capable of binding with high specificity PSMA-expressing tumor xenografts in nude mice, with a tumor-to-blood pool ratio of 8.3 at 60 min. |
| Müller et al. [31] | 2019 | Pre-clinical | 152Tb-PSMA-617 | PSMA | PSMA-617, labeled with the positron-emitter 152Tb, was successfully used to image PSMA-positive tumor xenografts in mice and to visualize metastases in a mCRPC patient. |
| Müller et al. [32] | 2019 | Pre-clinical | 161Tb-PSMA-617 | PSMA | PSMA-617, labeled with 161Tb, emitting both photons and Auger electrons, was tested as theranostic agent in vitro and in mice bearing PSMA-positive xenografts, showing superior results as compared to those obtained with 177Lu-PSMA. |
| Dehdashti et al. [33] | 2005 | Clinical trial | 18F-FDHT | AR | PET/CT with 18F-FDHT resulted in identifying AR status in patients with advanced prostate cancer; tracer binding was selectively blocked by the administration of flutamide. |
| Scher et al. [34] | 2010 | Phase I-II study | 18F-FDHT | AR | PET/CT with 18F-FDHT was applied for determining the safety and antitumor activity of enzalutamide. |
| Vargas et al. [35] | 2018 | Prospective, multi-center study | 18F-FDHT | AR | PET/CT with 18F-FDHT was proved to be highly repeatable with high inter-observer reproducibility, thus presenting potential usefulness for AR status monitoring during hormonal treatments. |
| Zhou et al. [36] | 2018 | pre-clinical | [18F]WC-DZ-F | PARP-1 | The synthesized PARP-1 radioligand resulted in being a suitable PET imaging agent for assessing PARP-1 expression in prostate cancer with high uptake in PC-3 cells and favorable biodistribution in xenograft tumor mice |
| Zhang-Yin et al. [37] | 2020 | Pre-clinical | 68Ga-AMBA | GRPR | 68Ga-AMBA showed good tumor uptake, high tumor-to-background contrast using PC3 cell line. |
| Dam et al. [38] | 2015 | Pre-clinical | 55Co-NOTA-AMBA | GRPR | 55Co-NOTA-AMBA in PC3 xenografted mice was found to be superior, for PET/CT imaging, compared to 68Ga-NOTA-AMBA, since it showed a better tumor-to-organ ratio |
| 68Ga-NOTA-AMBA | |||||
| Maddalena et al. [39] | 2009 | Pre-clinical | 177Lu-AMBA | GRPR | 177Lu-AMBA showed good radiotherapeutic efficacy in LNCaP, DU145, or PC-3 tumor–bearing male nude mice and was capable of identifying tumors in vivo. |
| Zhang et al. [40] | 2007 | Pre-clinical | 67Ga-DOTAPESIN | GRPR | 67Ga/177Lu-DOTAPESIN showed high uptake in human prostate tumor xenografts and in murine GRPR-positive organs, PET images demonstrated that 68Ga-DOTA-PESIN accumulates predominantly in PC-3 tumor, pancreas, and kidneys. |
| 68Ga-DOTAPESIN | |||||
| 177Lu-DOTAPESIN | |||||
| Wieser et al. [41] | 2014 | Clinical | 64Cu-CB-TE2A-AR06 | GRPR | In 3 out of 4 patients with newly diagnosed prostate cancer, 64Cu-CB-TE2A-AR06 was able to visualize tumors with high-contrast. |
| Maina et al. [42] | 2015 | Pre-clinicalClinical | 67Ga-SB3 | GRPR | 68Ga-SB3 showed, in patients affected by disseminated prostate and breast cancer submitted to PET/CT, pathological uptake in, respectively, 55% and 50% of patients. 67Ga-SB3 showed good pharmacokinetics in mice. |
| 68Ga-SB3 | |||||
| Lymperis et al. [43] | 2018 | Pre-clinical | 111In-SB3 | GRPR | The study aimed to explore the theranostic potential of 111In-SB3 for SPECT imaging and 177Lu-SB3 for radionuclide therapy in GRPR-positive PC-3 xenografts. |
| 177Lu-SB3 | |||||
| Chatalic et al. [44] | 2016 | Pre-clinical | 68Ga-JMV4168 | GRPR | In PC-3 tumor-bearing mice, the co-injection of a neutral endopeptidase inhibitor led to enhanced PC-3 tumor signal intensity in PET imaging with 68Ga-JMV4168, as well as regression of tumor size and increased survival rate following radionuclide therapy with 177Lu-JMV4168. |
| 177Lu-JMV4168 | |||||
| Dalm et al. [45] | 2017 | Pre-clinical | 68Ga-NeoBOMB1 | GRPR | In a mouse model, 68Ga-NeoBOMB1 for PET/CT imaging and 177Lu-NeoBOMB1 for radionuclide therapy reported, respectively, good visualization of the tumor tissue in PET images and high uptake in PC-3 cells. |
| 177Lu-NeoBOMB1 | |||||
| Khurt et al. [46] | 2019 | Clinical | 68Ga-RM2 | GRPR | Thirty-five patients with mCRPC underwent PET/CT with 68Ga-RM2. Among these, 4 underwent therapy with 177Lu-RM2, showing high tumor uptake and rapid clearance from normal organs and suggesting its suitability for radionuclide therapy in patients with mCRPC. |
| 177Lu-RM2 | |||||
| Yari et al. [47] | 2019 | Pre-clinical | P3-liposomes labeled with 99mTc and loaded with doxorubicin | PSMA | Liposomes carrying the lipopolymer P3 can be used for targeted delivery of therapeutics/diagnostics to advanced/metastatic PSMA+ prostate cancer tumors. |
| Loiseau et al. [48] | 2017 | Pre-clinical | TiONts-DTX-DOTA [111In] | 22Rv1 | After intratumoral injection, more than 70% of TiONts nanovectors were retained within the tumor for at least 7d with a significant reduction of tumor growth compared with free DTX |
| Jiménez-Mancilla et al. [49] | 2013 | Pre-clinical | 99mTc/177Lu-AuNP-Tat (49–57)-Lys3-BN | GRPR | The nanosystem showed properties suitable for both plasmonic photothermal therapy and targeted radiotherapy with β-particle, IC electrons and Auger electrons |
| Silva et al. [50] | 2019 | Pre-clinical | 67Ga-AuNP-Gd-BBN | GRPR | In addition to the favorable radiosensitization profile exhibited by bimodal MRI/SPECT AuNPs, the replacement of 67Ga with 68Ga, 90Y, 177Lu or 165Er could offer the possibility of combining SPECT, PET or MR imaging with β-particle targeted therapy |
| Moeendarbari et al. [51] | 2016 | Pre-clinical | 103Pd@Au-nanoseeds | Not applicable | Au-nanoseed-based brachytherapy has the potential to provide a theranostic solution for unresectable tumors exploiting the γ-emission of 103Pd for SPECT imaging |
| Chen at al. [52] | 2020 | Pre-clinical | 89Zr-DFO-PSMAi-PEG-Cy5-C’ dots | PSMA | PSMA-targeting C’ dots could represent a highly versatile theranostic tool for prostate cancer management, from imaging and image-guided surgery to treatment planning and α/β-particle targeted therapy |
| Xiang et al. [53] | 2019 | Pre-clinical | 18F-FAZA | Hypoxic regions of tumors | PET imaging with the hypoxia radiotracer 18F-FAZA can be used to assess reoxygenation of hypoxic tumors obtained with perfluorocarbon nanodroplets |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippi, L.; Frantellizzi, V.; Chiaravalloti, A.; Pontico, M.; De Feo, M.S.; Corica, F.; Montebello, M.; Schillaci, O.; De Vincentis, G.; Bagni, O. Prognostic and Theranostic Applications of Positron Emission Tomography for a Personalized Approach to Metastatic Castration-Resistant Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 3036. https://doi.org/10.3390/ijms22063036
Filippi L, Frantellizzi V, Chiaravalloti A, Pontico M, De Feo MS, Corica F, Montebello M, Schillaci O, De Vincentis G, Bagni O. Prognostic and Theranostic Applications of Positron Emission Tomography for a Personalized Approach to Metastatic Castration-Resistant Prostate Cancer. International Journal of Molecular Sciences. 2021; 22(6):3036. https://doi.org/10.3390/ijms22063036
Chicago/Turabian StyleFilippi, Luca, Viviana Frantellizzi, Agostino Chiaravalloti, Mariano Pontico, Maria Silvia De Feo, Ferdinando Corica, Melissa Montebello, Orazio Schillaci, Giuseppe De Vincentis, and Oreste Bagni. 2021. "Prognostic and Theranostic Applications of Positron Emission Tomography for a Personalized Approach to Metastatic Castration-Resistant Prostate Cancer" International Journal of Molecular Sciences 22, no. 6: 3036. https://doi.org/10.3390/ijms22063036
APA StyleFilippi, L., Frantellizzi, V., Chiaravalloti, A., Pontico, M., De Feo, M. S., Corica, F., Montebello, M., Schillaci, O., De Vincentis, G., & Bagni, O. (2021). Prognostic and Theranostic Applications of Positron Emission Tomography for a Personalized Approach to Metastatic Castration-Resistant Prostate Cancer. International Journal of Molecular Sciences, 22(6), 3036. https://doi.org/10.3390/ijms22063036







