Non-Coding RNAs in Hereditary Kidney Disorders
Abstract
1. Introduction
2. miRNAs
3. LncRNAs
4. Noncoding RNA in Polycystic Kidney Disease
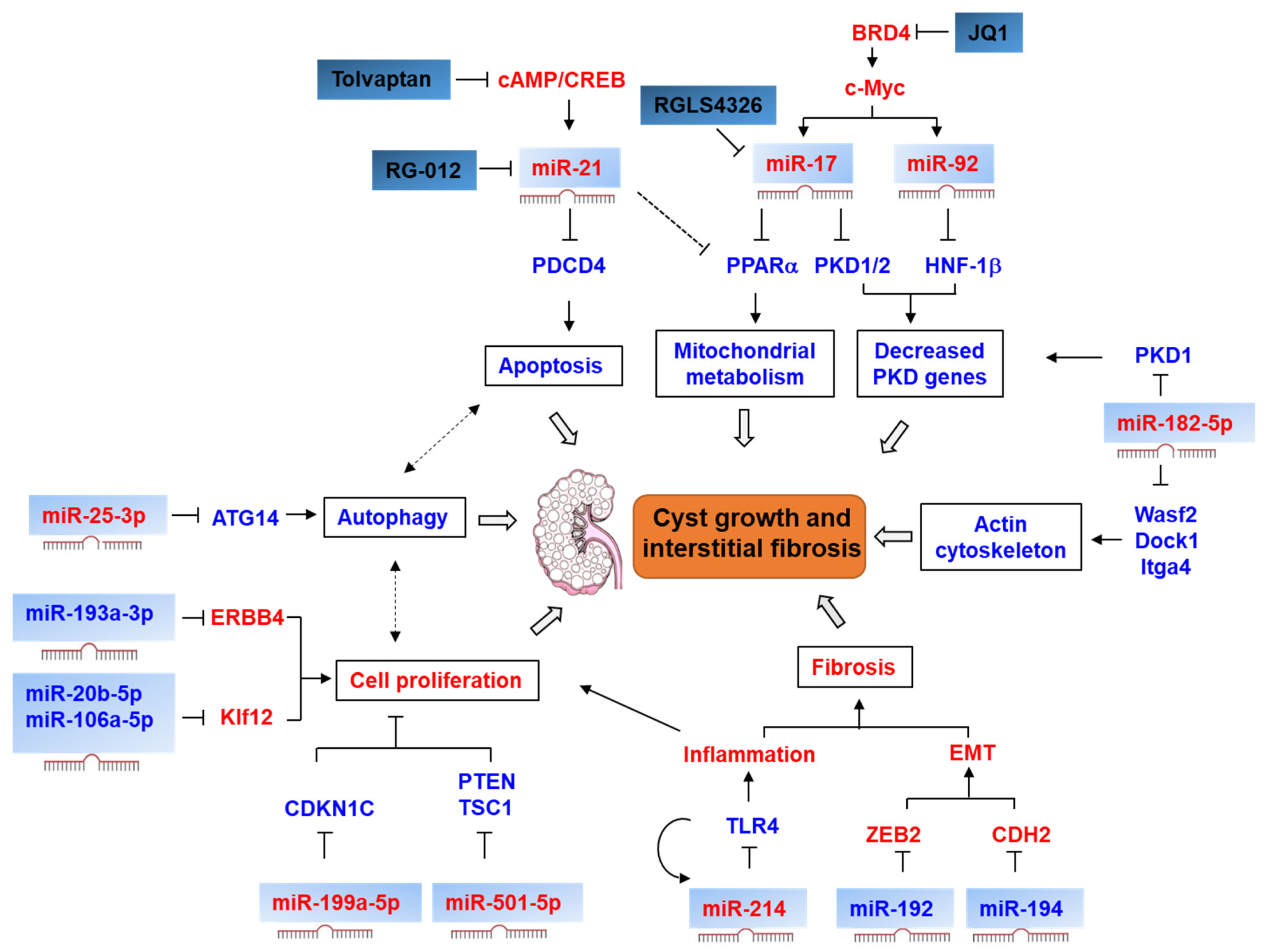
4.1. miRNAs and lncRNAs in ADPKD
4.1.1. mIR-17–92 Cluster
4.1.2. miR-21
4.1.3. miR-199a-5p
4.1.4. miR-200
4.1.5. miR-25-3p
4.1.6. miR-214
4.1.7. miR-192, miR-194, and miR-30
4.1.8. miR-193b-3p
4.1.9. miR-501-5p
4.1.10. miR-182-5p, miR-20b-5p and miR-106a-5p
4.1.11. LnRNAs in ADPKD
4.2. miRNAs in Autosomal Recessive Polycystic Kidney Disease (ARPKD)
5. miRNAs in HNF1β-Associated Kidney Disease
6. miRNAs in Alport Syndrome
7. miRNAs in Congenital Abnormalities of the Kidney and Urinary Tract (CAKUT)
8. Noncoding RNA in VHL Disease
9. miRNAs in Fabry Disease
10. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- The ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef]
- Brosnan, C.A.; Voinnet, O. The long and the short of noncoding RNAs. Curr. Opin. Cell Biol. 2009, 21, 416–425. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Paul, P.; Chakraborty, A.; Sarkar, D.; Langthasa, M.; Rahman, M.; Bari, M.; Singha, R.S.; Malakar, A.K.; Chakraborty, S. Interplay between miRNAs and human diseases. J. Cell Physiol. 2018, 233, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Huang, W. MicroRNAs: Biomarkers, Diagnostics, and Therapeutics. Methods Mol. Biol. 2017, 1617, 57–67. [Google Scholar] [PubMed]
- Connaughton, D.M.; Hildebrandt, F. Personalized medicine in chronic kidney disease by detection of monogenic mutations. Nephrol. Dial. Transpl. 2020, 35, 390–397. [Google Scholar] [CrossRef]
- Harris, P.C.; Torres, V.E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J. Clin. Investig. 2014, 124, 2315–2324. [Google Scholar] [CrossRef]
- Li, X. Epigenetics and cell cycle regulation in cystogenesis. Cell Signal. 2020, 68, 109509. [Google Scholar] [CrossRef]
- Ramalingam, H.; Yheskel, M.; Patel, V. Modulation of polycystic kidney disease by non-coding RNAs. Cell Signal. 2020, 71, 109548. [Google Scholar] [CrossRef]
- Ambros, V. microRNAs: Tiny regulators with great potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Denli, A.M.; Tops, B.B.; Plasterk, R.H.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235. [Google Scholar] [CrossRef]
- Zhang, H.; Kolb, F.A.; Jaskiewicz, L.; Westhof, E.; Filipowicz, W. Single processing center models for human Dicer and bacterial RNase III. Cell 2004, 118, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Yoda, M.; Kawamata, T.; Paroo, Z.; Ye, X.; Iwasaki, S.; Liu, Q.; Tomari, Y. ATP-dependent human RISC assembly pathways. Nat. Struct. Mol. Biol. 2010, 17, 17–23. [Google Scholar] [CrossRef]
- Khvorova, A.; Reynolds, A.; Jayasena, S.D. Functional siRNAs and miRNAs exhibit strand bias. Cell 2003, 115, 209–216. [Google Scholar] [CrossRef]
- Broughton, J.P.; Lovci, M.T.; Huang, J.L.; Yeo, G.W.; Pasquinelli, A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell 2016, 64, 320–333. [Google Scholar] [CrossRef]
- Jo, M.H.; Shin, S.; Jung, S.R.; Kim, E.; Song, J.J.; Hohng, S. Human Argonaute 2 Has Diverse Reaction Pathways on Target RNAs. Mol. Cell 2015, 59, 117–124. [Google Scholar] [CrossRef]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ipsaro, J.J.; Joshua-Tor, L. From guide to target: Molecular insights into eukaryotic RNA-interference machinery. Nat. Struct. Mol. Biol. 2015, 22, 20–28. [Google Scholar] [CrossRef]
- Forman, J.J.; Legesse-Miller, A.; Coller, H.A. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc. Natl. Acad. Sci. USA 2008, 105, 14879–14884. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, W.; Liu, Y.; Liu, T.; Li, C.; Wang, L. Oncogenic role of microRNA-532-5p in human colorectal cancer via targeting of the 5′UTR of. Oncol. Lett. 2018, 15, 7215–7220. [Google Scholar] [CrossRef]
- Dharap, A.; Pokrzywa, C.; Murali, S.; Pandi, G.; Vemuganti, R. MicroRNA miR-324-3p induces promoter-mediated expression of RelA gene. PLoS ONE 2013, 8, e79467. [Google Scholar]
- Ho, J.; Kreidberg, J.A. MicroRNAs in renal development. Pediatr. Nephrol. 2013, 28, 219–225. [Google Scholar] [CrossRef]
- Chen, H.; Fan, Y.; Jing, H.; Tang, S.; Zhou, J. Emerging role of lncRNAs in renal fibrosis. Arch. Biochem. Biophys. 2020, 692, 108530. [Google Scholar] [CrossRef]
- Nagalakshmi, V.K.; Ren, Q.; Pugh, M.M.; Valerius, M.T.; McMahon, A.P.; Yu, J. Dicer regulates the development of nephrogenic and ureteric compartments in the mammalian kidney. Kidney Int. 2011, 79, 317–330. [Google Scholar] [CrossRef]
- Ho, J.; Pandey, P.; Schatton, T.; Sims-Lucas, S.; Khalid, M.; Frank, M.H.; Hartwig, S.; Kreidberg, J.A. The pro-apoptotic protein Bim is a microRNA target in kidney progenitors. J. Am. Soc. Nephrol. 2011, 22, 1053–1063. [Google Scholar] [CrossRef]
- Brandenburger, T.; Lorenzen, J.M. Diagnostic and Therapeutic Potential of microRNAs in Acute Kidney Injury. Front. Pharmacol. 2020, 11, 657. [Google Scholar] [CrossRef]
- Peters, L.J.F.; Floege, J.; Biessen, E.A.L.; Jankowski, J.; van der Vorst, E.P.C. MicroRNAs in Chronic Kidney Disease: Four Candidates for Clinical Application. Int. J. Mol. Sci. 2020, 21, 6547. [Google Scholar] [CrossRef]
- Brandenburger, T.; Salgado Somoza, A.; Devaux, Y.; Lorenzen, J.M. Noncoding RNAs in acute kidney injury. Kidney Int. 2018, 94, 870–881. [Google Scholar] [CrossRef]
- Lorenzen, J.M.; Thum, T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat. Rev. Nephrol. 2016, 12, 360–373. [Google Scholar] [CrossRef]
- Devaux, Y.; Zangrando, J.; Schroen, B.; Creemers, E.E.; Pedrazzini, T.; Chang, C.P.; Dorn, G.W.; Thum, T.; Heymans, S.; Cardiolinc network. Long noncoding RNAs in cardiac development and ageing. Nat. Rev. Cardiol. 2015, 12, 415–425. [Google Scholar]
- Yao, R.W.; Wang, Y.; Chen, L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019, 21, 542–551. [Google Scholar] [CrossRef]
- Patel, V.; Williams, D.; Hajarnis, S.; Hunter, R.; Pontoglio, M.; Somlo, S.; Igarashi, P. miR-17~92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2013, 110, 10765–10770. [Google Scholar] [CrossRef]
- Hajarnis, S.; Lakhia, R.; Yheskel, M.; Williams, D.; Sorourian, M.; Liu, X.; Aboudelen, K.; Zhang, S.; Kersjes, K.; Galasso, R.; et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat. Commun. 2017, 8, 14395. [Google Scholar] [CrossRef] [PubMed]
- Yheskel, M.; Lakhia, R.; Cobo-Stark, P.; Flaten, A.; Patel, V. Anti-microRNA screen uncovers miR-17 family within miR-17~92 cluster as the primary driver of kidney cyst growth. Sci. Rep. 2019, 9, 1920. [Google Scholar] [CrossRef]
- Lakhia, R.; Hajarnis, S.; Williams, D.; Aboudehen, K.; Yheskel, M.; Xing, C.; Hatley, M.E.; Torres, V.E.; Wallace, D.P.; Patel, V. MicroRNA-21 Aggravates Cyst Growth in a Model of Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 2319–2330. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, J.; Wu, M.; Sun, H.; Zhou, C.; Fu, L.; Xu, C.; Mei, C. Inhibition of MiR-199a-5p reduced cell proliferation in autosomal dominant polycystic kidney disease through targeting CDKN1C. Med. Sci. Monit. 2015, 21, 195–200. [Google Scholar]
- Kurbegovic, A.; Côté, O.; Couillard, M.; Ward, C.J.; Harris, P.C.; Trudel, M. Pkd1 transgenic mice: Adult model of polycystic kidney disease with extrarenal and renal phenotypes. Hum. Mol. Genet. 2010, 19, 1174–1189. [Google Scholar] [CrossRef]
- Liu, G.; Kang, X.; Guo, P.; Shang, Y.; Du, R.; Wang, X.; Chen, L.; Yue, R.; Kong, F. miR-25-3p promotes proliferation and inhibits autophagy of renal cells in polycystic kidney mice by regulating ATG14-Beclin 1. Ren. Fail. 2020, 42, 333–342. [Google Scholar] [CrossRef]
- Lakhia, R.; Yheskel, M.; Flaten, A.; Ramalingam, H.; Aboudehen, K.; Ferrè, S.; Biggers, L.; Mishra, A.; Chaney, C.; Wallace, D.P.; et al. Interstitial microRNA miR-214 attenuates inflammation and polycystic kidney disease progression. JCI Insight 2020, 5, e133785. [Google Scholar] [CrossRef]
- Kim, D.Y.; Woo, Y.M.; Lee, S.; Oh, S.; Shin, Y.; Shin, J.O.; Park, E.Y.; Ko, J.Y.; Lee, E.; Bok, J.; et al. Impact of miR-192 and miR-194 on cyst enlargement through EMT in autosomal dominant polycystic kidney disease. Faseb. J. 2019, 33, 2870–2884. [Google Scholar] [CrossRef]
- Magayr, T.A.; Song, X.; Streets, A.J.; Vergoz, L.; Chang, L.; Valluru, M.K.; Yap, H.L.; Lannoy, M.; Haghighi, A.; Simms, R.J.; et al. Global microRNA profiling in human urinary exosomes reveals novel disease biomarkers and cellular pathways for autosomal dominant polycystic kidney disease. Kidney Int. 2020, 98, 420–435. [Google Scholar] [CrossRef]
- Streets, A.J.; Magayr, T.A.; Huang, L.; Vergoz, L.; Rossetti, S.; Simms, R.J.; Harris, P.C.; Peter, D.J.; Ong, A.C. Parallel microarray profiling identifies ErbB4 as a determinant of cyst growth in ADPKD and a prognostic biomarker for disease progression. Am. J. Physiol. Renal. Physiol. 2017, 312, F577–F588. [Google Scholar] [CrossRef]
- de Stephanis, L.; Mangolini, A.; Servello, M.; Harris, P.C.; Dell’Atti, L.; Pinton, P.; Aguiari, G. MicroRNA501-5p induces p53 proteasome degradation through the activation of the mTOR/MDM2 pathway in ADPKD cells. J. Cell Physiol. 2018, 233, 6911–6924. [Google Scholar] [CrossRef]
- Woo, Y.M.; Kim, D.Y.; Koo, N.J.; Kim, Y.M.; Lee, S.; Ko, J.Y.; Shin, Y.; Kim, B.H.; Mun, H.; Choi, S.; et al. Profiling of miRNAs and target genes related to cystogenesis in ADPKD mouse models. Sci. Rep. 2017, 7, 14151. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, D.Y.; Ko, J.Y.; Woo, Y.M.; Park, J.H. Regulation of KLF12 by microRNA-20b and microRNA-106a in cystogenesis. Faseb. J. 2018, 32, 3574–3582. [Google Scholar] [CrossRef]
- Ilatovskaya, D.V.; Levchenko, V.; Pavlov, T.S.; Isaeva, E.; Klemens, C.A.; Johnson, J.; Liu, P.; Kriegel, A.J.; Staruschenko, A. Salt-deficient diet exacerbates cystogenesis in ARPKD via epithelial sodium channel (ENaC). EBioMedicine 2019, 40, 663–674. [Google Scholar] [CrossRef]
- Lee, S.O.; Masyuk, T.; Splinter, P.; Banales, J.M.; Masyuk, A.; Stroope, A.; Larusso, N. MicroRNA15a modulates expression of the cell-cycle regulator Cdc25A and affects hepatic cystogenesis in a rat model of polycystic kidney disease. J. Clin. Investig. 2008, 118, 3714–3724. [Google Scholar] [CrossRef] [PubMed]
- de Pontual, L.; Yao, E.; Callier, P.; Faivre, L.; Drouin, V.; Cariou, S.; Van Haeringen, A.; Geneviève, D.; Goldenberg, A.; Oufadem, M.; et al. Germline deletion of the miR-17∼92 cluster causes skeletal and growth defects in humans. Nat. Genet. 2011, 43, 1026–1030. [Google Scholar] [CrossRef]
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008, 132, 875–886. [Google Scholar] [CrossRef]
- Marrone, A.K.; Stolz, D.B.; Bastacky, S.I.; Kostka, D.; Bodnar, A.J.; Ho, J. MicroRNA-17~92 is required for nephrogenesis and renal function. J. Am. Soc. Nephrol. 2014, 25, 1440–1452. [Google Scholar] [CrossRef]
- Brinkmann, K.; Ng, A.P.; de Graaf, C.A.; Di Rago, L.; Hyland, C.D.; Morelli, E.; Rautela, J.; Huntington, N.D.; Strasser, A.; Alexander, W.S.; et al. miR17~92 restrains pro-apoptotic BIM to ensure survival of haematopoietic stem and progenitor cells. Cell Death Differ. 2020, 27, 1475–1488. [Google Scholar] [CrossRef]
- Trudel, M.; D’Agati, V.; Costantini, F. C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int. 1991, 39, 665–671. [Google Scholar] [CrossRef]
- Zhou, X.; Fan, L.X.; Peters, D.J.; Trudel, M.; Bradner, J.E.; Li, X. Therapeutic targeting of BET bromodomain protein, Brd4, delays cyst growth in ADPKD. Hum. Mol. Genet. 2015, 24, 3982–3993. [Google Scholar] [CrossRef]
- Sun, H.; Li, Q.W.; Lv, X.Y.; Ai, J.Z.; Yang, Q.T.; Duan, J.J.; Bian, G.H.; Xiao, Y.; Wang, Y.D.; Zhang, Z.; et al. MicroRNA-17 post-transcriptionally regulates polycystic kidney disease-2 gene and promotes cell proliferation. Mol. Biol. Rep. 2010, 37, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Chan, S.C.; Igarashi, P. Role of transcription factor hepatocyte nuclear factor-1β in polycystic kidney disease. Cell Signal. 2020, 71, 109568. [Google Scholar] [CrossRef]
- Hopp, K.; Ward, C.J.; Hommerding, C.J.; Nasr, S.H.; Tuan, H.F.; Gainullin, V.G.; Rossetti, S.; Torres, V.E.; Harris, P.C. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J. Clin. Investig. 2012, 122, 4257–4273. [Google Scholar] [CrossRef]
- Trott, J.F.; Hwang, V.J.; Ishimaru, T.; Chmiel, K.J.; Zhou, J.X.; Shim, K.; Stewart, B.J.; Mahjoub, M.R.; Jen, K.Y.; Barupal, D.K.; et al. Arginine reprogramming in ADPKD results in arginine-dependent cystogenesis. Am. J. Physiol. Renal. Physiol. 2018, 315, F1855–F1868. [Google Scholar] [CrossRef]
- Nowak, K.L.; Hopp, K. Metabolic Reprogramming in Autosomal Dominant Polycystic Kidney Disease: Evidence and Therapeutic Potential. Clin. J. Am. Soc. Nephrol. 2020, 15, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Rowe, I.; Chiaravalli, M.; Mannella, V.; Ulisse, V.; Quilici, G.; Pema, M.; Song, X.W.; Xu, H.; Mari, S.; Qian, F.; et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 2013, 19, 488–493. [Google Scholar] [CrossRef]
- Lee, E.C.; Valencia, T.; Allerson, C.; Schairer, A.; Flaten, A.; Yheskel, M.; Kersjes, K.; Li, J.; Gatto, S.; Takhar, M.; et al. Discovery and preclinical evaluation of anti-miR-17 oligonucleotide RGLS4326 for the treatment of polycystic kidney disease. Nat. Commun. 2019, 10, 4148. [Google Scholar] [CrossRef]
- Krichevsky, A.M.; Gabriely, G. miR-21: A small multi-faceted RNA. J. Cell Mol. Med. 2009, 13, 39–53. [Google Scholar] [CrossRef]
- Feng, Y.H.; Tsao, C.J. Emerging role of microRNA-21 in cancer. Biomed. Rep. 2016, 5, 395–402. [Google Scholar] [CrossRef]
- Seeger-Nukpezah, T.; Geynisman, D.M.; Nikonova, A.S.; Benzing, T.; Golemis, E.A. The hallmarks of cancer: Relevance to the pathogenesis of polycystic kidney disease. Nat. Rev. Nephrol. 2015, 11, 515–534. [Google Scholar] [CrossRef]
- Loboda, A.; Sobczak, M.; Jozkowicz, A.; Dulak, J. TGF-β1/Smads and miR-21 in Renal Fibrosis and Inflammation. Mediators Inflamm. 2016, 2016, 8319283. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Torres, V.E. Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013, 22, 459–470. [Google Scholar] [CrossRef]
- Fan, L.X.; Zhou, X.; Sweeney, W.E.; Wallace, D.P.; Avner, E.D.; Grantham, J.J.; Li, X. Smac-mimetic-induced epithelial cell death reduces the growth of renal cysts. J. Am. Soc. Nephrol. 2013, 24, 2010–2022. [Google Scholar] [CrossRef]
- Buscaglia, L.E.; Li, Y. Apoptosis and the target genes of microRNA-21. Chin. J. Cancer 2011, 30, 371–380. [Google Scholar] [CrossRef]
- Hilliard, A.; Hilliard, B.; Zheng, S.J.; Sun, H.; Miwa, T.; Song, W.; Göke, R.; Chen, Y.H. Translational regulation of autoimmune inflammation and lymphoma genesis by programmed cell death 4. J. Immunol. 2006, 177, 8095–8102. [Google Scholar] [CrossRef]
- Pandey, P.; Brors, B.; Srivastava, P.K.; Bott, A.; Boehn, S.N.; Groene, H.J.; Gretz, N. Microarray-based approach identifies microRNAs and their target functional patterns in polycystic kidney disease. BMC Genom. 2008, 9, 624. [Google Scholar] [CrossRef]
- Dweep, H.; Sticht, C.; Kharkar, A.; Pandey, P.; Gretz, N. Parallel analysis of mRNA and microRNA microarray profiles to explore functional regulatory patterns in polycystic kidney disease: Using PKD/Mhm rat model. PLoS ONE 2013, 8, e53780. [Google Scholar] [CrossRef]
- Besson, A.; Dowdy, S.F.; Roberts, J.M. CDK inhibitors: Cell cycle regulators and beyond. Dev. Cell 2008, 14, 159–169. [Google Scholar] [CrossRef]
- Pandey, P.; Qin, S.; Ho, J.; Zhou, J.; Kreidberg, J.A. Systems biology approach to identify transcriptome reprogramming and candidate microRNA targets during the progression of polycystic kidney disease. BMC Syst. Biol. 2011, 5, 56. [Google Scholar] [CrossRef]
- Patel, V.; Hajarnis, S.; Williams, D.; Hunter, R.; Huynh, D.; Igarashi, P. MicroRNAs regulate renal tubule maturation through modulation of Pkd1. J. Am. Soc. Nephrol. 2012, 23, 1941–1948. [Google Scholar] [CrossRef]
- Nowak, K.L.; Edelstein, C.L. Apoptosis and autophagy in polycystic kidney disease (PKD). Cell Signal. 2020, 68, 109518. [Google Scholar] [CrossRef] [PubMed]
- Song, C.J.; Zimmerman, K.A.; Henke, S.J.; Yoder, B.K. Inflammation and Fibrosis in Polycystic Kidney Disease. Results Probl. Cell Differ. 2017, 60, 323–344. [Google Scholar]
- Pan, Z.; Tian, Y.; Niu, G.; Cao, C. Role of microRNAs in remodeling the tumor microenvironment (Review). Int. J. Oncol. 2020, 56, 407–416. [Google Scholar] [CrossRef]
- Fragiadaki, M.; Mason, R.M. Epithelial-mesenchymal transition in renal fibrosis—Evidence for and against. Int. J. Exp. Pathol. 2011, 92, 143–150. [Google Scholar] [CrossRef]
- Schieren, G.; Rumberger, B.; Klein, M.; Kreutz, C.; Wilpert, J.; Geyer, M.; Faller, D.; Timmer, J.; Quack, I.; Rump, L.C.; et al. Gene profiling of polycystic kidneys. Nephrol. Dial. Transpl. 2006, 21, 1816–1824. [Google Scholar] [CrossRef]
- Mets, E.; Van der Meulen, J.; Van Peer, G.; Boice, M.; Mestdagh, P.; Van de Walle, I.; Lammens, T.; Goossens, S.; De Moerloose, B.; Benoit, Y.; et al. MicroRNA-193b-3p acts as a tumor suppressor by targeting the MYB oncogene in T-cell acute lymphoblastic leukemia. Leukemia 2015, 29, 798–806. [Google Scholar] [CrossRef]
- Zhou, X.; Fan, L.X.; Sweeney, W.E.; Denu, J.M.; Avner, E.D.; Li, X. Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J. Clin. Investig. 2013, 123, 3084–3098. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Fan, L.X.; Zhou, J.X.; Grantham, J.J.; Calvet, J.P.; Sage, J.; Li, X. Lysine methyltransferase SMYD2 promotes cyst growth in autosomal dominant polycystic kidney disease. J. Clin. Investig. 2017, 127, 2751–2764. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.X.; Zhou, J.X.; Harris, P.C.; Calvet, J.P.; Li, X. RNA helicase p68 inhibits the transcription and post-transcription of. Theranostics 2020, 10, 8281–8297. [Google Scholar] [CrossRef]
- Aboudehen, K.; Farahani, S.; Kanchwala, M.; Chan, S.C.; Avdulov, S.; Mickelson, A.; Lee, D.; Gearhart, M.D.; Patel, V.; Xing, C.; et al. Long noncoding RNA Hoxb3os is dysregulated in autosomal dominant polycystic kidney disease and regulates mTOR signaling. J. Biol. Chem. 2018, 293, 9388–9398. [Google Scholar] [CrossRef]
- Hartung, E.A.; Guay-Woodford, L.M. Autosomal recessive polycystic kidney disease: A hepatorenal fibrocystic disorder with pleiotropic effects. Pediatrics 2014, 134, e833–e845. [Google Scholar] [CrossRef]
- Duan, J.; Huang, H.; Lv, X.; Wang, H.; Tang, Z.; Sun, H.; Li, Q.; Ai, J.; Tan, R.; Liu, Y.; et al. PKHD1 post-transcriptionally modulated by miR-365-1 inhibits cell-cell adhesion. Cell Biochem. Funct. 2012, 30, 382–389. [Google Scholar] [CrossRef]
- Ferrè, S.; Igarashi, P. New insights into the role of HNF-1β in kidney (patho)physiology. Pediatr. Nephrol. 2019, 34, 1325–1335. [Google Scholar] [CrossRef]
- Yang, L.; Frindt, G.; Palmer, L.G. Magnesium modulates ROMK channel-mediated potassium secretion. J. Am. Soc. Nephrol. 2010, 21, 2109–2116. [Google Scholar] [CrossRef]
- Hajarnis, S.S.; Patel, V.; Aboudehen, K.; Attanasio, M.; Cobo-Stark, P.; Pontoglio, M.; Igarashi, P. Transcription Factor Hepatocyte Nuclear Factor-1β (HNF-1β) Regulates MicroRNA-200 Expression through a Long Noncoding RNA. J. Biol. Chem. 2015, 290, 24793–24805. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.; Madzio, J.; Kozinski, K.; Patel, K.; Janikiewicz, J.; Szopa, M.; Tracz, A.; Borowiec, M.; Jarosz-Chobot, P.; Mysliwiec, M.; et al. Differential regulation of serum microRNA expression by HNF1β and HNF1α transcription factors. Diabetologia 2016, 59, 1463–1473. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Y.; Ni, D.; Liu, J.; Xia, H.; Xu, L.; Zhou, Q.; Xie, Y. miR-194 regulates the proliferation and migration via targeting Hnf1β in mouse metanephric mesenchyme cells. Vitr. Cell Dev. Biol. Anim. 2019, 55, 512–521. [Google Scholar] [CrossRef]
- Kornfeld, J.W.; Baitzel, C.; Könner, A.C.; Nicholls, H.T.; Vogt, M.C.; Herrmanns, K.; Scheja, L.; Haumaitre, C.; Wolf, A.M.; Knippschild, U.; et al. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature 2013, 494, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Nozu, K.; Takaoka, Y.; Kai, H.; Takasato, M.; Yabuuchi, K.; Yamamura, T.; Horinouchi, T.; Sakakibara, N.; Ninchoji, T.; Nagano, C.; et al. Genetic background, recent advances in molecular biology, and development of novel therapy in Alport syndrome. Kidney Res. Clin. Pract. 2020.
- Cosgrove, D.; Liu, S. Collagen IV diseases: A focus on the glomerular basement membrane in Alport syndrome. Matrix Biol. 2017, 57–58, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Gomez, I.G.; MacKenna, D.A.; Johnson, B.G.; Kaimal, V.; Roach, A.M.; Ren, S.; Nakagawa, N.; Xin, C.; Newitt, R.; Pandya, S.; et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J. Clin. Investig. 2015, 125, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Song, W.; Boulanger, J.; Xu, E.Y.; Wang, F.; Zhang, Y.; He, Q.; Wang, S.; Yang, L.; Pryce, C.; et al. Dysregulated Expression of microRNA-21 and Disease-Related Genes in Human Patients and in a Mouse Model of Alport Syndrome. Hum. Gene Ther. 2019, 30, 865–881. [Google Scholar] [CrossRef]
- Chau, B.N.; Xin, C.; Hartner, J.; Ren, S.; Castano, A.P.; Linn, G.; Li, J.; Tran, P.T.; Kaimal, V.; Huang, X.; et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci. Transl. Med. 2012, 4, 121ra18. [Google Scholar] [CrossRef]
- Sun, I.O.; Lerman, L.O. Urinary microRNA in kidney disease: Utility and roles. Am. J. Physiol. Renal. Physiol. 2019, 316, F785–F793. [Google Scholar] [CrossRef]
- Chen, W.; Tang, D.; Dai, Y.; Diao, H. Establishment of microRNA, transcript and protein regulatory networks in Alport syndrome induced pluripotent stem cells. Mol. Med. Rep. 2019, 19, 238–250. [Google Scholar] [CrossRef]
- Stonebrook, E.; Hoff, M.; Spencer, J.D. Congenital Anomalies of the Kidney and Urinary Tract: A Clinical Review. Curr. Treat. Options Pediatr. 2019, 5, 223–235. [Google Scholar] [CrossRef]
- van der Ven, A.T.; Vivante, A.; Hildebrandt, F. Novel Insights into the Pathogenesis of Monogenic Congenital Anomalies of the Kidney and Urinary Tract. J. Am. Soc. Nephrol. 2018, 29, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Marrone, A.K.; Ho, J. MicroRNAs: Potential regulators of renal development genes that contribute to CAKUT. Pediatr. Nephrol. 2014, 29, 565–574. [Google Scholar] [CrossRef][Green Version]
- Bartram, M.P.; Höhne, M.; Dafinger, C.; Völker, L.A.; Albersmeyer, M.; Heiss, J.; Göbel, H.; Brönneke, H.; Burst, V.; Liebau, M.C.; et al. Conditional loss of kidney microRNAs results in congenital anomalies of the kidney and urinary tract (CAKUT). J. Mol. Med. 2013, 91, 739–748. [Google Scholar] [CrossRef]
- Bartram, M.P.; Dafinger, C.; Habbig, S.; Benzing, T.; Schermer, B.; Müller, R.U. Loss of Dgcr8-mediated microRNA expression in the kidney results in hydronephrosis and renal malformation. BMC Nephrol. 2015, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, I.; Zivkovic, M.; Kostic, M.; Krstic, Z.; Djuric, T.; Kolic, I.; Alavantic, D.; Stankovic, A. Transcriptome-wide based identification of miRs in congenital anomalies of the kidney and urinary tract (CAKUT) in children: The significant upregulation of tissue miR-144 expression. J. Transl. Med. 2016, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Chen, J.; Vivante, A.; Hwang, D.Y.; Shril, S.; Dworschak, G.C.; Van Der Ven, A.; Sanna-Cherichi, S.; Bauer, S.B.; Lee, R.S.; et al. Targeted sequencing of 96 renal developmental microRNAs in 1213 individuals from 980 families with congenital anomalies of the kidney and urinary tract. Nephrol. Dial. Transpl. 2016, 31, 1280–1283. [Google Scholar] [CrossRef]
- Kim, E.; Zschiedrich, S. Renal Cell Carcinoma in von Hippel-Lindau Disease-From Tumor Genetics to Novel Therapeutic Strategies. Front. Pediatr. 2018, 6, 16. [Google Scholar] [CrossRef]
- Clark, P.E. The role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapy. Kidney Int. 2009, 76, 939–945. [Google Scholar] [CrossRef]
- Braga, E.A.; Fridman, M.V.; Loginov, V.I.; Dmitriev, A.A.; Morozov, S.G. Molecular Mechanisms in Clear Cell Renal Cell Carcinoma: Role of miRNAs and Hypermethylated miRNA Genes in Crucial Oncogenic Pathways and Processes. Front. Genet. 2019, 10, 320. [Google Scholar] [CrossRef]
- Mehdi, A.; Riazalhosseini, Y. Epigenome Aberrations: Emerging Driving Factors of the Clear Cell Renal Cell Carcinoma. Int. J. Mol. Sci. 2017, 18, 1774. [Google Scholar] [CrossRef]
- Schanza, L.M.; Seles, M.; Stotz, M.; Fosselteder, J.; Hutterer, G.C.; Pichler, M.; Stiegelbauer, V. MicroRNAs Associated with Von Hippel-Lindau Pathway in Renal Cell Carcinoma: A Comprehensive Review. Int. J. Mol. Sci. 2017, 18, 2495. [Google Scholar] [CrossRef]
- Gattolliat, C.H.; Couvé, S.; Meurice, G.; Oréar, C.; Droin, N.; Chiquet, M.; Ferlicot, S.; Verkarre, V.; Vasiliu, V.; Molinié, V.; et al. Integrative analysis of dysregulated microRNAs and mRNAs in multiple recurrent synchronized renal tumors from patients with von Hippel-Lindau disease. Int. J. Oncol. 2018, 53, 1455–1468. [Google Scholar] [CrossRef] [PubMed]
- Ivan, M.; Huang, X. miR-210: Fine-tuning the hypoxic response. Adv. Exp. Med. Biol. 2014, 772, 205–227. [Google Scholar]
- Kong, W.; He, L.; Richards, E.J.; Challa, S.; Xu, C.X.; Permuth-Wey, J.; Lancaster, J.M.; Coppola, D.; Sellers, T.A.; Djeu, J.Y.; et al. Upregulation of miRNA-155 promotes tumour angiogenesis by targeting VHL and is associated with poor prognosis and triple-negative breast cancer. Oncogene 2014, 33, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Bruning, U.; Cerone, L.; Neufeld, Z.; Fitzpatrick, S.F.; Cheong, A.; Scholz, C.C.; Simpon, D.A.; Leonard, M.O.; Tambuwala, M.M.; Cummins, E.P.; et al. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol. Cell Biol. 2011, 31, 4087–4096. [Google Scholar] [CrossRef]
- Mathew, L.K.; Lee, S.S.; Skuli, N.; Rao, S.; Keith, B.; Nathanson, K.L.; Lal, P.; Simon, M.C. Restricted expression of miR-30c-2-3p and miR-30a-3p in clear cell renal cell carcinomas enhances HIF2α activity. Cancer Discov. 2014, 4, 53–60. [Google Scholar] [CrossRef]
- Jaurretche, S.; Perez, G.; Antongiovanni, N.; Perretta, F.; Venera, G. Variables Associated with a Urinary MicroRNAs Excretion Profile Indicative of Renal Fibrosis in Fabry Disease Patients. Int. J. Chronic Dis. 2019, 2019, 4027606. [Google Scholar] [CrossRef]
- Cammarata, G.; Scalia, S.; Colomba, P.; Zizzo, C.; Pisani, A.; Riccio, E.; Montalbano, M.; Alessandro, R.; Giordano, A.; Duro, G. A pilot study of circulating microRNAs as potential biomarkers of Fabry disease. Oncotarget 2018, 9, 27333–27345. [Google Scholar] [CrossRef]
- Xiao, K.; Lu, D.; Hoepfner, J.; Santer, L.; Gupta, S.; Pfanne, A.; Thum, S.; Lenders, M.; Brand, E.; Nordbeck, P.; et al. Circulating microRNAs in Fabry Disease. Sci. Rep. 2019, 9, 15277. [Google Scholar] [CrossRef]
- Janssen, H.L.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; Van Der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef]



| Kidney Disorder or Syndrome | Characteristic Signs and Features | Genes and Protein | Involved Kidney Structure |
|---|---|---|---|
| Autosomal dominant polycystic kidney disease, type 1 | Polycystic kidneys, liver cysts, brain aneurysms, CKD | PKD1, Polycystin 1 | Renal tubules |
| Autosomal dominant polycystic kidney disease 1, type 2 | Polycystic kidneys, CKD | PKD2, Polycystin 2 | Renal tubules |
| Autosomal recessive polycystic kidney disease | Polycystic kidneys, liver fibrosis, CKD | PKHD1, Fibrocystin | Renal tubules |
| HNF1β-associated kidney disease (autosomal dominant) | Renal cyst, diabetes, CAKUT, and other renal manifestations | HNF1B, hepatocyte nuclear factor-1 beta | Renal tubules |
| Alport syndrome (X-linked) | Nephritis, SND, CKD | COL4A5, Type IV collagen α5 chain | Basement membrane |
| Alport Syndrome (autosomal recessive) | Alport syndrome or benign familial hematuria | COL4A3, Type IV collagen α3 chain | Basement membrane |
| Nephritis, SND, CKD | COL4A4, Type IV collagen α4 chain | Basement membrane | |
| Alport syndrome with leiomyomatosis (X-linked) | Alport syndrom with leiomyomatosis, CKD | COL4A5 and COL4A6, Type IV collagen α5 and α6 chain | Basement membrane |
| Congenital abnormalities of the kidney and urinary tract (CAKUT) (autosomal dominant or autosomal recessive) | CAKUT, hypodysplasia, cystic kidney disease, dysplastic kidney, hydronephrosis, ureteropelvic junction obstruction, ureter malformations, vesicoureteral reflux | FOXC1, forkhead transcription factor C1 | Renal tubules, podocytes, and basement membrane |
| HNF1B, hepatocyte nuclear factor-1 beta | Renal tubules | ||
| PAX2, paired box gene 2 | Renal tubules | ||
| Other more than 100 genes | |||
| Von-Lippel-Lindau (VHL) disease (autosomal dominant) | Lindau tumor, retinal angiomatosis, pheochromocytoma, renal tumor | VHL, Tumor suppressor gene g7 | Renal tubules |
| Fabry disease (X linked) | Angiokeratoma, FSGS, adult-onset CKD | GLA, α-galactosidaseA (α-galA) | Renal tubules, interstitium, and glomeruli |
| miRNA | Model | Expression | Target | Function | Ref. |
|---|---|---|---|---|---|
| miR17–92 cluster | Pkd1 mouse Pkd2 mouse Hnf1B mouse Pkhd1 mouse | upregulated | Pkd1, Pkd2, Hnf1B | Decrease the expression of PKD genes | [33] |
| miR-17 | Pkd1 mouse Pkd2 mouse Human ADPKD | upregulated | Pparα | Regulate mitochondrial metabolism, promote cystic cell proliferation and inflammation | [34,35] |
| miR-21 | Pkd1 mouse Pkd2 mouse Hnf1b mouse Pkhd1 mouse | upregulated | Pdcd4 | Inhibit cystic cell apoptosis | [36] |
| miR-199a-5p | Human ADPKD | upregulated | CDKN1C | Promote cell proliferation and inhibit apoptosis of cystic epithelia | [37] |
| miR-200 | Dicer mouse | downregulated | Pkd1 | Increase the expression of Pkd1 | [38] |
| miR-25-3p | Pkd1 mouse | upregulated | Atg14 | Suppress autophagy and increase cell proliferation | [39] |
| miR-214 | Pkd1 mouse Pkd2 mouse Human ADPKD | upregulated | TLR4 | Promote cyst growth and interstitial inflammation | [40] |
| miR-192 | Human ADPKD | downregulated | ZEB2 | Promote epithelial–mesenchymal transition | [41] |
| miR-194 | Human ADPKD | downregulated | CDH2 | Promote epithelial–mesenchymal transition | [41] |
| miR-194 | Human ADPKD | downregulated | PIK3R1, ANO1 | Promote cyst growth | [42] |
| miR-193b-3p | Human ADPKD | downregulated | ErbB4 | Promote cell proliferation | [43] |
| miR-501-5p | Human ADPKD | upregulated | PTEN, TSC1 | Promote proliferation and inhibit apoptosis of cystic epithelia | [44] |
| miR-182-5p | Pkd1 mouse | upregulated | Wasf2, Dock1, Itga4 | Modulate the actin cytoskeleton | [45] |
| miR-20b-5p, miR-106a-5p | Pkd2 mouse | downregulated | Klf12 | Promote cell proliferation | [46] |
| miR-9a-5p | PCK rat | downregulated by salt deficient diet | ENaC | Promote cyst growth | [47] |
| miR-15a | PCK rat | downregulated | Cdc25a | Promote proliferation of cholangiocyte cells | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.X.; Li, X. Non-Coding RNAs in Hereditary Kidney Disorders. Int. J. Mol. Sci. 2021, 22, 3014. https://doi.org/10.3390/ijms22063014
Zhou JX, Li X. Non-Coding RNAs in Hereditary Kidney Disorders. International Journal of Molecular Sciences. 2021; 22(6):3014. https://doi.org/10.3390/ijms22063014
Chicago/Turabian StyleZhou, Julie Xia, and Xiaogang Li. 2021. "Non-Coding RNAs in Hereditary Kidney Disorders" International Journal of Molecular Sciences 22, no. 6: 3014. https://doi.org/10.3390/ijms22063014
APA StyleZhou, J. X., & Li, X. (2021). Non-Coding RNAs in Hereditary Kidney Disorders. International Journal of Molecular Sciences, 22(6), 3014. https://doi.org/10.3390/ijms22063014





