RNA Helicases as Shadow Modulators of Cell Cycle Progression
Abstract
1. Introduction
2. Basic Regulation of Cell Cycle
3. RNA Helicases in the Regulation of Cell Cycle
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morgan, D.O. Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 1997, 13, 261–291. [Google Scholar] [CrossRef]
- Hochegger, H.; Takeda, S.; Hunt, T. Cyclin-dependent kinases and cell-cycle transitions: Does one fit all? Nat. Rev. Mol. Cell Biol. 2008, 9, 910–916. [Google Scholar] [CrossRef]
- Hopkins, M.; Tyson, J.J.; Novák, B. Cell-cycle transitions: A common role for stoichiometric inhibitors. Mol. Biol. Cell 2017, 28, 3437–3446. [Google Scholar] [CrossRef]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef]
- Black, A.R.; Black, J.D. Protein kinase C signaling and cell cycle regulation. Front. Immunol. 2013, 3, 423. [Google Scholar] [CrossRef]
- Teixeira, L.K.; Carrossini, N.; Sécca, C.; Kroll, J.E.; DaCunha, D.C.; Faget, D.V.; Carvalho, L.D.S.; de Souza, S.J.; Violaa, J.P.B. NFAT1 transcription factor regulates cell cycle progression and cyclin E expression in B lymphocytes. Cell Cycle 2016, 15, 2346–2359. [Google Scholar] [CrossRef] [PubMed]
- Daga, R.R.; Jimenez, J. Translational control of the cdc25 cell cycle phosphatase: A molecular mechanism coupling mitosis to cell growth. J. Cell. Sci. 1999, 18, 3137–3146. [Google Scholar]
- Zhivotovsky, B.; Orrenius, S. Cell cycle and cell death in disease: Past, present and future. J. Intern. Med. 2010, 268, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer. 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Swanton, C. Cell-cycle targeted therapies. Lancet Oncol. 2004, 5, 27–36. [Google Scholar] [CrossRef]
- van Bijsterveldt, L.; Durley, S.C.; Maughan, T.S.; Humphrey, T.C. The Challenge of Combining Chemo- and Radiotherapy with Checkpoint Kinase Inhibitors. Clin. Cancer Res. 2021, 27, 937–962. [Google Scholar] [CrossRef] [PubMed]
- Steimer, L.; Klostermeier, D. RNA helicases in infection and disease. RNA Biol. 2012, 9, 751–771. [Google Scholar] [CrossRef] [PubMed]
- Kronja, I.; Orr-Weaver, T.L. Translational regulation of the cell cycle: When, where, how and why? Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2011, 366, 3638–3652. [Google Scholar] [CrossRef] [PubMed]
- Van Voss, M.R.H.; Van Diest, P.J.; Raman, V. Targeting RNA helicases in cancer: The translation trap. Biochim. Biophys. Acta. 2017, 1868, 510–520. [Google Scholar] [CrossRef]
- Yoneyama-Hirozane, M.; Kondo, M.; Matsumoto, S.; Morikawa-Oki, A.; Morishita, D.; Nakanishi, A.; Kawamoto, T.; Nakayama, M. High-Throughput Screening to Identify Inhibitors of DEAD Box Helicase DDX41. SLAS Discov. 2017, 22, 1084–1092. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakao, S.; Nogami, M.; Iwatani, M.; Imaeda, T.; Ito, M.; Tanaka, T.; Tawada, M.; Endo, S.; Cary, D.R.; Ohori, M.; et al. Identification of a selective DDX3X inhibitor with newly developed quantitative high-throughput RNA helicase assays. Biochem. Biophys. Res. Commun. 2020, 523, 795–801. [Google Scholar] [CrossRef]
- Lindqvist, L.; Oberer, M.; Reibarkh, M.; Cencic, R.; Bordeleau, M.-E.; Vogt, E.; Marintchev, A.; Tanaka, J.; Fagotto, F.; Altmann, M.; et al. Selective Pharmacological Targeting of a DEAD Box RNA Helicase. PLoS ONE 2008, 3, e1583. [Google Scholar] [CrossRef]
- Belon, C.A.; Frick, D.N. Helicase inhibitors as specifically targeted antiviral therapy for hepatitis C. Future Virol. 2009, 4, 277–293. [Google Scholar] [CrossRef]
- Daibler, R.W.; Kirschner, M.W. Quantitative reconstitution of mitotic CDK1 activation in somatic cell extracts. Moll. Cell 2010, 37, 753–767. [Google Scholar] [CrossRef]
- Parry, D.H.; O’Farrell, P.H. The schedule of destruction of three mitotic cyclins can dictate the timing of events during exit from mitosis. Curr. Biol. 2001, 11, 671–683. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; De Bruin, R.A.M. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef]
- Hofmann, F.; Livingston, D.M. Differential effects of cdk2 and cdk3 on the control of pRb and E2F function during G1 exit. Genes Dev. 1996, 10, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Topacio, B.R.; Zatulovskiy, E.; Cristea, S.; Xie, S.; Tambo, C.S.; Rubin, S.M.; Sage, J.; Kõivomägi, M.; Skotheim, J.M. Cyclin D-Cdk4,6 drives cell-cycle progression via the retinoblastoma protein’s C-terminal helix. Mol. Cell. 2019, 74, 758–770. [Google Scholar] [CrossRef]
- Tyson, J.J.; Novak, B. Temporal organization of the cell cycle. Curr. Biol. 2008, 18, R759–R768. [Google Scholar] [CrossRef]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122–132. [Google Scholar] [CrossRef]
- Gookin, S.; Min, M.; Phadke, H.; Chung, M.; Moser, J.; Miller, I.; Carter, D.; Spencer, S.L. A map of protein dynamics during cell-cycle progression and cell-cycle exit. PloS Biol. 2007, 15, e2003268. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer. 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, S.; Roussel-Gervais, A.; Drouin, J. Distinct Developmental Roles of Cell Cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Mol. Cell. Biol. 2009, 29, 1895–1908. [Google Scholar] [CrossRef]
- Dannenberg, J.-H.; Van Rossum, A.; Schuijff, L.; Riele, H.T. Ablation of the retinoblastoma gene family deregulates G1 control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev. 2000, 14, 3051–3064. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Tan, Y.; Zhou, C.; Melmed, S. Pituitary tumor transforming gene interacts with Sp1 to modulate G1/S cell phase transition. Oncogene 2007, 26, 5596–5605. [Google Scholar] [CrossRef] [PubMed]
- Prives, C. Doing the right thing: Feedback control and p53. Curr. Opin. Cell Biol. 1993, 5, 214–218. [Google Scholar] [CrossRef]
- Murray, A. Cell cycle checkpoints. Curr. Opin. Cell Biol. 1994, 6, 872–876. [Google Scholar] [CrossRef]
- Fairman-Williams, M.E.; Guenther, U.-P.; Jankowsky, E. SF1 and SF2 helicases: Family matters. Curr. Opin. Struct. Biol. 2010, 20, 313–324. [Google Scholar] [CrossRef]
- Caruthers, J.M.; McKay, D.B. Helicase structure and mechanism. Curr. Opin. Struct. Biol. 2002, 12, 123–133. [Google Scholar] [CrossRef]
- Jarmoskaite, I.; Russell, R. DEAD-box proteins as RNA helicases and chaperones. Wiley Interdiscip. Rev. RNA 2011, 2, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Pace, F.V. DExD/H box RNA helicases: Multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Res. 2006, 34, 4206–4215. [Google Scholar] [CrossRef]
- Jankowsky, E. RNA Helicases at work: Binding and rearranging. Trends Biochem. Sci. 2011, 36, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Straub, A.U.; Doebele, C.; Bohnsack, M.T. DExD/H-box RNA helicases in ribosome biogenesis. RNA Biol. 2013, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.F.; Mortreux, F.; Auboeuf, D. The multiple functions of RNA helicases as drivers and regulators of gene expression. Nat. Rev. Mol. Cell Biol. 2016, 17, 426–438. [Google Scholar] [CrossRef]
- Cencic, R.; Carrier, M.; Galicia-Vázquez, G.; Bordeleau, M.-E.; Sukarieh, R.; Bourdeau, A.; Brem, B.; Teodoro, J.G.; Greger, H.; Tremblay, M.L.; et al. Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, silvestrol. PLoS ONE 2009, 4, e5223. [Google Scholar] [CrossRef] [PubMed]
- Bol, G.M.; Vesuna, F.; Xie, M.; Zeng, J.; Aziz, K.; Gandhi, N.; Levine, A.; Irving, A.; Korz, D.; Tantravedi, S.; et al. Targeting DDX3 with a small molecule inhibitor for lung cancer therapy. EMBO Mol. Med. 2015, 7, 648–669. [Google Scholar] [CrossRef]
- Ranji, A.; Boris-Lawrie, K. RNA helicases: Emerging roles in viral replication and the host innate response. RNA Biol. 2010, 7, 775–787. [Google Scholar] [CrossRef]
- Chaar, W.; Ibrahim, H.; Kozah, J.; Chamieh, H. Comparative analysis data of SF1 and SF2 helicases from three domains of life. Data Brief. 2017, 11, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.-W.; Lee, Y.-H.W. Human DExD/H RNA helicases: Emerging roles in stress survival regulation. Clin. Chim. Acta. 2014, 436, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Frick, D.N. Helicases as antiviral drug targets. Drug News Perspect. 2003, 16, 355–362. [Google Scholar] [CrossRef]
- Lai, M.-C.; Lee, Y.-H.W.; Tarn, W.-Y. The DEAD-box RNA helicase DDX3 associates with export messenger ribonucleoproteins as well as tip-associated protein and participates in translational control. Mol. Biol. Cell. 2008, 19, 3847–3858. [Google Scholar] [CrossRef]
- Soto-Rifo, R.; Rubilar, P.S.; Limousin, T.; De Breyne, S.; Décimo, D.; Ohlmann, T. DEAD-box protein DDX3 associates with eIF4F to promote translation of selected mRNAs. EMBO J. 2012, 31, 3745–3756. [Google Scholar] [CrossRef]
- Lee, C.-S.; Dias, A.P.; Jedrychowski, M.; Patel, A.H.; Hsu, J.L.; Reed, R. Human DDX3 functions in translation and interacts with the translation initiation factor eIF3. Nucleic Acids Res. 2008, 36, 4708–4718. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, P.; Zhang, C.; Wang, Y.; Wan, R.; Yang, Y.; Guo, X.; Huo, R.; Lin, M.; Zhou, Z.; et al. DDX3X regulates cell survival and cell cycle during mouse early embryonic development. J. Biomed. Res. 2014, 28, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhou, T.; Jonasch, E.; Jope, R. DDX3 regulates DNA damage-induced apoptosis and p53 stabilization. Biochim. Biophys. Acta. 2013, 1833, 1489–1497. [Google Scholar] [CrossRef]
- Lai, M.-C.; Chang, W.-C.; Shieh, S.-Y.; Tarn, W.-Y. DDX3 regulates cell growth through translational control of cyclin E1. Mol. Cell. Biol. 2010, 30, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, E.; Bannister, A.J.; Han, N.; Alendar, A.; Kouzarides, T. DDX3X RNA helicase affects breast cancer cell cycle progression by regulating expression of KLF4. FEBS Lett. 2018, 592, 2308–2322. [Google Scholar] [CrossRef] [PubMed]
- Calviello, L.; Venkataramanan, S.; Rogowski, K.J.; Wyler, E.; Wilkins, K.; Tejura, M.; Thai, B.; Krol, J.; Filipowicz, W.; Landthaler, M.; et al. DDX3 depletion represses translation of mRNAs with complex 5′ UTRs. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kosowski, T.R.; Keys, H.R.; Quan, T.K.; Ruby, S.W. DExD/H-box Prp5 protein is in the spliceosome during most of the splicing cycle. RNA N. Y. N. 2009, 15, 1345–1362. [Google Scholar] [CrossRef][Green Version]
- Zheng, Q.; Hou, J.; Zhou, Y.; Li, Z.; Cao, X. The RNA helicase DDX46 inhibits innate immunity by entrapping m 6 A-demethylated antiviral transcripts in the nucleus. Nat. Immunol. 2017, 18, 1094–1103. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.-M.; He, W.-T.; Chen, H.; Zhu, H.-W.; Liu, T.; Zhang, J.-H.; Song, T.-N.; Zhou, Y.-L. Knockdown of DDX46 inhibits proliferation and induces apoptosis in esophageal squamous cell carcinoma cells. Oncol. Rep. 2016, 36, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Marcon, B.H.; Rebelatto, C.K.; Cofré, A.R.; Dallagiovanna, B.; Correa, A. DDX6 helicase behavior and protein partners in human adipose tissue-derived stem cells during early adipogenesis and osteogenesis. Int. J. Mol. Sci. 2020, 21, 2607. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.; Sommerville, J. Xp54 and related (DDX6-like) RNA helicases: Roles in messenger RNP assembly, translation regulation and RNA degradation. Nucleic Acids Res. 2006, 34, 3082–3094. [Google Scholar] [CrossRef]
- De Vries, S.; Vries, I.S.N.; Urlaub, H.; Lue, H.; Bernhagen, J.; Ostareck, D.H.; Ostareck-Lederer, A. Identification of DEAD-box RNA helicase 6 (DDX6) as a cellular modulator of vascular endothelial growth factor expression under hypoxia. J. Biol. Chem. 2013, 288, 5815–5827. [Google Scholar] [CrossRef]
- Smillie, D.A.; Sommerville, J. RNA helicase p54 (DDX6) is a shuttling protein involved in nuclear assembly of stored mRNP particles. J. Cell Sci. 2002, 115, 395–407. [Google Scholar]
- Bergkessel, M.; Reese, J.C. An essential role for the Saccharomyces cerevisiae DEAD-box helicase DHH1 in G1/S DNA-damage checkpoint recovery. Genetics 2004, 167, 21–33. [Google Scholar] [CrossRef]
- Coller, J.; Parker, R. General translational repression by activators of mRNA decapping. Cell 2005, 122, 875–886. [Google Scholar] [CrossRef]
- Akao, Y.; Matsumoto, K.; Ohguchi, K.; Nakagawa, Y.; Yoshida, H. Human DEAD-box/RNA unwindase rck/p54 contributes to maintenance of cell growth by affecting cell cycle in cultured cells. Int. J. Oncol. 2006, 29, 41–48. [Google Scholar] [CrossRef]
- Lin, F.; Wang, R.; Shen, J.-J.; Wang, X.; Gao, P.; Dong, K.; Zhang, H.-Z. Knockdown of RCK/p54 expression by RNAi inhibits proliferation of human colorectal cancer cells in vitro and in vivo. Cancer Biol. Ther. 2008, 7, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Nakagawa, Y.; Morikawa, H.; Niki, M.; Egashira, Y.; Hirata, I.; Katsu, K.; Akao, Y. Co-overexpression of DEAD box protein rck/p54 and c-myc protein in human colorectal adenomas and the relevance of their expression in cultured cell lines. Carcinogenesis 2001, 22, 1965–1970. [Google Scholar] [CrossRef]
- Akao, Y.; Mizoguchi, H.; Ohishi, N.; Yagi, K. Growth inhibition by overexpression of human DEAD box protein rck/p54 in cells of a guinea pig cell line. FEBS Lett. 1998, 429, 279–283. [Google Scholar] [CrossRef]
- Tajirika, T.; Tokumaru, Y.; Taniguchi, K.; Sugito, N.; Matsuhashi, N.; Futamura, M.; Yanagihara, K.; Akao, Y.; Yoshida, K. DEAD-Box protein RNA-helicase DDX6 regulates the expression of HER2 and FGFR2 at the post-transcriptional step in gastric cancer cells. Int. J. Mol. Sci. 2017, 19, 2005. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Kang, W.; Kim, S.H.; Sa, J.K.; Kim, N.; Paddison, P.J.; Kim, M.; Joo, K.M.; Hwang, Y.-I.; Nam, D.-H. Involvement of DDX6 gene in radio- and chemoresistance in glioblastoma. Int. J. Oncol. 2016, 48, 1053–1062. [Google Scholar] [CrossRef]
- Henning, D.; So, R.B.; Jin, R.; Lau, L.F.; Valdez, B.C. Silencing of RNA helicase II/Gualpha inhibits mammalian ribosomal RNA production. J. Biol. Chem. 2003, 278, 52307–52314. [Google Scholar] [CrossRef]
- Holmström, T.H.; Mialon, A.; Kallio, M.; Nymalm, Y.; Mannermaa, L.; Holm, T.; Johansson, H.; Black, E.; Gillespie, D.; Salminen, T.A.; et al. c-Jun supports ribosomal RNA processing and nucleolar localization of RNA helicase DDX21. J. Biol. Chem. 2008, 283, 7046–7053. [Google Scholar] [CrossRef]
- Song, C.; Hotz-Wagenblatt, A.; Voit, R.; Grummt, I. SIRT7 and the DEAD-box helicase DDX21 cooperate to resolve genomic R loops and safeguard genome stability. Genes Dev. 2017, 31, 1370–1381. [Google Scholar] [CrossRef]
- Zhang, Y.; Baysac, K.C.; Yee, L.-F.; Saporita, A.J.; Weber, J.D. Elevated DDX21 regulates c-Jun activity and rRNA processing in human breast cancers. Breast Cancer Res. BCR 2014, 16, 449. [Google Scholar] [CrossRef]
- Cao, J.; Wu, N.; Han, Y.; Hou, Q.; Zhao, Y.; Pan, Y.; Xie, X.; Chen, F. DDX21 promotes gastric cancer proliferation by regulating cell cycle, Biochem. Biophys. Res. Commun. 2018, 505, 1189–1194. [Google Scholar] [CrossRef]
- Kim, D.-S.; Camacho, C.V.; Nagari, A.; Malladi, V.S.; Challa, S.; Kraus, W.L. Activation of PARP-1 by snoRNAs controls ribosome biogenesis and cell growth via the RNA helicase DDX21. Mol. Cell. 2019, 75, 1270–1285.e14. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, L.; Lapik, Y.R.; Wang, M.; Pestov, D.G. Mammalian DEAD box protein Ddx51 acts in 3′ end maturation of 28S rRNA by promoting the release of U8 snoRNA. Mol. Cell. Biol. 2010, 30, 2947–2956. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, Z.; Zhou, L.; Li, X.; Jiang, T.; Fu, E. DDX5 promotes proliferation and tumorigenesis of non-small-cell lung cancer cells by activating β-catenin signaling pathway. Cancer Sci. 2015, 106, 1303–1312. [Google Scholar] [CrossRef]
- Sun, W.; Cang, S.; Lv, X.; Wang, P.; Lin, Q.; Zhang, Q.; Yan, Z.; Liu, Z.; Song, Y. DDX51 gene promotes proliferation by activating Wnt/β-catenin signaling in breast cancer. Int. J. Clin. Exp. Pathol. 2017, 10, 10892–10900. [Google Scholar] [PubMed]
- Wang, X.; Liu, H.; Zhao, C.; Li, W.; Xu, H.; Chen, Y. The DEAD-box RNA helicase 51 controls non-small cell lung cancer proliferation by regulating cell cycle progression via multiple pathways. Sci. Rep. 2016, 6, 26108. [Google Scholar] [CrossRef]
- Zonta, E.; Bittencourt, D.; Samaan, S.; Germann, S.; Dutertre, M.; Auboeuf, D. The RNA helicase DDX5/p68 is a key factor promoting c-fos expression at different levels from transcription to mRNA export. Nucleic Acids Res. 2013, 41, 554–564. [Google Scholar] [CrossRef]
- Kar, A.; Fushimi, K.; Zhou, X.; Ray, P.; Shi, C.; Chen, X.; Liu, Z.; Chen, S.; Wu, J.Y. RNA helicase p68 (DDX5) regulates tau exon 10 splicing by modulating a stem-loop structure at the 5’ splice site. Mol Cell Biol. 2011, 31, 1812–1821. [Google Scholar] [CrossRef]
- Jalal, C.; Uhlmann-Schiffler, H.; Stahl, H. Redundant role of DEAD box proteins p68 (Ddx5) and p72/p82 (Ddx17) in ribosome biogenesis and cell proliferation. Nucleic Acids Res. 2007, 35, 3590–3601. [Google Scholar] [CrossRef]
- Salzman, D.W.; Shubert-Coleman, J.; Furneaux, H. P68 RNA helicase unwinds the human let-7 microRNA precursor duplex and is required for let-7-directed silencing of gene expression. J. Biol. Chem. 2007, 282, 32773–32779. [Google Scholar] [CrossRef]
- Sithole, N.; Williams, C.A.; Abbink, T.E.M.; Lever, A.M.L. DDX5 potentiates HIV-1 transcription as a co-factor of Tat. Retrovirology 2020, 17, 1–16. [Google Scholar] [CrossRef]
- Clark, E.L.; Coulson, A.; Dalgliesh, C.; Rajan, P.; Nicol, S.M.; Fleming, S.; Heer, R.; Gaughan, L.; Leung, H.Y.; Elliott, D.J.; et al. The RNA helicase p68 is a novel androgen receptor coactivator involved in splicing and is overexpressed in prostate cancer. Cancer Res. 2008, 68, 7938–7946. [Google Scholar] [CrossRef]
- Jensen, E.D.; Niu, L.; Caretti, G.; Nicol, S.M.; Teplyuk, N.; Stein, G.S.; Sartorelli, V.; van Wijnen, A.J.; Fuller-Pace, F.V.; Westendorf, J.J. p68 (Ddx5) interacts with Runx2 and regulates osteoblast differentiation. J. Cell. Biochem. 2008, 103, 1438–1451. [Google Scholar] [CrossRef]
- Wang, R.; Jiao, Z.; Li, R.; Yue, H.; Chen, L. p68 RNA helicase promotes glioma cell proliferation in vitro and in vivo via direct regulation of NF-κB transcription factor p50. Neuro-Oncol. 2012, 14, 1116–1124. [Google Scholar] [CrossRef]
- Yang, L.; Lin, C.; Liu, Z.-R. P68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing Axin from beta-catenin. Cell 2006, 127, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.L.; Lin, C.; Liu, C.-Y.; Yang, L.; Liu, Z.-R. Phosphorylated p68 RNA helicase activates Snail1 transcription by promoting HDAC1 dissociation from the Snail1 promoter. Oncogene 2010, 29, 5427–5436. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Rossow, K.L.; Grande, J.P.; Janknecht, R. Involvement of RNA Helicases p68 and p72 in Colon Cancer. Cancer Res. 2007, 67, 7572–7578. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.K.K.; Yan, B.; Cui, Z.; Sun, J.; Utturkar, S.; Foca, A.; Fares, N.; Durantel, D.; Lanman, N.; Merle, P.; et al. Restoration of RNA helicase DDX5 suppresses hepatitis B virus (HBV) biosynthesis and Wnt signaling in HBV-related hepatocellular carcinoma. Theranostics 2020, 10, 10957–10972. [Google Scholar] [CrossRef]
- Zhang, M.; Weng, W.; Zhang, Q.; Wu, Y.; Ni, S.; Tan, C.; Xu, M.; Sun, H.; Liu, C.; Wei, P.; et al. The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5. J. Hepatol. Oncol. 2018, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Legrand, J.M.D.; Chan, A.-L.; La, H.M.; Rossello, F.J.; Änkö, M.-L.; Fuller-Pace, F.V.; Hobbs, R.M. DDX5 plays essential transcriptional and post-transcriptional roles in the maintenance and function of spermatogonia. Nat. Commun. 2019, 10, 2278. [Google Scholar] [CrossRef]
- Hao, Q.; Zong, X.; Sun, Q.; Lin, Y.-C.; Song, Y.J.; Hashemikhabir, S.; Hsu, R.Y.; Kamran, M.; Chaudhary, R.; Tripathi, V.; et al. The S-phase-induced lncRNA SUNO1 promotes cell proliferation by controlling YAP1/Hippo signaling pathway. ELife 2020, 9, e55102. [Google Scholar] [CrossRef]
- Caretti, G.; Schiltz, R.L.; Dilworth, F.J.; Di Padova, M.; Zhao, P.; Ogryzko, V.; Fuller-Pace, F.V.; Hoffman, E.P.; Tapscott, S.J.; Sartorelli, V. The RNA helicases p68/p72 and the noncoding RNA SRA are coregulators of MyoD and skeletal muscle differentiation. Dev. Cell. 2006, 11, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.J.; Nicol, S.M.; Wilson, B.J.; Jacobs, A.-M.F.; Bourdon, J.-C.; Wardrop, J.; Gregory, D.J.; Lane, D.P.; Perkins, N.D.; Fuller-Pace, F.V. The DEAD box protein p68: A novel transcriptional coactivator of the p53 tumour suppressor. EMBO J. 2005, 24, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Nicol, S.M.; Bray, S.E.; Black, H.D.; Lorimore, S.A.; Wright, E.G.; Lane, D.P.; Meek, D.W.; Coates, P.J.; Fuller-Pace, F.V. The RNA helicase p68 (DDX5) is selectively required for the induction of p53-dependent p21 expression and cell-cycle arrest after DNA damage. Oncogene 2013, 32, 3461–3469. [Google Scholar] [CrossRef]
- Yang, L.; Lin, C.; Zhao, S.; Wang, H.; Liu, Z.-R. Phosphorylation of p68 RNA helicase plays a role in platelet-derived growth factor-induced cell proliferation by up-regulating cyclin D1 and c-Myc expression. J. Biol. Chem. 2007, 282, 16811–16819. [Google Scholar] [CrossRef]
- Mazurek, A.; Luo, W.; Krasnitz, A.; Hicks, J.; Powers, R.S.; Stillman, B. DDX5 regulates DNA replication and is required for cell proliferation in a subset of breast cancer cells. Cancer Discov. 2012, 2, 812–825. [Google Scholar] [CrossRef]
- Zhang, Y.; Forys, J.; Miceli, A.; Gwinn, A.; Weber, J. Identification of DHX33 as a mediator of rRNA synthesis and cell growth. Mol. Cell. Biol. 2011, 31, 4676–4691. [Google Scholar] [CrossRef]
- Zhang, Y.; You, J.; Wang, X.; Weber, J. The DHX33 RNA helicase promotes mRNA translation initiation. Mol. Cell. Biol. 2015, 35, 2918–2931. [Google Scholar] [CrossRef][Green Version]
- Fu, J.; Liu, Y.; Wang, X.; Yuan, B.; Zhang, Y. Role of DHX33 in c-Myc-induced cancers. Carcinogenesis 2017, 38, 649–660. [Google Scholar] [CrossRef]
- Mitoma, H.; Hanabuchi, S.; Kim, T.; Bao, M.; Zhang, Z.; Sugimoto, N.; Liu, Y.-J. The DHX33 RNA helicase senses cytosolic RNA and activates the NLRP3 inflammasome. Immunity 2013, 39, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wang, X.; Fan, C.; You, J.; Liu, Y.; Weber, J.D.; Zhong, H.; Zhang, Y. DHX33 transcriptionally controls genes involved in the cell cycle. Mol. Cell. Biol. 2016, 36, 2903–2917. [Google Scholar] [CrossRef]
- Wang, H.; Yu, J.; Wang, X.; Zhang, Y. The RNA helicase DHX33 is required for cancer cell proliferation in human glioblastoma and confers resistance to PI3K/mTOR inhibition. Cell Signal. 2019, 54, 170–178. [Google Scholar] [CrossRef]
- Feng, W.; Chen, S.; Wang, J.; Wang, X.; Chen, H.; Ning, W.; Zhang, Y. DHX33 Recruits Gadd45a To Cause DNA Demethylation and Regulates a Subset of Gene Transcription. Mol. Cell. Biol. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, W.; Peng, C.; Chen, S.; Ji, H.; Zhong, H.; Ge, W.; Zhang, Y. Targeting RNA helicase DHX33 blocks Ras-driven lung tumorigenesis in vivo. Cancer Sci. 2020, 111, 3564–3575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yuan, B.; Bao, M.; Lu, N.; Kim, T.; Liu, Y.-J. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat. Immunol. 2011, 12, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Polprasert, C.; Schulze, I.; Sekeres, M.A.; Makishima, H.; Przychodzen, B.; Hosono, N.; Singh, J.; Padgett, R.A.; Gu, X.; Phillips, J.G.; et al. Inherited and somatic defects in DDX41 in myeloid neoplasms. Cancer Cell. 2015, 27, 658–670. [Google Scholar] [CrossRef]
- Peters, D.; Radine, C.; Reese, A.; Budach, W.; Sohn, D.; Jänicke, R.U. The DEAD-box RNA helicase DDX41 is a novel repressor of p21WAF1/CIP1 mRNA translation. J. Biol. Chem. 2017, 292, 8331–8341. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.W.; Komar, A.A.; Merrick, W.C. eIF4A: The godfather of the DEAD box helicases. Prog. Nucleic Acid Res. Mol. Biol. 2002, 72, 307–331. [Google Scholar] [CrossRef]
- Sokabe, M.; Fraser, C.S. A helicase-independent activity of eIF4A in promoting mRNA recruitment to the human ribosome. Proc. Natl. Acad. Sci. USA 2017, 114, 6304–6309. [Google Scholar] [CrossRef]
- Rubio, C.A.; Weisburd, B.; Holderfield, M.; Arias, C.; Fang, E.; DeRisi, J.L.; Fanidi, A. Transcriptome-wide characterization of the eIF4A signature highlights plasticity in translation regulation. Genome Biol. 2015, 15, 476. [Google Scholar] [CrossRef] [PubMed]
- Montero, H.; Pérez-Gil, G.; Sampieri, C.L. Eukaryotic initiation factor 4A (eIF4A) during viral infections. Virus Genes 2019, 55, 267–273. [Google Scholar] [CrossRef]
- Naineni, S.K.; Maiga, R.I.; Cencic, R.; Putnam, A.A.; Amador, L.A.; Rodriguez, A.D.; Jankowsky, E.; Pelletier, J. A comparative study of small molecules targeting eIF4A. RNA 2020, 26, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Wang, Y.-N.; Xia, W.; Hsu, S.-C.; Lai, C.-C.; Li, L.-Y.; Chang, W.-C.; Wang, Y.; Hsu, M.-C.; Yu, Y.-L.; et al. RNA helicase A is a DNA-binding partner for EGFR-mediated transcriptional activation in the nucleus. Proc. Natl. Acad. Sci. USA 2010, 107, 16125-30. [Google Scholar] [CrossRef]
- Fidaleo, M.; Svetoni, F.; Volpe, E.; Miñana, B.; Caporossi, D.; Paronetto, M.P. Genotoxic stress inhibits Ewing sarcoma cell growth by modulating alternative pre-mRNA processing of the RNA helicase DHX9. Oncotarget 2015, 6, 31740–31757. [Google Scholar] [CrossRef] [PubMed]
- Palombo, R.; Frisone, P.; Fidaleo, M.; Mercatelli, N.; Sette, C.; Paronetto, M.P. The Promoter-Associated Noncoding RNA pncCCND1_B Assembles a Protein–RNA Complex to Regulate Cyclin D1 Transcription in Ewing Sarcoma. Cancer Res. 2019, 79, 3570–3582. [Google Scholar] [CrossRef]
- Myöhänen, S.; Baylin, S. Sequence-specific DNA Binding Activity of RNA Helicase A to the p16INK4a Promoter. J. Biol. Chem. 2001, 276, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, B.P.; Starita, L.M.; Parvin, J.D. Overexpression of a protein fragment of RNA helicase A causes inhibition of endogenous BRCA1 function and defects in ploidy and cytokinesis in mammary epithelial cells. Oncogene 2003, 22, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Paquet, M.; Larsson, O.; Pelletier, J. Tumor cell survival dependence on the DHX9 DExH-box helicase. Oncogene 2016, 35, 5093–5105. [Google Scholar] [CrossRef] [PubMed]
- Thacker, U.; Pauzaite, T.; Tollitt, J.; Twardowska, M.; Harrison, C.; Dowle, A.; Coverley, D.; Copeland, N. Identification of DHX9 as a cell cycle regulated nucleolar recruitment factor for CIZ1. Sci. Rep. 2020, 10, 18103. [Google Scholar] [CrossRef] [PubMed]
- Kouyama, Y.; Masuda, T.; Fujii, A.; Ogawa, Y.; Sato, K.; Tobo, T.; Wakiyama, H.; Yoshikawa, Y.; Noda, M.; Tsuruda, Y.; et al. Oncogenic splicing abnormalities induced by DEAD-Box Helicase 56 amplification in colorectal cancer. Cancer Sci. 2019, 110, 3132–3144. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, X.; Kourkoumelis, N.; Shen, Y.; Huang, W. Integrated Analysis of DEAD-Box Helicase 56: A Potential Oncogene in Osteosarcoma. Front Bioeng. Biotechnol. 2020, 8, 588. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Calero, C.; Bayona-Feliu, A.; Xue, X.; Barroso, S.I.; Muñoz, S.; González-Basallote, V.M.; Sung, P.; Aguilera, A. UAP56/DDX39B is a major cotranscriptional RNA–DNA helicase that unwinds harmful R loops genome-wide. Genes Dev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.; Fedoryszak-Kuśka, N.; Tkaczuk, K.; Dobrucki, J.; Waligórska, A.; Stępień, P.P. Human SUV3 helicase regulates growth rate of the HeLa cells and can localize in the nucleoli. Acta Biochim. Pol. 2017, 64, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, Z.; Lu, J.Y.; Huang, B.; Zhou, H.; Xie, W.; Wang, J.; Shen, X. DEAD-box helicase 18 counteracts PRC2 to safeguard ribosomal DNA in pluripotency regulation. Cell Rep. 2020, 30, 81–97. [Google Scholar] [CrossRef]
- Ehsani, A.; Alluin, J.V.; Rossi, J.J. Cell cycle abnormalities associated with differential perturbations of the human U5 snRNP associated U5-200kD RNA helicase. PLoS ONE 2013, 8, e62125. [Google Scholar] [CrossRef] [PubMed]
- Hasgall, P.A.; Hoogewijs, D.; Faza, M.B.; Panse, V.G.; Wenger, R.H.; Camenisch, G. The putative RNA helicase HELZ promotes cell proliferation, translation initiation and ribosomal protein S6 phosphorylation. PLoS ONE 2011, 6, e22107. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Chen, C.-M.; Cheng, P.-L.; Shih, J.-W.; Tsou, A.-P.; Lee, Y.-H.W. DDX3, a DEAD box RNA helicase with tumor growth-suppressive property and transcriptional regulation activity of the p21waf1/cip1 promoter, is a candidate tumor suppressor. Cancer Res. 2006, 66, 6579–6588. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Ping, P.; Wang, G.; Zhang, X.; Sun, F. Imsnc761 and DDX6 synergistically suppress cell proliferation and promote apoptosis via p53 in testicular embryonal carcinoma cells. Biosci. Rep. 2018, 38, BSR20180271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Arribas-Layton, Y.; Chen, J.; Lykke-Andersen, G.L. DDX6 Orchestrates Mammalian Progenitor Function through the mRNA Degradation and Translation Pathways. Mol. Cell. 2015, 60, 118–130. [Google Scholar] [CrossRef]
- Barkovich, K.J.; Moore, M.K.; Hu, Q.; Shokat, K.M. Chemical genetic inhibition of DEAD-box proteins using covalent complementarity. Nucleic Acids Res. 2018, 46, 8689–8699. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chen, Z.X.; Rane, G.; Singh, S.S.; Choo, Z.; Wang, C.; Yuan, Y.; Tan, T.Z.; Arfuso, F.; Yap, C.T.; et al. Wanted DEAD/H or alive: Helicases winding up in cancers. JNCI J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.D.; Rao, B.G.; Jeang, K.-T. Viral and cellular RNA helicases as antiviral targets. Nat. Rev. Drug Discov. 2005, 4, 845–853. [Google Scholar] [CrossRef]
- Cencic, R.; Galicia-Vázquez, G.; Pelletier, J. Inhibitors of translation targeting eukaryotic translation initiation factor 4A. Methods Enzymol. 2012, 511, 437–461. [Google Scholar] [CrossRef]
- Tsumuraya, T.; Ishikawa, C.; Machijima, Y.; Nakachi, S.; Senba, M.; Tanaka, J.; Mori, N. Effects of hippuristanol, an inhibitor of eIF4A, on adult T-cell leukemia. Biochem. Pharmacol. 2011, 81, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Low, W.-K.; Dang, Y.; Schneider-Poetsch, T.; Shi, Z.; Choi, N.S.; Merrick, W.C.; Romo, D.; Liu, J.O. Inhibition of eukaryotic translation initiation by the marine natural product pateamine A. Mol. Cell. 2005, 20, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hwang, B.Y.; Su, B.-N.; Chai, H.; Mi, Q.; Kinghorn, A.D.; Wild, R.; Swanson, S.M. Silvestrol, a potential anticancer rocaglate derivative from Aglaia foveolata, induces apoptosis in LNCaP cells through the mitochondrial/apoptosome pathway without activation of executioner caspase-3 or -7. Anticancer Res. 2007, 27, 2175–2183. [Google Scholar]
- Bol, G.M.; Khan, R.; van Voss, M.R.H.; Tantravedi, S.; Korz, D.; Kato, Y.; Raman, V. PLGA nanoparticle formulation of RK-33: An RNA helicase inhibitor against DDX3. Cancer Chemother. Pharmacol. 2015, 76, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Mazhari, R.; Kim, D.J. The anticancer effects of supinoxin (RX-5902) in pancreatic carcinoma. J. Clin. Oncol. 2016, 34, 238. [Google Scholar] [CrossRef]
- Kukhanova, M.K.; Karpenko, I.L.; Ivanov, A.V. DEAD-box RNA Helicase DDX3: Functional Properties and Development of DDX3 Inhibitors as Antiviral and Anticancer Drugs. Molecules 2020, 25, 1015. [Google Scholar] [CrossRef]
- Biegel, J.M.; Henderson, E.; Cox, E.M.; Bonenfant, G.; Netzband, R.; Kahn, S.; Eager, R.; Pager, C.T. Cellular DEAD-box RNA helicase DDX6 modulates interaction of miR-122 with the 5′ untranslated region of hepatitis C virus RNA. Virology 2017, 507, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Han, T.; Xuan, B.; Sun, Y.; Tang, S.; Yue, N.; Qian, Z. Dissecting the role of DDX21 in regulating human cytomegalovirus replication. J. Virol. 2019, 93, e01222-19. [Google Scholar] [CrossRef]
- Dong, Y.; Ye, W.; Yang, J.; Han, P.; Wang, Y.; Ye, C.; Weng, D.; Zhang, F.; Xu, Z.; Lei, Y. DDX21 translocates from nucleus to cytoplasm and stimulates the innate immune response due to dengue virus infection. Biochem. Biophys. Res. Comm. 2016, 473, 648–653. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ohtaki, N.; Hayashi, Y.; Ikuta, K.; Tomonaga, K. Autogenous translational regulation of the borna disease virus negative control Factor X from polycistronic mRNA using host RNA helicases. PLoS Pathog. 2009, 5, e1000654. [Google Scholar] [CrossRef]
- Ariumi, Y. Multiple functions of DDX3 RNA helicase in gene regulation, tumorigenesis, and viral infection. Front. Genet. 2014, 5. [Google Scholar] [CrossRef]
- Giraud, G.; Terrone, S.; Bourgeois, C.F. Functions of DEAD box RNA helicases DDX5 and DDX17 in chromatin organization and transcriptional regulation. BMB Rep. 2018, 51, 613–622. [Google Scholar] [CrossRef]
- Sergeeva, O.; Sviridov, E.; Zatsepin, T. Noncoding RNA in liver regeneration-from molecular mechanisms to clinical applications. Semin Liver Dis. 2020, 40, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Stojic, L.; Lun, A.T.L.; Mascalchi, P.; Ernst, C.; Redmond, A.M.; Mangei, J.; Barr, A.R.; Bousgouni, V.; Bakal, C.; Marioni, J.C.; et al. A high-content RNAi screen reveals multiple roles for long noncoding RNAs in cell division. Nat Commun. 2020, 11, 1851. [Google Scholar] [CrossRef] [PubMed]
- Abulwerdi, F.A.; Xu, W.; Ageeli, A.A.; Yonkunas, M.J.; Arun, G.; Nam, H.; Schneekloth, J.S.; Dayie, T.K.; Spector, D.; Baird, N.; et al. Selective small-molecule targeting of a triple helix encoded by the long noncoding RNA, MALAT1. ACS Chem. Biol. 2019, 14, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, R.P.; Salah-Uddin, S.; Modarresi, F.; Khoury, N.; Wahlestedt, C.; Faghihi, M.A. Screening for small-molecule modulators of long noncoding RNA-protein interactions using AlphaScreen. J. Biomol. Screen. 2015, 20, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Kutova, O.M.; Guryev, E.L.; Sokolova, E.A.; Alzeibak, R.; Balalaeva, I.V. Targeted Delivery to Tumors: Multidirectional Strategies to Improve Treatment Efficiency. Cancers 2019, 11, 68. [Google Scholar] [CrossRef]
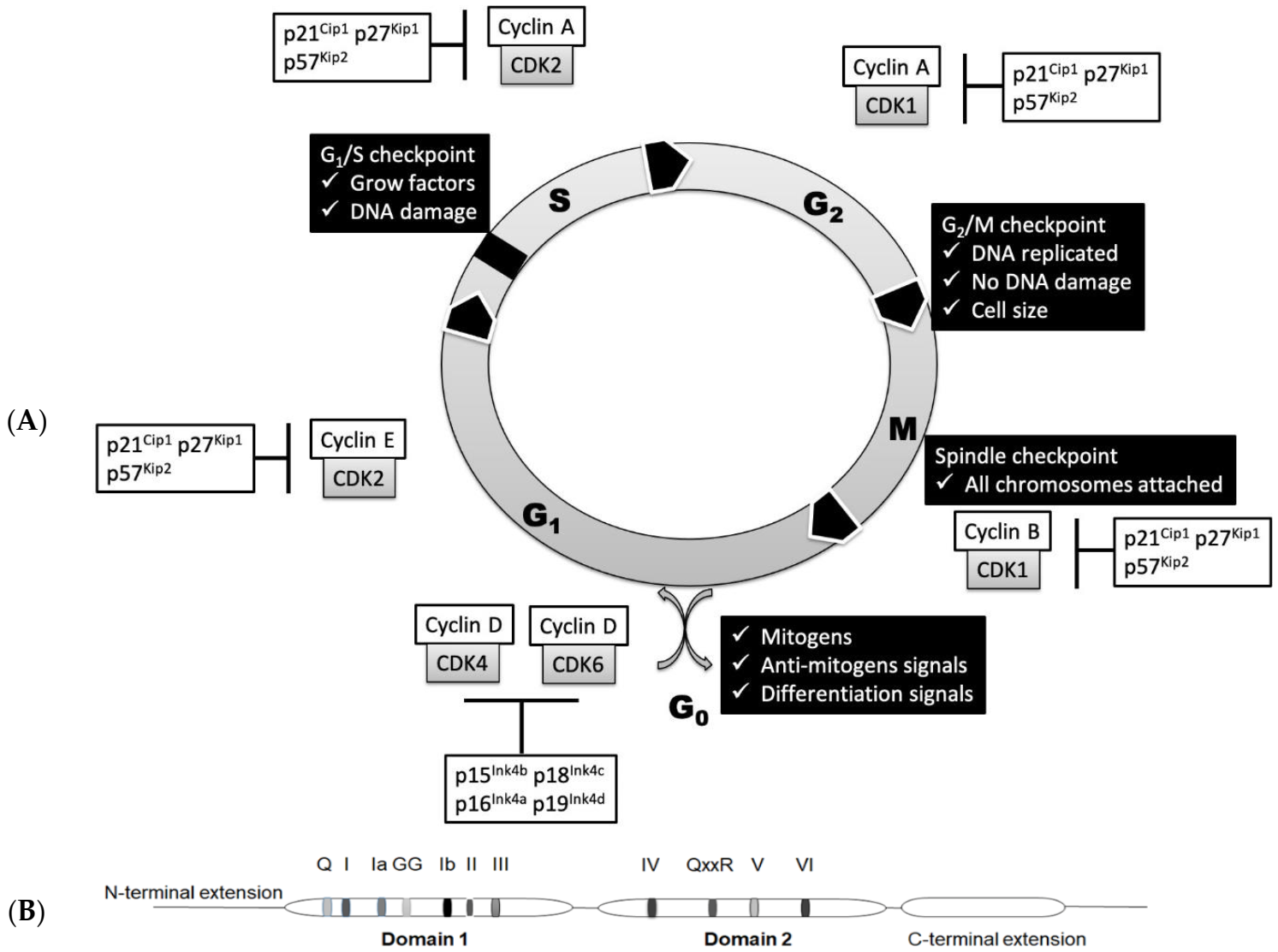
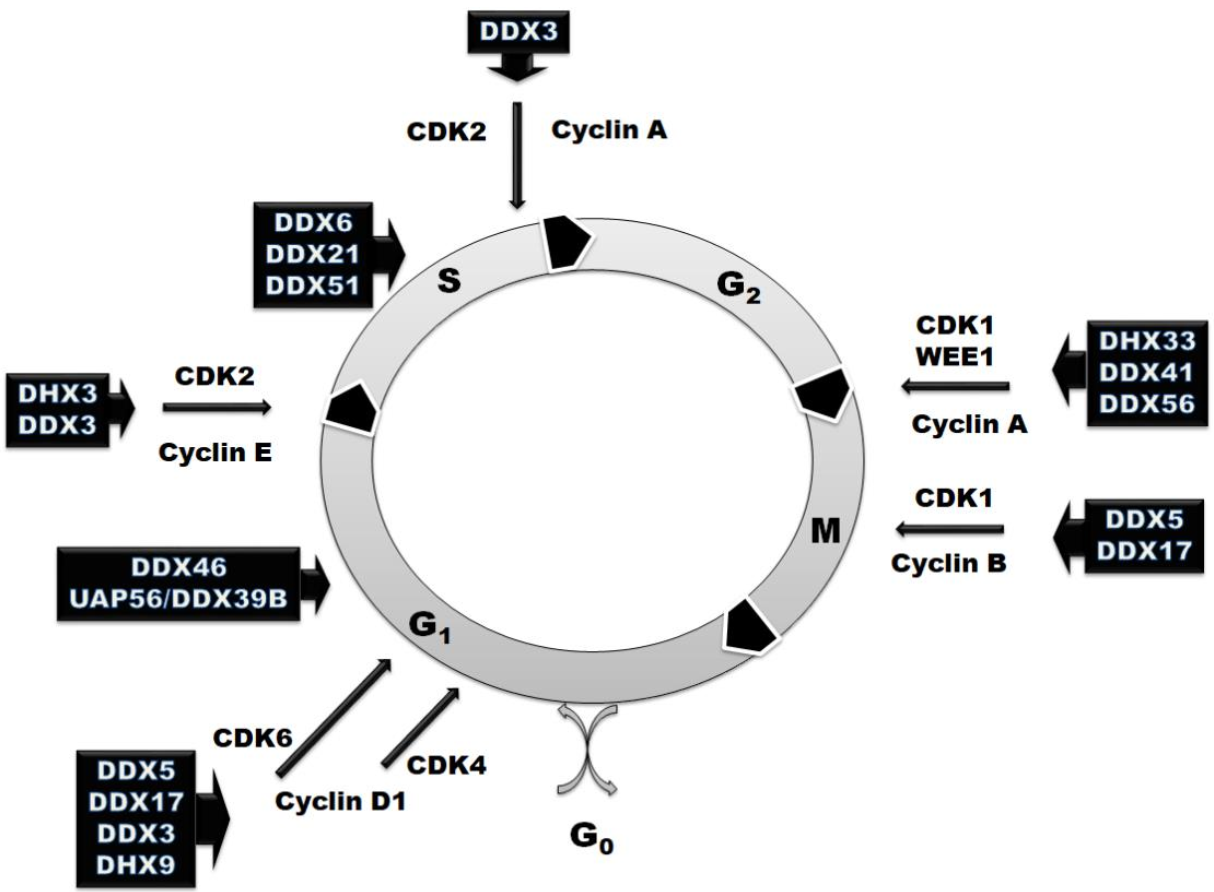
| Helicase | Target Crucial for the Cell Cycle | Cell Cycle Phase or Transition |
|---|---|---|
| DDX3 | cyclin E1, cyclin A1, cyclin D1, CDK2 | G1–S [39,52,53,130] |
| DDX46 | phosphorylation of Akt1 and IkBa inhibitor | Arrest at G1 phase [57] |
| DDX6 | transcription factor Tcf, target genes of Wnt/β-catenin—c-Myc, cyclin D1, cox-2, livin, survivin and VEGF | Arrest at S phase [130,131] |
| DDX21 | c-Jun, required for the synthesis of cyclin D1 | Arrest at S phase [73,74] |
| DDX51 | TGFBR2 | Arrest at S phase [132] |
| DDX5 | cyclin D1, c-Myc, β-catenin activation, Cdc45/Mcm2-7/GINS | Arrest at G1 and M phases [85,86,92,99] |
| DDX41 | p21WAF1/CIP1 | G2–M [16,110] |
| DHX33 | cyclin A2, cyclin B2, cyclin E2, MCM4, MCM7, cdc6, cdc20, and E2F1, cdc6, cyclin D1 and Ki-67 | G1–S; G2–M [104,105,106,107] |
| eIF4A | cyclin D1, cyclin D2, CDK6, CDK8 and Bcl2 | All stages [111,113,122] |
| DHX9 | Cyclin D1, zinc finger protein 1 | G1–S [117,118,119,120,121,122] |
| DDX56 | WEE1 | G2–M [123,124] |
| UAP56/DDX39B | R-loop | Arrest at G1 phase [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergeeva, O.; Zatsepin, T. RNA Helicases as Shadow Modulators of Cell Cycle Progression. Int. J. Mol. Sci. 2021, 22, 2984. https://doi.org/10.3390/ijms22062984
Sergeeva O, Zatsepin T. RNA Helicases as Shadow Modulators of Cell Cycle Progression. International Journal of Molecular Sciences. 2021; 22(6):2984. https://doi.org/10.3390/ijms22062984
Chicago/Turabian StyleSergeeva, Olga, and Timofei Zatsepin. 2021. "RNA Helicases as Shadow Modulators of Cell Cycle Progression" International Journal of Molecular Sciences 22, no. 6: 2984. https://doi.org/10.3390/ijms22062984
APA StyleSergeeva, O., & Zatsepin, T. (2021). RNA Helicases as Shadow Modulators of Cell Cycle Progression. International Journal of Molecular Sciences, 22(6), 2984. https://doi.org/10.3390/ijms22062984







