Response Criteria in Advanced Systemic Mastocytosis: Evolution in the Era of KIT Inhibitors
Abstract
1. Introduction
2. Response Criteria in AdvSM
2.1. Valent Criteria
2.2. Mayo Criteria
2.3. International Working Group-Myeloproliferative Neoplasms Research and Treatment & European Competence Network on Mastocytosis (IWG-MRT-ECNM) Response Criteria
3. Tyrosine Kinase Inhibitors in AdvSM and Challenges for Response Criteria
3.1. Imatinib
3.2. Midostaurin
3.3. Avapritinib
4. Challenges of Response Adjudication and Concerns of the Regulatory Authorities
5. Pure Pathologic Response (PPR) Criteria
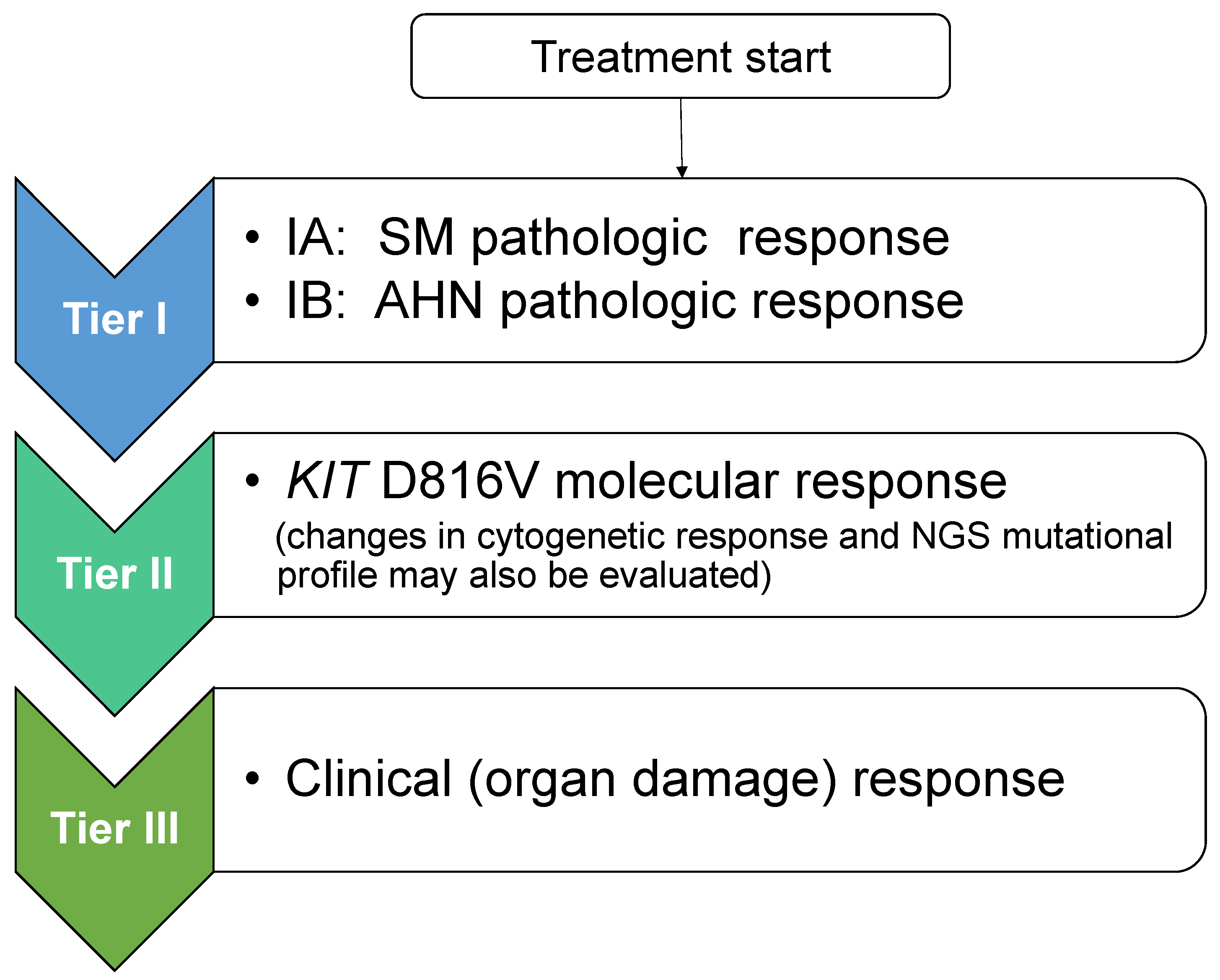
6. Proposed Response Criteria to Meet the Era of KIT Inhibitors
7. Conclusions and Future Challenges
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AdvSM | Advanced systemic mastocytosis |
| BM | Bone marrow |
| CR | Complete remission/response |
| CRh | Complete response with partial hematologic recovery |
| ECNM | European Competence Network on Mastocytosis |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| HαT | Hereditary alpha-tryptasemia |
| ISM | Indolent systemic mastocytosis |
| IWG-MRT-ECN | International Working Group-Myeloproliferative Neoplasms Research and Treatment |
| MC | Mast cells |
| MCL | Mast cell leukemia |
| mIWG-MRT-ECNM | Modified International Working Group-Myeloproliferative Neoplasms Research and Treatment |
| MR | Major response |
| PPR | Pure pathologic response |
| PR | Partial response |
| SM | Systemic mastocytosis |
| SM-AHN | Systemic mastocytosis with an associated hematologic neoplasm |
| SSM | Smoldering systemic mastocytosis |
| WHO | World Health Organization |
References
- Shomali, W.; Gotlib, J. The new tool “KIT” in advanced systemic mastocytosis. Hematology 2018, 2018, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, J.; Pardanani, A.; Akin, C.; Reiter, A.; George, T.; Hermine, O.; Kluin-Nelemans, H.; Hartmann, K.; Sperr, W.R.; Brockow, K.; et al. International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) & European Competence Network on Mastocytosis (ECNM) consensus response criteria in advanced systemic mastocytosis. Blood 2013, 121, 2393–2401. [Google Scholar] [PubMed]
- Lim, K.-H.; Tefferi, A.; Lasho, T.L.; Finke, C.; Patnaik, M.; Butterfield, J.H.; McClure, R.F.; Li, C.Y.; Pardanani, A. Systemic mastocytosis in 342 consecutive adults: Survival studies and prognostic factors. Blood 2009, 113, 5727–5736. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Tefferi, A. A critical reappraisal of treatment response criteria in systemic mastocytosis and a proposal for revisions. Eur. J. Haematol. 2010, 84, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Sperr, W.R.; Escribano, L.; Arock, M.; Horny, H.P.; Bennett, J.M.; Metcalfe, D.D. Aggressive systemic mastocytosis and related mast cell disorders: Current treatment options and proposed response criteria. Leuk. Res. 2003, 27, 635–641. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Escribano, L.; Födinger, M.; Hartmann, K.; Brockow, K.; Castells, M.; Sperr, W.R.; Kluin-Nelemans, H.C.; Hamdy, N.A.; et al. Standards and standardization in mastocytosis: Consensus statements on diagnostics, treatment recommendations and response criteria. Eur. J. Clin. Investig. 2007, 37, 435–453. [Google Scholar] [CrossRef]
- Valent, P.; Horny, H.-P.; Escribano, L.; Longley, B.J.; Li, C.Y.; Schwartz, L.B.; Marone, G.; Nuñez, R.; Akin, C.; Sotlar, K.; et al. Diagnostic criteria and classification of mastocytosis: A consensus proposal. Leuk. Res. 2001, 25, 603–625. [Google Scholar] [CrossRef]
- Kluin-Nelemans, H.C.; Oldhoff, J.M.; van Doormaal, J.J.; Van’t Wout, J.W.; Verhoef, G.; Gerrits, W.B.; van Dobbenburgh, O.A.; Pasmans, S.G.; Fijnheer, R. Cladribine therapy for systemic mastocytosis. Blood 2003, 102, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Vega-Ruiz, A.; Cortes, J.E.; Sever, M.; Manshouri, T.; Quintás-Cardama, A.; Luthra, R.; Kantarjian, H.M.; Verstovsek, S. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk. Res. 2009, 33, 1481–1484. [Google Scholar] [CrossRef]
- Verstovsek, S.; Tefferi, A.; Cortes, J.; O’Brien, S.; Garcia-Manero, G.; Pardanani, A.; Akin, C.; Faderl, S.; Manshouri, T.; Thomas, D.; et al. Phase II study of dasatinib in Philadelphia chromosome-negative acute and chronic myeloid diseases, including systemic mastocytosis. Clin. Cancer Res. 2008, 14, 3906–3915. [Google Scholar] [CrossRef]
- Hochhaus, A.; Baccarani, M.; Giles, F.J.; le Coutre, P.D.; Müller, M.C.; Reiter, A.; Santanastasio, H.; Leung, M.; Novick, S.; Kantarjian, H.M. Nilotinib in patients with systemic mastocytosis: Analysis of the phase 2, open-label, single-arm nilotinib registration study. J. Cancer Res. Clin. Oncol. 2015, 141, 2047–2060. [Google Scholar] [CrossRef]
- Cheson, B.D.; Greenberg, P.L.; Bennett, J.M.; Lowenberg, B.; Wijermans, P.W.; Nimer, S.D.; Pinto, A.; Beran, M.; de Witte, T.M.; Stone, R.M.; et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006, 108, 419–425. [Google Scholar] [CrossRef] [PubMed]
- DeAngelo, D.J.; George, T.I.; Linder, A.; Langford, C.; Perkins, C.; Ma, J.; Westervelt, P.; Merker, J.D.; Berube, C.; Coutre, S.; et al. Efficacy and safety of midostaurin in patients with advanced systemic mastocytosis: 10-year median follow-up of a phase II trial. Leukemia 2018, 32, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, J.; Kluin-Nelemans, H.C.; George, T.I.; Akin, C.; Sotlar, K.; Hermine, O.; Awan, F.T.; Hexner, E.; Mauro, M.J.; Sternberg, D.W.; et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N. Engl. J. Med. 2016, 374, 2530–2541. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, J.; Baird, J.H.; George, T.I.; Langford, C.; Reyes, I.; Abuel, J.; Perkins, C.; Schroeder, K.; Bose, P.; Verstovsek, S. A phase 2 study of brentuximab vedotin in patients with CD30-positive advanced systemic mastocytosis. Blood Adv. 2019, 3, 2264–2271. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Joensuu, H.; Demetri, G.D.; Corless, C.L.; Apperley, J.; Fletcher, J.A.; Soulieres, D.; Dirnhofer, S.; Harlow, A.; Town, A.; et al. Imatinib Target Exploration Consortium Study B2225. Phase II, open-label study evaluating the activity of imatinib in treating life-threatening malignancies known to be associated with imatinib sensitive tyrosine kinases. Clin. Cancer Res. 2008, 14, 2717–2725. [Google Scholar] [CrossRef]
- Imatinib Drug Label, Revised 8/2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021588s056s057lbl.pdf (accessed on 9 November 2020).
- Alvarez-Twose, I.; Matito, A.; Morgado, J.M.; Sánchez-Muñoz, L.; Jara-Acevedo, M.; García-Montero, A.; Mayado, A.; Caldas, C.; Teodósio, C.; Muñoz-González, J.I.; et al. Imatinib in systemic mastocytosis: A phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget 2016, 8, 68950–68963. [Google Scholar] [CrossRef]
- Reiter, A.; Kluin-Nelemans, H.C.; George, T.; Akin, C.; DeAngelo, D.; Hermine, O.; Awan, F.; Hexner, E.; Mauro, M.; Schwaab, J.; et al. Pooled survival analysis of midostaurin clinical study data (D2201 + A2213) in patients with advanced systemic mastocytosis compared with historical controls. Haematologica 2017, 102 (Suppl. 2), 321–323. [Google Scholar]
- Valent, P.; Akin, C.; Hartmann, K.; George, T.I.; Sotlar, K.; Peter, B.; Gleixner, K.V.; Blatt, K.; Sperr, W.R.; Manley, P.W.; et al. Midostaurin: A magic bullet that blocks mast cell expansion and activation. Ann. Oncol. 2017, 28, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- FDA Medical Review. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/207997Orig1Orig2s000MedR.pdf (accessed on 16 November 2020).
- EMA Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/rydapt-epar-product-information_en.pdfformidostaurin (accessed on 16 November 2020).
- Evans, E.K.; Gardino, A.K.; Kim, J.L.; Hodous, B.L.; Shutes, A.; Davis, A.; Zhu, X.J.; Schmidt-Kittler, O.; Wilson, D.; Wilson, K.; et al. A precision therapy against cancers driven by KIT/PDGFRA mutations. Sci. Transl. Med. 2017, 9, eaao1690. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Jones, R.L.; von Mehren, M.; Schöffski, P.; Serrano, C.; Kang, Y.K.; Cassier, P.A.; Mir, O.; Eskens, F.; Tap, W.D.; et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): A multicentre, open-label, phase I trial. Lancet Oncol. 2020, 21, 935–946. [Google Scholar] [CrossRef]
- Alzofon, N.; Jimeno, A. Avapritinib for metastatic or unresectable gastrointestinal stromal tumors. Drugs Today 2020, 56, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, J.; Radia, D.H.; George, T.I.; Robinson, W.; Quiery, A.; Drummond, M.; Bose, P.; Hexner, E.O.; Winton, E.; Horny, H.; et al. Pure pathologic response is associated with improved overall survival in patients with advanced systemic mastocytosis receiving avapritinib in the phase I EXPLORER study. Blood 2020, 136 (Suppl. 1), 37–38. [Google Scholar] [CrossRef]
- Gotlib, J.; Radia, D.H.; George, T.I.; Robinson, W.A.; Query, A.T.; Drummon, M.W.; Bose, P.; Hexner, E.O.; Winton, E.F.; Horny, H.-P.; et al. Avapritinib induces responses in patients with advanced systemic mastocytosis regardless of prior midostaurin therapy. HemaSphere 2020, 4 (Suppl. 1), 496. [Google Scholar]
- Jawhar, M.; Schwaab, J.; Naumann, N.; Horny, H.P.; Sotlar, K.; Haferlach, T.; Metzgeroth, G.; Fabarius, A.; Valent, P.; Hofmann, W.K.; et al. Response and progression on midostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers. Blood 2017, 130, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montero, A.C.; Jara-Acevedo, M.; Teodosio, C.; Sanchez, M.L.; Nunez, R.; Prados, A.; Aldanondo, I.; Sanchez, L.; Dominguez, M.; Botana, L.M.; et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: A prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood 2006, 108, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Schwaab, J.; Schnittger, S.; Sotlar, K.; Walz, C.; Fabarius, A.; Pfirrmann, M.; Kohlmann, A.; Grossmann, V.; Meggendorfer, M.; Horny, H.P.; et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood 2013, 122, 2460–2466. [Google Scholar] [CrossRef]
- Jawhar, M.; Schwaab, J.; Schnittger, S.; Sotlar, K.; Horny, H.P.; Metzgeroth, G.; Müller, N.; Schneider, S.; Naumann, N.; Walz, C.; et al. Molecular profiling of myeloid progenitor cells in multi-mutated advanced systemic mastocytosis identifies KIT D816V as a distinct and late event. Leukemia 2015, 29, 1115–1122. [Google Scholar] [CrossRef]
- Sotlar, K.; Colak, S.; Bache, A.; Berezowska, S.; Krokowski, M.; Bültmann, B.; Valent, P.; Horny, H.P. Variable presence of KIT D816V in clonal haematological non-mast cell lineage diseases associated with systemic mastocytosis (SM-AHNMD). J. Pathol. 2010, 220, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, R.M.; Dueck, A.C.; Geyer, H.L.; Kiladjian, J.J.; Slot, S.; Zweegman, S.; te Boekhorst, P.A.; Commandeur, S.; Schouten, H.C.; Sackmann, F.; et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: Prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J. Clin. Oncol. 2012, 30, 4098–4103. [Google Scholar] [CrossRef]
- Gotlib, J.; Radia, D.; DeAngelo, D.J.; Bose, P.; Drummond, M.W.; Hexner, E.O.; Robinson, W.A.; Conlan, M.G.; Oren, R.G.; Shi, H.; et al. Avapritinib, a potent and selective inhibitor of KIT D816V, improves symptoms of advanced systemic mastocytosis (AdvSM): Analyses of patient reported outcomes (PROs) from the phase 1 (EXPLORER) study using the AdvSM symptom assessment form (AdvSM-SAF), a new PRO questionnaire for AdvSM. Blood 2018, 132 (Suppl. 1), 351. [Google Scholar]
- Lyons, J.J.; Yu, X.; Hughes, J.D.; Le, Q.T.; Jamil, A.; Bai, Y.; Ho, N.; Zhao, M.; Liu, Y.; O’Connell, M.P.; et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat. Genet. 2016, 48, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Greiner, G.; Sprinzl, B.; Górska, A.; Ratzinger, F.; Gurbisz, M.; Witzeneder, N.; Schmetterer, K.G.; Gisslinger, B.; Uyanik, G.; Hadzijusufovic, E.; et al. Hereditary alpha tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood 2021, 137, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Chovanec, J.; O’Connell, M.P.; Liu, Y.; Šelb, J.; Zanotti, R.; Bai, Y.; Kim, J.; Le, Q.T.; DiMaggio, T.; et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase-encoding germline copy number at TPSAB1. J. Allergy Clin. Immunol. 2021, 147, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, J. What your HαT says about you. Blood 2021, 137, 151–153. [Google Scholar] [CrossRef] [PubMed]
| Major Response |
Complete resolution of at least one (one or more) C-finding(s) and no progression in other C-findings
|
| Partial Response |
Incomplete regression of one or more C-finding(s) a, without complete regression, and no progression in other C-findings
|
| No Response |
C-finding(s) persistent or progressive b
|
| Response Category | Disease-Related Symptoms 1 | Organomegaly/ Lymphadenopathy 2 | Disease-Related Organopathy 3 | Bone Marrow (BM) Findings 4 |
|---|---|---|---|---|
| A | B | C | D | |
| Complete response (CR): A+B+C+D required (when present) | Complete resolution for 3 months | Complete resolution 2 | Complete resolution 5 | Absence of abnormal mast cell (MC) infiltration 7 |
| Major response (MR): A+B+C+D required (when present) | No progression (at a minimum) | No progression (at a minimum) | Complete resolution of at least 1 element of organopathy 3,6 | >50% decrease in BM MC (%) |
| Partial response (PR): A or B or C (without progression of others) | Complete resolution for 3 months | Complete resolution 2 | ≥2 grade improvement in at least 1 element of organopathy 6,8 | No progression (at a minimum) |
| Stable disease (SD) | None of the above responses | |||
| Progressive disease (PD): B or C required | Not applicable 9 | >50% increase from baseline 2 | ≥2 grade worsening from baseline | Not applicable |
| IWG-MRT-ECNM Definition | IWG-MRT-ECNM Response Criteria | mIWG-MRT-ECNM Modifications | |
|---|---|---|---|
| Non-hematologic organ damage | |||
| Ascites or pleural effusions | Symptomatic ascites or pleural effusion requiring medical intervention such as: Use of diuretics (grade 2) or ≥2 therapeutic paracenteses or thoracenteses (grade 3) at least 28 days apart over 12 weeks before the start of treatment with one procedure performed 6 weeks before the start of treatment | Complete resolution of symptomatic ascites or pleural effusion (including trace/minimal on radiographic imaging) and no longer in need of diuretics for ≥12 weeks and No longer in need of diuretics for ≥12 weeks or No therapeutic paracenteses or thoracentesis for ≥12 weeks | Same as IWG-MRT-ECNM |
| Liver function abnormalities | ≥Grade 2 abnormalities in direct bilirubin (>1.5 × ULN), AST (>3.0 × ULN), ALT (>3.0 × ULN), or ALP (>2.5 × ULN) in the presence of: Ascites and/or Clinically relevant portal hypertension, and/or Liver MC infiltration that is biopsy-proven or No other identified cause of abnormal liver function | Reversion of ≥1 LFTs to normal range for ≥12 weeks | Same as IWG-MRT-ECNM |
| Hypoalbuminemia | ≥Grade 2 hypoalbuminemia (<3.0 g/dL) | Reversion of albumin to normal range for ≥12 weeks | Same as IWG-MRT-ECNM |
| Marked symptomatic splenomegaly | A spleen that is palpable >5 cm below the left costal margin and patient endorses symptoms of discomfort and/or early satiety | ≥50% reduction in palpable splenomegaly (or ≥35% reduction in spleen volume based on 3D MRI or CT scan) and no endorsement of discomfort and/or early satiety for ≥12 weeks | Definition: Symptomatic or non- symptomatic splenomegaly palpable ≥5 cm below left costal margin. Response criteria: ≥35% reduction in spleen volume based on 3D MRI or CT scan for ≥12 weeks |
| Weight loss | N/A | N/A | Definition: Medically documented >10% weight loss in last 24 weeks ( ± 12 weeks) Response criteria: Reversion of >50% of weight loss in the 24 weeks preceding treatment |
| Hematologic organ damage | |||
| Neutropenia | ≥Grade 3 ANC (<1.0 × 10⁹/L) | ≥100% increase and an absolute increase ≥0.5 × 109/L for ≥12 weeks | Same as IWG-MRT-ECNM with allowance of CRh * |
| Anemia (transfusion-independent) | ≥Grade 2 Hgb (<10 g/dL) | An increase in Hgb ≥2 g/dL that is maintained for ≥12 weeks | Same as IWG-MRT-ECNM with allowance of CRh * |
| Anemia (transfusion- dependent) | Transfusion of ≥6 units PRBCs in the 12 weeks before the start of treatment and Most recent transfusion occurring during the 4 weeks before the start of treatment and Transfusions administered for Hgb ≤8.5 g/dL and Reason for transfusions is not bleeding, hemolysis, or therapy-related | Transfusion independence for ≥12 weeks and maintenance of Hgb ≥8.5 g/dL at the end of the 12-week period of response duration | Same as IWG-MRT-ECNM with allowance of CRh * |
| Thrombocytopenia (transfusion- independent) | ≥Grade 2 thrombocytopenia (<75 × 10⁹/L) | ≥100% increase and an absolute increase ≥50 × 109/L and no need for platelet transfusion for ≥12 weeks | Same as IWG-MRT-ECNM with allowance of CRh * |
| Thrombocytopenia (transfusion- dependent) | Transfusion of ≥6 units of apheresed platelets during 12 weeks preceding treatment and ≥2 units transfused during 4 weeks preceding treatment and Transfusions administered for platelet count <20 × 10⁹/L | Transfusion independence for ≥12 weeks and maintenance of platelet count ≥20 × 109/L | Same as IWG-MRT-ECNM with allowance of CRh * |
| Response Category | Definition |
|---|---|
| Complete remission with full (CR) or partial (CRh) hematologic recovery a | Bone marrow mast cell aggregates eliminated and serum tryptase <20 ng/mL |
| Molecular complete remission (molecular CR/molecular CRh) | KIT D816V mutant allele fraction falls below limit of detection by sensitive assay b |
| Partial remission (PR) | ≥50% reduction in bone marrow mast cells and serum tryptase level |
| Stable disease (SD) | Not in a CR, PR, or PD |
| Progressive disease (PD) | Transformation to acute myeloid leukemia (AML) or mast cell leukemia (MCL) |
Primary Objective
|
Secondary Objectives
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shomali, W.; Gotlib, J. Response Criteria in Advanced Systemic Mastocytosis: Evolution in the Era of KIT Inhibitors. Int. J. Mol. Sci. 2021, 22, 2983. https://doi.org/10.3390/ijms22062983
Shomali W, Gotlib J. Response Criteria in Advanced Systemic Mastocytosis: Evolution in the Era of KIT Inhibitors. International Journal of Molecular Sciences. 2021; 22(6):2983. https://doi.org/10.3390/ijms22062983
Chicago/Turabian StyleShomali, William, and Jason Gotlib. 2021. "Response Criteria in Advanced Systemic Mastocytosis: Evolution in the Era of KIT Inhibitors" International Journal of Molecular Sciences 22, no. 6: 2983. https://doi.org/10.3390/ijms22062983
APA StyleShomali, W., & Gotlib, J. (2021). Response Criteria in Advanced Systemic Mastocytosis: Evolution in the Era of KIT Inhibitors. International Journal of Molecular Sciences, 22(6), 2983. https://doi.org/10.3390/ijms22062983





