The Current Challenges for Drug Discovery in CNS Remyelination
Abstract
1. Introduction
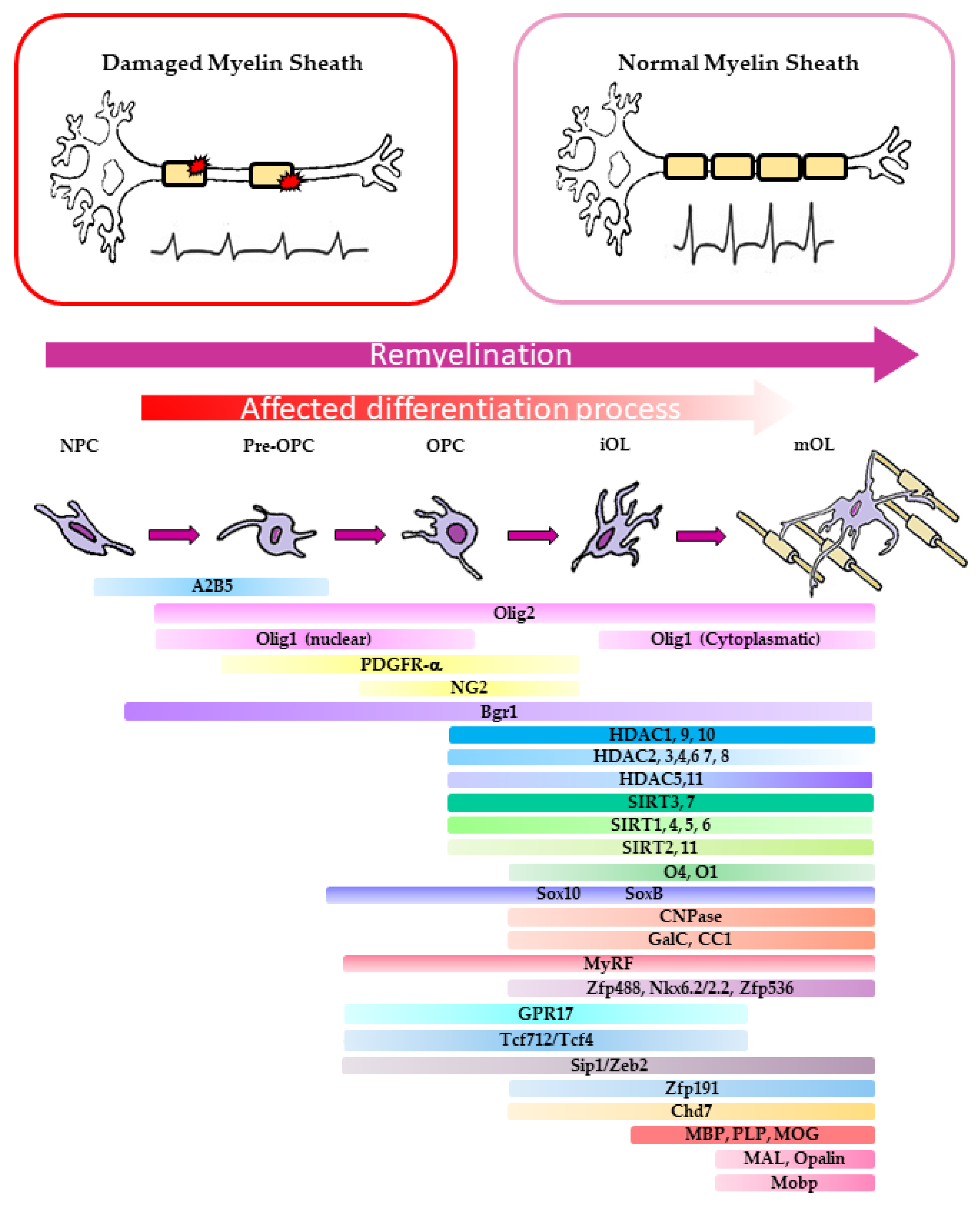
2. The Biological Basis of CNS Remyelination
2.1. Molecular Aspects of OPC Differentiation in Murine Models of Demyelination
2.2. Molecular Basis of Remyelination Defects in MS Patients
3. Influence of the Three-Dimensional (3D) Environment on the Axon Myelination
3.1. In Vitro Mimicking of OPC Development in a 3D Environment
3.1.1. Synthetic Axons
3.1.2. Extracellular Matrix Function in Axon Recognition and NPC Differentiation
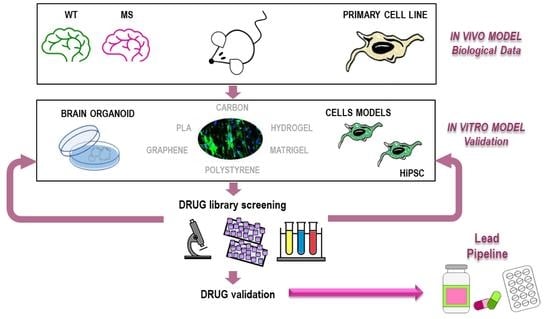
4. Identification of Promyelinating Drugs Using Phenotypical Screen
4.1. Cellular Models to Study Remyelination and Suitable for Phenotypical Drug Library Screens
| Model for Drugs Screening | Advantages | Disadvantages | Drugs Identified in Phenotypical Screen | Ref. |
|---|---|---|---|---|
| Zebrafish Danio rerio larva | Excellent visualization of myelination; easy genetic manipulation | Biochemical and genetic difference among species | Src kinase inhibitor PP2; a biogenic ammine and a Thioxanthene, Fenofibrate, Gemfibrozil. | [181,182,183,184,193] |
| Primary Cells Culture | Genetic and biological fidelity | Complex isolation; difficult to scale up for drug screening | Regulators of muscarinic acetylcholine signaling: Benztropine, Clemastine, Donepezil, Oxybutynin, Vesamicol, Ipratropium. Estrogen receptor modulators (SERMs): Raloxifene, Toremifene, and Tamoxifen. Tricyclic antidepressant molecules: Perphenazine, Prochlorperazine, Fluphenazine, Trifluorperazine, Quetiapine fumarate. Non-tricyclic antidepressants: Perospirone, Escitalopram, Citalopram, Metylperon, and Bupropion. Regulators of adrenergic receptor pathway: Salmeterol, Betaxolol, Esmolol. Ion channel modulators: Ifenprodil, Benproperine, Proxymetacaine, Dofetilide, Dimethylphenylpiperazinium. Antifungal agent: Bifonazole. | [6,29,30,34] |
| Oli-neu, immortalized oligodendrocyte cell line | Well characterized at molecular level for Myelin protein trafficking and lipid signaling | Difficult to culture; Pro-apoptotic behaviour. Cannot be used in axon engagement tests. Mouse cell line | PKA pathway agonist: dbcAMP, Forskolin. Nuclear receptor ligands: Nr3C1: Dexamethasone, Hydrocortisone, Budesonide, 1,3-cis-Retinoic acid: RxRs. ErbB inhibitor: PD174265, 4557W. Nucleoside analogs: Ribavirin, Lefluonomide. | [186,187,188,192] |
| Oli-neuM, immortalized oligodendrocyte cell line | Easy to culture in drug screening; stably expressing MyrF gene. It differentiates till axon engagement. Suitable for in vitro synthetic axon engagement tests | Mouse cell line | Glucocorticoids acting as Smo agonists: Clobetasol, Halcinonide. EGFR inhibitors: Gefitinib Erlotinib. Antifungal agents: Clotrimazole, Itraconazole. Immunosuppressant: Azatriopina. | [33,37,186,187,188] |
| OPCs from mouse epiblast stem cells | Easy manipulation; Fast results; high reproducibility; easy to scale up for drug screening | Mouse cell line | Glucocorticoids: Clobetasol, Bethamethasone, Methilprendisolone. Antifungal agents: Clotrimazole, Miconazole, Bifonazole, Ketoconazole. | [7] |
| Human iPSC-derived OPC (HiPSC) | Genetic and biological fidelity; iPSC from patient; they can recapitulate disease defects | Derivation of OPC from iPSC requires good technical skill; it requires time. Large drug screens not performed as yet | Immunomodulatory treatment of PBMC | [4,194,195,196] |
| Organoids | Promising close model to resemble the development, composition, architecture, and partially the function ex vivo of the original human tissue | Require long differentiation time, technology for culturing; expensive. Do not recapitulate brain structures | [197,198] |
4.2. Drug Repurposing Strategies in Remyelination
4.3. Human iPSC-Derived OPCs and Organoids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bechler, M.E.; Byrne, L.; Ffrench-Constant, C. CNS Myelin Sheath Lengths Are an Intrinsic Property of Oligodendrocytes. Curr. Biol. 2015, 25, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, M.M.; Brendel, M.B.; Melendez-Vasquez, C.V. Acute and Chronic Demyelinated CNS Lesions Exhibit Opposite Elastic Properties. Sci. Rep. 2019, 9, 999. [Google Scholar] [CrossRef] [PubMed]
- Bribián, A.; Medina-Rodríguez, E.M.; Josa-Prado, F.; García-Álvarez, I.; Machín-Díaz, I.; Esteban, P.F.; Murcia-Belmonte, V.; Vega-Zelaya, L.; Pastor, J.; Garrido, L.; et al. Functional Heterogeneity of Mouse and Human Brain OPCs: Relevance for Preclinical Studies in Multiple Sclerosis. J. Clin. Med. 2020, 9, 1681. [Google Scholar] [CrossRef] [PubMed]
- Starost, L.; Lindner, M.; Herold, M.; Xu, Y.K.T.; Drexler, H.C.A.; Heß, K.; Ehrlich, M.; Ottoboni, L.; Ruffini, F.; Stehling, M.; et al. Extrinsic Immune Cell-Derived, but Not Intrinsic Oligodendroglial Factors Contribute to Oligodendroglial Differentiation Block in Multiple Sclerosis. Acta Neuropathol. 2020, 140, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Traiffort, E.; Kassoussi, A.; Zahaf, A.; Laouarem, Y. Astrocytes and Microglia as Major Players of Myelin Production in Normal and Pathological Conditions. Front. Cell. Neurosci. 2020, 14, 79. [Google Scholar] [CrossRef]
- Deshmukh, V.A.; Tardif, V.; Lyssiotis, C.A.; Green, C.C.; Kerman, B.; Kim, H.J.; Padmanabhan, K.; Swoboda, J.G.; Ahmad, I.; Kondo, T.; et al. A Regenerative Approach to the Treatment of Multiple Sclerosis. Nature 2013, 502, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Najm, F.J.; Madhavan, M.; Zaremba, A.; Shick, E.; Karl, R.T.; Factor, D.C.; Miller, T.E.; Nevin, Z.S.; Kantor, C.; Sargent, A.; et al. Drug-Based Modulation of Endogenous Stem Cells Promotes Functional Remyelination in Vivo. Nature 2015, 522, 216–220. [Google Scholar] [CrossRef]
- Moffatt, J.G.; Vincent, F.; Lee, J.A.; Eder, J.; Prunotto, M. Opportunities and Challenges in Phenotypic Drug Discovery: An Industry Perspective. Nat. Rev. Drug Discov. 2017, 16, 531–543. [Google Scholar] [CrossRef]
- Plemel, J.R.; Liu, W.-Q.; Yong, V.W. Remyelination Therapies: A New Direction and Challenge in Multiple Sclerosis. Nat. Rev. Drug Discov. 2017, 16, 617–634. [Google Scholar] [CrossRef]
- Lubetzki, C.; Zalc, B.; Williams, A.; Stadelmann, C.; Stankoff, B. Remyelination in Multiple Sclerosis: From Basic Science to Clinical Translation. Lancet Neurol. 2020, 19, 678–688. [Google Scholar] [CrossRef]
- Melchor, G.S.; Khan, T.; Reger, J.F.; Huang, J.K. Remyelination Pharmacotherapy Investigations Highlight Diverse Mechanisms Underlying Multiple Sclerosis Progression. ACS Pharmacol. Transl. Sci. 2019, 2, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Ahmad, T.K.; Gozda, K.; Truong, J.; Kong, J.; Namaka, M. Implications of White Matter Damage in Amyotrophic Lateral Sclerosis (Review). Mol. Med. Rep. 2017, 16, 4379–4392. [Google Scholar] [CrossRef]
- Davis, K.L.; Stewart, D.G.; Friedman, J.I.; Buchsbaum, M.; Harvey, P.D.; Hof, P.R.; Buxbaum, J.; Haroutunian, V. White Matter Changes in Schizophrenia: Evidence for Myelin-Related Dysfunction. Arch. Gen. Psychiatry 2003, 60, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Tkachev, D.; Mimmack, M.L.; Ryan, M.M.; Wayland, M.; Freeman, T.; Jones, P.B.; Starkey, M.; Webster, M.J.; Yolken, R.H.; Bahn, S. Oligodendrocyte Dysfunction in Schizophrenia and Bipolar Disorder. Lancet 2003, 362, 798–805. [Google Scholar] [CrossRef]
- Wan, C.; Yang, Y.; Feng, G.; Gu, N.; Liu, H.; Zhu, S.; He, L.; Wang, L. Polymorphisms of Myelin-Associated Glycoprotein Gene Are Associated with Schizophrenia in the Chinese Han Population. Neurosci. Lett. 2005, 388, 126–131. [Google Scholar] [CrossRef]
- Yao, L.; Lui, S.; Liao, Y.; Du, M.-Y.; Hu, N.; Thomas, J.A.; Gong, Q.-Y. White Matter Deficits in First Episode Schizophrenia: An Activation Likelihood Estimation Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 100–106. [Google Scholar] [CrossRef]
- Rahi, S.; Mehan, S. Understanding Abnormal SMO-SHH Signaling in Autism Spectrum Disorder: Potential Drug Target and Therapeutic Goals. Cell. Mol. Neurobiol. 2020. [Google Scholar] [CrossRef]
- Neumann, B.; Baror, R.; Zhao, C.; Segel, M.; Dietmann, S.; Rawji, K.S.; Foerster, S.; McClain, C.R.; Chalut, K.; van Wijngaarden, P.; et al. Metformin Restores CNS Remyelination Capacity by Rejuvenating Aged Stem Cells. Cell Stem Cell 2019, 25, 473–485.e8. [Google Scholar] [CrossRef]
- Kremer, D.; Göttle, P.; Hartung, H.-P.; Küry, P. Pushing Forward: Remyelination as the New Frontier in CNS Diseases. Trends Neurosci. 2016, 39, 246–263. [Google Scholar] [CrossRef] [PubMed]
- Bodini, B.; Veronese, M.; García-Lorenzo, D.; Battaglini, M.; Poirion, E.; Chardain, A.; Freeman, L.; Louapre, C.; Tchikviladze, M.; Papeix, C.; et al. Dynamic Imaging of Individual Remyelination Profiles in Multiple Sclerosis. Ann. Neurol. 2016, 79, 726–738. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Wang, Z.; Wang, Z.J.; Ward, R.K.; Wang, X. Deep Learning for Pixel-Level Image Fusion: Recent Advances and Future Prospects. Inf. Fusion 2018, 42, 158–173. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain Development in Rodents and Humans: Identifying Benchmarks of Maturation and Vulnerability to Injury across Species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Nave, K.-A.; Ehrenreich, H. Time to Revisit Oligodendrocytes in Multiple Sclerosis. Nat. Med. 2019, 25, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Cunniffe, N.; Coles, A. Promoting Remyelination in Multiple Sclerosis. J. Neurol. 2021, 268, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; ffrench-Constant, C.; Edgar, J.M.; Smith, K.J. Neuroprotection and Repair in Multiple Sclerosis. Nat. Rev. Neurol. 2012, 8, 624–634. [Google Scholar] [CrossRef]
- Lee, S.; Leach, M.K.; Redmond, S.A.; Chong, S.Y.C.; Mellon, S.H.; Tuck, S.J.; Feng, Z.-Q.; Corey, J.M.; Chan, J.R. A Culture System to Study Oligodendrocyte Myelination Processes Using Engineered Nanofibers. Nat. Methods 2012, 9, 917–922. [Google Scholar] [CrossRef]
- Espinosa-Hoyos, D.; Jagielska, A.; Homan, K.A.; Du, H.; Busbee, T.; Anderson, D.G.; Fang, N.X.; Lewis, J.A.; Van Vliet, K.J. Engineered 3D-Printed Artificial Axons. Sci. Rep. 2018, 8, 478. [Google Scholar] [CrossRef]
- Xu, Y.K.T.; Chitsaz, D.; Brown, R.A.; Cui, Q.L.; Dabarno, M.A.; Antel, J.P.; Kennedy, T.E. Deep Learning for High-Throughput Quantification of Oligodendrocyte Ensheathment at Single-Cell Resolution. Commun. Biol. 2019, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Fancy, S.P.J.; Shen, Y.-A.A.; Niu, J.; Zhao, C.; Presley, B.; Miao, E.; Lee, S.; Mayoral, S.R.; Redmond, S.A.; et al. Micropillar Arrays as a High-Throughput Screening Platform for Therapeutics in Multiple Sclerosis. Nat. Med. 2014, 20, 954–960. [Google Scholar] [CrossRef]
- Mei, F.; Lehmann-Horn, K.; Shen, Y.-A.A.; Rankin, K.A.; Stebbins, K.J.; Lorrain, D.S.; Pekarek, K.; Sagan, S.A.; Xiao, L.; Teuscher, C.; et al. Accelerated Remyelination during Inflammatory Demyelination Prevents Axonal Loss and Improves Functional Recovery. Elife 2016, 5, e18246. [Google Scholar] [CrossRef] [PubMed]
- Makhija, E.P.; Espinosa-Hoyos, D.; Jagielska, A.; Van Vliet, K.J. Mechanical Regulation of Oligodendrocyte Biology. Neurosci. Lett. 2020, 717, 134673. [Google Scholar] [CrossRef]
- Sharma, R.; Smits, I.P.M.; De La Vega, L.; Lee, C.; Willerth, S.M. 3D Bioprinting Pluripotent Stem Cell Derived Neural Tissues Using a Novel Fibrin Bioink Containing Drug Releasing Microspheres. Front. Bioeng. Biotechnol. 2020, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Porcu, G.; Serone, E.; De Nardis, V.; Di Giandomenico, D.; Lucisano, G.; Scardapane, M.; Poma, A.; Ragnini-Wilson, A. Clobetasol and Halcinonide Act as Smoothened Agonists to Promote Myelin Gene Expression and RxRγ Receptor Activation. PLoS ONE 2015, 10, e0144550. [Google Scholar] [CrossRef] [PubMed]
- Lariosa-Willingham, K.D.; Rosler, E.S.; Tung, J.S.; Dugas, J.C.; Collins, T.L.; Leonoudakis, D. A High Throughput Drug Screening Assay to Identify Compounds That Promote Oligodendrocyte Differentiation Using Acutely Dissociated and Purified Oligodendrocyte Precursor Cells. BMC Res. Notes 2016, 9, 419. [Google Scholar] [CrossRef]
- Göttle, P.; Förster, M.; Weyers, V.; Küry, P.; Rejdak, K.; Hartung, H.-P.; Kremer, D. An Unmet Clinical Need: Roads to Remyelination in MS. Neurol. Res. Pract. 2019, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Hlavica, M.; Schuler, F.A.F.; Good, N.; Good, A.; Baumgartner, L.; Galeno, G.; Schneider, M.P.; Jung, T.; de Vries, R.; et al. Remyelination Promoting Therapies in Multiple Sclerosis Animal Models: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 822. [Google Scholar] [CrossRef] [PubMed]
- Nocita, E.; Del Giovane, A.; Tiberi, M.; Boccuni, L.; Fiorelli, D.; Sposato, C.; Romano, E.; Basoli, F.; Trombetta, M.; Rainer, A.; et al. EGFR/ErbB Inhibition Promotes OPC Maturation up to Axon Engagement by Co-Regulating PIP2 and MBP. Cells 2019, 8, 844. [Google Scholar] [CrossRef]
- Huang, J.K.; Jarjour, A.A.; Oumesmar, B.N.; Kerninon, C.; Williams, A.; Krezel, W.; Kagechika, H.; Bauer, J.; Zhao, C.; Baron-Van Evercooren, A.; et al. Retinoid X Receptor Gamma Signaling Accelerates CNS Remyelination. Nat. Neurosci. 2011, 14, 45–53. [Google Scholar] [CrossRef]
- Chandraratna, R.A.; Noelle, R.J.; Nowak, E.C. Treatment with Retinoid X Receptor Agonist IRX4204 Ameliorates Experimental Autoimmune Encephalomyelitis. Am. J. Transl. Res. 2016, 8, 1016–1026. [Google Scholar]
- Farsetti, A.; Mitsuhashi, T.; Desvergne, B.; Robbins, J.; Nikodem, V.M. Molecular Basis of Thyroid Hormone Regulation of Myelin Basic Protein Gene Expression in Rodent Brain. J. Biol. Chem. 1991, 266, 23226–23232. [Google Scholar] [CrossRef]
- Farsetti, A.; Desvergne, B.; Hallenbeck, P.; Robbins, J.; Nikodem, V.M. Characterization of Myelin Basic Protein Thyroid Hormone Response Element and Its Function in the Context of Native and Heterologous Promoter. J. Biol. Chem. 1992, 267, 15784–15788. [Google Scholar] [CrossRef]
- Wooliscroft, L.; Altowaijri, G.; Hildebrand, A.; Samuels, M.; Oken, B.; Bourdette, D.; Cameron, M. Phase I Randomized Trial of Liothyronine for Remyelination in Multiple Sclerosis: A Dose-Ranging Study with Assessment of Reliability of Visual Outcomes. Mult. Scler. Relat. Disord. 2020, 41, 102015. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Lee, D.-H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric Acid Esters Exert Neuroprotective Effects in Neuroinflammation via Activation of the Nrf2 Antioxidant Pathway. Brain 2011, 134 Pt 3, 678–692. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, Y.; Yan, B.; Fang, S.; He, J.; Zhang, D.; Zhang, Z.; Kong, J.; Tan, Q.; Li, X.-M. Quetiapine Prevents Oligodendrocyte and Myelin Loss and Promotes Maturation of Oligodendrocyte Progenitors in the Hippocampus of Global Cerebral Ischemia Mice. J. Neurochem. 2012, 123, 14–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, L.; Jiang, W.; Xu, H.; Xiao, L.; Bi, X.; Wang, J.; Zhu, S.; Zhang, R.; et al. Quetiapine Enhances Oligodendrocyte Regeneration and Myelin Repair after Cuprizone-Induced Demyelination. Schizophr. Res. 2012, 138, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Zhornitsky, S.; Wee Yong, V.; Koch, M.W.; Mackie, A.; Potvin, S.; Patten, S.B.; Metz, L.M. Quetiapine Fumarate for the Treatment of Multiple Sclerosis: Focus on Myelin Repair. CNS Neurosci. Ther. 2013, 19, 737–744. [Google Scholar] [CrossRef]
- Negrotto, L.; Farez, M.F.; Correale, J. Immunologic Effects of Metformin and Pioglitazone Treatment on Metabolic Syndrome and Multiple Sclerosis. JAMA Neurol. 2016, 73, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Schwartzbach, C.J.; Grove, R.A.; Brown, R.; Tompson, D.; Then Bergh, F.; Arnold, D.L. Lesion Remyelinating Activity of GSK239512 versus Placebo in Patients with Relapsing-Remitting Multiple Sclerosis: A Randomised, Single-Blind, Phase II Study. J. Neurol. 2017, 264, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Cadavid, D.; Mellion, M.; Hupperts, R.; Edwards, K.R.; Calabresi, P.A.; Drulović, J.; Giovannoni, G.; Hartung, H.-P.; Arnold, D.L.; Fisher, E.; et al. Safety and Efficacy of Opicinumab in Patients with Relapsing Multiple Sclerosis (SYNERGY): A Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Neurol. 2019, 18, 845–856. [Google Scholar] [CrossRef]
- Hanf, K.J.M.; Arndt, J.W.; Liu, Y.; Gong, B.J.; Rushe, M.; Sopko, R.; Massol, R.; Smith, B.; Gao, Y.; Dalkilic-Liddle, I.; et al. Functional Activity of Anti-LINGO-1 Antibody Opicinumab Requires Target Engagement at a Secondary Binding Site. MAbs 2020, 12, 1713648. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; Gelfand, J.M.; Cree, B.A.; Bevan, C.; Boscardin, W.J.; Mei, F.; Inman, J.; Arnow, S.; Devereux, M.; Abounasr, A.; et al. Clemastine Fumarate as a Remyelinating Therapy for Multiple Sclerosis (ReBUILD): A Randomised, Controlled, Double-Blind, Crossover Trial. Lancet 2017, 390, 2481–2489. [Google Scholar] [CrossRef]
- Cramer, P.E.; Cirrito, J.R.; Wesson, D.W.; Daniel Lee, C.Y.; Karlo, J.C.; Zinn, A.E.; Casali, B.T.; Restivo, J.L.; Goebel, W.D.; James, M.J.; et al. ApoE-Directed Therapeutics Rapidly Clear β-Amyloid and Reverse Deficits in AD Mouse Models. Science 2012, 335, 1503–1506. [Google Scholar] [CrossRef]
- Cummings, J.L.; Zhong, K.; Kinney, J.W.; Heaney, C.; Moll-Tudla, J.; Joshi, A.; Pontecorvo, M.; Devous, M.; Tang, A.; Bena, J. Double-Blind, Placebo-Controlled, Proof-of-Concept Trial of Bexarotene Xin Moderate Alzheimer’s Disease. Alzheimers Res. Ther. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Kato, H.; Yamashita, S.; Aramaki, S.; Morioka, H.; Koresawa, M.; Miyauchi, F.; Toyoshima, H.; Torigoe, T. Effects of Domperidone on Serum Prolactin Levels in Human Beings. Endocrinol. Jpn. 1980, 27, 521–525. [Google Scholar] [CrossRef]
- Koch, M.W.; Liu, W.-Q.; Camara-Lemarroy, C.; Zhang, Y.; Pike, G.B.; Metz, L.; Yong, V.W. Domperidone-Induced Elevation of Serum Prolactin Levels and Immune Response in Multiple Sclerosis. J. Neuroimmunol. 2019, 334, 576974. [Google Scholar] [CrossRef] [PubMed]
- Pershadsingh, H.A.; Heneka, M.T.; Saini, R.; Amin, N.M.; Broeske, D.J.; Feinstein, D.L. Effect of Pioglitazone Treatment in a Patient with Secondary Multiple Sclerosis. J. Neuroinflamm. 2004, 1, 3. [Google Scholar] [CrossRef]
- Schmidt, S.; Moric, E.; Schmidt, M.; Sastre, M.; Feinstein, D.L.; Heneka, M.T. Anti-Inflammatory and Antiproliferative Actions of PPAR-Gamma Agonists on T Lymphocytes Derived from MS Patients. J. Leukoc. Biol. 2004, 75, 478–485. [Google Scholar] [CrossRef]
- Goodkin, D.E. Interferon Beta Therapy for Multiple Sclerosis. Lancet 1998, 352, 1486–1487. [Google Scholar] [CrossRef]
- Kieseier, B.C. The Mechanism of Action of Interferon-β in Relapsing Multiple Sclerosis. CNS Drugs 2011, 25, 491–502. [Google Scholar] [CrossRef]
- Chataway, J.; Schuerer, N.; Alsanousi, A.; Chan, D.; MacManus, D.; Hunter, K.; Anderson, V.; Bangham, C.R.M.; Clegg, S.; Nielsen, C.; et al. Effect of High-Dose Simvastatin on Brain Atrophy and Disability in Secondary Progressive Multiple Sclerosis (MS-STAT): A Randomised, Placebo-Controlled, Phase 2 Trial. Lancet 2014, 383, 2213–2221. [Google Scholar] [CrossRef]
- Chan, D.; Binks, S.; Nicholas, J.M.; Frost, C.; Cardoso, M.J.; Ourselin, S.; Wilkie, D.; Nicholas, R.; Chataway, J. Effect of High-Dose Simvastatin on Cognitive, Neuropsychiatric, and Health-Related Quality-of-Life Measures in Secondary Progressive Multiple Sclerosis: Secondary Analyses from the MS-STAT Randomised, Placebo-Controlled Trial. Lancet Neurol. 2017, 16, 591–600. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Ohayon, D.; Li, H.; de Faria, J.P.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor Skill Learning Requires Active Central Myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.J.; Plemel, J.R.; Assinck, P.; Manesh, S.B.; Muir, F.G.W.; Hirata, R.; Berson, M.; Liu, J.; Wegner, M.; Emery, B.; et al. Myelin Regulatory Factor Drives Remyelination in Multiple Sclerosis. Acta Neuropathol. 2017, 134, 403–422. [Google Scholar] [CrossRef] [PubMed]
- Kaller, M.S.; Lazari, A.; Blanco-Duque, C.; Sampaio-Baptista, C.; Johansen-Berg, H. Myelin Plasticity and Behaviour—Connecting the Dots. Curr. Opin. Neurobiol. 2017, 47, 86–92. [Google Scholar] [CrossRef]
- Bejanin, A.; Desgranges, B.; La Joie, R.; Landeau, B.; Perrotin, A.; Mézenge, F.; Belliard, S.; de La Sayette, V.; Eustache, F.; Chételat, G. Distinct White Matter Injury Associated with Medial Temporal Lobe Atrophy in Alzheimer’s versus Semantic Dementia. Hum. Brain Mapp. 2017, 38, 1791–1800. [Google Scholar] [CrossRef]
- Papuć, E.; Rejdak, K. The Role of Myelin Damage in Alzheimer’s Disease Pathology. Arch. Med. Sci. 2020, 16, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Magliozzi, R.; Howell, O.; Vora, A.; Serafini, B.; Nicholas, R.; Puopolo, M.; Reynolds, R.; Aloisi, F. Meningeal B-Cell Follicles in Secondary Progressive Multiple Sclerosis Associate with Early Onset of Disease and Severe Cortical Pathology. Brain 2007, 130 Pt 4, 1089–1104. [Google Scholar] [CrossRef]
- Howell, O.W.; Reeves, C.A.; Nicholas, R.; Carassiti, D.; Radotra, B.; Gentleman, S.M.; Serafini, B.; Aloisi, F.; Roncaroli, F.; Magliozzi, R.; et al. Meningeal Inflammation Is Widespread and Linked to Cortical Pathology in Multiple Sclerosis. Brain 2011, 134 Pt 9, 2755–2771. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- McMurran, C.E.; Jones, C.A.; Fitzgerald, D.C.; Franklin, R.J.M. CNS Remyelination and the Innate Immune System. Front. Cell Dev. Biol. 2016, 4. [Google Scholar] [CrossRef]
- Miron, V.E.; Boyd, A.; Zhao, J.-W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 Microglia and Macrophages Drive Oligodendrocyte Differentiation during CNS Remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef]
- Ruckh, J.M.; Zhao, J.-W.; Shadrach, J.L.; van Wijngaarden, P.; Rao, T.N.; Wagers, A.J.; Franklin, R.J.M. Rejuvenation of Regeneration in the Aging Central Nervous System. Cell Stem Cell 2012, 10, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.E.; Hanak, S.; Pu, S.-F.; Liang, J.; Dang, C.; Iglesias-Bregna, D.; Harvey, B.; Zhu, B.; McMonagle-Strucko, K. Teriflunomide Reduces Behavioral, Electrophysiological, and Histopathological Deficits in the Dark Agouti Rat Model of Experimental Autoimmune Encephalomyelitis. J. Neurol. 2009, 256, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Orthmann-Murphy, J.; Call, C.L.; Molina-Castro, G.C.; Hsieh, Y.C.; Rasband, M.N.; Calabresi, P.A.; Bergles, D.E. Remyelination Alters the Pattern of Myelin in the Cerebral Cortex. Elife 2020, 9, e56621. [Google Scholar] [CrossRef]
- Hughes, E.G.; Orthmann-Murphy, J.L.; Langseth, A.J.; Bergles, D.E. Myelin Remodeling through Experience-Dependent Oligodendrogenesis in the Adult Somatosensory Cortex. Nat. Neurosci. 2018, 21, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Zuccaro, E.; Arlotta, P. The Quest for Myelin in the Adult Brain. Nat Cell Biol 2013, 15, 572–575. [Google Scholar] [CrossRef]
- Del Giovane, A.; Ragnini-Wilson, A. Targeting Smoothened as a New Frontier in the Functional Recovery of Central Nervous System Demyelinating Pathologies. Int. J. Mol. Sci. 2018, 19, 3677. [Google Scholar] [CrossRef]
- Ruat, M.; Hoch, L.; Faure, H.; Rognan, D. Targeting of Smoothened for Therapeutic Gain. Trends Pharmacol. Sci. 2014, 35, 237–246. [Google Scholar] [CrossRef]
- Lopez Juarez, A.; He, D.; Richard Lu, Q. Oligodendrocyte Progenitor Programming and Reprogramming: Toward Myelin Regeneration. Brain Res. 2016, 1638 Pt B, 209–220. [Google Scholar] [CrossRef]
- Sun, X.; Gao, L.; Yu, R.K.; Zeng, G. Down-Regulation of WNK1 Protein Kinase in Neural Progenitor Cells Suppresses Cell Proliferation and Migration. J. Neurochem. 2006, 99, 1114–1121. [Google Scholar] [CrossRef]
- Pan, S.; Chan, J.R. Regulation and Dysregulation of Axon Infrastructure by Myelinating Glia. J. Cell Biol. 2017, 216, 3903–3916. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Compston, A.; Jauniaux, E.; Gilson, J.; Blakemore, W.; Svendsen, C. Differential Generation of Oligodendrocytes from Human and Rodent Embryonic Spinal Cord Neural Precursors. Glia 2004, 47, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Ferent, J.; Zimmer, C.; Durbec, P.; Ruat, M.; Traiffort, E. Sonic Hedgehog Signaling Is a Positive Oligodendrocyte Regulator during Demyelination. J. Neurosci. 2013, 33, 1759–1772. [Google Scholar] [CrossRef]
- Ferent, J.; Traiffort, E. Hedgehog: Multiple Paths for Multiple Roles in Shaping the Brain and Spinal Cord. Neuroscientist 2015, 21, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Hoch, L.; Faure, H.; Roudaut, H.; Schoenfelder, A.; Mann, A.; Girard, N.; Bihannic, L.; Ayrault, O.; Petricci, E.; Taddei, M.; et al. MRT-92 Inhibits Hedgehog Signaling by Blocking Overlapping Binding Sites in the Transmembrane Domain of the Smoothened Receptor. FASEB J. 2015, 29, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Daynac, M.; Tirou, L.; Faure, H.; Mouthon, M.-A.; Gauthier, L.R.; Hahn, H.; Boussin, F.D.; Ruat, M. Hedgehog Controls Quiescence and Activation of Neural Stem Cells in the Adult Ventricular-Subventricular Zone. Stem Cell Rep. 2016, 7, 735–748. [Google Scholar] [CrossRef]
- Loulier, K.; Ruat, M.; Traiffort, E. Increase of Proliferating Oligodendroglial Progenitors in the Adult Mouse Brain upon Sonic Hedgehog Delivery in the Lateral Ventricle. J. Neurochem. 2006, 98, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Petrova, R.; Joyner, A.L. Roles for Hedgehog Signaling in Adult Organ Homeostasis and Repair. Development 2014, 141, 3445–3457. [Google Scholar] [CrossRef]
- Samanta, J.; Grund, E.M.; Silva, H.M.; Lafaille, J.J.; Fishell, G.; Salzer, J.L. Inhibition of Gli1 Mobilizes Endogenous Neural Stem Cells for Remyelination. Nature 2015, 526, 448–452. [Google Scholar] [CrossRef]
- Niewiadomski, P.; Niedziółka, S.M.; Markiewicz, Ł.; Uśpieński, T.; Baran, B.; Chojnowska, K. Gli Proteins: Regulation in Development and Cancer. Cells 2019, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, T.K.; Carpenter, R.L.; Qasem, S.; Chan, M.; Lo, H.-W. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Gregath, A.; Lu, Q.R. Epigenetic Modifications-Insight into Oligodendrocyte Lineage Progression, Regeneration, and Disease. FEBS Lett. 2018, 592, 1063–1078. [Google Scholar] [CrossRef]
- Marie, C.; Clavairoly, A.; Frah, M.; Hmidan, H.; Yan, J.; Zhao, C.; Van Steenwinckel, J.; Daveau, R.; Zalc, B.; Hassan, B.; et al. Oligodendrocyte Precursor Survival and Differentiation Requires Chromatin Remodeling by Chd7 and Chd8. Proc. Natl. Acad. Sci. USA 2018, 115, E8246–E8255. [Google Scholar] [CrossRef]
- Ming, X.; Dupree, J.L.; Gallo, V.; Chew, L.-J. Sox17 Promotes Oligodendrocyte Regeneration by Dual Modulation of Hedgehog and Wnt Signaling. iScience 2020, 23, 101592. [Google Scholar] [CrossRef] [PubMed]
- Emery, B.; Agalliu, D.; Cahoy, J.D.; Watkins, T.A.; Dugas, J.C.; Mulinyawe, S.B.; Ibrahim, A.; Ligon, K.L.; Rowitch, D.H.; Barres, B.A. Myelin Gene Regulatory Factor Is a Critical Transcriptional Regulator Required for CNS Myelination. Cell 2009, 138, 172–185. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Itoh, N.; Tassoni, A.; Matsukawa, M.A.; Ren, E.; Tse, V.; Jang, E.; Suen, T.T.; Itoh, Y. Gene Expression in Oligodendrocytes during Remyelination Reveals Cholesterol Homeostasis as a Therapeutic Target in Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 10130–10139. [Google Scholar] [CrossRef]
- Hubler, Z.; Allimuthu, D.; Bederman, I.; Elitt, M.S.; Madhavan, M.; Allan, K.C.; Shick, H.E.; Garrison, E.; Karl, M.T.; Factor, D.C.; et al. Accumulation of 8,9-Unsaturated Sterols Drives Oligodendrocyte Formation and Remyelination. Nature 2018, 560, 372–376. [Google Scholar] [CrossRef]
- Nawaz, S.; Sánchez, P.; Schmitt, S.; Snaidero, N.; Mitkovski, M.; Velte, C.; Brückner, B.R.; Alexopoulos, I.; Czopka, T.; Jung, S.Y.; et al. Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev. Cell 2015, 34, 139–151. [Google Scholar] [CrossRef]
- Zuchero, J.B.; Fu, M.-M.; Sloan, S.A.; Ibrahim, A.; Olson, A.; Zaremba, A.; Dugas, J.C.; Wienbar, S.; Caprariello, A.V.; Kantor, C.; et al. CNS Myelin Wrapping Is Driven by Actin Disassembly. Dev. Cell 2015, 34, 152–167. [Google Scholar] [CrossRef]
- Lee, X.; Shao, Z.; Sheng, G.; Pepinsky, B.; Mi, S. LINGO-1 Regulates Oligodendrocyte Differentiation by Inhibiting ErbB2 Translocation and Activation in Lipid Rafts. Mol. Cell. Neurosci. 2014, 60, 36–42. [Google Scholar] [CrossRef]
- Brugarolas, P.; Popko, B. Remyelination Therapy Goes to Trial for Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2014, 1, e26. [Google Scholar] [CrossRef] [PubMed]
- Gobert, R.P.; Joubert, L.; Curchod, M.-L.; Salvat, C.; Foucault, I.; Jorand-Lebrun, C.; Lamarine, M.; Peixoto, H.; Vignaud, C.; Frémaux, C.; et al. Convergent Functional Genomics of Oligodendrocyte Differentiation Identifies Multiple Autoinhibitory Signaling Circuits. Mol. Cell. Biol. 2009, 29, 1538–1553. [Google Scholar] [CrossRef] [PubMed]
- Keough, M.B.; Yong, V.W. Remyelination Therapy for Multiple Sclerosis. Neurotherapeutics 2013, 10, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Villoslada, P.; Alonso, C.; Agirrezabal, I.; Kotelnikova, E.; Zubizarreta, I.; Pulido-Valdeolivas, I.; Saiz, A.; Comabella, M.; Montalban, X.; Villar, L.; et al. Metabolomic Signatures Associated with Disease Severity in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e321. [Google Scholar] [CrossRef]
- Chamberlain, K.A.; Nanescu, S.E.; Psachoulia, K.; Huang, J.K. Oligodendrocyte Regeneration: Its Significance in Myelin Replacement and Neuroprotection in Multiple Sclerosis. Neuropharmacology 2016, 110 Pt B, 633–643. [Google Scholar] [CrossRef]
- Jäkel, S.; Agirre, E.; Mendanha Falcão, A.; van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; Ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered Human Oligodendrocyte Heterogeneity in Multiple Sclerosis. Nature 2019, 566, 543–547. [Google Scholar] [CrossRef]
- Foerster, S.; Hill, M.F.E.; Franklin, R.J.M. Diversity in the oligodendrocyte lineage: Plasticity or heterogeneity? Glia 2019, 67, 1797–1805. [Google Scholar] [CrossRef]
- Marques, S.; Zeisel, A.; Codeluppi, S.; van Bruggen, D.; Mendanha Falcão, A.; Xiao, L.; Li, H.; Häring, M.; Hochgerner, H.; Romanov, R.A.; et al. Oligodendrocyte Heterogeneity in the Mouse Juvenile and Adult Central Nervous System. Science 2016, 352, 1326–1329. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Ffrench-Constant, C. Regenerating CNS Myelin—From Mechanisms to Experimental Medicines. Nat. Rev. Neurosci. 2017, 18, 753–769. [Google Scholar] [CrossRef]
- Esmonde-White, C.; Yaqubi, M.; Bilodeau, P.A.; Cui, Q.L.; Pernin, F.; Larochelle, C.; Ghadiri, M.; Xu, Y.K.T.; Kennedy, T.E.; Hall, J.; et al. Distinct Function-Related Molecular Profile of Adult Human A2B5-Positive Pre-Oligodendrocytes Versus Mature Oligodendrocytes. J. Neuropathol. Exp. Neurol. 2019, 78, 468–479. [Google Scholar] [CrossRef]
- Yeung, M.S.Y.; Djelloul, M.; Steiner, E.; Bernard, S.; Salehpour, M.; Possnert, G.; Brundin, L.; Frisén, J. Dynamics of Oligodendrocyte Generation in Multiple Sclerosis. Nature 2019, 566, 538–542. [Google Scholar] [CrossRef]
- Oh, J.; Alikhani, K.; Bruno, T.; Devonshire, V.; Giacomini, P.S.; Giuliani, F.; Nakhaipour, H.R.; Schecter, R.; Larochelle, C. Diagnosis and Management of Secondary-Progressive Multiple Sclerosis: Time for Change. Neurodegener. Dis. Manag. 2019, 9, 301–317. [Google Scholar] [CrossRef]
- Bacmeister, C.M.; Barr, H.J.; McClain, C.R.; Thornton, M.A.; Nettles, D.; Welle, C.G.; Hughes, E.G. Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat Neurosci. 2020, 23, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Jan, E.; Kotov, N.A. Successful Differentiation of Mouse Neural Stem Cells on Layer-by-Layer Assembled Single-Walled Carbon Nanotube Composite. Nano Lett. 2007, 7, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Zuchowska, A.; Chudy, M.; Dybko, A.; Brzozka, Z. Graphene as a New Material in Anticancer Therapy-in Vitro Studies. Sens. Actuators B Chem. 2017, 243, 152–165. [Google Scholar] [CrossRef]
- Bunge, M.B.; Bunge, R.P.; Ris, H. Ultrastructural Study of Remyelination in an Experimental Lesion in Adult Cat Spinal Cord. J. Biophys. Biochem. Cytol. 1961, 10, 67–94. [Google Scholar] [CrossRef] [PubMed]
- Bunge, R.P.; Bunge, M.B.; Bates, M. Movements of the Schwann Cell Nucleus Implicate Progression of the Inner (Axon-Related) Schwann Cell Process during Myelination. J. Cell Biol. 1989, 109, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Knobler, R.L.; Stempak, J.G.; Laurencin, M. Nonuniformity of the Oligodendroglial Ensheathment of Axons during Myelination in the Developing Rat Central Nervous System. A Serial Section Electron Microscopical Study. J. Ultrastruct. Res. 1976, 55, 417–432. [Google Scholar] [CrossRef]
- Sobottka, B.; Ziegler, U.; Kaech, A.; Becher, B.; Goebels, N. CNS Live Imaging Reveals a New Mechanism of Myelination: The Liquid Croissant Model. Glia 2011, 59, 1841–1849. [Google Scholar] [CrossRef]
- Snaidero, N.; Möbius, W.; Czopka, T.; Hekking, L.H.P.; Mathisen, C.; Verkleij, D.; Goebbels, S.; Edgar, J.; Merkler, D.; Lyons, D.A.; et al. Myelin Membrane Wrapping of CNS Axons by PI(3,4,5)P3-Dependent Polarized Growth at the Inner Tongue. Cell 2014, 156, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Geren, B.B.; Schmitt, F.O. The structure of the schwann cell and its relation to the axon in certain invertebrate nerve fibers. Proc. Natl. Acad. Sci. USA 1954, 40, 863–870. [Google Scholar] [CrossRef]
- Beyer, B.A.; Fang, M.; Sadrian, B.; Montenegro-Burke, J.R.; Plaisted, W.C.; Kok, B.P.C.; Saez, E.; Kondo, T.; Siuzdak, G.; Lairson, L.L. Metabolomics-Based Discovery of a Metabolite That Enhances Oligodendrocyte Maturation. Nat. Chem. Biol. 2018, 14, 22–28. [Google Scholar] [CrossRef]
- Nordengen, K.; Heuser, C.; Rinholm, J.E.; Matalon, R.; Gundersen, V. Localisation of N-Acetylaspartate in Oligodendrocytes/Myelin. Brain Struct. Funct. 2015, 220, 899–917. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Hadera, M.G.; Kotter, M.; Sonnewald, U. Oligodendrocytes Do Not Export NAA-Derived Aspartate In Vitro. Neurochem. Res. 2017, 42, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Appu, A.P.; Moffett, J.R.; Arun, P.; Moran, S.; Nambiar, V.; Krishnan, J.K.S.; Puthillathu, N.; Namboodiri, A.M.A. Increasing N-Acetylaspartate in the Brain during Postnatal Myelination Does Not Cause the CNS Pathologies of Canavan Disease. Front. Mol. Neurosci. 2017, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mattan, N.S.; de Vellis, J. Canavan Disease: A White Matter Disorder. Ment. Retard. Dev. Disabil. Res. Rev. 2006, 12, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.K.; Huang, H.; Li, S.; Clements, R.; Gadd, J.; Daniels, A.; Kooijman, E.E.; Bannerman, P.; Burns, T.; Guo, F.; et al. The Neuronal Metabolite NAA Regulates Histone H3 Methylation in Oligodendrocytes and Myelin Lipid Composition. Exp. Brain Res. 2017, 235, 279–292. [Google Scholar] [CrossRef]
- Umemori, H.; Sato, S.; Yagi, T.; Aizawa, S.; Yamamoto, T. Initial Events of Myelination Involve Fyn Tyrosine Kinase Signalling. Nature 1994, 367, 572–576. [Google Scholar] [CrossRef]
- White, R.; Gonsior, C.; Krämer-Albers, E.-M.; Stöhr, N.; Hüttelmaier, S.; Trotter, J. Activation of Oligodendroglial Fyn Kinase Enhances Translation of MRNAs Transported in HnRNP A2-Dependent RNA Granules. J. Cell Biol. 2008, 181, 579–586. [Google Scholar] [CrossRef]
- Chun, S.J.; Rasband, M.N.; Sidman, R.L.; Habib, A.A.; Vartanian, T. Integrin-Linked Kinase Is Required for Laminin-2-Induced Oligodendrocyte Cell Spreading and CNS Myelination. J. Cell Biol. 2003, 163, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Relucio, J.; Tzvetanova, I.D.; Ao, W.; Lindquist, S.; Colognato, H. Laminin Alters Fyn Regulatory Mechanisms and Promotes Oligodendrocyte Development. J. Neurosci. 2009, 29, 11794–11806. [Google Scholar] [CrossRef]
- Bergles, D.E.; Roberts, J.D.; Somogyi, P.; Jahr, C.E. Glutamatergic Synapses on Oligodendrocyte Precursor Cells in the Hippocampus. Nature 2000, 405, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Bergles, D.E. Synaptic Signaling between GABAergic Interneurons and Oligodendrocyte Precursor Cells in the Hippocampus. Nat. Neurosci. 2004, 7, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.N.; Appel, B. Oligodendrocytes Express Synaptic Proteins That Modulate Myelin Sheath Formation. Nat. Commun. 2019, 10, 4125. [Google Scholar] [CrossRef]
- Wake, H.; Ortiz, F.C.; Woo, D.H.; Lee, P.R.; Angulo, M.C.; Fields, R.D. Nonsynaptic Junctions on Myelinating Glia Promote Preferential Myelination of Electrically Active Axons. Nat. Commun. 2015, 6, 7844. [Google Scholar] [CrossRef]
- Friede, R.L. Control of Myelin Formation by Axon Caliber (with a Model of the Control Mechanism). J. Comp. Neurol. 1972, 144, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Althaus, H.H.; Montz, H.; Neuhoff, V.; Schwartz, P. Isolation and Cultivation of Mature Oligodendroglial Cells. Naturwissenschaften 1984, 71, 309–315. [Google Scholar] [CrossRef]
- Voyvodic, J.T. Target Size Regulates Calibre and Myelination of Sympathetic Axons. Nature 1989, 342, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Gard, A.L.; Pfeiffer, S.E. Stimulation of Oligodendrocyte Differentiation in Culture by Growth in the Presence of a Monoclonal Antibody to Sulfated Glycolipid. J. Neurosci. Res. 1988, 21, 260–267. [Google Scholar] [CrossRef]
- Bullock, P.N.; Rome, L.H. Glass Micro-Fibers: A Model System for Study of Early Events in Myelination. J. Neurosci. Res. 1990, 27, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Howe, C.L. Coated Glass and Vicryl Microfibers as Artificial Axons. Cells Tissues Organs 2006, 183, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.L.; LaRocca, P.J. Method of Production and Control of a Commercial Tissue Culture Surface. J. Tissue Cult. Methods 1994, 16, 151–153. [Google Scholar] [CrossRef]
- Young, E.W.K.; Berthier, E.; Guckenberger, D.J.; Sackmann, E.; Lamers, C.; Meyvantsson, I.; Huttenlocher, A.; Beebe, D.J. Rapid Prototyping of Arrayed Microfluidic Systems in Polystyrene for Cell-Based Assays. Anal. Chem. 2011, 83, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Lerman, M.J.; Lembong, J.; Muramoto, S.; Gillen, G.; Fisher, J.P. The Evolution of Polystyrene as a Cell Culture Material. Tissue Eng. Part B Rev. 2018, 24, 359–372. [Google Scholar] [CrossRef]
- Chong, S.Y.C.; Rosenberg, S.S.; Fancy, S.P.J.; Zhao, C.; Shen, Y.-A.A.; Hahn, A.T.; McGee, A.W.; Xu, X.; Zheng, B.; Zhang, L.I.; et al. Neurite Outgrowth Inhibitor Nogo-A Establishes Spatial Segregation and Extent of Oligodendrocyte Myelination. Proc. Natl. Acad. Sci. USA 2012, 109, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Sakiyama-Elbert, S.; Johnson, P.J.; Hodgetts, S.I.; Plant, G.W.; Harvey, A.R. Scaffolds to Promote Spinal Cord Regeneration. Handb. Clin. Neurol. 2012, 109, 575–594. [Google Scholar] [CrossRef]
- Estrada, V.; Tekinay, A.; Müller, H.W. Neural ECM Mimetics. Prog. Brain Res. 2014, 214, 391–413. [Google Scholar] [CrossRef]
- Pajevic, S.; Basser, P.J.; Fields, R.D. Role of Myelin Plasticity in Oscillations and Synchrony of Neuronal Activity. Neuroscience 2014, 276, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Jagielska, A.; Norman, A.L.; Whyte, G.; Vliet, K.J.V.; Guck, J.; Franklin, R.J.M. Mechanical Environment Modulates Biological Properties of Oligodendrocyte Progenitor Cells. Stem Cells Dev. 2012, 21, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Levental, I.; Georges, P.C.; Janmey, P.A. Soft Biological Materials and Their Impact on Cell Function. Soft Matter 2007, 3, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-B.; Franze, K.; Seifert, G.; Steinhäuser, C.; Kirchhoff, F.; Wolburg, H.; Guck, J.; Janmey, P.; Wei, E.-Q.; Käs, J.; et al. Viscoelastic Properties of Individual Glial Cells and Neurons in the CNS. Proc. Natl. Acad. Sci. USA 2006, 103, 17759–17764. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Tuohy, T.M.F.; Chen, H.; Wallingford, N.; Craig, A.; Struve, J.; Luo, N.L.; Banine, F.; Liu, Y.; Chang, A.; et al. Hyaluronan Accumulates in Demyelinated Lesions and Inhibits Oligodendrocyte Progenitor Maturation. Nat. Med. 2005, 11, 966–972. [Google Scholar] [CrossRef]
- Russell, L.N.; Lampe, K.J. Oligodendrocyte Precursor Cell Viability, Proliferation, and Morphology Is Dependent on Mesh Size and Storage Modulus in 3D Poly(Ethylene Glycol)-Based Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3459–3468. [Google Scholar] [CrossRef]
- Stoffels, J.M.J.; de Jonge, J.C.; Stancic, M.; Nomden, A.; van Strien, M.E.; Ma, D.; Sisková, Z.; Maier, O.; Ffrench-Constant, C.; Franklin, R.J.M.; et al. Fibronectin Aggregation in Multiple Sclerosis Lesions Impairs Remyelination. Brain 2013, 136 Pt 1, 116–131. [Google Scholar] [CrossRef]
- Bershadsky, A.D.; Balaban, N.Q.; Geiger, B. Adhesion-Dependent Cell Mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003, 19, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Giera, S.; Deng, Y.; Luo, R.; Ackerman, S.D.; Mogha, A.; Monk, K.R.; Ying, Y.; Jeong, S.-J.; Makinodan, M.; Bialas, A.R.; et al. The Adhesion G Protein-Coupled Receptor GPR56 Is a Cell-Autonomous Regulator of Oligodendrocyte Development. Nat. Commun. 2015, 6, 6121. [Google Scholar] [CrossRef]
- Colognato, H.; Baron, W.; Avellana-Adalid, V.; Relvas, J.B.; Baron-Van Evercooren, A.; Georges-Labouesse, E.; ffrench-Constant, C. CNS Integrins Switch Growth Factor Signalling to Promote Target-Dependent Survival. Nat. Cell Biol. 2002, 4, 833–841. [Google Scholar] [CrossRef]
- Spiegel, I.; Peles, E. A Novel Method for Isolating Schwann Cells Using the Extracellular Domain of Necl1. J. Neurosci. Res. 2009, 87, 3288–3296. [Google Scholar] [CrossRef]
- Li, Y.; Ceylan, M.; Shrestha, B.; Wang, H.; Lu, Q.R.; Asmatulu, R.; Yao, L. Nanofibers Support Oligodendrocyte Precursor Cell Growth and Function as a Neuron-Free Model for Myelination Study. Biomacromolecules 2014, 15, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement Membrane Matrix with Biological Activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From Discovery and ECM Mimicry to Assays and Models for Cancer Research. Adv. Drug Deliv. Rev. 2014, 79–80, 3–18. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A Complex Protein Mixture Required for Optimal Growth of Cell Culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Taub, M.; Wang, Y.; Szczesny, T.M.; Kleinman, H.K. Epidermal Growth Factor or Transforming Growth Factor Alpha Is Required for Kidney Tubulogenesis in Matrigel Cultures in Serum-Free Medium. Proc. Natl. Acad. Sci. USA 1990, 87, 4002–4006. [Google Scholar] [CrossRef]
- Basic, N.; Basic, V.; Bulic, K.; Grgic, M.; Kleinman, H.K.; Luyten, F.P.; Vukicevic, S. TGF-Beta and Basement Membrane Matrigel Stimulate the Chondrogenic Phenotype in Osteoblastic Cells Derived from Fetal Rat Calvaria. J. Bone Miner. Res. 1996, 11, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Cui, L.; Snider, B.J.; Rivkin, M.; Yu, S.S.; Lee, C.-S.; Adams, L.D.; Gottlieb, D.I.; Johnson, E.M.; Yu, S.P.; et al. Transplantation of Embryonic Stem Cells Overexpressing Bcl-2 Promotes Functional Recovery after Transient Cerebral Ischemia. Neurobiol. Dis. 2005, 19, 183–193. [Google Scholar] [CrossRef]
- Jin, K.; Mao, X.; Xie, L.; Galvan, V.; Lai, B.; Wang, Y.; Gorostiza, O.; Wang, X.; Greenberg, D.A. Transplantation of Human Neural Precursor Cells in Matrigel Scaffolding Improves Outcome from Focal Cerebral Ischemia after Delayed Postischemic Treatment in Rats. J. Cereb. Blood Flow Metab. 2010, 30, 534–544. [Google Scholar] [CrossRef]
- Aurand, E.R.; Lampe, K.J.; Bjugstad, K.B. Defining and Designing Polymers and Hydrogels for Neural Tissue Engineering. Neurosci. Res. 2012, 72, 199–213. [Google Scholar] [CrossRef]
- Licht, C.; Rose, J.C.; Anarkoli, A.O.; Blondel, D.; Roccio, M.; Haraszti, T.; Gehlen, D.B.; Hubbell, J.A.; Lutolf, M.P.; De Laporte, L. Synthetic 3D PEG-Anisogel Tailored with Fibronectin Fragments Induce Aligned Nerve Extension. Biomacromolecules 2019, 20, 4075–4087. [Google Scholar] [CrossRef]
- Unal, D.B.; Caliari, S.R.; Lampe, K.J. 3D Hyaluronic Acid Hydrogels for Modeling Oligodendrocyte Progenitor Cell Behavior as a Function of Matrix Stiffness. Biomacromolecules 2020, 21, 4962–4971. [Google Scholar] [CrossRef] [PubMed]
- Seidlits, S.K.; Khaing, Z.Z.; Petersen, R.R.; Nickels, J.D.; Vanscoy, J.E.; Shear, J.B.; Schmidt, C.E. The Effects of Hyaluronic Acid Hydrogels with Tunable Mechanical Properties on Neural Progenitor Cell Differentiation. Biomaterials 2010, 31, 3930–3940. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Thomas, A.; Kotov, N.A.; Haag, R. Functional Graphene Nanomaterials Based Architectures: Biointeractions, Fabrications, and Emerging Biological Applications. Chem. Rev. 2017, 117, 1826–1914. [Google Scholar] [CrossRef]
- Barahuie, F.; Saifullah, B.; Dorniani, D.; Fakurazi, S.; Karthivashan, G.; Hussein, M.Z.; Elfghi, F.M. Graphene Oxide as a Nanocarrier for Controlled Release and Targeted Delivery of an Anticancer Active Agent, Chlorogenic Acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Mollazade, M.; Nejati-Koshki, K.; Akbarzadeh, A.; Zarghami, N.; Nasiri, M.; Jahanban-Esfahlan, R.; Alibakhshi, A. PAMAM Dendrimers Augment Inhibitory Effects of Curcumin on Cancer Cell Proliferation: Possible Inhibition of Telomerase. Asian Pac. J. Cancer Prev. 2013, 14, 6925–6928. [Google Scholar] [CrossRef]
- Abbasi, E.; Akbarzadeh, A.; Kouhi, M.; Milani, M. Graphene: Synthesis, Bio-Applications, and Properties. Artif. Cells Nanomed. Biotechnol. 2016, 44, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.L.; Cui, X.T. Directed Neural Stem Cell Differentiation with a Functionalized Graphene Oxide Nanocomposite. Adv. Healthc. Mater. 2015, 4, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Yin, P.T.; Uehara, T.M.; Chueng, S.-T.D.; Yang, L.; Lee, K.-B. Guiding Stem Cell Differentiation into Oligodendrocytes Using Graphene-Nanofiber Hybrid Scaffolds. Adv. Mater. 2014, 26, 3673–3680. [Google Scholar] [CrossRef]
- Han, S.S.W.; Liu, Y.; Tyler-Polsz, C.; Rao, M.S.; Fischer, I. Transplantation of Glial-Restricted Precursor Cells into the Adult Spinal Cord: Survival, Glial-Specific Differentiation, and Preferential Migration in White Matter. Glia 2004, 45, 1–16. [Google Scholar] [CrossRef]
- Cao, Q.; Xu, X.-M.; Devries, W.H.; Enzmann, G.U.; Ping, P.; Tsoulfas, P.; Wood, P.M.; Bunge, M.B.; Whittemore, S.R. Functional Recovery in Traumatic Spinal Cord Injury after Transplantation of Multineurotrophin-Expressing Glial-Restricted Precursor Cells. J. Neurosci. 2005, 25, 6947–6957. [Google Scholar] [CrossRef]
- Buckley, C.E.; Marguerie, A.; Roach, A.G.; Goldsmith, P.; Fleming, A.; Alderton, W.K.; Franklin, R.J.M. Drug Reprofiling Using Zebrafish Identifies Novel Compounds with Potential Pro-Myelination Effects. Neuropharmacology 2010, 59, 149–159. [Google Scholar] [CrossRef]
- Buckley, C.E.; Goldsmith, P.; Franklin, R.J.M. Zebrafish Myelination: A Transparent Model for Remyelination? Dis. Model. Mech. 2008, 1, 221–228. [Google Scholar] [CrossRef]
- Jeserich, G.; Strelau, J.; Lanwert, C. Partial Characterization of the 5′-Flanking Region of Trout IP: A Po-like Gene Containing a PLP-like Promoter. J. Neurosci. Res. 1997, 50, 781–790. [Google Scholar] [CrossRef]
- Jeserich, G.; Klempahn, K.; Pfeiffer, M. Features and Functions of Oligodendrocytes and Myelin Proteins of Lower Vertebrate Species. J. Mol. Neurosci. 2008, 35, 117–126. [Google Scholar] [CrossRef]
- Xing, Y.L.; Röth, P.T.; Stratton, J.A.; Chuang, B.H.; Danne, J.; Ellis, S.L.; Ng, S.W.; Kilpatrick, T.J.; Merson, T.D. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J Neurosci. 2014, 34, 14128–14146. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Krämer, E.; Grzenkowski, M.; Tang, K.; Blakemore, W.; Aguzzi, A.; Khazaie, K.; Chlichlia, K.; von Blankenfeld, G.; Kettenmann, H. Lines of Murine Oligodendroglial Precursor Cells Immortalized by an Activated Neu Tyrosine Kinase Show Distinct Degrees of Interaction with Axons in Vitro and in Vivo. Eur. J. Neurosci. 1995, 7, 1245–1265. [Google Scholar] [CrossRef]
- White, R.; Krämer-Albers, E.-M. Axon-Glia Interaction and Membrane Traffic in Myelin Formation. Front. Cell. Neurosci. 2014, 7, 284. [Google Scholar] [CrossRef]
- De Vries, G.H.; Boullerne, A.I. Glial Cell Lines: An Overview. Neurochem. Res. 2010, 35, 1978–2000. [Google Scholar] [CrossRef]
- Chenoweth, J.G.; Tesar, P.J. Isolation and Maintenance of Mouse Epiblast Stem Cells. Methods Mol. Biol. 2010, 636, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.C.; Mason, E.A.; Pera, M.F. The Pluripotent State in Mouse and Human. Development 2015, 142, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Tesar, P.J.; Chenoweth, J.G.; Brook, F.A.; Davies, T.J.; Evans, E.P.; Mack, D.L.; Gardner, R.L.; McKay, R.D.G. New Cell Lines from Mouse Epiblast Share Defining Features with Human Embryonic Stem Cells. Nature 2007, 448, 196–199. [Google Scholar] [CrossRef]
- Joubert, L.; Foucault, I.; Sagot, Y.; Bernasconi, L.; Duval, F.; Alliod, C.; Frossard, M.-J.; Pescini Gobert, R.; Curchod, M.-L.; Salvat, C.; et al. Chemical Inducers and Transcriptional Markers of Oligodendrocyte Differentiation. J. Neurosci. Res. 2010, 88, 2546–2557. [Google Scholar] [CrossRef]
- Ashikawa, Y.; Nishimura, Y.; Okabe, S.; Sasagawa, S.; Murakami, S.; Yuge, M.; Kawaguchi, K.; Kawase, R.; Tanaka, T. Activation of Sterol Regulatory Element Binding Factors by Fenofibrate and Gemfibrozil Stimulates Myelination in Zebrafish. Front. Pharmacol. 2016, 7, 206. [Google Scholar] [CrossRef]
- Elitt, M.S.; Barbar, L.; Tesar, P.J. Drug Screening for Human Genetic Diseases Using IPSC Models. Hum. Mol. Genet. 2018, 27, R89–R98. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.G.; Daley, G.Q. Induced Pluripotent Stem Cells in Disease Modelling and Drug Discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef]
- Chanoumidou, K.; Mozafari, S.; Baron-Van Evercooren, A.; Kuhlmann, T. Stem Cell Derived Oligodendrocytes to Study Myelin Diseases. Glia 2020, 68, 705–720. [Google Scholar] [CrossRef]
- Lee, C.-T.; Bendriem, R.M.; Wu, W.W.; Shen, R.-F. 3D Brain Organoids Derived from Pluripotent Stem Cells: Promising Experimental Models for Brain Development and Neurodegenerative Disorders. J. Biomed. Sci. 2017, 24, 59. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of Cerebral Organoids from Human Pluripotent Stem Cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Padhy, B.M.; Gupta, Y.K. Drug Repositioning: Re-Investigating Existing Drugs for New Therapeutic Indications. J. Postgrad. Med. 2011, 57, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug Repurposing: A Promising Tool to Accelerate the Drug Discovery Process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Iorio, F.; Bosotti, R.; Scacheri, E.; Belcastro, V.; Mithbaokar, P.; Ferriero, R.; Murino, L.; Tagliaferri, R.; Brunetti-Pierri, N.; Isacchi, A.; et al. Discovery of Drug Mode of Action and Drug Repositioning from Transcriptional Responses. Proc. Natl. Acad. Sci. USA 2010, 107, 14621–14626. [Google Scholar] [CrossRef]
- Oprea, T.I.; Bauman, J.E.; Bologa, C.G.; Buranda, T.; Chigaev, A.; Edwards, B.S.; Jarvik, J.W.; Gresham, H.D.; Haynes, M.K.; Hjelle, B.; et al. Drug Repurposing from an Academic Perspective. Drug Discov. Today Ther. Strateg. 2011, 8, 61–69. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Wang, S.; Bates, J.; Li, X.; Schanz, S.; Chandler-Militello, D.; Levine, C.; Maherali, N.; Studer, L.; Hochedlinger, K.; Windrem, M.; et al. Human IPSC-Derived Oligodendrocyte Progenitor Cells Can Myelinate and Rescue a Mouse Model of Congenital Hypomyelination. Cell Stem Cell 2013, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Windrem, M.S.; Osipovitch, M.; Liu, Z.; Bates, J.; Chandler-Militello, D.; Zou, L.; Munir, J.; Schanz, S.; McCoy, K.; Miller, R.H.; et al. Human IPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia. Cell Stem Cell 2017, 21, 195–208.e6. [Google Scholar] [CrossRef] [PubMed]
- Joung, D.; Truong, V.; Neitzke, C.C.; Guo, S.-Z.; Walsh, P.J.; Monat, J.R.; Meng, F.; Park, S.H.; Dutton, J.R.; Parr, A.M.; et al. 3D Printed Stem-Cell Derived Neural Progenitors Generate Spinal Cord Scaffolds. Adv. Funct. Mater. 2018, 28, 1801850. [Google Scholar] [CrossRef]
- Huch, M.; Knoblich, J.A.; Lutolf, M.P.; Martinez-Arias, A. The Hope and the Hype of Organoid Research. Development 2017, 144, 938–941. [Google Scholar] [CrossRef]
- Xu, C.; Inokuma, M.S.; Denham, J.; Golds, K.; Kundu, P.; Gold, J.D.; Carpenter, M.K. Feeder-Free Growth of Undifferentiated Human Embryonic Stem Cells. Nat. Biotechnol. 2001, 19, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Schwank, G.; Koo, B.-K.; Sasselli, V.; Dekkers, J.F.; Heo, I.; Demircan, T.; Sasaki, N.; Boymans, S.; Cuppen, E.; van der Ent, C.K.; et al. Functional Repair of CFTR by CRISPR/Cas9 in Intestinal Stem Cell Organoids of Cystic Fibrosis Patients. Cell Stem Cell 2013, 13, 653–658. [Google Scholar] [CrossRef]
- Chhibber, T.; Bagchi, S.; Lahooti, B.; Verma, A.; Al-Ahmad, A.; Paul, M.K.; Pendyala, G.; Jayant, R.D. CNS Organoids: An Innovative Tool for Neurological Disease Modeling and Drug Neurotoxicity Screening. Drug Discov. Today 2020, 25, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Xu, R.; Padmashri, R.; Dunaevsky, A.; Liu, Y.; Dreyfus, C.F.; Jiang, P. Pluripotent Stem Cell-Derived Cerebral Organoids Reveal Human Oligodendrogenesis with Dorsal and Ventral Origins. Stem Cell Rep. 2019, 12, 890–905. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Song, L.; Bejoy, J.; Zhao, J.; Kanekiyo, T.; Bu, G.; Zhou, Y.; Li, Y. Modeling Neurodegenerative Microenvironment Using Cortical Organoids Derived from Human Stem Cells. Tissue Eng. Part A 2018, 24, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
| Drug Name | Drug Class | Target/Evidence of Action in Remyelination | Drug Identification Method | Clinical Trial Phase | Ref. |
|---|---|---|---|---|---|
| IRX4204 | Second generation RXR agonist | It agonizes RXR-γ, a positive regulator of the differentiation and remyelination of endogenous precursor cells of oligodendrocytes | Target-based/In vivo studies | Phase 1 ClinicalTrials.gov Identifier: NCT02438215 | [38,39] |
| T3 | Thyroid Hormone | T3 is required for central CNS myelination during development, and CNS remyelination in animal models of MS | Micropillar-based, drug repurposing phenotypical screen | Phase 1 ClinicalTrials.gov Identifier: NCT02760056 | [29,40,41,42] |
| Quetiapine fumarate | Dibenzothiazepine | Atypical antipsychotic drug Used in the symptomatic treatment of schizophrenia and bipolar disorders. It has a role as a serotonergic antagonist, a dopaminergic antagonist, a histamine antagonist, an adrenergic antagonist, and a second generation antipsychotic. It stimulates proliferation of OPC and their differentiation oligodendrocytes. It increases SOD1 activity and the scavenging of free radicals, alleviating oxidative stress. | Micropillar-based, Drug repurposing phenotypical screen | Phase 1/2 ClinicalTrials.gov Identifier: NCT02087631 | [29,43,44,45,46] |
| Metformin | Biguanide hypoglycemic agent used in the treatment of non-insulin-dependent diabetes mellitus | Metformin is associated with a reduction in MS disease activity, but the potential mechanisms underlying this anti-inflammatory effect have not yet been clarified. | Not specified/in vivo validation | Phase 1/2 ClinicalTrials.gov Identifier: NCT04121468 | [47] |
| GSK239512 | Small molecule | Histamine H3 antagonist, orally available, assessed for modulating cholinergic and monoaminergic neurotransmission in AD. In RRMS, it has been tested for enhancing remyelination. | Not specified | Phase 2 ClinicalTrials.gov Identifier: NCT01772199 | [48] |
| Opicinumab | Monoclonal antibody | LINGO-1 antagonist. SYNERGY study did not show a significant dose-linear improvement in disability compared with placebo in patients with RRMS. | Target-based. LINGO-1 is a cell-surface glycoprotein selectively expressed on CNS neurons and OL. It inhibits oligodendrocyte differentiation, myelination. | Phase 2 ClinicalTrials.gov Identifier: NCT01864148 Phase 2 ClinicalTrials.gov Identifier: NCT03222973 | [49,50] |
| Clemastine | Anti-histamine. | Competitively bind to histamine receptor H1 sites. It promotes oligodendrocytes differentiation and myelination by inhibitor of cytochromeP450. It may interfere with other drugs metabolized by this isozyme. | Micropillar-based, drug repurposing phenotypical screen | Phase 2 ClinicalTrials.gov Identifier: NCT02040298 | [29,51] |
| Bexarotene | Synthetic retinoid- RXR agonist | It agonizes RXR-γ, a positive regulator of the differentiation and remyelination of endogenous precursor cells of oligodendrocytes | In vitro/in vivo Studies | Phase 2 EudraCT Number: 2014-003145-99 * | [38,52,53] |
| Domperidone | Peripheral Dopamine receptor antagonist | It increases prolactin levels and this may improve remyelination | Not specified, validated in vivo | Phase 2 ClinicalTrials.gov Identifier: NCT02493049 (RRMS) Phase 2 ClinicalTrials.gov Identifier: NCT02308137 (SPMS) | [54,55] |
| Pioglitazone | PPARƴ agonist | Anti-inflammatory effects in glial cells. Delays onset and reduces severity of clinical symptoms in EAE mice | In vitro/in vivo studies | Phase 2 ClinicalTrials.gov Identifier: NCT03109288 | [56,57] |
| BIIB061 ** | Small molecule produced by Biogen | Undisclosed | Not specified | Phase 2 ClinicalTrials.gov Identifier: NCT04079088 ** | [58,59] |
| Simvastatin | Statin | Inhibits 3-hydroxy-3-methylglutaryl co-enzyme A reductase, restricting synthesis of cholesterol and the post-translational lipid attachments, isoprenoids. Reduces initial disease severity in an EAE animal mode following short-term statin therapy. 24-month MS-STAT phase 2 trial. High dose simvastatin significantly reduced the annualized rate of whole brain atrophy in patients with secondary progressive multiple sclerosis (SPMS). | Synthetic derivative of a fermentation product of Aspergillus terreus | Phase 2 ClinicalTrials.gov Identifier: NCT00647348 Phase 3 ClinicalTrials.gov Identifier: NCT03387670 | [60,61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestri, S.; Del Giovane, A.; Sposato, C.; Ferrarelli, M.; Ragnini-Wilson, A. The Current Challenges for Drug Discovery in CNS Remyelination. Int. J. Mol. Sci. 2021, 22, 2891. https://doi.org/10.3390/ijms22062891
Balestri S, Del Giovane A, Sposato C, Ferrarelli M, Ragnini-Wilson A. The Current Challenges for Drug Discovery in CNS Remyelination. International Journal of Molecular Sciences. 2021; 22(6):2891. https://doi.org/10.3390/ijms22062891
Chicago/Turabian StyleBalestri, Sonia, Alice Del Giovane, Carola Sposato, Marta Ferrarelli, and Antonella Ragnini-Wilson. 2021. "The Current Challenges for Drug Discovery in CNS Remyelination" International Journal of Molecular Sciences 22, no. 6: 2891. https://doi.org/10.3390/ijms22062891
APA StyleBalestri, S., Del Giovane, A., Sposato, C., Ferrarelli, M., & Ragnini-Wilson, A. (2021). The Current Challenges for Drug Discovery in CNS Remyelination. International Journal of Molecular Sciences, 22(6), 2891. https://doi.org/10.3390/ijms22062891