Comparisons of Extracellular Vesicles from Human Epidural Fat-Derived Mesenchymal Stem Cells and Fibroblast Cells
Abstract
1. Introduction
2. Results
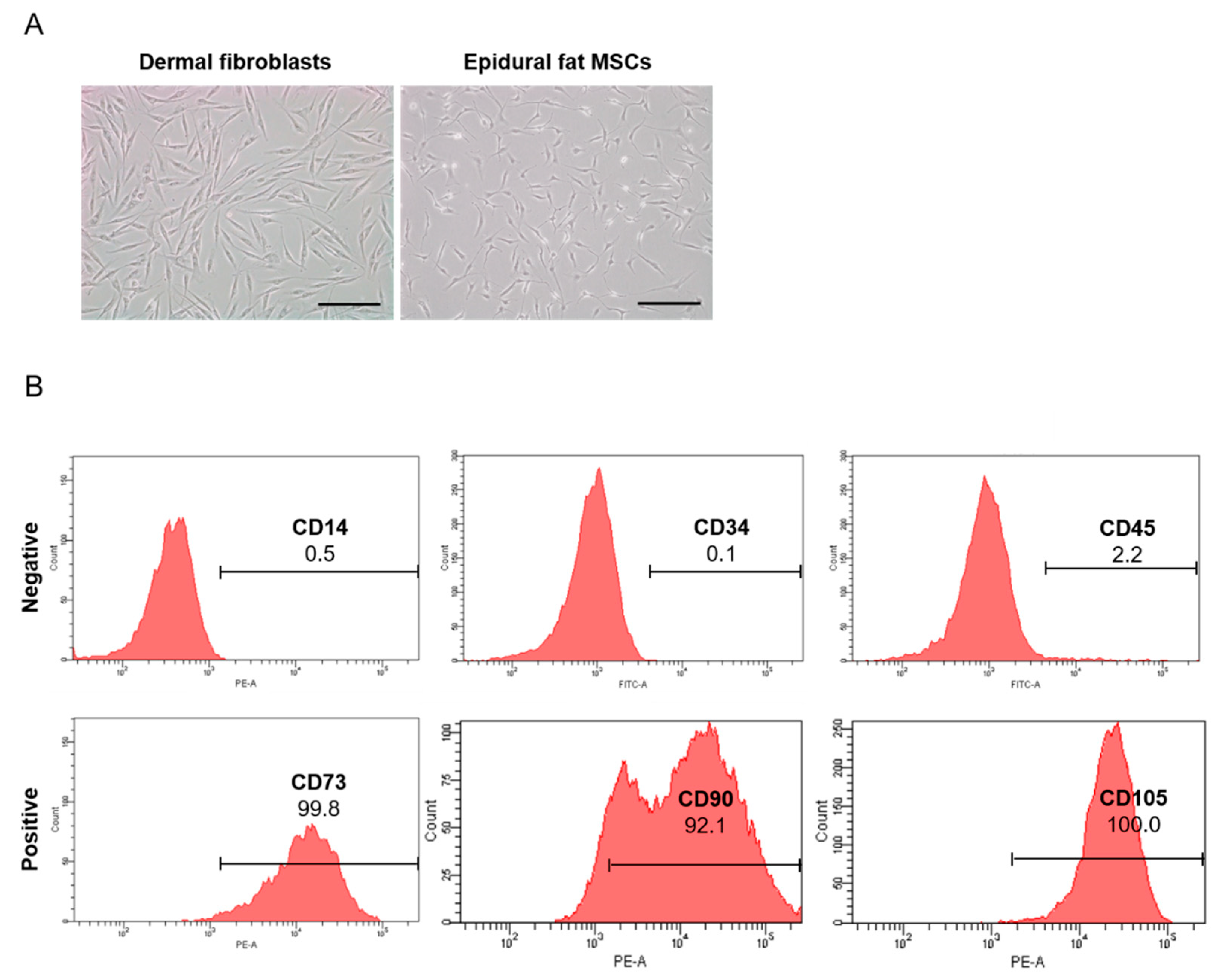
2.1. Isolation and Characterization of Human Epidural Fat MSCs
2.2. EVs from Human Dermal Fibroblasts and Human Epidural Fat MSCs
2.3. Pro-Inflammatory Molecule Levels in Human Epidural Fat MSC-Derived EVs
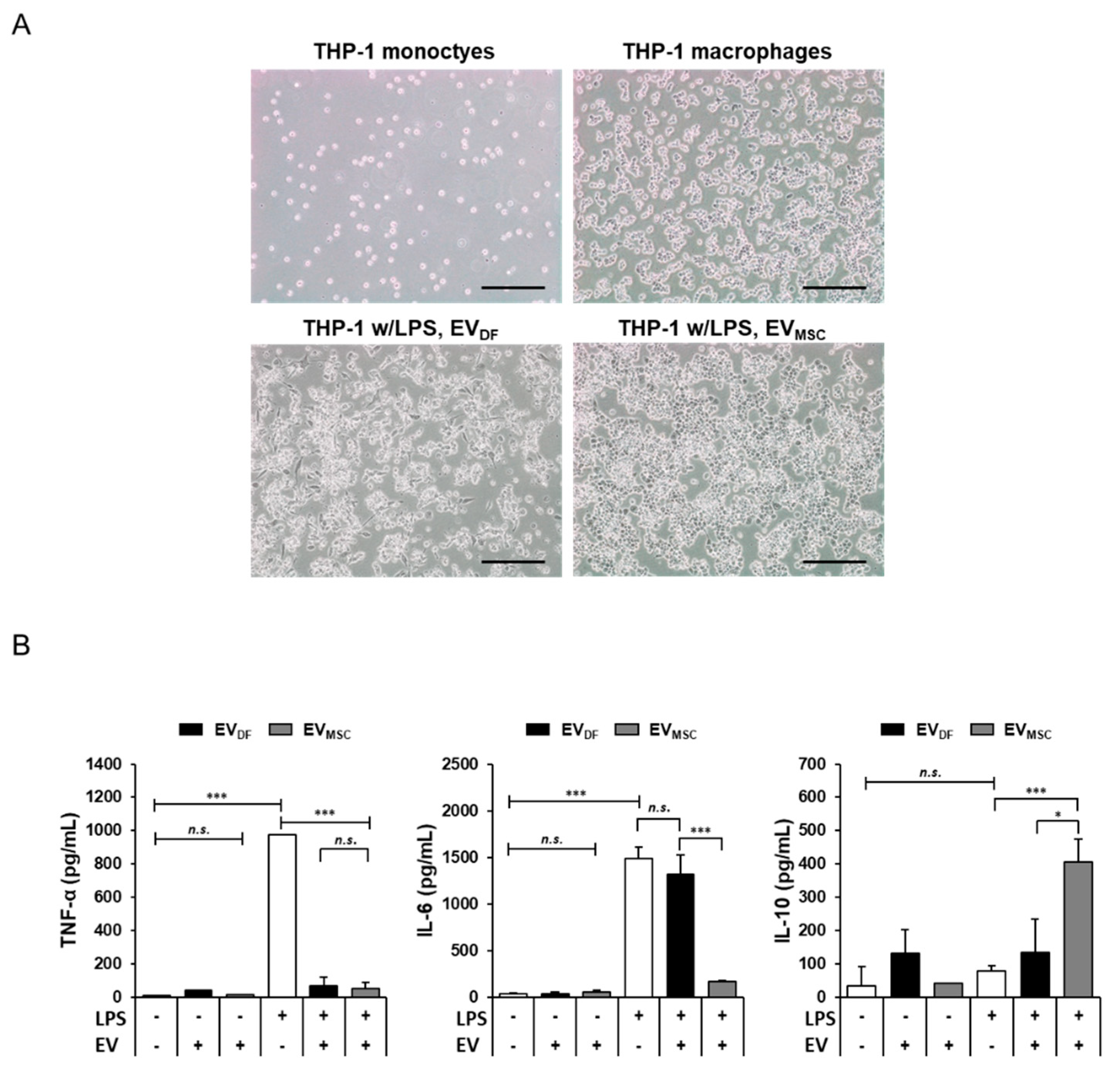
2.4. Anti-Inflammatory Effects of Human Epidural Fat MSC-Derived EVs
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Culture
4.2. EV Isolation
4.3. Flow Cytometry
4.4. TEM
4.5. NTA
4.6. Cytokine Assay
4.7. LPS and EV Treatment of THP-1 Cells
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A Protocol for Exosome Isolation and Characterization: Evaluation of Ultracentrifugation, Density-Gradient Separation, and Immunoaffinity Capture Methods. Methods Mol. Biol. 2015, 1295, 179–209. [Google Scholar]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Lu, M.; Xing, H.; Xun, Z.; Yang, T.; Ding, P.; Cai, C.; Wang, D.; Zhao, X. Exosome-Based Small RNA Delivery: Progress and Prospects. Asian J. Pharm. Sci. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Nair, S.; Tang, K.D.; Kenny, L.; Punyadeera, C. Salivary Exosomes as Potential Biomarkers in Cancer. Oral. Oncol. 2018, 84, 31–40. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, F.; Zhang, Q.; Liu, Y.; You, P.; Sun, S.; Lin, J.; Chen, N. Salivary Exosomal PSMA7: A Promising Biomarker of Inflammatory Bowel Disease. Protein Cell 2017, 8, 686–695. [Google Scholar] [CrossRef]
- Fries, G.R.; Quevedo, J. Exosomal MicroRNAs as Potential Biomarkers in Neuropsychiatric Disorders. Methods Mol. Biol. 2018, 1733, 79–85. [Google Scholar]
- Seo, Y.; Kim, H.-S.; Hong, I.-S. Stem Cell-Derived Extracellular Vesicles as Immunomodulatory Therapeutics. Stem. Cells Int. 2019, 2019, 5126156. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Lee, G.W.; Seo, M.-S.; Kang, K.-K.; Oh, S.-K. Epidural Fat-Derived Mesenchymal Stem Cell: First Report of Epidural Fat-Derived Mesenchymal Stem Cell. Asian Spine J. 2019, 13, 361–367. [Google Scholar] [CrossRef]
- Cook, D.; Genever, P. Regulation of Mesenchymal Stem Cell Differentiation. Adv. Exp. Med. Biol. 2013, 786, 213–229. [Google Scholar]
- Jin, R.; Shen, M.; Yu, L.; Wang, X.; Lin, X. Adipose-Derived Stem Cells Suppress Inflammation Induced by IL-1β through Down-Regulation of P2X7R Mediated by MiR-373 in Chondrocytes of Osteoarthritis. Mol. Cells 2017, 40, 222–229. [Google Scholar]
- Qi, Y.; Ma, J.; Li, S.; Liu, W. Applicability of Adipose-Derived Mesenchymal Stem Cells in Treatment of Patients with Type 2 Diabetes. Stem. Cell Res. Ther. 2019, 10, 274. [Google Scholar] [CrossRef]
- Sun, C.-K.; Yen, C.-H.; Lin, Y.-C.; Tsai, T.-H.; Chang, L.-T.; Kao, Y.-H.; Chua, S.; Fu, M.; Ko, S.-F.; Leu, S.; et al. Autologous Transplantation of Adipose-Derived Mesenchymal Stem Cells Markedly Reduced Acute Ischemia-Reperfusion Lung Injury in a Rodent Model. J. Transl. Med. 2011, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Al-Jezani, N.; Cho, R.; Masson, A.O.; Lenehan, B.; Krawetz, R.; Lyons, F.G. Isolation and Characterization of an Adult Stem Cell Population from Human Epidural Fat. Stem Cells Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Toward Cell-Free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Börger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int. J. Mol. Sci. 2017, 18, 1450. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [PubMed]
- Harting, M.T.; Srivastava, A.K.; Zhaorigetu, S.; Bair, H.; Prabhakara, K.S.; Toledano Furman, N.E.; Vykoukal, J.V.; Ruppert, K.A.; Cox, C.S.; Olson, S.D. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem. Cells 2018, 36, 79–90. [Google Scholar] [CrossRef]
- Huang, J.-H.; Fu, C.-H.; Xu, Y.; Yin, X.-M.; Cao, Y.; Lin, F.-Y. Extracellular Vesicles Derived from Epidural Fat-Mesenchymal Stem Cells Attenuate NLRP3 Inflammasome Activation and Improve Functional Recovery After Spinal Cord Injury. Neurochem. Res. 2020, 45, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Y.; Yue, Y.; Sun, J.; Cui, L. Reconstruction of Epidural Fat with Engineered Adipose Tissue from Adipose Derived Stem Cells and PLGA in the Rabbit Dorsal Laminectomy Model. Biomaterials 2012, 33, 6965–6973. [Google Scholar] [CrossRef]
- Lee, G.W.; Mun, J.-U.; Ahn, M.-W. The Impact of Posterior Epidural Adipose Tissue on Postoperative Outcomes after Posterior Decompression Surgery for Lumbar Spinal Stenosis: A Prospectively Randomized Non-Inferiority Trial. J. Orthop. Surg. (Hong Kong) 2020, 28. [Google Scholar] [CrossRef]
- EL Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Soundararajan, M.; Kannan, S. Fibroblasts and Mesenchymal Stem Cells: Two Sides of the Same Coin? J. Cell Physiol. 2018, 233, 9099–9109. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 Differentiation Is Required for the Detection of Responses to Weak Stimuli. Inflamm. Res. 2007, 56, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Xanthou, G.; Petrakou, E.; Zacharioudaki, V.; Tsatsanis, C.; Fotopoulos, S.; Xanthou, M. Distinct Roles of TLR4 and CD14 in LPS-Induced Inflammatory Responses of Neonates. Pediatr. Res. 2009, 66, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Scambler, T.E.; Smallie, T.; Cunliffe, H.E.; Ross, E.A.; Rosner, D.R.; O’Neil, J.D.; Clark, A.R. Macrophage Responses to Lipopolysaccharide Are Modulated by a Feedback Loop Involving Prostaglandin E2, Dual Specificity Phosphatase 1 and Tristetraprolin. Sci. Rep. 2017, 7, 4350. [Google Scholar] [CrossRef]
- Volarevic, V.; Gazdic, M.; Simovic Markovic, B.; Jovicic, N.; Djonov, V.; Arsenijevic, N. Mesenchymal Stem Cell-Derived Factors: Immuno-Modulatory Effects and Therapeutic Potential. Biofactors 2017, 43, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Halabian, R.; Imani Fooladi, A.A. A Revealing Review of Mesenchymal Stem Cells Therapy, Clinical Perspectives and Modification Strategies. Stem. Cell Investig. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Wei, S.; Wang, Y.; Wang, Y.; Man, Y.; Qu, Y. Extracellular Vesicle and Mesenchymal Stem Cells in Bone Regeneration: Recent Progress and Perspectives. J. Biomed. Mater. Res. A 2019, 107, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Yeo, R.W.Y.; Lim, S.K. Mesenchymal Stem Cell Exosomes. Semin Cell Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef]
- Qing, L.; Chen, H.; Tang, J.; Jia, X. Exosomes and Their MicroRNA Cargo: New Players in Peripheral Nerve Regeneration. Neurorehabil. Neural. Repair 2018, 32, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.L.; et al. Comprehensive Toxicity and Immunogenicity Studies Reveal Minimal Effects in Mice Following Sustained Dosing of Extracellular Vesicles Derived from HEK293T Cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef]
- Yang, Y.-H.K. Aging of Mesenchymal Stem Cells: Implication in Regenerative Medicine. Regen. Ther. 2018, 9, 120–122. [Google Scholar] [CrossRef]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chu, Y.; Ren, X.; Xiang, H.; Xi, Y.; Ma, X.; Zhu, K.; Guo, Z.; Zhou, C.; Zhang, G.; et al. Epidural Adipose Tissue-Derived Mesenchymal Stem Cell Activation Induced by Lung Cancer Cells Promotes Malignancy and EMT of Lung Cancer. Stem. Cell Res. Ther. 2019, 10, 168. [Google Scholar] [CrossRef]
- Shah, S.; Mudigonda, S.; Mitha, A.P.; Salo, P.; Krawetz, R.J. Epidural Fat Mesenchymal Stem Cells: Important Microenvironmental Regulators in Health, Disease, and Regeneration: Do EF-MSCs Play a Role in Dural Homeostasis/Maintenance? BioEssays 2020, 2000215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.-T.; Tomkins, J.E.; Denecke, B.; Musante, L.; et al. Secretome of Adipose-Derived Mesenchymal Stem Cells Promotes Skeletal Muscle Regeneration through Synergistic Action of Extracellular Vesicle Cargo and Soluble Proteins. Stem Cell Res. Ther. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; DePalo, V.A. Anti-Inflammatory Cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic Balance of Pro- and Anti-Inflammatory Signals Controls Disease and Limits Pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Dalirfardouei, R.; Jamialahmadi, K.; Jafarian, A.H.; Mahdipour, E. Promising Effects of Exosomes Isolated from Menstrual Blood-Derived Mesenchymal Stem Cell on Wound-Healing Process in Diabetic Mouse Model. J. Tissue Eng. Regen. Med. 2019, 13, 555–568. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem. Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef]
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, S.-E.; Kang, K.-K.; Choi, J.-H.; Lee, S.-J.; Kim, K.; Lim, J.-H.; Yang, S.Y.; Kim, S.-K.; Seo, M.-S.; Lee, G.W. Comparisons of Extracellular Vesicles from Human Epidural Fat-Derived Mesenchymal Stem Cells and Fibroblast Cells. Int. J. Mol. Sci. 2021, 22, 2889. https://doi.org/10.3390/ijms22062889
Sung S-E, Kang K-K, Choi J-H, Lee S-J, Kim K, Lim J-H, Yang SY, Kim S-K, Seo M-S, Lee GW. Comparisons of Extracellular Vesicles from Human Epidural Fat-Derived Mesenchymal Stem Cells and Fibroblast Cells. International Journal of Molecular Sciences. 2021; 22(6):2889. https://doi.org/10.3390/ijms22062889
Chicago/Turabian StyleSung, Soo-Eun, Kyung-Ku Kang, Joo-Hee Choi, Si-Joon Lee, KilSoo Kim, Ju-Hyeon Lim, Seung Yun Yang, Seul-Ki Kim, Min-Soo Seo, and Gun Woo Lee. 2021. "Comparisons of Extracellular Vesicles from Human Epidural Fat-Derived Mesenchymal Stem Cells and Fibroblast Cells" International Journal of Molecular Sciences 22, no. 6: 2889. https://doi.org/10.3390/ijms22062889
APA StyleSung, S.-E., Kang, K.-K., Choi, J.-H., Lee, S.-J., Kim, K., Lim, J.-H., Yang, S. Y., Kim, S.-K., Seo, M.-S., & Lee, G. W. (2021). Comparisons of Extracellular Vesicles from Human Epidural Fat-Derived Mesenchymal Stem Cells and Fibroblast Cells. International Journal of Molecular Sciences, 22(6), 2889. https://doi.org/10.3390/ijms22062889







