Dietary Micronutrient Management to Treat Mitochondrial Dysfunction in Diet-Induced Obese Mice
Abstract
1. Introduction
2. Results
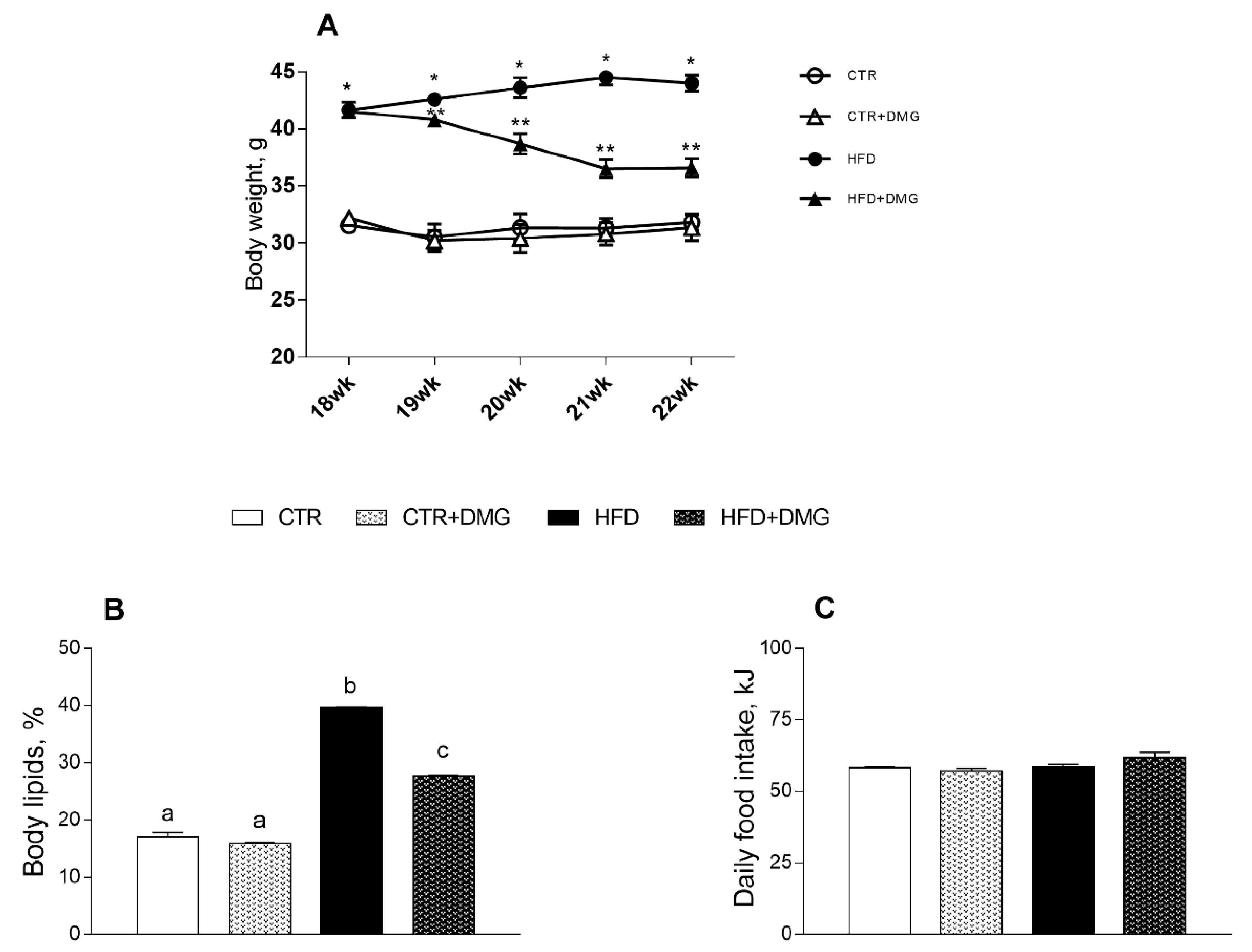
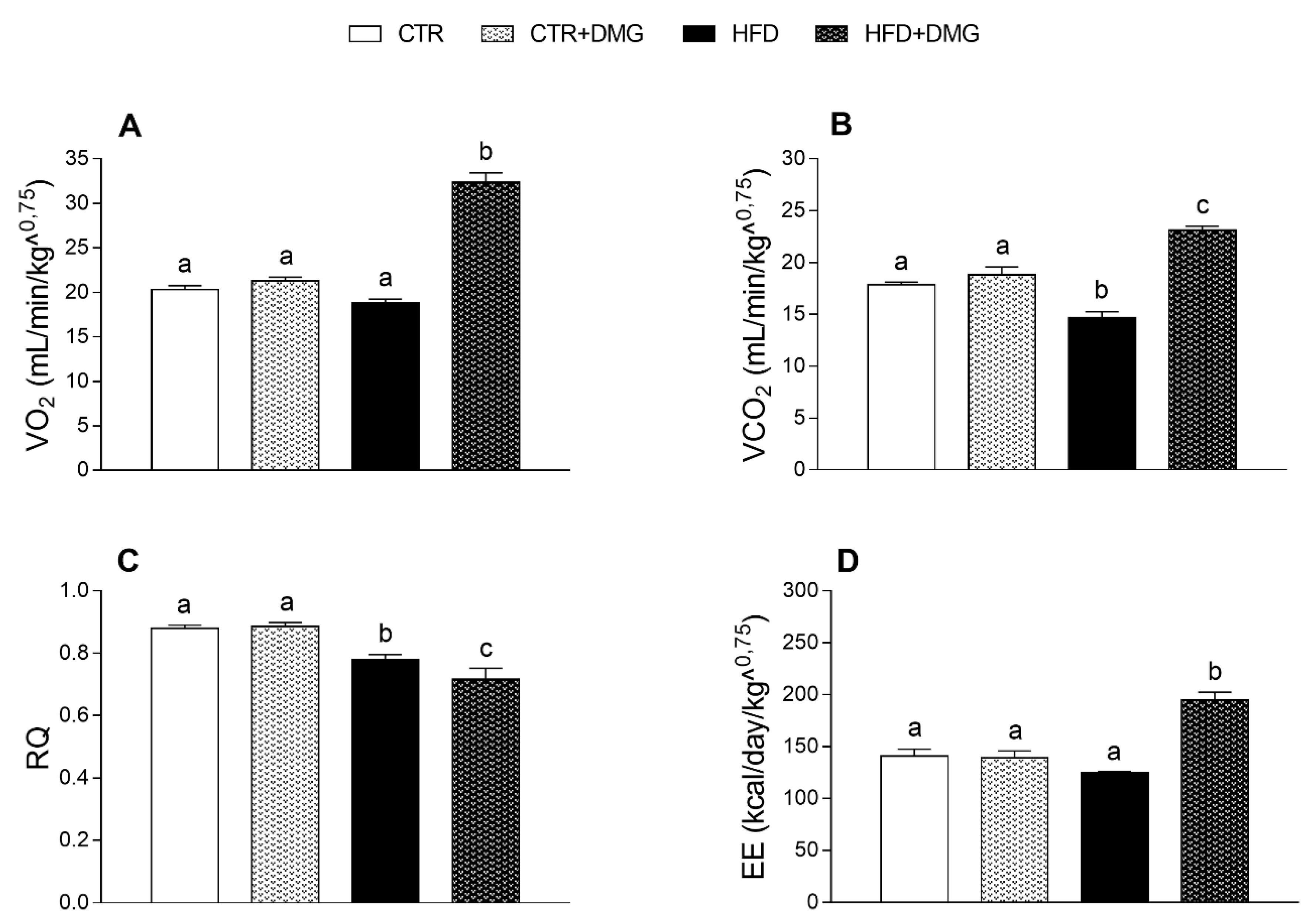
2.1. DMG-Gold Supplementation Reduces Lipid Accumulation and Increases Energy Expenditure and Resting Metabolic Rate
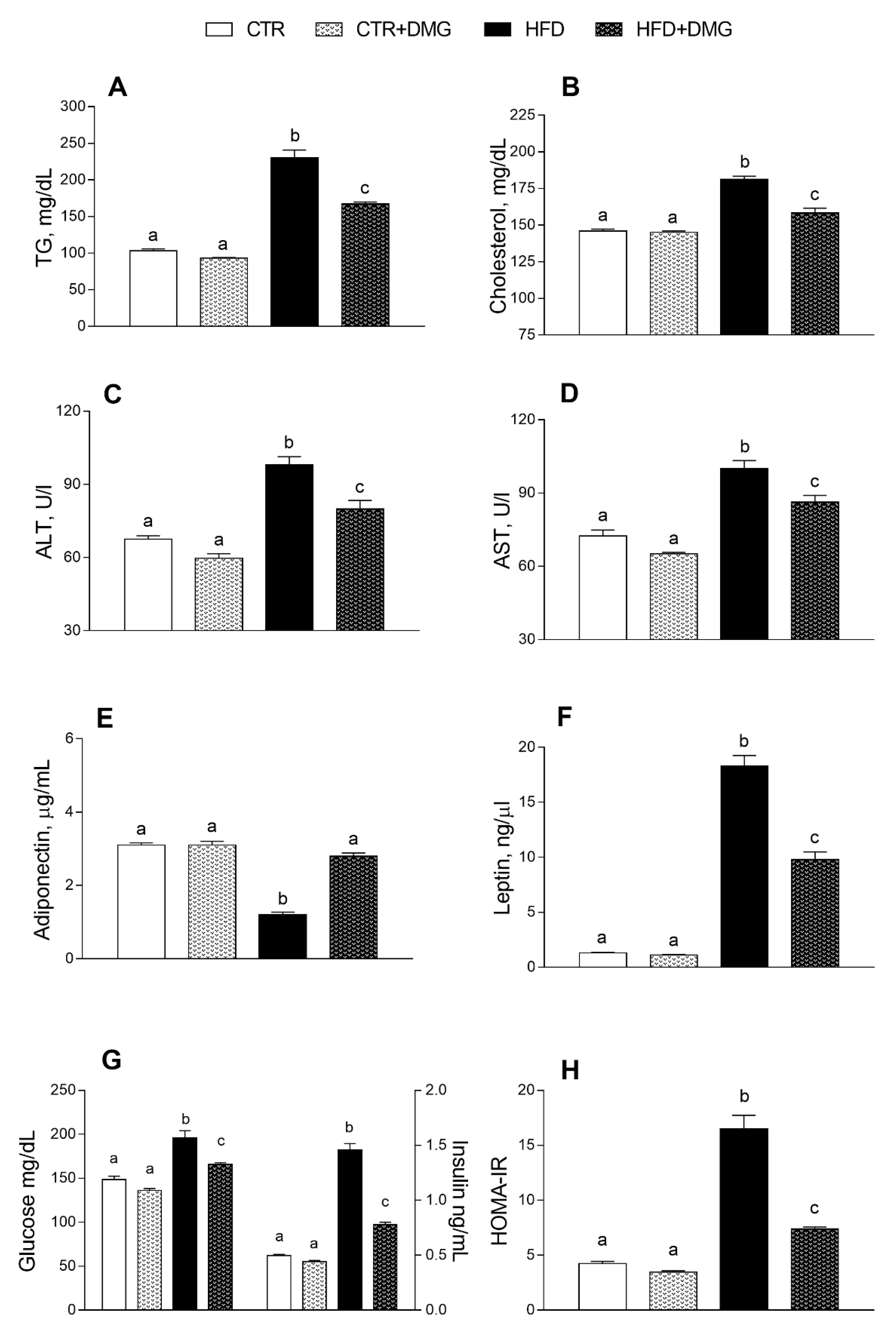
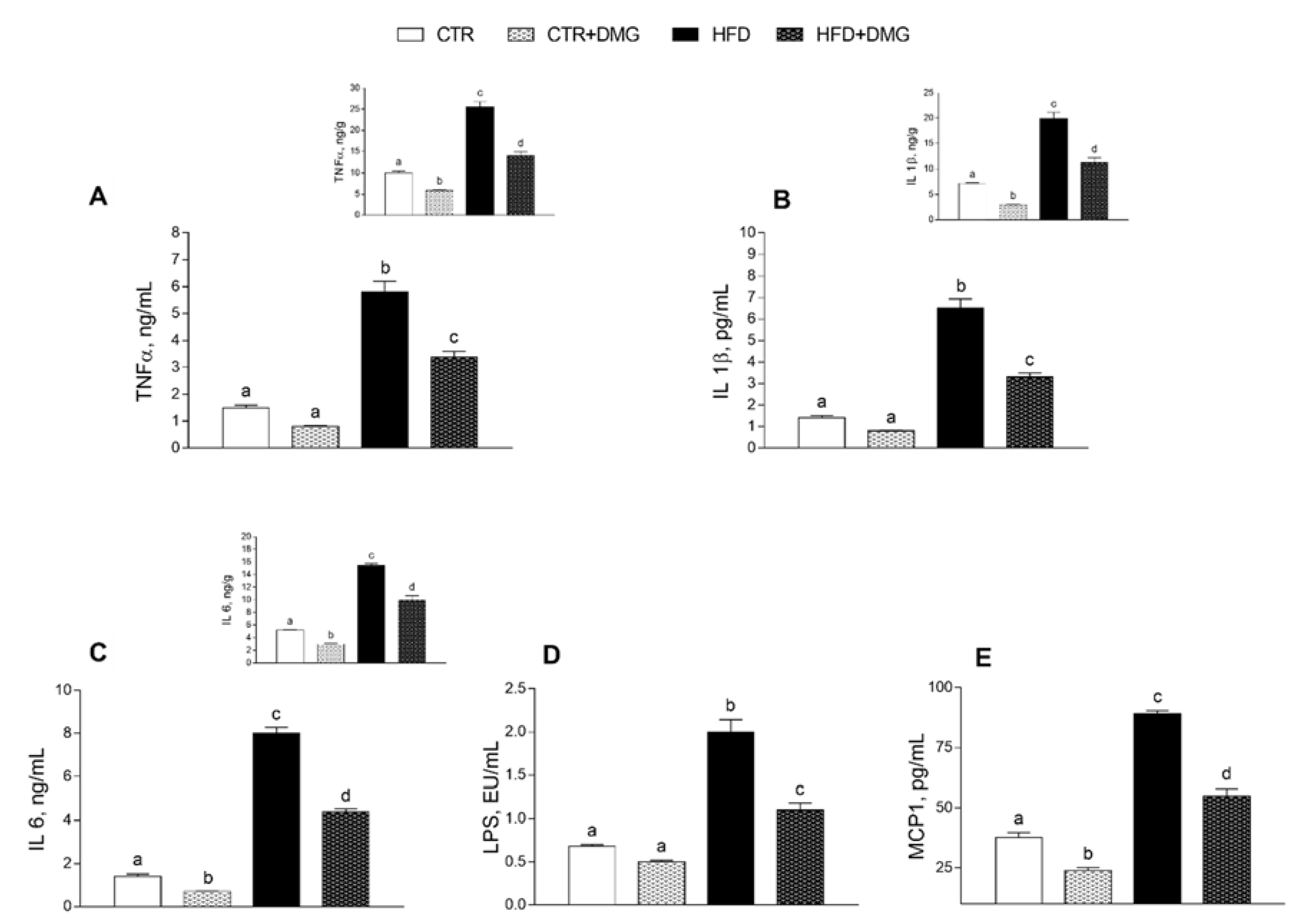
2.2. DMG-Gold Supplementation Modulates Serum and Hepatic Inflammatory Markers and Metabolic Parameters
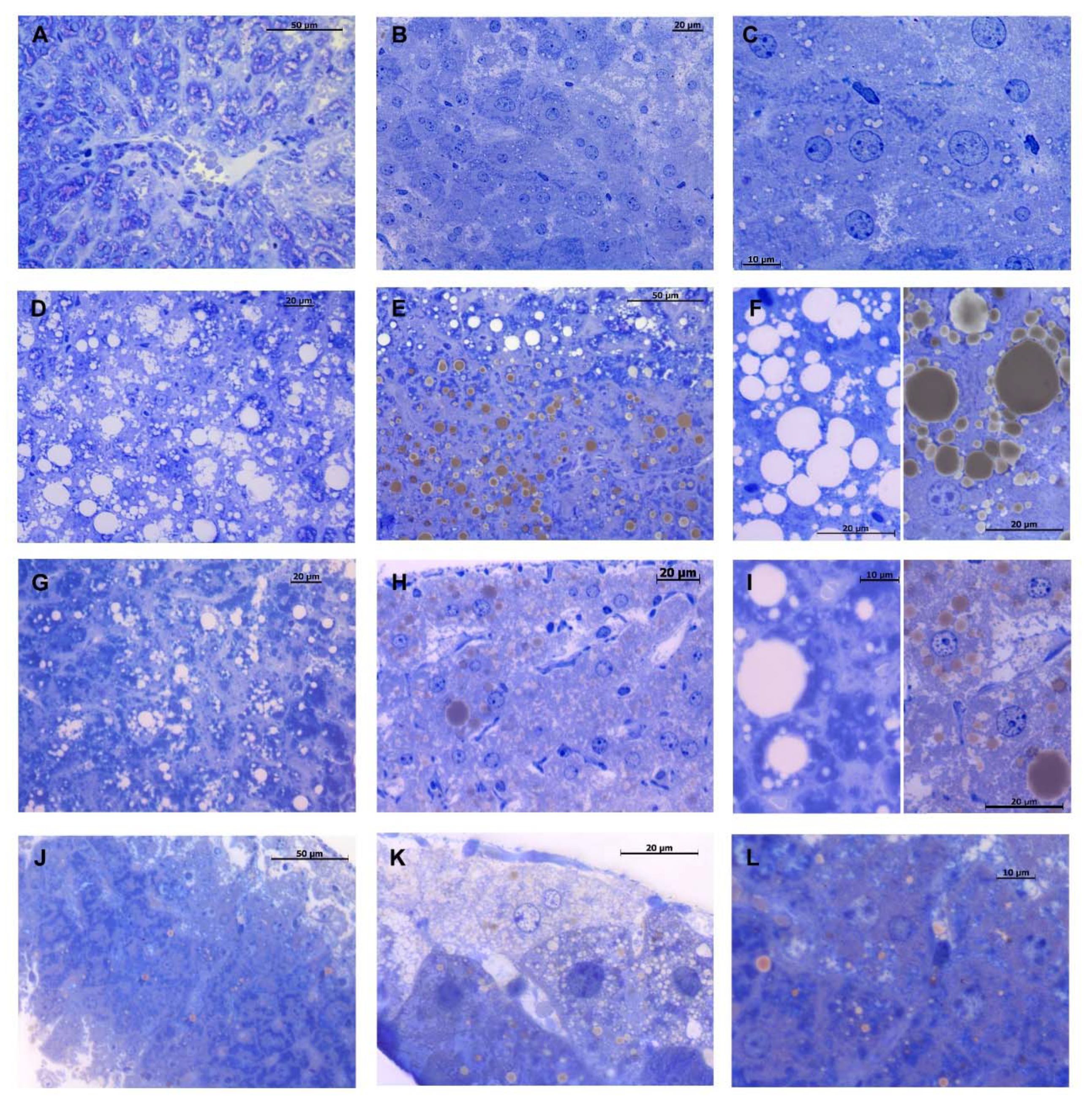
2.3. DMG-Gold Supplementation Decreases Hepatic Lipid Depots
2.4. Modulation of Hepatic Mitochondrial Efficiency and Oxidative Stress by DMG-Gold Treatment
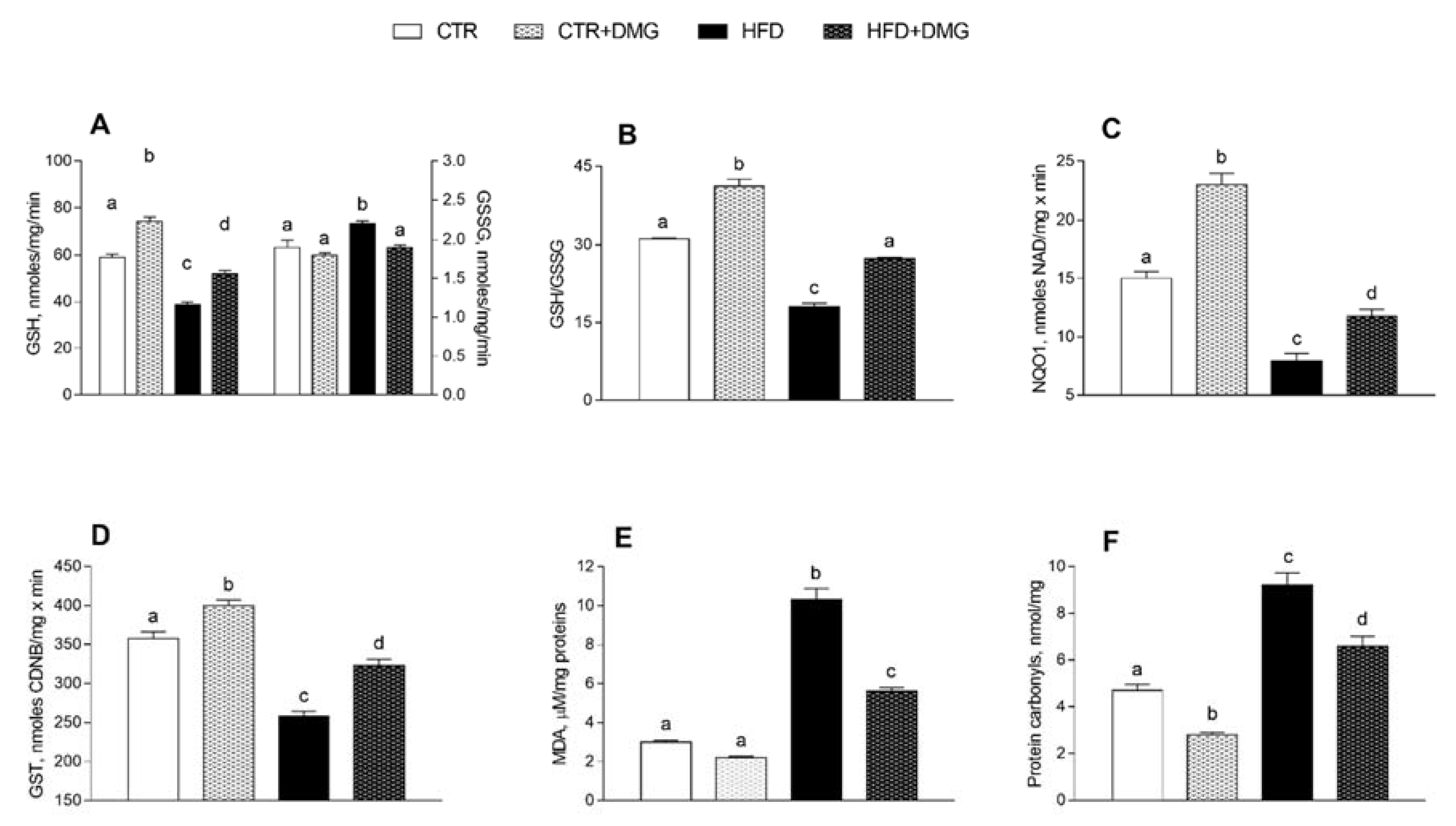
2.5. DMG-Gold Supplementation Increases Antioxidant/Detoxifying Defenses
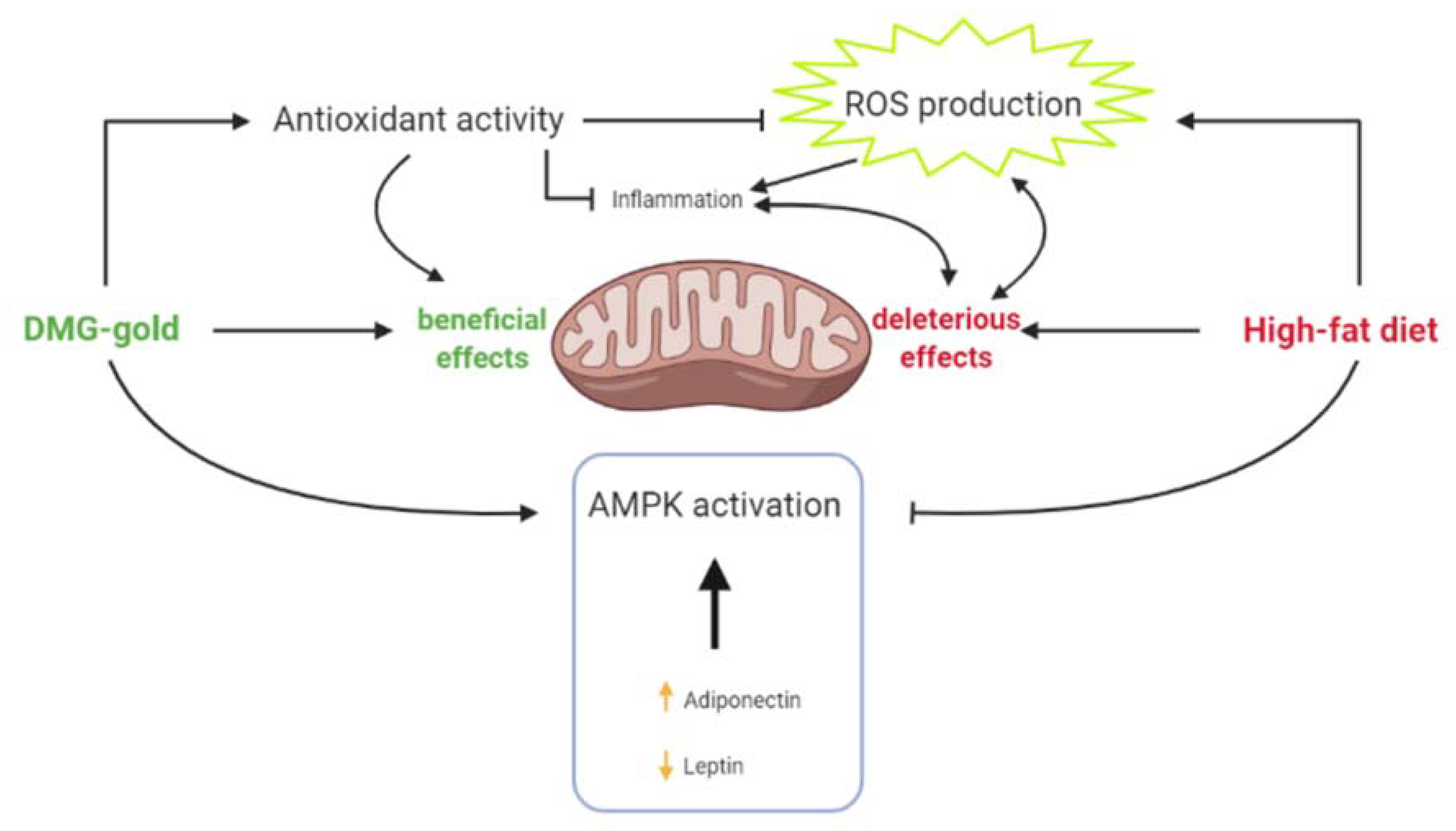
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Ethics Statement
4.3. Animals
4.4. Measurement of Oxygen Consumption (VO2), Carbon Dioxide Production (VCO2) and Respiratory Quotient (RQ)
4.5. Evaluation of Markers in Blood and in Tissue
4.6. Hepatic Histological Analyses
4.7. Mitochondria Preparation and Analysis
4.8. Western Blot Analysis
4.9. Hepatic Lipid Peroxidation, Inflammatory Markers and Antioxidant/Detoxifying Defenses
4.10. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NCD | Non-communicable diseases |
| DMG | Dimethylglycine |
| TMG | Trimethylglycine |
| NADPH | Nicotinamide adenine dinucleotide phosphate-hydrogen |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| FAD | Flavin adenine dinucleotide |
| NAD+ | Nicotinamide adenine dinucleotide |
| NADP+ | Nicotinamide adenine dinucleotide phosphate |
| ATP | Adenosine triphosphate |
| ADP | Adenosine diphosphate |
| ROS | Reactive oxygen species |
| HFD | High-fat diet |
| STD | Standard diet |
| CTR | Control |
| VO2 | Oxygen consumption |
| VCO2 | Carbon dioxide production |
| RQ | Respiratory quotient |
| EE | Energy expenditure |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| TG | Triglycerides |
| IR-HOMA | Insulin resistance-homoeostatic model assessment |
| TNF-α | Tumor necrosis factor-α |
| IL-1-β | Interleukin 1-β |
| IL-6 | Interleukin-6 |
| LPS | Lipopolysaccharide |
| MCP-1 | Monocyte chemoattractant protein-1 |
| CPT | Carnitine palmitoyl transferase |
| AMPK | Adenosine monophosphate-activated protein kinase |
| p-AMPK | Phosphorylated adenosine monophosphate-activated protein kinase |
| H2O2 | Hydrogen peroxide |
| SOD | Superoxide dismutase |
| NQO1 | NADPH quinone dehydrogenase |
| GST | Glutathione transferase |
| MDA | Malondialdehyde |
| PC | Protein carbonyls |
| FFAs | Free fatty acids |
| EDTA | Ethylenediaminetetraacetic acid |
| PBS | Phosphate-buffered saline |
| TBAR | Thiobarbituric acid |
| DTNB | Dithionitrobenzoic acid |
| Nrf2 | NF-E2-related factor 2 |
References
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation–Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nat. Cell Biol. 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- GBD Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Mollica, M.P.; Lionetti, L.; Putti, R.; Cavaliere, G.; Gaita, M.; Barletta, A. From chronic overfeeding to hepatic injury: Role of endoplasmic reticulum stress and inflammation. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 222–230. [Google Scholar] [CrossRef]
- Lionetti, L.; Mollica, M.; Lombardi, A.; Cavaliere, G.; Gifuni, G.; Barletta, A. From chronic overnutrition to insulin resistance: The role of fat-storing capacity and inflammation. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Cavaliere, G.; Viggiano, E.; Trinchese, G.; De Filippo, C.; Messina, A.; Monda, V.; Valenzano, A.; Cincione, R.I.; Zammit, C.; Cimmino, F.; et al. Long Feeding High-Fat Diet Induces Hypothalamic Oxidative Stress and Inflammation, and Prolonged Hypothalamic AMPK Activation in Rat Animal Model. Front. Physiol. 2018, 9, 818. [Google Scholar] [CrossRef]
- Micha, R.; Kalantarian, S.; Wirojratana, P.; Byers, T.; Danaei, G.; Elmadfa, I.; Ding, E.; Giovannucci, E.; Powles, J.; Smith-Warner, S.; et al. Estimating the global and regional burden of suboptimal nutrition on chronic disease: Methods and inputs to the analysis. Eur. J. Clin. Nutr. 2011, 66, 119–129. [Google Scholar] [CrossRef]
- Willett, W.C.; Hu, F.B.; Thun, M. Overweight, Obesity, and All-Cause Mortality. JAMA 2013, 309, 1681–1682. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of B vitamins on the one-carbon transfer pathways. Chem. Interact. 2006, 163, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Friesen, R.W.; Novak, E.M.; Hasman, D.; Innis, S.M. Relationship of Dimethylglycine, Choline, and Betaine with Oxoproline in Plasma of Pregnant Women and Their Newborn Infants. J. Nutr. 2007, 137, 2641–2646. [Google Scholar] [CrossRef] [PubMed]
- Cools, A.; Maes, D.; Buyse, J.; Kalmar, I.; Vandermeiren, J.-A.; Janssens, G. Effect of N,N-dimethylglycine supplementation in parturition feed for sows on metabolism, nutrient digestibility and reproductive performance. Animal 2010, 4, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Hariganesh, K.; Prathiba, J. Effect of Dimethylglycine on Gastric Ulcers in Rats. J. Pharm. Pharmacol. 2000, 52, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.A.; Galan, D.T.; Ribeiro, A.M.; Cruz, J.S. Thiamine deficiency during pregnancy leads to cerebellar neuronal death in rat offspring: Role of voltage-dependent K+ channels. Brain Res. 2007, 1134, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gralak, M.A.; Dębski, B.; Drywień, M. Thiamine deficiency affects glucose transport and β-oxidation in rats. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1629–1635. [Google Scholar] [CrossRef]
- Gershoff, S.N.; Andrus, S.B.; Hegsted, D.M. The Effect of the Carbohydrate and Fat Content of the Diet upon the Riboflavin Requirement of the Cat. J. Nutr. 1959, 68, 75–88. [Google Scholar] [CrossRef]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Meyers, C.D.; Kamanna, V.S.; Kashyap, M.L. Niacin therapy in atherosclerosis. Curr. Opin. Lipidol. 2004, 15, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Cao, Z.; Lai, X.; Shi, Y.; Zhou, N. Niacin Ameliorates Hepatic Steatosis by Inhibiting De Novo Lipogenesis Via a GPR109A-Mediated PKC–ERK1/2–AMPK Signaling Pathway in C57BL/6 Mice Fed a High-Fat Diet. J. Nutr. 2019, 150, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Lukasova, M.; Hanson, J.; Tunaru, S.; Offermanns, S. Nicotinic acid (niacin): New lipid-independent mechanisms of action and therapeutic potentials. Trends Pharmacol. Sci. 2011, 32, 700–707. [Google Scholar] [CrossRef]
- Ganji, S.H.; Kukes, G.D.; Lambrecht, N.; Kashyap, M.L.; Kamanna, V.S. Therapeutic role of niacin in the prevention and regression of hepatic steatosis in rat model of nonalcoholic fatty liver disease. Am. J. Physiol. Liver Physiol. 2014, 306, G320–G327. [Google Scholar] [CrossRef]
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B6 and Its Role in Cell Metabolism and Physiology. Cells 2018, 7, 84. [Google Scholar] [CrossRef]
- Liu, Z.; Li, P.; Zhao, Z.-H.; Zhang, Y.; Ma, Z.-M.; Wang, S.-X. Vitamin B6 Prevents Endothelial Dysfunction, Insulin Resistance, and Hepatic Lipid Accumulation inApoe−/−Mice Fed with High-Fat Diet. J. Diabetes Res. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Li, F.-J.; Zheng, S.-R.; Wang, D.-M. Adrenomedullin: An important participant in neurological diseases. Neural Regen. Res. 2020, 15, 1199–1207. [Google Scholar] [CrossRef]
- Hernández-Aguilera, A.; Rull, A.; Rodríguez-Gallego, E.; Riera-Borrull, M.; Luciano-Mateo, F.; Camps, J.; Menéndez, J.A.; Joven, J. Mitochondrial Dysfunction: A Basic Mechanism in Inflammation-Related Non-Communicable Diseases and Therapeutic Opportunities. Mediat. Inflamm. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Takamura, T.; Matsuzawa-Nagata, N.; Takayama, H.; Misu, H.; Noda, H.; Nabemoto, S.; Kurita, S.; Ota, T.; Ando, H.; et al. Palmitate Induces Insulin Resistance in H4IIEC3 Hepatocytes through Reactive Oxygen Species Produced by Mitochondria. J. Biol. Chem. 2009, 284, 14809–14818. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Nolte, L.A.; Ju, J.-S.; Han, D.H.; Coleman, T.; Holloszy, J.O.; Semenkovich, C.F. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat. Med. 2000, 6, 1115–1120. [Google Scholar] [CrossRef]
- Mollica, M.P.; Raso, G.M.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Iossa, S.; Lionetti, L.; Mollica, M.P.; Crescenzo, R.; Barletta, A.; Liverini, G. Effect of long-term high-fat feeding on energy balance and liver oxidative activity in rats. Br. J. Nutr. 2000, 84, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Kim, Y.-B.; Peroni, O.D.; Fryer, L.G.D.; Müller, C.; Carling, D.; Kahn, B.B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Cell Biol. 2002, 415, 339–343. [Google Scholar] [CrossRef]
- Long, Y.C. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Investig. 2006, 116, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, B.; Karuna, R.; Sreenivasa, R.S.; Haritha, K.; Sai, M.D.; Sasis, B.R.B.; Saralakumari, D. Effect of Commiphora mukul gum resin on hepatic marker enzymes, lipid peroxidation and antioxidants status in pancreas and heart of streptozotocin induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012, 2, 895–900. [Google Scholar] [CrossRef]
- Papac-Milicevic, N.; Busch, C.-L.; Binder, C. Malondialdehyde Epitopes as Targets of Immunity and the Implications for Atherosclerosis. Adv. Immunol. 2016, 131, 1–59. [Google Scholar] [CrossRef]
- Gonos, E.S.; Kapetanou, M.; Sereikaite, J.; Bartosz, G.; Naparło, K.; Grzesik, M.; Sadowska-Bartosz, I. Origin and pathophysiology of protein carbonylation, nitration and chlorination in age-related brain diseases and aging. Aging 2018, 10, 868–901. [Google Scholar] [CrossRef]
- Szendroedi, J.; Phielix, E.; Roden, M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 8, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Park, E.-J.; Joe, Y.; Kang, S.; Kim, M.-S.; Hong, S.-H.; Park, M.-K.; Kim, D.K.; Koh, H.; Lee, H.-J. Overexpression of AMPKα1 Ameliorates Fatty Liver in Hyperlipidemic Diabetic Rats. Korean J. Physiol. Pharmacol. 2009, 13, 449–454. [Google Scholar] [CrossRef][Green Version]
- Gaidhu, M.P.; Fediuc, S.; Anthony, N.M.; So, M.; Mirpourian, M.; Perry, R.L.S.; Ceddia, R.B. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: Novel mechanisms integrating HSL and ATGL. J. Lipid Res. 2009, 50, 704–715. [Google Scholar] [CrossRef]
- Han, Y.; Hu, Z.; Cui, A.; Liu, Z.; Ma, F.; Xue, Y.; Liu, Y.; Zhang, F.; Zhao, Z.; Yu, Y.; et al. Post-translational regulation of lipogenesis via AMPK-dependent phosphorylation of insulin-induced gene. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Rikans, L.L.; Arata, D.; Cederquist, D.C. Fatty Livers Produced in Albino Rats by Excess Niacin in High Fat Diets. J. Nutr. 1964, 82, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Li, Z.; Graff, E.C.; McCafferty, K.J.; Judd, R.L. Niacin increases diet-induced hepatic steatosis in B6129 mice. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158731. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef]
- Levine, S.; Myhre, G.D.; Smith, G.L.; Burns, J.G.; Erb, H. Effect of a nutritional supplement containing N, N-Dimethylglycine (DMG) on the racing standardbred [Horses]. Equine Pract. 1982. [Google Scholar]
- Bai, K.; Xu, W.; Zhang, J.; Kou, T.; Niu, Y.; Wan, X.; Zhang, L.; Wang, C.; Wang, T. Assessment of Free Radical Scavenging Activity of Dimethylglycine Sodium Salt and Its Role in Providing Protection against Lipopolysaccharide-Induced Oxidative Stress in Mice. PLoS ONE 2016, 11, e0155393. [Google Scholar] [CrossRef] [PubMed]
- Hamoud, S.; Hayek, T.; Hassan, A.; Meilin, E.; Kaplan, M.; Torgovicky, R.; Cohen, R. Niacin Administration Significantly Reduces Oxidative Stress in Patients with Hypercholesterolemia and Low Levels of High-Density Lipoprotein Cholesterol. Am. J. Med. Sci. 2013, 345, 195–199. [Google Scholar] [CrossRef]
- Hart, P.C.; Mao, M.; De Abreu, A.L.P.; Ansenberger-Fricano, K.; Ekoue, D.N.; Ganini, D.; Kajdacsy-Balla, A.; Diamond, A.M.; Minshall, R.D.; Consolaro, M.E.L.; et al. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Dominguez, J.F.; Guo, L.; Molnar, M.A.C.; Escobedo, A.B.; Dunphy, T.; Lund, T.D.; Turman, J.E. Novel Indirect Calorimetry Technology to Analyze Metabolism in Individual Neonatal Rodent Pups. PLoS ONE 2009, 4, e6790. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.; Sevillano, J.; De Castro, J.; Herrera, E.; Ramos, M.P. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Physiol. Metab. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef] [PubMed]
- Tarantola, E.; Bertone, V.; Milanesi, G.; Capelli, E.; Ferrigno, A.; Neri, D.; Vairetti, M.; Barni, S.; Freitas, I. Dipeptidylpeptidase--IV, a key enzyme for the degradation of incretins and neuropeptides: Activity and expression in the liver of lean and obese rats. Eur. J. Histochem. 2012, 56, e41. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Mollica, M.P.; Sica, R.; Donizzetti, I.; Gifuni, G.; Pignalosa, A.; Cavaliere, G.; Putti, R. Differential Effects of High-Fish Oil and High-Lard Diets on Cells and Cytokines Involved in the Inflammatory Process in Rat Insulin-Sensitive Tissues. Int. J. Mol. Sci. 2014, 15, 3040–3063. [Google Scholar] [CrossRef] [PubMed]
- Hartree, E. Determination of protein: A modification of the lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- Estabrook, R.W. Mitochondrial respiratory control and the polarographic measurement of ADP: O ratios. Methods Enzymol. 1967, 10, 41–47. [Google Scholar] [CrossRef]
- Cavaliere, G.; Trinchese, G.; Bergamo, P.; De Filippo, C.; Mattace Raso, G.; Gifuni, G.; Putti, R.; Moni, B.H.; Canani, R.B.; Meli, R.; et al. Polyunsaturated Fatty Acids Attenuate Diet Induced Obesity and Insulin Resistance, Modulating Mitochondrial Respiratory Uncoupling in Rat Skeletal Muscle. PLoS ONE 2016, 11, e0149033. [Google Scholar] [CrossRef] [PubMed]
- Alexson, S.E.; Nedergaard, J. A novel type of short- and medium-chain acyl-CoA hydrolases in brown adipose tissue mitochondria. J. Biol. Chem. 1988, 263, 13564–13571. [Google Scholar] [CrossRef]
- Barja, G. Mitochondrial Free Radical Production and Aging in Mammals and Birdsa. Ann. N. Y. Acad. Sci. 1998, 854, 224–238. [Google Scholar] [CrossRef]
- Flohé, L.; Ötting, F. Superoxide dismutase assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar] [CrossRef]
- Penna, E.; Cerciello, A.; Chambery, A.; Russo, R.; Cernilogar, F.M.; Pedone, E.M.; Perrone-Capano, C.; Cappello, S.; Di Giaimo, R.; Crispino, M. Cystatin B Involvement in Synapse Physiology of Rodent Brains and Human Cerebral Organoids. Front. Mol. Neurosci. 2019, 12, 195. [Google Scholar] [CrossRef]
- Lü, H.; Zhang, D.-M.; Chen, H.-L.; Lin, Y.-X.; Hang, C.-H.; Yin, H.-X.; Shi, J.-X. N-acetylcysteine suppresses oxidative stress in experimental rats with subarachnoid hemorrhage. J. Clin. Neurosci. 2009, 16, 684–688. [Google Scholar] [CrossRef]
- Cavaliere, G.; Trinchese, G.; Penna, E.; Cimmino, F.; Pirozzi, C.; Lama, A.; Annunziata, C.; Catapano, A.; Mattace Raso, G.; Meli, R.; et al. High-Fat Diet Induces Neuroinflammation and Mitochondrial Impairment in Mice Cerebral Cortex and Synaptic Fraction. Front. Cell. Neurosci. 2019, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, P.; Maurano, F.; Rossi, M. Phase 2 enzyme induction by conjugated linoleic acid improves lupus-associated oxidative stress. Free Radic. Biol. Med. 2007, 43, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.M.; Hunkeler, M.J.; Talalay, P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: Possible role in protection against carcinogenesis and toxicity. Proc. Natl. Acad. Sci. USA 1980, 77, 5216–5220. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Jakoby, W.B. Assays for differentiation of glutathione S-Transferases. Cellulases 1981, 77, 398–405. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]


| Fatty Acids | STD | HFD |
|---|---|---|
| %/Total Fat | %/Total Fat | |
| C10, Capric | - | 0.056 |
| C12, Lauric | 0.232 | 0.085 |
| C14, Myristic | 1.16 | 1.101 |
| C15 | - | 0.073 |
| C16, Palmitic | 14.83 | 19.29 |
| C16:1, Palmitoleic | 0.929 | 1.311 |
| C17 | - | 0.353 |
| C18, Stearic | 3.252 | 10.38 |
| C18:1, Oleic | 19.783 | 33.61 |
| C18:2, Linoleic | 50.25 | 29.47 |
| C18:3, Linolenic | 9.562 | 2.202 |
| C20, Arachidic | - | 0.209 |
| C20:1 | - | 0.608 |
| C20:2 | - | 0.734 |
| C20:3, n6 | - | 0.105 |
| C20:4, Arachidonic | - | 0.262 |
| C22, Behenic | - | 0.052 |
| C22:5, Docosapentaenoic | - | 0.080 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimmino, F.; Catapano, A.; Trinchese, G.; Cavaliere, G.; Culurciello, R.; Fogliano, C.; Penna, E.; Lucci, V.; Crispino, M.; Avallone, B.; et al. Dietary Micronutrient Management to Treat Mitochondrial Dysfunction in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2021, 22, 2862. https://doi.org/10.3390/ijms22062862
Cimmino F, Catapano A, Trinchese G, Cavaliere G, Culurciello R, Fogliano C, Penna E, Lucci V, Crispino M, Avallone B, et al. Dietary Micronutrient Management to Treat Mitochondrial Dysfunction in Diet-Induced Obese Mice. International Journal of Molecular Sciences. 2021; 22(6):2862. https://doi.org/10.3390/ijms22062862
Chicago/Turabian StyleCimmino, Fabiano, Angela Catapano, Giovanna Trinchese, Gina Cavaliere, Rosanna Culurciello, Chiara Fogliano, Eduardo Penna, Valeria Lucci, Marianna Crispino, Bice Avallone, and et al. 2021. "Dietary Micronutrient Management to Treat Mitochondrial Dysfunction in Diet-Induced Obese Mice" International Journal of Molecular Sciences 22, no. 6: 2862. https://doi.org/10.3390/ijms22062862
APA StyleCimmino, F., Catapano, A., Trinchese, G., Cavaliere, G., Culurciello, R., Fogliano, C., Penna, E., Lucci, V., Crispino, M., Avallone, B., Pizzo, E., & Mollica, M. P. (2021). Dietary Micronutrient Management to Treat Mitochondrial Dysfunction in Diet-Induced Obese Mice. International Journal of Molecular Sciences, 22(6), 2862. https://doi.org/10.3390/ijms22062862












