Structural and Functional Characterization of the ABCC6 Transporter in Hepatic Cells: Role on PXE, Cancer Therapy and Drug Resistance
Abstract
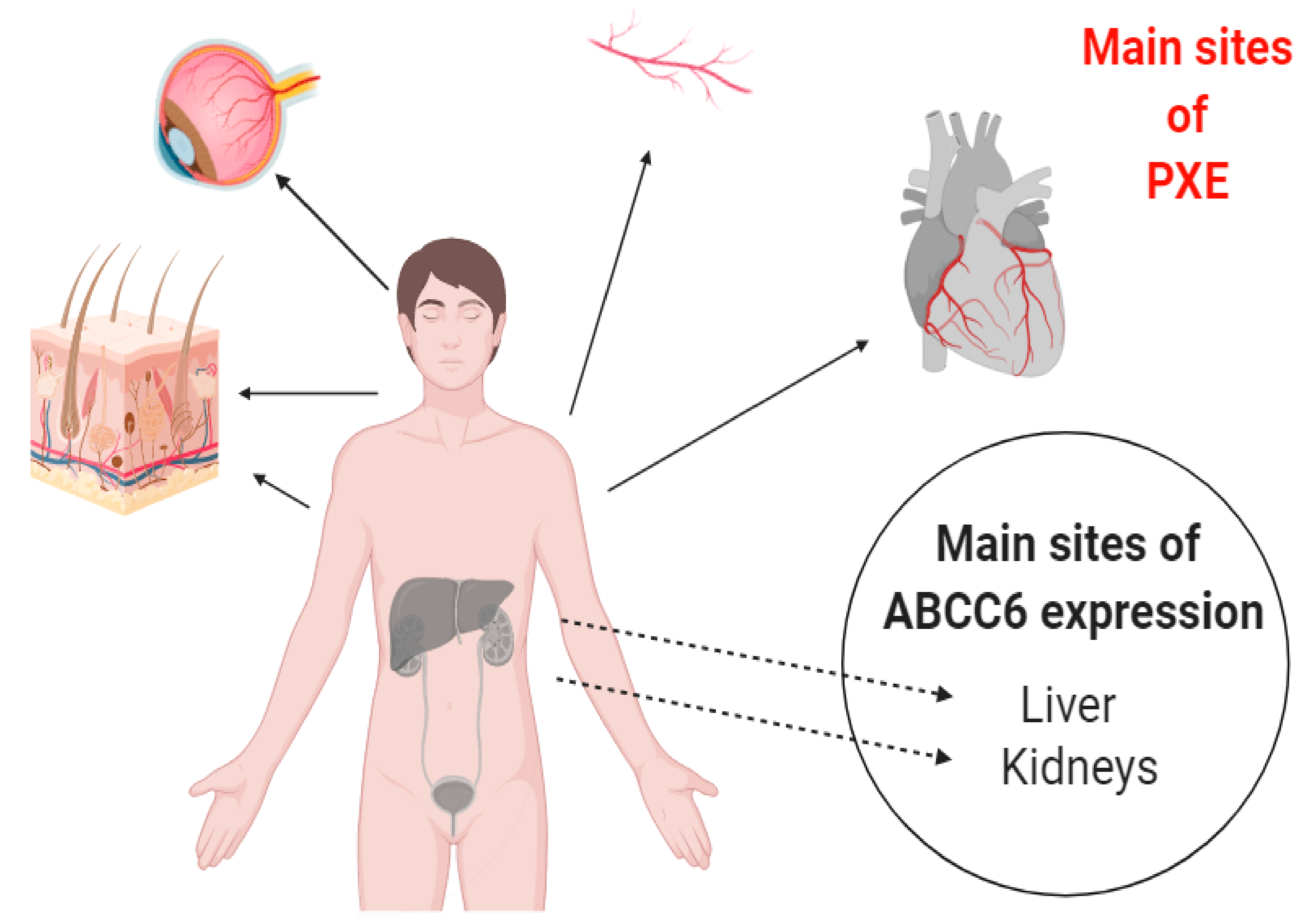
1. Introduction
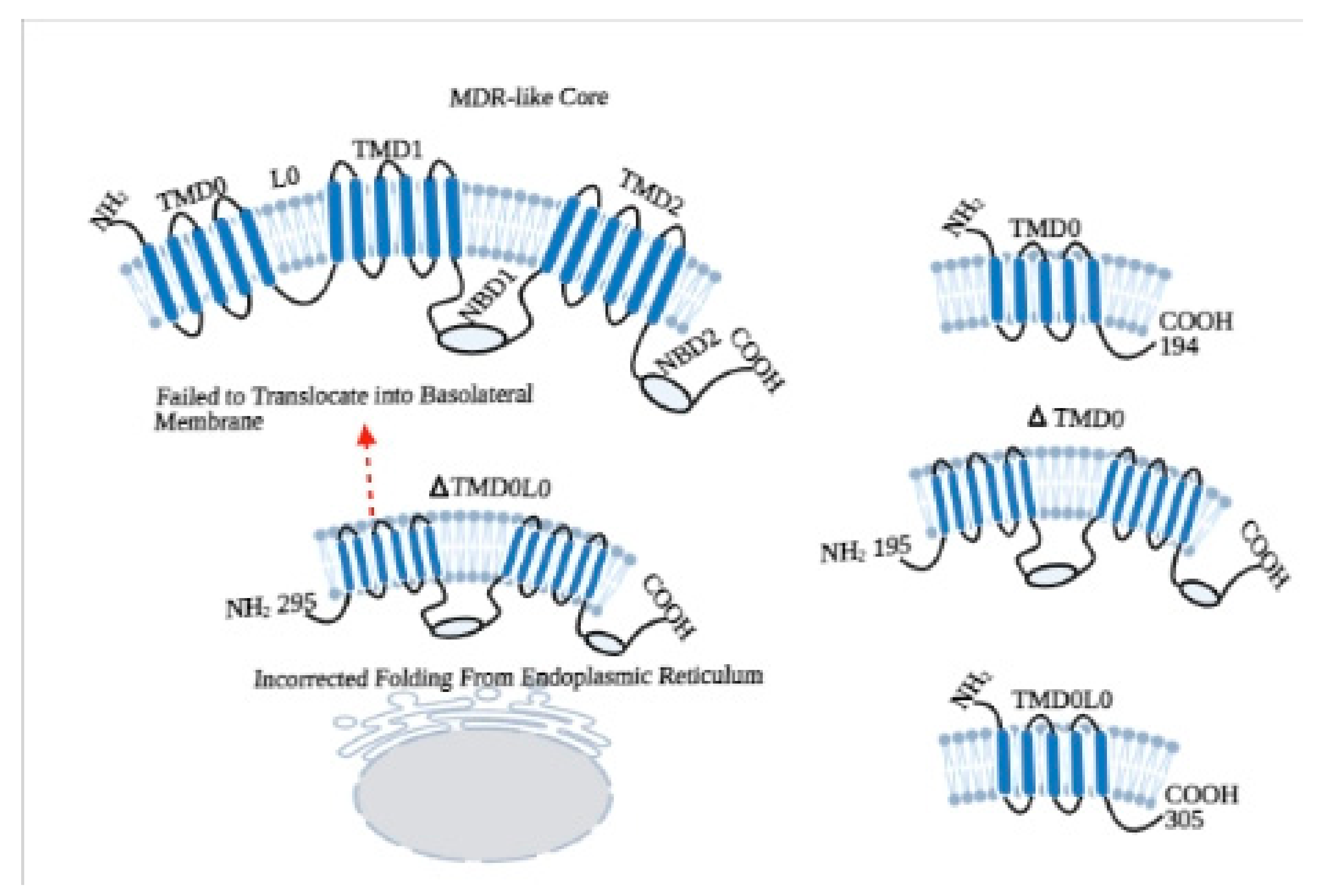
2. Structural Properties of ABCC6 Transporter
3. Roles of Additional TMD0 and L0 Domains
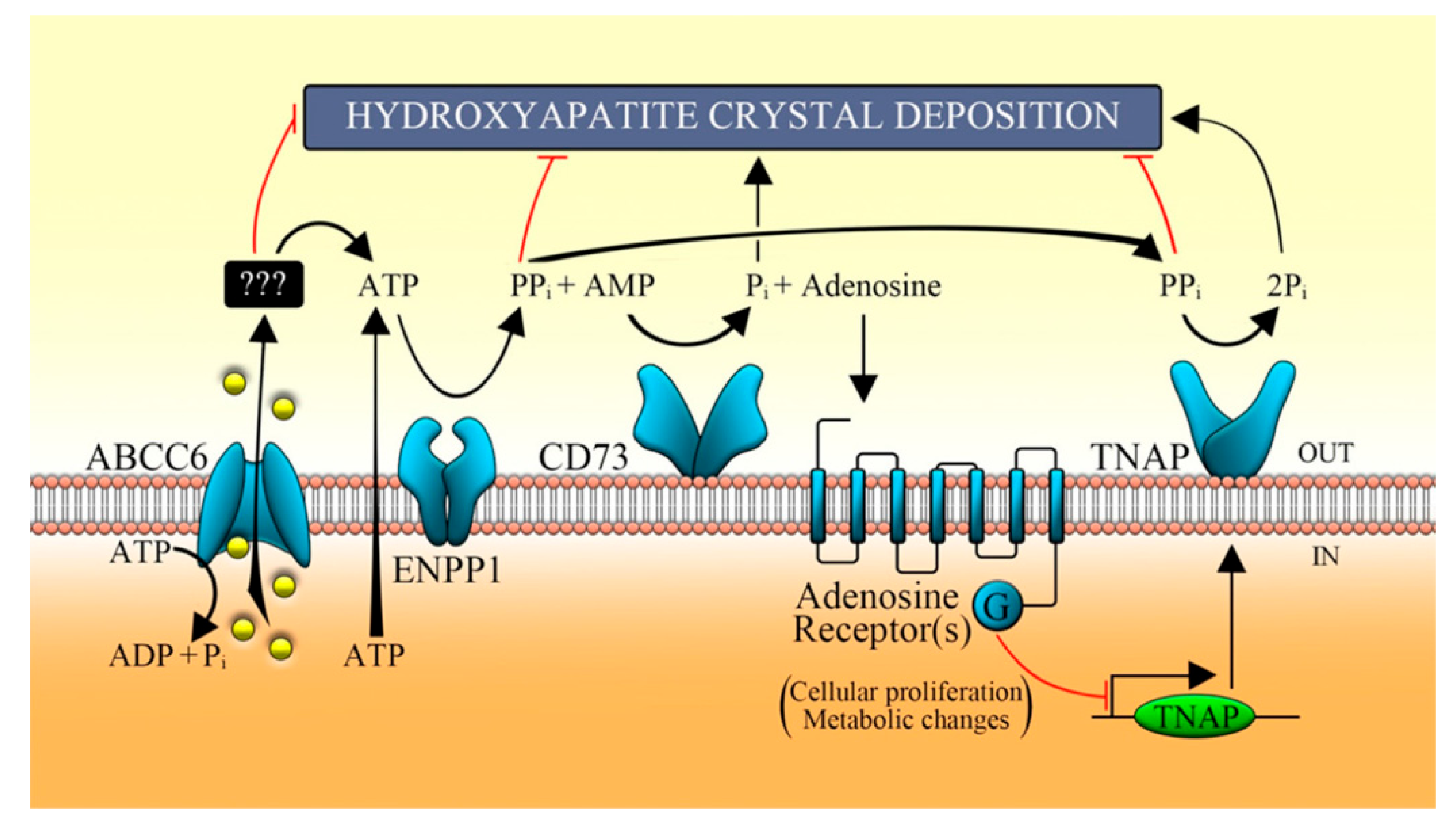
4. The Decrease or Loss of ABCC6 Functionality Changes Extracellular Environment
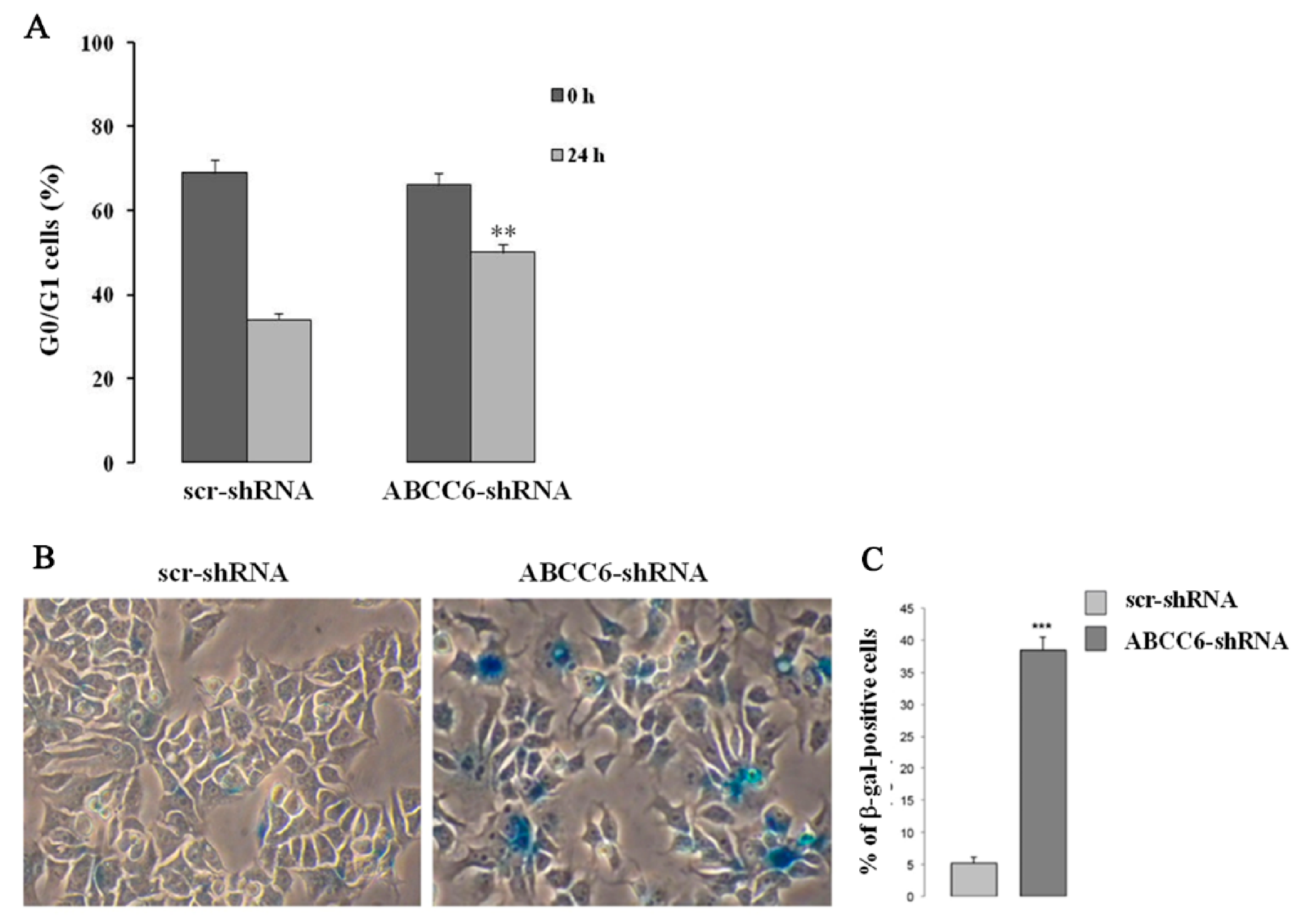
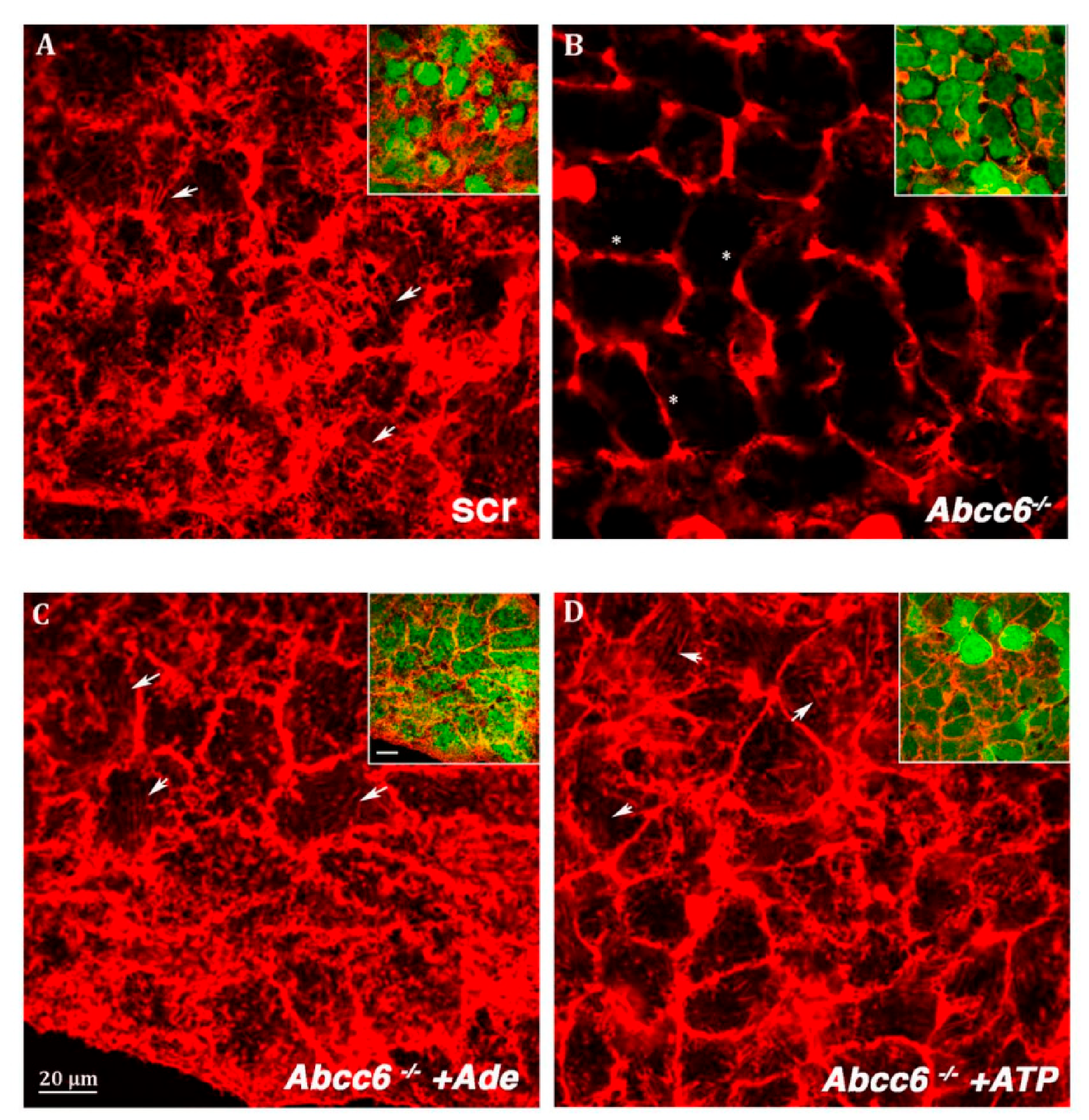
5. Intracellular Consequences Associated with ABCC6 Transporter Activity in HepG2 Cells
6. Involvement of ABCC6/MRP6 in Drug Resistance
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moitra, K.; Garcia, S.; Jaldin, M.; Etoundi, C.; Cooper, D.; Roland, A.; Dixon, P.; Reyes, S.; Turan, S.; Terry, S.; et al. ABCC6 and pseudoxanthoma elasticum: The face of a rare disease from genetics to advocacy. Int. J. Mol. Sci. 2017, 18, 1488. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.S.; Küçükosmanoglu, A.; De Haas, M.; Sapthu, S.; Otero, J.A.; Hegman, I.E.M.; Bergen, A.A.B.; Gorgels, T.G.M.F.; Borst, P.; Van De Wetering, K. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc. Natl. Acad. Sci. USA 2013, 110, 20206–20211. [Google Scholar] [CrossRef]
- Luo, H.; Li, Q.; Cao, Y.; Uitto, J. Therapeutics Development for Pseudoxanthoma Elasticum and Related Ectopic Mineralization Disorders: Update 2020. J. Clin. Med. 2021, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- De Vilder, E.Y.; Hosen, M.J.; Vanakker, O.M. The ABCC6 transporter as a paradigm for networking from an orphan disease to complex disorders. Biomed. Res. Int. 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Lau, W.L.; Liu, S.; Vaziri, N.D. Chronic kidney disease results in deficiency of ABCC6, the novel inhibitor of vascular calcification. Am. J. Nephrol. 2014, 40, 51–55. [Google Scholar] [CrossRef]
- Jiang, Q.; Endo, M.; Dibra, F.; Wang, K.; Uitto, J. Pseudoxanthoma elasticum is a metabolic disease. J. Investig. Dermatol. 2009, 129, 348–354. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, Q.; Pfendner, E.; Váradi, A.; Uitto, J. Pseudoxanthoma elasticum: Clinical phenotypes, molecular genetics and putative pathomechanisms. Exp. Dermatol. 2009, 18, 1–11. [Google Scholar] [CrossRef]
- Klement, J.F.; Matsuzaki, Y.; Jiang, Q.-J.; Terlizzi, J.; Choi, H.Y.; Fujimoto, N.; Li, K.; Pulkkinen, L.; Birk, D.E.; Sundberg, J.P.; et al. Targeted ablation of the abcc6 gene results in ectopic mineralization of connective tissues. Mol. Cell. Biol. 2005, 25, 8299–8310. [Google Scholar] [CrossRef]
- Xiong, J.; Feng, J.; Yuan, D.; Zhou, J.; Miao, W. Tracing the structural evolution of eukaryotic ATP binding cassette transporter superfamily. Sci. Rep. 2015, 5, 16724. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pan, G. Drug Transporters in Drug Disposition, Effects and Toxicity; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Favre, G.; Laurain, A.; Aranyi, T.; Szeri, F.; Fulop, K.; Le Saux, O.; Duranton, C.; Kauffenstein, G.; Martin, L.; Lefthériotis, G. The ABCC6 transporter: A new player in biomineralization. Int. J. Mol. Sci. 2017, 18, 1941. [Google Scholar] [CrossRef]
- Ostuni, A.; Miglionico, R.; Castiglione Morelli, M.A.; Bisaccia, F. Study of the nucleotide-binding domain 1 of the human transporter protein MRP6. Protein Pept. Lett. 2010, 17, 1553–1558. [Google Scholar] [CrossRef]
- Ostuni, A.; Castiglione Morelli, M.A.; Cuviello, F.; Bavoso, A.; Bisaccia, F. Structural characterization of the L0 cytoplasmic loop of human multidrug resistance protein 6 (MRP6). Biochim. Biophys. Acta Biomembr. 2019, 1861, 380–386. [Google Scholar] [CrossRef]
- Thomas, C.; Tampé, R. Multifaceted structures and mechanisms of ABC transport systems in health and disease. Curr. Opin. Struct. Biol. 2018, 51, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Thibodeau, P.H. Stabilization of nucleotide binding domain dimers rescues ABCC6 mutants associated with pseudoxanthoma elasticum. J. Biol. Chem. 2017, 292, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C.; Karpowich, N.; Millen, L.; Moody, J.E.; Rosen, J.; Thomas, P.J.; Hunt, J.F. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol. Cell 2002, 10, 139–149. [Google Scholar] [CrossRef]
- Ran, Y.; Zheng, A.; Thibodeau, P.H. Structural analysis reveals pathomechanisms associated with pseudoxanthoma elasticum–causing mutations in the ABCC6 transporter. J. Biol. Chem. 2018, 293, 15855–15866. [Google Scholar] [CrossRef]
- Dhasmana, D.; Singh, A.; Shukla, R.; Tripathi, T.; Garg, N. Targeting nucleotide binding domain of multidrug resistance-associated protein-1 (MRP1) for the reversal of multi drug resistance in cancer. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.L.; Chen, J. Structural basis of substrate recognition by the multidrug resistance protein MRP1. Cell 2017, 168, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Miglionico, R.; Gerbino, A.; Ostuni, A.; Armentano, M.F.; Monné, M.; Carmosino, M.; Bisaccia, F. New insights into the roles of the N-terminal region of the ABCC6 transporter. J. Bioenerg. Biomembr. 2016, 48, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, A.; Miglionico, R.; Bisaccia, F.; Castiglione Morelli, M.A. Biochemical characterization and NMR study of the region E748-A785 of the human protein MRP6/ABCC6. Protein Pept. Lett. 2010, 17, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Beck, K.; Sachsinger, C.; Silvestri, C.; Treiber, C.; Göring, H.H.H.; Johnson, E.W.; De Paepe, A.; Pope, F.M.; Pasquali-Ronchetti, I.; et al. A spectrum of ABCC6 mutations is responsible for pseudoxanthoma elasticum. Am. J. Hum. Genet. 2001, 69, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, A.; Miglionico, R.; Monné, M.; Castiglione Morelli, M.A.; Bisaccia, F. The nucleotide-binding domain 2 of the human transporter protein MRP6. J. Bioenerg. Biomembr. 2011, 43, 465. [Google Scholar] [CrossRef]
- Goda, K.; Dönmez-Cakil, Y.; Tarapcsák, S.; Szalóki, G.; Szöllősi, D.; Parveen, Z.; Türk, D.; Szakács, G.; Chiba, P.; Stockner, T. Human ABCB1 with an ABCB11-like degenerate nucleotide binding site maintains transport activity by avoiding nucleotide occlusion. PLoS Genet. 2020, 16, e1009016. [Google Scholar] [CrossRef]
- Cuviello, F.; Tellgren-Roth, Å.; Lara, P.; Selin, F.R.; Monné, M.; Bisaccia, F.; Nilsson, I.; Ostuni, A. Membrane insertion and topology of the amino-terminal domain TMD0 of multidrug-resistance associated protein 6 (MRP6). FEBS Lett. 2015, 589, 3921–3928. [Google Scholar] [CrossRef]
- Iliás, A.; Urbán, Z.; Seidl, T.L.; Le Saux, O.; Sinkó, E.; Boyd, C.D.; Sarkadi, B.; Váradi, A. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6). J. Biol. Chem. 2002, 277, 16860–16867. [Google Scholar] [CrossRef]
- Belinsky, M.G.; Chen, Z.-S.; Shchaveleva, I.; Zeng, H.; Kruh, G.D. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6). Cancer Res. 2002, 62, 6172–6177. [Google Scholar]
- Borst, P.; van de Wetering, K.; Schlingemann, R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with Pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle 2008, 7, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Gorgels, T.G.M.F.; Waarsing, J.H.; Herfs, M.; Versteeg, D.; Schoensiegel, F.; Sato, T.; Schlingemann, R.O.; Ivandic, B.; Vermeer, C.; Schurgers, L.J.; et al. Vitamin K supplementation increases vitamin K tissue levels but fails to counteract ectopic calcification in a mouse model for pseudoxanthoma elasticum. J. Mol. Med. 2011, 89, 1125. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, K.; Jiang, Q.; Wetering, K.V.; Pomozi, V.; Szabó, P.T.; Arányi, T.; Sarkadi, B.; Borst, P.; Uitto, J.; Váradi, A. ABCC6 does not transport vitamin K3-glutathione conjugate from the liver: Relevance to pathomechanisms of pseudoxanthoma elasticum. Biochem. Biophys. Res. Commun. 2011, 415, 468–471. [Google Scholar] [CrossRef]
- Miglionico, R.; Armentano, M.F.; Carmosino, M.; Salvia, A.M.; Cuviello, F.; Bisaccia, F.; Ostuni, A. Dysregulation of gene expression in ABCC6 knockdown HepG2 cells. Cell. Mol. Biol. Lett. 2014, 19, 517–526. [Google Scholar] [CrossRef]
- Ostuni, A.; Carmosino, M.; Miglionico, R.; Abruzzese, V.; Martinelli, F.; Russo, D.; Laurenzana, I.; Petillo, A.; Bisaccia, F. Inhibition of ABCC6 Transporter Modifies Cytoskeleton and Reduces Motility of HepG2 Cells via Purinergic Pathway. Cells 2020, 9, 1410. [Google Scholar] [CrossRef]
- Miglionico, R.; Ostuni, A.; Armentano, M.F.; Milella, L.; Crescenzi, E.; Carmosino, M.; Bisaccia, F. ABCC6 knockdown in HepG2 cells induces a senescent-like cell phenotype. Cell. Mol. Biol. Lett. 2017, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Pasquali-Ronchetti, I.; Garcia-Fernandez, M.I.; Boraldi, F.; Quaglino, D.; Gheduzzi, D.; Paolinelli, C.D.V.; Tiozzo, R.; Bergamini, S.; Ceccarelli, D.; Muscatello, U. Oxidative stress in fibroblasts from patients with pseudoxanthoma elasticum: Possible role in the pathogenesis of clinical manifestations. J. Pathol. J. Pathol. Soc. Great Br. Irel. 2006, 208, 54–61. [Google Scholar] [CrossRef]
- Martinelli, F.; Cuviello, F.; Pace, M.C.; Armentano, M.F.; Miglionico, R.; Ostuni, A.; Bisaccia, F. Extracellular ATP regulates CD73 and ABCC6 expression in HepG2 Cells. Front. Mol. Biosci. 2018, 5, 75. [Google Scholar] [CrossRef]
- Kristiani, L.; Kim, M.; Kim, Y. Role of the Nuclear Lamina in Age-Associated Nuclear Reorganization and Inflammation. Cells 2020, 9, 718. [Google Scholar] [CrossRef]
- Honoki, K.; Fujii, H.; Tsukamoto, S.; Kishi, S.; Tsujiuchi, T.; Tanaka, Y. Crossroads of hallmarks in aging and cancer: Anti-aging and anti-cancer target pathways can be shared. Tre Can Res 2016, 11, 39–59. [Google Scholar]
- Irianto, J.; Pfeifer, C.R.; Ivanovska, I.L.; Swift, J.; Discher, D.E. Nuclear lamins in cancer. Cell. Mol. Bioeng. 2016, 9, 258–267. [Google Scholar] [CrossRef]
- Dean, M.; Allikmets, R. Complete characterization of the human ABC gene family. J. Bioenerg. Biomembr. 2001, 33, 475–479. [Google Scholar] [CrossRef]
- Eadie, L.N.; Dang, P.; Goyne, J.M.; Hughes, T.P.; White, D.L. ABCC6 plays a significant role in the transport of nilotinib and dasatinib, and contributes to TKI resistance in vitro, in both cell lines and primary patient mononuclear cells. PLoS ONE 2018, 13, e0192180. [Google Scholar] [CrossRef]
- Salvia, A.M.; Cuviello, F.; Coluzzi, S.; Nuccorini, R.; Attolico, I.; Pascale, S.P.; Bisaccia, F.; Pizzuti, M.; Ostuni, A. Expression of some ATP-binding cassette transporters in acute myeloid leukemia. Hematol. Rep. 2017, 9, 7406. [Google Scholar] [CrossRef]
- Jeon, H.-M.; Sohn, Y.-W.; Oh, S.-Y.; Kim, S.-H.; Beck, S.; Kim, S.; Kim, H. ID4 imparts chemoresistance and cancer stemness to glioma cells by derepressing miR-9*–mediated suppression of SOX2. Cancer Res. 2011, 71, 3410–3421. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Morton, D.J.; Carey, J.; Havrda, M.C.; Chaudhary, J. Inhibitor of differentiation 4 (ID4): From development to cancer. Biochim. Biophys. Acta Rev. Cancer 2015, 1855, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Cuviello, F.; Bisaccia, F.; Spinelli, D.; Armentano, M.F.; Bufo, S.A.; Cosimelli, B.; Ostuni, A. The P-glycoprotein inhibitor diltiazem-like 8-(4-chlorophenyl)-5-methyl-8-[(2Z)-pent-2-en-1-yloxy]-8H-[1,2,4] oxadiazolo [3,4-c][1,4] thiazin-3-one inhibits esterase activity and H3 histone acetylation. Eur. J. Med. Chem. 2019, 164, 1–7. [Google Scholar] [CrossRef]
- Váradi, A.; Szabó, Z.; Pomozi, V.; de Boussac, H.; Fulop, K.; Arányi, T. ABCC6 as a target in pseudoxanthoma elasticum. Curr. Drug Targets 2011, 12, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Armentano, M.F.; Ostuni, A.; Infantino, V.; Iacobazzi, V.; Castiglione Morelli, M.A.; Bisaccia, F. Identification of a new splice variant of the human ABCC6 transporter. Res. Lett. Biochem. 2008, 2008, 1–4. [Google Scholar] [CrossRef]
- Le Saux, O.; Martin, L.; Aherrahrou, Z.; Leftheriotis, G.; Váradi, A.; Brampton, C.N. The molecular and physiological roles of ABCC6: More than meets the eye. Front. Genet. 2012, 3, 289. [Google Scholar] [CrossRef] [PubMed]
- Arányi, T.; Bacquet, C.; De Boussac, H.; Ratajewski, M.; Pomozi, V.; Fülöp, K.; Brampton, C.N.; Pulaski, L.; Le Saux, O.; Váradi, A. Transcriptional regulation of the ABCC6 gene and the background of impaired function of missense disease-causing mutations. Front. Genet. 2013, 4, 27. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisaccia, F.; Koshal, P.; Abruzzese, V.; Castiglione Morelli, M.A.; Ostuni, A. Structural and Functional Characterization of the ABCC6 Transporter in Hepatic Cells: Role on PXE, Cancer Therapy and Drug Resistance. Int. J. Mol. Sci. 2021, 22, 2858. https://doi.org/10.3390/ijms22062858
Bisaccia F, Koshal P, Abruzzese V, Castiglione Morelli MA, Ostuni A. Structural and Functional Characterization of the ABCC6 Transporter in Hepatic Cells: Role on PXE, Cancer Therapy and Drug Resistance. International Journal of Molecular Sciences. 2021; 22(6):2858. https://doi.org/10.3390/ijms22062858
Chicago/Turabian StyleBisaccia, Faustino, Prashant Koshal, Vittorio Abruzzese, Maria Antonietta Castiglione Morelli, and Angela Ostuni. 2021. "Structural and Functional Characterization of the ABCC6 Transporter in Hepatic Cells: Role on PXE, Cancer Therapy and Drug Resistance" International Journal of Molecular Sciences 22, no. 6: 2858. https://doi.org/10.3390/ijms22062858
APA StyleBisaccia, F., Koshal, P., Abruzzese, V., Castiglione Morelli, M. A., & Ostuni, A. (2021). Structural and Functional Characterization of the ABCC6 Transporter in Hepatic Cells: Role on PXE, Cancer Therapy and Drug Resistance. International Journal of Molecular Sciences, 22(6), 2858. https://doi.org/10.3390/ijms22062858





