Metabolic Glycoengineering in hMSC-TERT as a Model for Skeletal Precursors by Using Modified Azide/Alkyne Monosaccharides
Abstract
1. Introduction
2. Results
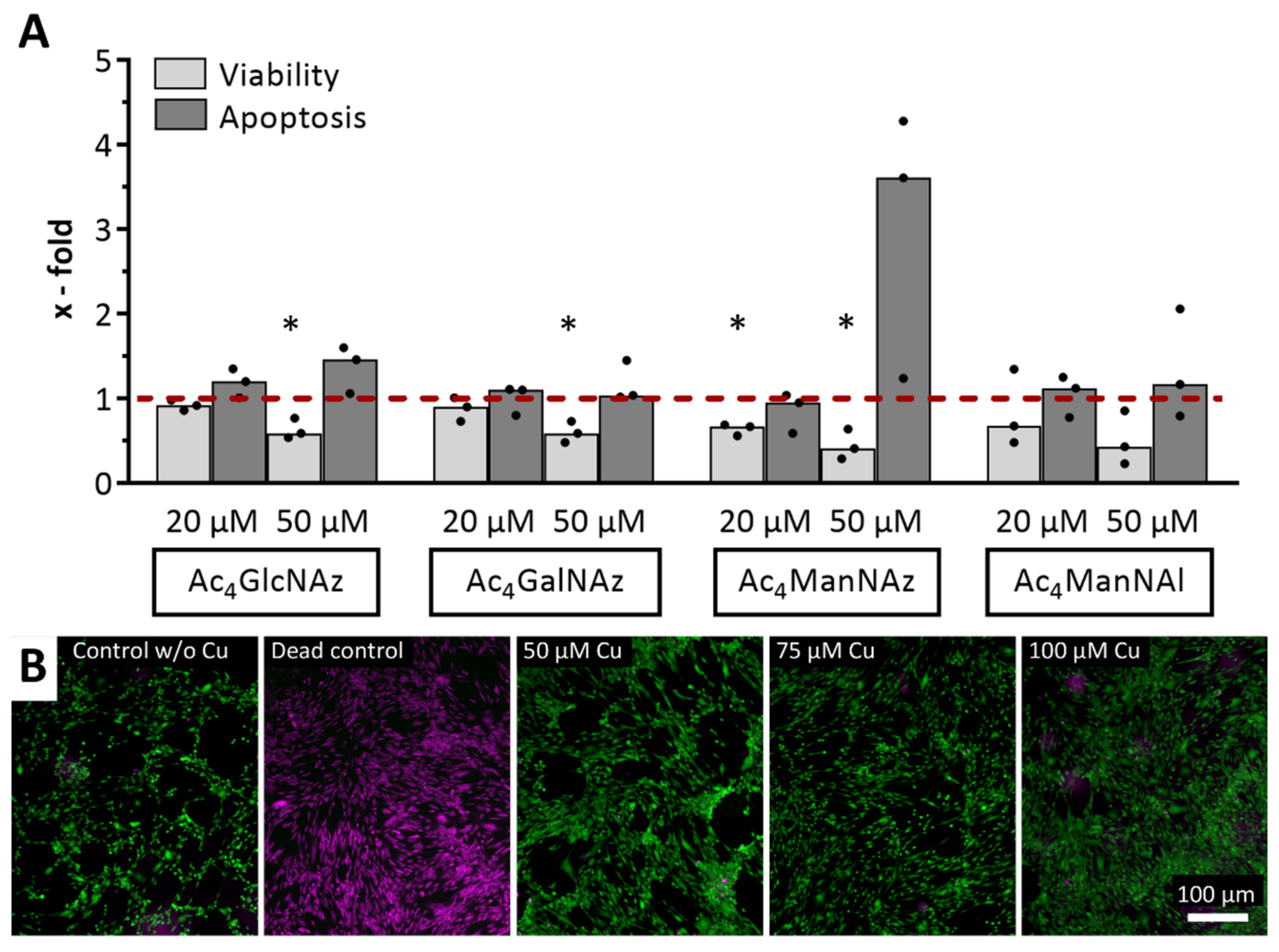
2.1. Impact of Click Reagents on the Cell Viability
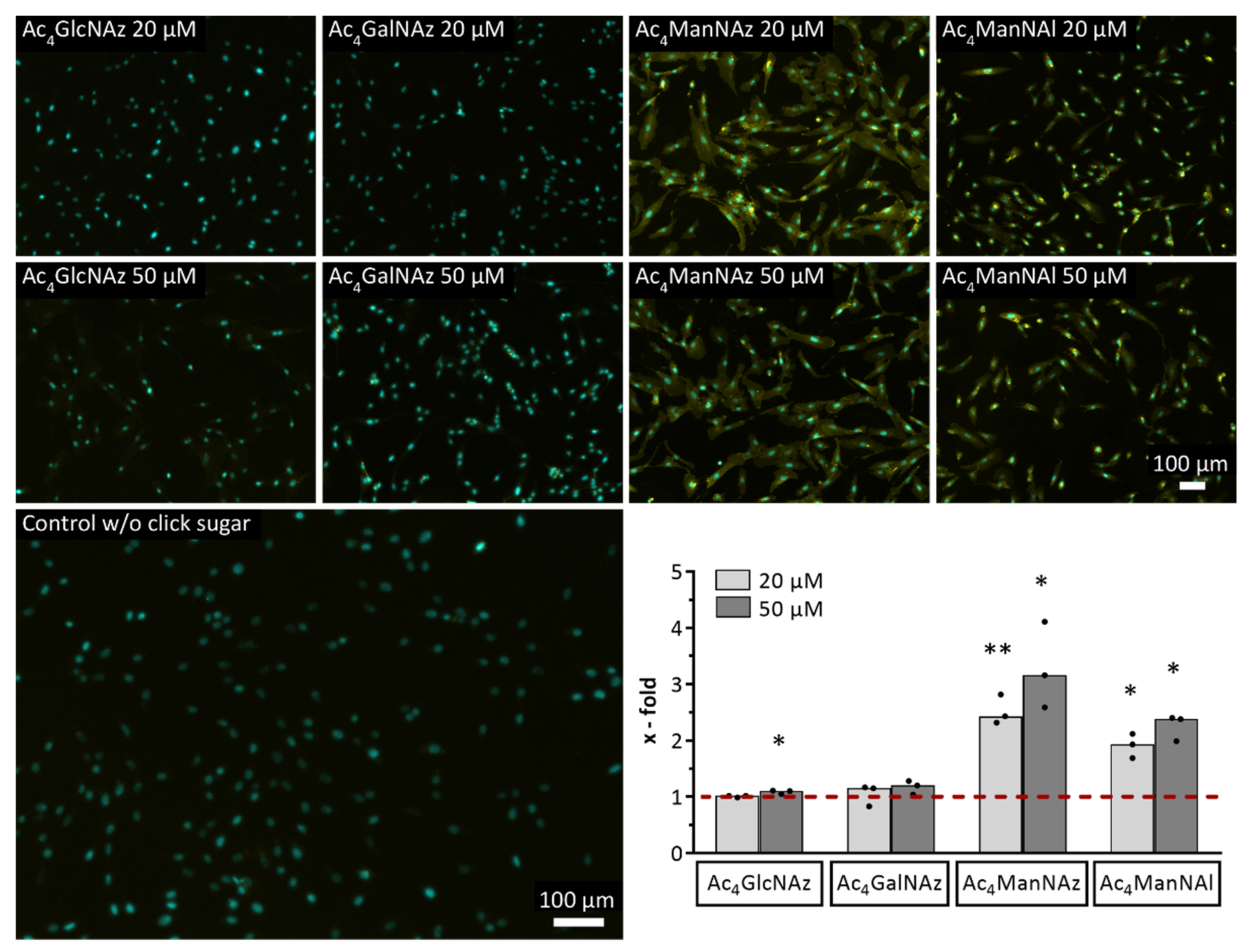
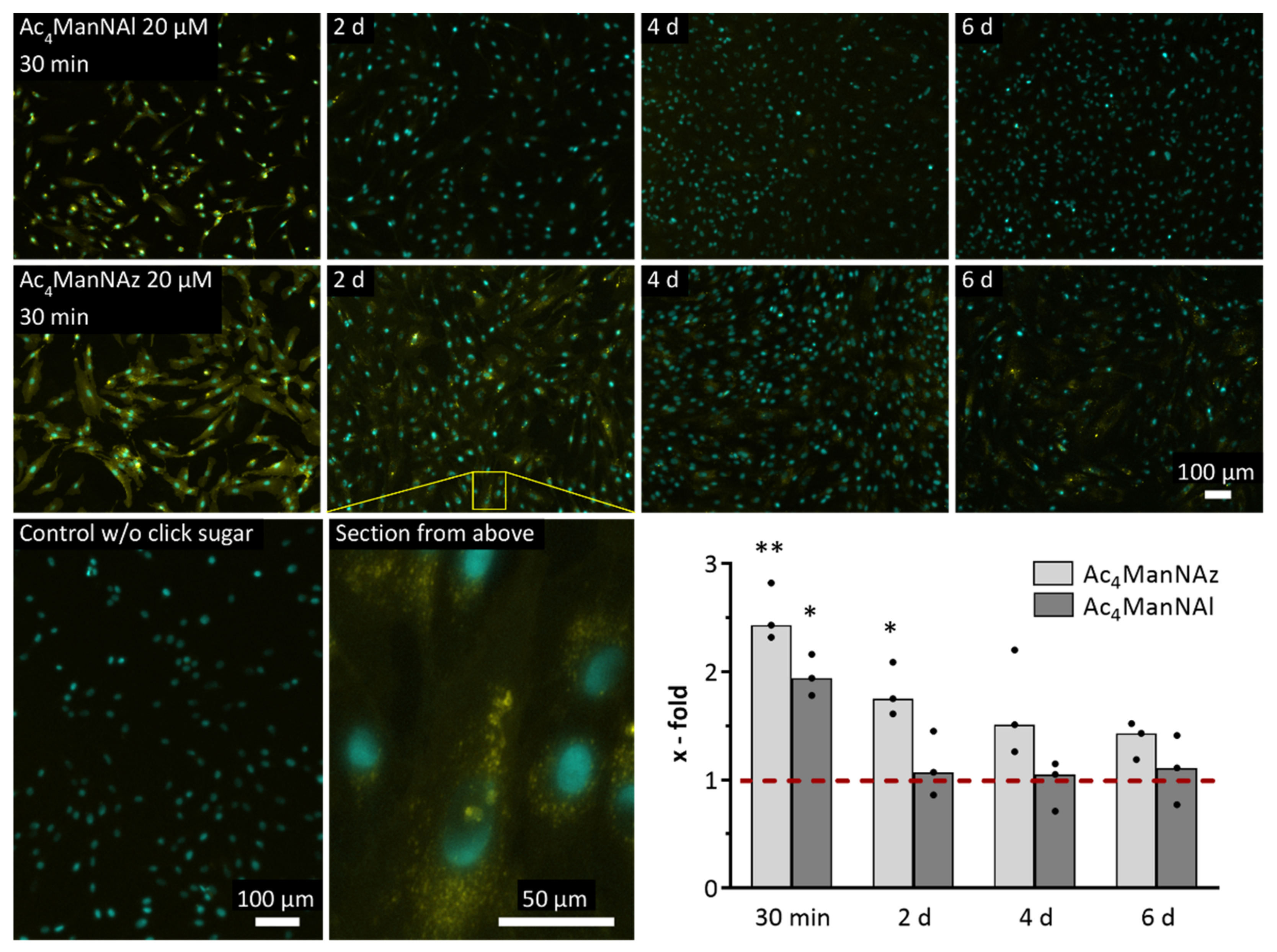
2.2. Evaluation of Different Modified Monosaccharides Regarding Their Incorporation and Retention in the Glycocalyx
2.3. Impact of Modified Monosaccharides on Mesenchymal Differentiation and Gene Regulation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
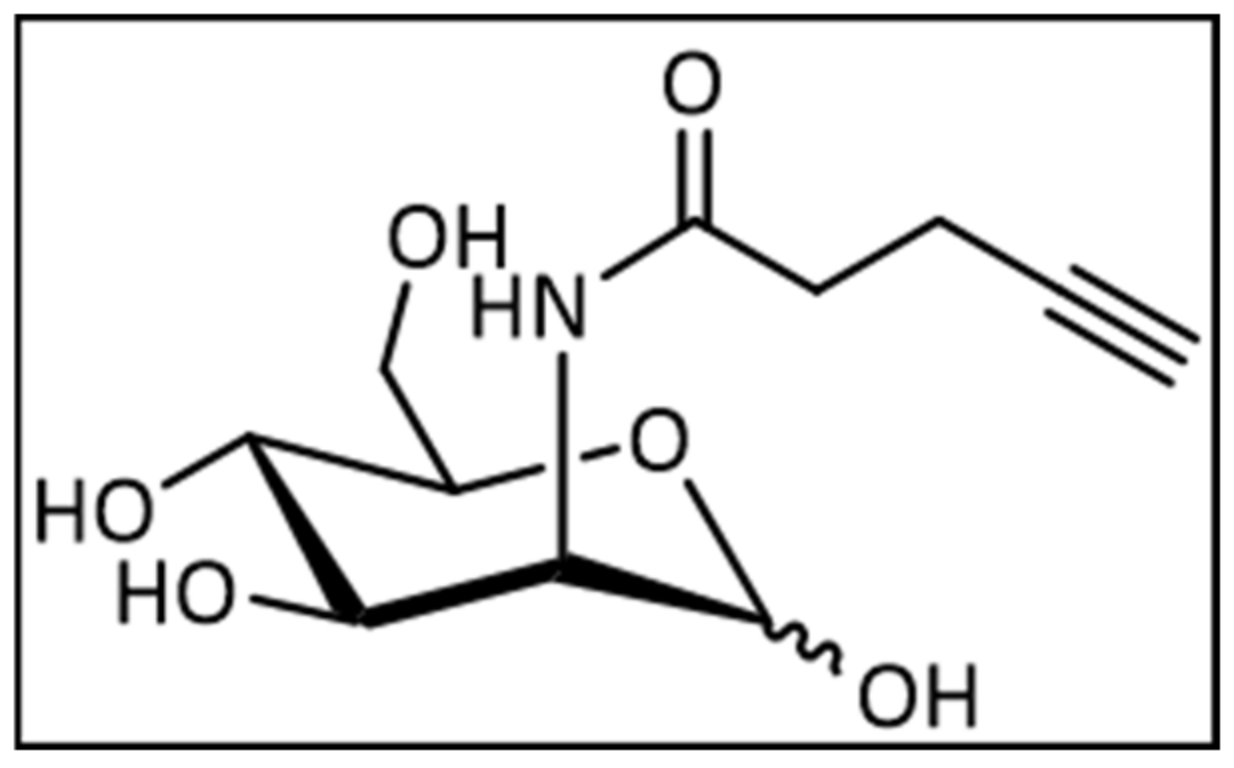
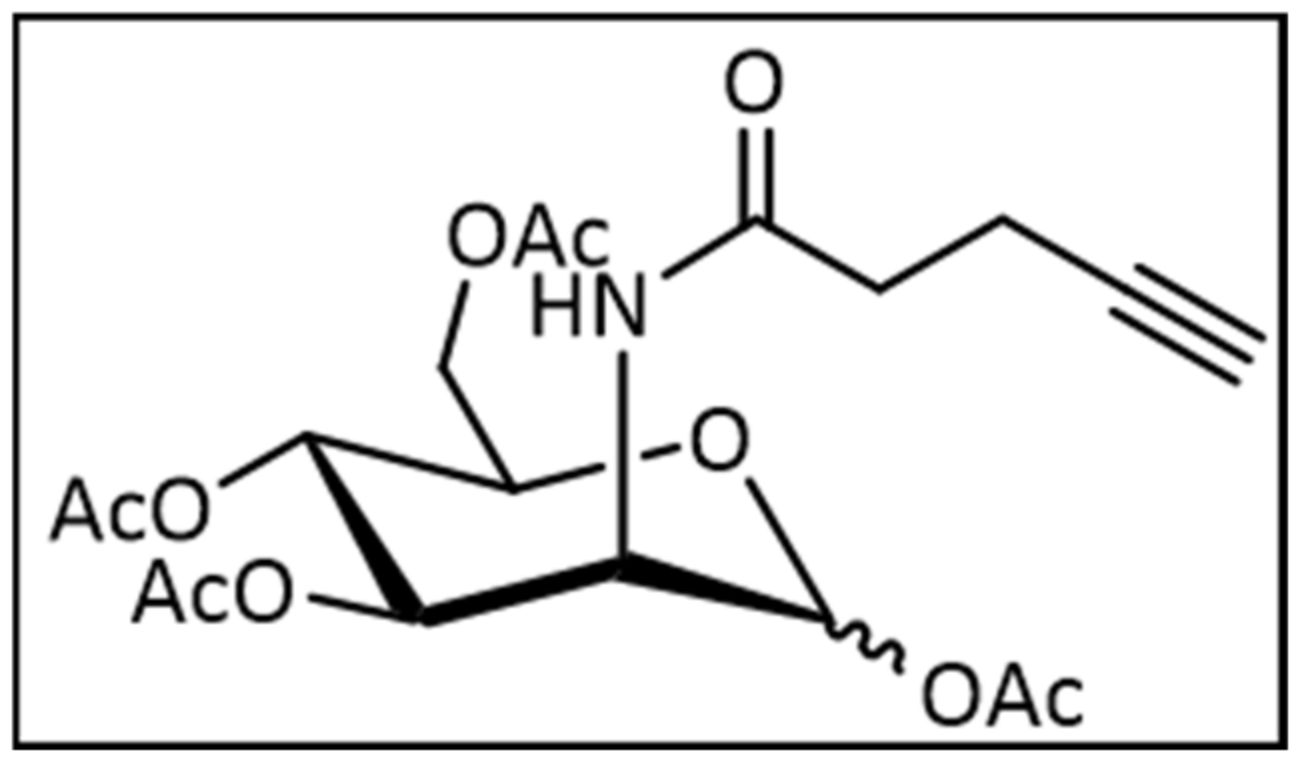
4.1.1. Synthesis of 2-(N-4-Pentynoyl)-2-Deoxy-d-Mannopyranoside
4.1.2. Synthesis of 2-(N-4-Pentynoyl)-2-Deoxy-(1,3,4,6)-Tetra-O-Acetyl-d-Mannopyranoside
4.2. Cell Viability and Apoptosis Assays
4.3. Live/Dead Assay
4.4. Adipogenic Differentiation
4.5. Osteogenic Differentiation
4.6. Histochemical Staining
4.7. RNA Isolation, Reverse Transcription and qPCR Analysis
4.8. Fluorescence Imaging after Click Reaction on the Cell Surface
4.9. Data Processing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Herrmann, M.; Jakob, F. Bone Marrow Niches for Skeletal Progenitor Cells and their Inhabitants in Health and Disease. Curr. Stem Cell Res. Ther. 2019, 14, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: Implications for further clinical uses. Cell. Mol. Life Sci. 2020, 1–21. [Google Scholar] [CrossRef]
- Twine, N.A.; Harkness, L.; Adjaye, J.; Aldahmash, A.; Wilkins, M.R.; Kassem, M. Molecular Phenotyping of Telomerized Human Bone Marrow Skeletal Stem Cells Reveals a Genetic Program of Enhanced Proliferation and Maintenance of Differentiation Responses. JBMR Plus 2018, 2, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Rapraeger, A.; Jalkanen, M.; Endo, E.; Koda, J.; Bernfield, M. The cell surface proteoglycan from mouse mammary epithelial cells bears chondroitin sulfate and heparan sulfate glycosaminoglycans. J. Biol. Chem. 1985, 260, 11046–11052. [Google Scholar] [CrossRef]
- Gromnicova, R.; Kaya, M.; Romero, I.A.; Williams, P.; Satchell, S.; Sharrack, B.; Male, D. Transport of Gold Nanoparticles by Vascular Endothelium from Different Human Tissues. PLoS ONE 2016, 11, e0161610. [Google Scholar] [CrossRef]
- Möckl, L.; Hirn, S.; Torrano, A.A.; Uhl, B.; Bräuchle, C.; Krombach, F. The glycocalyx regulates the uptake of nanoparticles by human endothelial cells in vitro. Nanomedicine 2017, 12, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Uhl, B.; Hirn, S.; Immler, R.; Mildner, K.; Möckl, L.; Sperandio, M.; Bräuchle, C.; Reichel, C.A.; Zeuschner, D.; Krombach, F. The Endothelial Glycocalyx Controls Interactions of Quantum Dots with the Endothelium and Their Translocation across the Blood-Tissue Border. ACS Nano 2017, 11, 1498–1508. [Google Scholar] [CrossRef]
- Xiao, H.; Woods, E.C.; Vukojicic, P.; Bertozzi, C.R. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2016, 113, 10304–10309. [Google Scholar] [CrossRef]
- Blaum, B.S.; Hannan, J.P.; Herbert, A.P.; Kavanagh, D.; Uhrín, D.; Stehle, T. Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat. Chem. Biol. 2015, 11, 77–82. [Google Scholar] [CrossRef]
- Jandus, C.; Boligan, K.F.; Chijioke, O.; Liu, H.; Dahlhaus, M.; Démoulins, T.; Schneider, C.; Wehrli, M.; Hunger, R.E.; Baerlocher, G.M.; et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J. Clin. Investig. 2014, 124, 1810–1820. [Google Scholar] [CrossRef]
- Läubli, H.; Pearce, O.M.; Schwarz, F.; Siddiqui, S.S.; Deng, L.; Stanczak, M.A.; Deng, L.; Verhagen, A.; Secrest, P.; Lusk, C.; et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 14211–14216. [Google Scholar] [CrossRef]
- Soler, M.; Desplat-Jego, S.; Vacher, B.; Ponsonnet, L.; Fraterno, M.; Bongrand, P.; Martin, J.M.; Foa, C. Adhesion-related glycocalyx study: Quantitative approach with imaging-spectrum in the energy filtering transmission electron microscope (EFTEM). FEBS Lett. 1998, 429, 89–94. [Google Scholar] [CrossRef]
- Shurer, C.R.; Colville, M.J.; Gupta, V.K.; Head, S.E.; Kai, F.; Lakins, J.N.; Paszek, M.J. Genetically Encoded Toolbox for Glycocalyx Engineering: Tunable Control of Cell Adhesion, Survival, and Cancer Cell Behaviors. ACS Biomater. Sci. Eng. 2018, 4, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Memmel, E.; Homann, A.; Oelschlaeger, T.A.; Seibel, J. Metabolic glycoengineering of Staphylococcus aureus reduces its adherence to human T24 bladder carcinoma cells. Chem. Commun. 2013, 49, 7301–7303. [Google Scholar] [CrossRef] [PubMed]
- Hart, G.W.; Housley, M.P.; Slawson, C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007, 446, 1017–1022. [Google Scholar] [CrossRef]
- Thi, M.M.; Tarbell, J.M.; Weinbaum, S.; Spray, D.C. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: A “bumper-car” model. Proc. Natl. Acad. Sci. USA 2004, 101, 16483–16488. [Google Scholar] [CrossRef]
- Pahakis, M.Y.; Kosky, J.R.; Dull, R.O.; Tarbell, J.M. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem. Biophys. Res. Commun. 2007, 355, 228–233. [Google Scholar] [CrossRef]
- Florian, J.A.; Kosky, J.R.; Ainslie, K.; Pang, Z.; Dull, R.O.; Tarbell, J.M. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 2003, 93, e136–e142. [Google Scholar] [CrossRef]
- Chen, J.; Gao, J.; Wu, J.; Zhang, M.; Cai, M.; Xu, H.; Jiang, J.; Tian, Z.; Wang, H. Revealing the carbohydrate pattern on a cell surface by super-resolution imaging. Nanoscale 2015, 7, 3373–3380. [Google Scholar] [CrossRef]
- Mockl, L.; Pedram, K.; Roy, A.R.; Krishnan, V.; Gustavsson, A.K.; Dorigo, O.; Bertozzi, C.R.; Moerner, W.E. Quantitative Super-Resolution Microscopy of the Mammalian Glycocalyx. Dev. Cell 2019, 50, 57–72.e56. [Google Scholar] [CrossRef]
- Ebong, E.E.; Macaluso, F.P.; Spray, D.C.; Tarbell, J.M. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1908–1915. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. Engl. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. From mechanism to mouse: A tale of two bioorthogonal reactions. Acc. Chem. Res. 2011, 44, 666–676. [Google Scholar] [CrossRef]
- Du, J.; Meledeo, M.A.; Wang, Z.; Khanna, H.S.; Paruchuri, V.D.; Yarema, K.J. Metabolic glycoengineering: Sialic acid and beyond. Glycobiology 2009, 19, 1382–1401. [Google Scholar] [CrossRef]
- Layek, B.; Sadhukha, T.; Prabha, S. Glycoengineered mesenchymal stem cells as an enabling platform for two-step targeting of solid tumors. Biomaterials 2016, 88, 97–109. [Google Scholar] [CrossRef]
- Dykstra, B.; Lee, J.; Mortensen, L.J.; Yu, H.; Wu, Z.L.; Lin, C.P.; Rossi, D.J.; Sackstein, R. Glycoengineering of E-Selectin Ligands by Intracellular versus Extracellular Fucosylation Differentially Affects Osteotropism of Human Mesenchymal Stem Cells. Stem Cells 2016, 34, 2501–2511. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.; Lee, J.; Yhee, J.Y.; Yoon, H.I.; Park, S.J.; Koo, H.; Moon, S.H.; Lee, H.; Cho, Y.W.; et al. Non-invasive stem cell tracking in hindlimb ischemia animal model using bio-orthogonal copper-free click chemistry. Biochem. Biophys. Res. Commun. 2016, 479, 779–786. [Google Scholar] [CrossRef]
- Kellman, B.P.; Lewis, N.E. Big-Data Glycomics: Tools to Connect Glycan Biosynthesis to Extracellular Communication. Trends Biochem. Sci. 2020. [Google Scholar] [CrossRef]
- Smith, B.A.H.; Bertozzi, C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021, 1–27. [Google Scholar] [CrossRef]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Nischan, N.; Kohler, J.J. Advances in cell surface glycoengineering reveal biological function. Glycobiology 2016, 26, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Fan, X.; Shi, Y.; Zhang, C.; Sun, D.E.; Qin, K.; Qin, W.; Zhou, W.; Chen, X. Next-generation unnatural monosaccharides reveal that ESRRB O-GlcNAcylation regulates pluripotency of mouse embryonic stem cells. Nat. Commun. 2019, 10, 4065. [Google Scholar] [CrossRef]
- Pecori, F.; Yokota, I.; Hanamatsu, H.; Miura, T.; Ogura, C.; Ota, H.; Furukawa, J.I.; Oki, S.; Yamamoto, K.; Yoshie, O.; et al. A defined glycosylation regulatory network modulates total glycome dynamics during pluripotency state transition. Sci. Rep. 2021, 11, 1276. [Google Scholar] [CrossRef]
- Ragni, E.; Lommel, M.; Moro, M.; Crosti, M.; Lavazza, C.; Parazzi, V.; Saredi, S.; Strahl, S.; Lazzari, L. Protein O-mannosylation is crucial for human mesencyhmal stem cells fate. Cell. Mol. Life Sci. 2016, 73, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.M.; Jagger, A.M.; Walker, M.; Seinkmane, E.; Fox, J.M.; Kröger, R.; Genever, P.; Ungar, D. Glycans modify mesenchymal stem cell differentiation to impact on the function of resulting osteoblasts. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Zhang, C.; Kenry; Liu, J.; Wang, X.; Li, B.; Yan, H.; Hu, F.; Kong, D.; Wang, Z.; et al. Bio-orthogonal click reaction-enabled highly specific in situ cellularization of tissue engineering scaffolds. Biomaterials 2020, 230, 119615. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Yang, S.; Choi, S.; Sabean, M.; Roberts, E.A. In vitro assessment of copper-induced toxicity in the human hepatoma line, Hep G2. Toxicol. In Vitro 2004, 18, 501–509. [Google Scholar] [CrossRef]
- Bai, K.; Wang, W. Shear stress-induced redistribution of the glycocalyx on endothelial cells in vitro. Biomech. Model. Mechanobiol. 2014, 13, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Seyrek, K.; Richter, M.; Lavrik, I.N. Decoding the sweet regulation of apoptosis: The role of glycosylation and galectins in apoptotic signaling pathways. Cell Death Differ. 2019, 26, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Sampathkumar, S.G.; Jones, M.B.; Rhee, J.K.; Baskaran, G.; Goon, S.; Yarema, K.J. Characterization of the metabolic flux and apoptotic effects of O-hydroxyl- and N-acyl-modified N-acetylmannosamine analogs in Jurkat cells. J. Biol. Chem. 2004, 279, 18342–18352. [Google Scholar] [CrossRef] [PubMed]
- Besanceney-Webler, C.; Jiang, H.; Zheng, T.; Feng, L.; Soriano del Amo, D.; Wang, W.; Klivansky, L.M.; Marlow, F.L.; Liu, Y.; Wu, P. Increasing the efficacy of bioorthogonal click reactions for bioconjugation: A comparative study. Angew. Chem. Int. Ed. Engl. 2011, 50, 8051–8056. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, M.; Memmel, E.; Braun, A.C.; Seibel, J.; Meinel, L.; Luhmann, T. Biocompatible Azide-Alkyne "Click" Reactions for Surface Decoration of Glyco-Engineered Cells. Chembiochem 2016, 17, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Shim, H.E.; Park, S.J.; Kim, B.C.; Lee, D.E.; Chung, H.M.; Moon, S.H.; Kang, S.W. Safety and Optimization of Metabolic Labeling of Endothelial Progenitor Cells for Tracking. Sci. Rep. 2018, 8, 13212. [Google Scholar] [CrossRef]
- Chen, S.C.; Huang, C.H.; Lai, S.J.; Yang, C.S.; Hsiao, T.H.; Lin, C.H.; Fu, P.K.; Ko, T.P.; Chen, Y. Mechanism and inhibition of human UDP-GlcNAc 2-epimerase, the key enzyme in sialic acid biosynthesis. Sci. Rep. 2016, 6, 23274. [Google Scholar] [CrossRef]
- Homann, A.; Qamar, R.U.; Serim, S.; Dersch, P.; Seibel, J. Bioorthogonal metabolic glycoengineering of human larynx carcinoma (HEp-2) cells targeting sialic acid. Beilstein J. Org. Chem. 2010, 6, 24. [Google Scholar] [CrossRef]
- Letschert, S.; Gohler, A.; Franke, C.; Bertleff-Zieschang, N.; Memmel, E.; Doose, S.; Seibel, J.; Sauer, M. Super-resolution imaging of plasma membrane glycans. Angew. Chem. Int. Ed. Engl. 2014, 53, 10921–10924. [Google Scholar] [CrossRef]
- Dold, J.; Wittmann, V. Metabolic Glycoengineering with Azide- and Alkene-Modified Hexosamines: Quantification of Sialic Acid Levels. Chembiochem 2020. [Google Scholar] [CrossRef]
- Almaraz, R.T.; Aich, U.; Khanna, H.S.; Tan, E.; Bhattacharya, R.; Shah, S.; Yarema, K.J. Metabolic oligosaccharide engineering with N-Acyl functionalized ManNAc analogs: Cytotoxicity, metabolic flux, and glycan-display considerations. Biotechnol. Bioeng. 2012, 109, 992–1006. [Google Scholar] [CrossRef]
- Chang, P.V.; Chen, X.; Smyrniotis, C.; Xenakis, A.; Hu, T.; Bertozzi, C.R.; Wu, P. Metabolic labeling of sialic acids in living animals with alkynyl sugars. Angew. Chem. Int. Ed. Engl. 2009, 48, 4030–4033. [Google Scholar] [CrossRef]
- Wang, G.; Kostidis, S.; Tiemeier, G.L.; Sol, W.; de Vries, M.R.; Giera, M.; Carmeliet, P.; van den Berg, B.M.; Rabelink, T.J. Shear Stress Regulation of Endothelial Glycocalyx Structure Is Determined by Glucobiosynthesis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Wright, M.H.; Sieber, S.A. Making a Long Journey Short: Alkyne Functionalization of Natural Product Scaffolds. Chemistry 2016, 22, 4666–4678. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.M.; Haack-Sorensen, M.; Burns, J.S.; Elsnab, B.; Jakob, F.; Hokland, P.; Kassem, M. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochem. Biophys. Res. Commun. 2005, 326, 527–538. [Google Scholar] [CrossRef]
- Simonsen, J.L.; Rosada, C.; Serakinci, N.; Justesen, J.; Stenderup, K.; Rattan, S.I.; Jensen, T.G.; Kassem, M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 2002, 20, 592–596. [Google Scholar] [CrossRef]
- Fernandez-Rebollo, E.; Mentrup, B.; Ebert, R.; Franzen, J.; Abagnale, G.; Sieben, T.; Ostrowska, A.; Hoffmann, P.; Roux, P.F.; Rath, B.; et al. Human Platelet Lysate versus Fetal Calf Serum: These Supplements Do Not Select for Different Mesenchymal Stromal Cells. Sci. Rep. 2017, 7, 5132. [Google Scholar] [CrossRef] [PubMed]
- Ebert, R.; Ulmer, M.; Zeck, S.; Meissner-Weigl, J.; Schneider, D.; Stopper, H.; Schupp, N.; Kassem, M.; Jakob, F. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells 2006, 24, 1226–1235. [Google Scholar] [CrossRef]
- Bloemendal, V.; Moons, S.J.; Heming, J.J.A.; Chayoua, M.; Niesink, O.; van Hest, J.C.M.; Boltje, T.J.; Rutjes, F. Chemoenzymatic Synthesis of Sialic Acid Derivatives Using Immobilized N-Acetylneuraminate Lyase in a Continuous Flow Reactor. Adv. Synth. Catal. 2019, 361, 2443–2447. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Wolf, N.; Kersting, L.; Herok, C.; Mihm, C.; Seibel, J. High-Yielding Water-Soluble Asymmetric Cyanine Dyes for Labeling Applications. J. Org. Chem. 2020, 85, 9751–9760. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Aigouy, B.; Mirouse, V. ScientiFig: A tool to build publication-ready scientific figures. Nat. Methods 2013, 10, 1048. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altmann, S.; Mut, J.; Wolf, N.; Meißner-Weigl, J.; Rudert, M.; Jakob, F.; Gutmann, M.; Lühmann, T.; Seibel, J.; Ebert, R. Metabolic Glycoengineering in hMSC-TERT as a Model for Skeletal Precursors by Using Modified Azide/Alkyne Monosaccharides. Int. J. Mol. Sci. 2021, 22, 2820. https://doi.org/10.3390/ijms22062820
Altmann S, Mut J, Wolf N, Meißner-Weigl J, Rudert M, Jakob F, Gutmann M, Lühmann T, Seibel J, Ebert R. Metabolic Glycoengineering in hMSC-TERT as a Model for Skeletal Precursors by Using Modified Azide/Alkyne Monosaccharides. International Journal of Molecular Sciences. 2021; 22(6):2820. https://doi.org/10.3390/ijms22062820
Chicago/Turabian StyleAltmann, Stephan, Jürgen Mut, Natalia Wolf, Jutta Meißner-Weigl, Maximilian Rudert, Franz Jakob, Marcus Gutmann, Tessa Lühmann, Jürgen Seibel, and Regina Ebert. 2021. "Metabolic Glycoengineering in hMSC-TERT as a Model for Skeletal Precursors by Using Modified Azide/Alkyne Monosaccharides" International Journal of Molecular Sciences 22, no. 6: 2820. https://doi.org/10.3390/ijms22062820
APA StyleAltmann, S., Mut, J., Wolf, N., Meißner-Weigl, J., Rudert, M., Jakob, F., Gutmann, M., Lühmann, T., Seibel, J., & Ebert, R. (2021). Metabolic Glycoengineering in hMSC-TERT as a Model for Skeletal Precursors by Using Modified Azide/Alkyne Monosaccharides. International Journal of Molecular Sciences, 22(6), 2820. https://doi.org/10.3390/ijms22062820







