IgGs-Abzymes from the Sera of Patients with Multiple Sclerosis Recognize and Hydrolyze miRNAs
Abstract
1. Introduction
2. Results
2.1. Characteristic of Patients
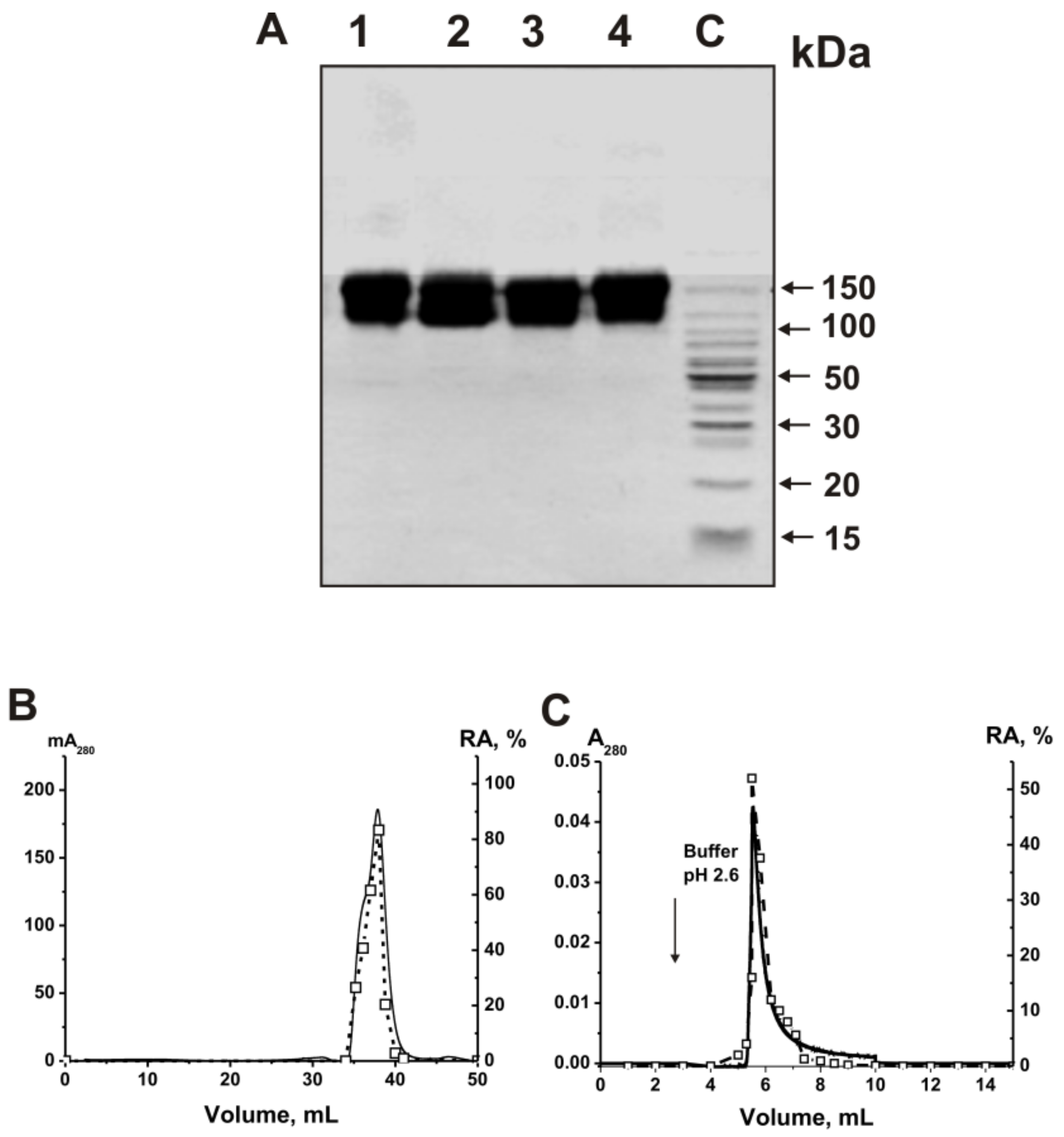
2.2. Purification and Characterizing of IgGs
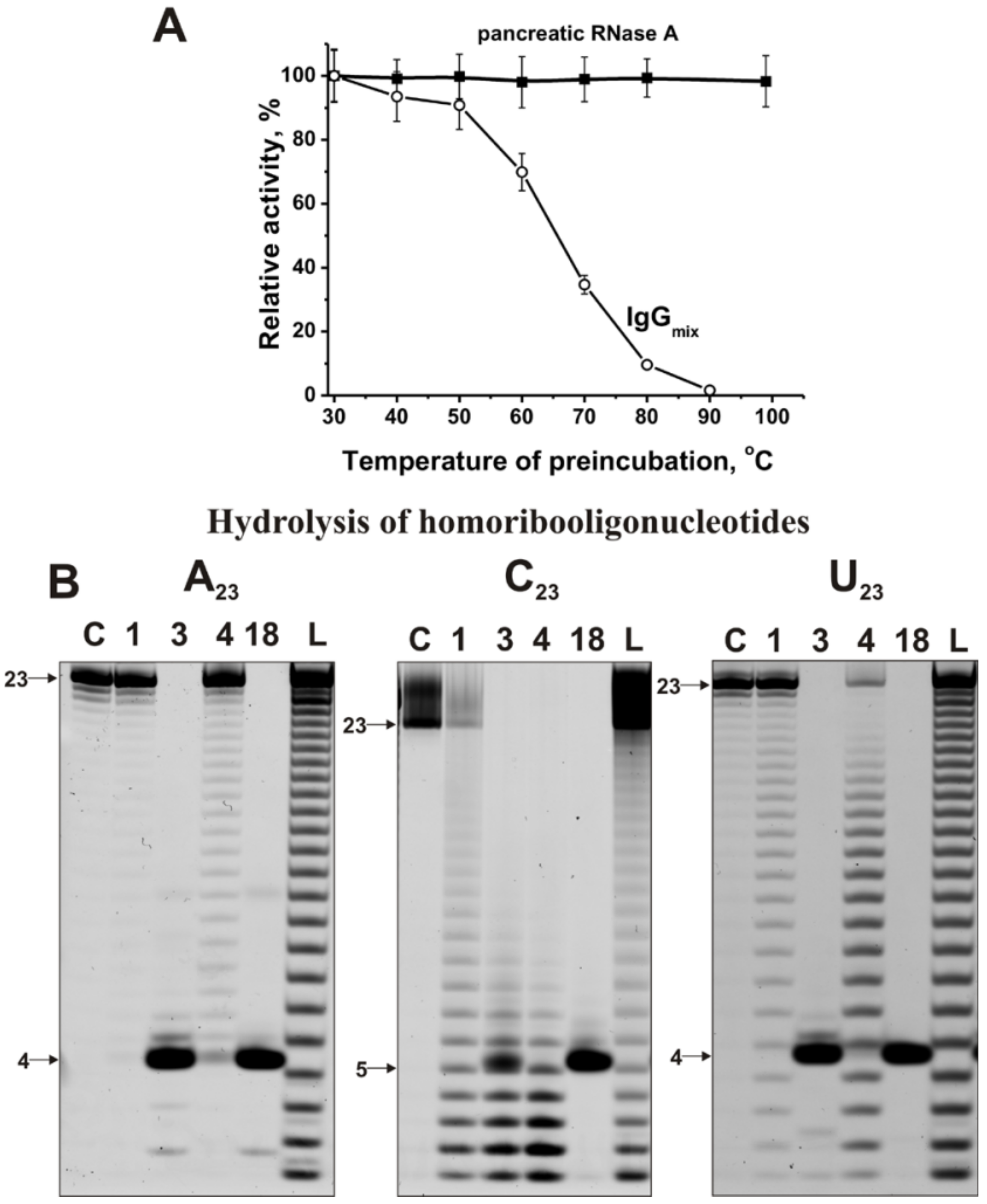
2.3. Application of Strict Criteria
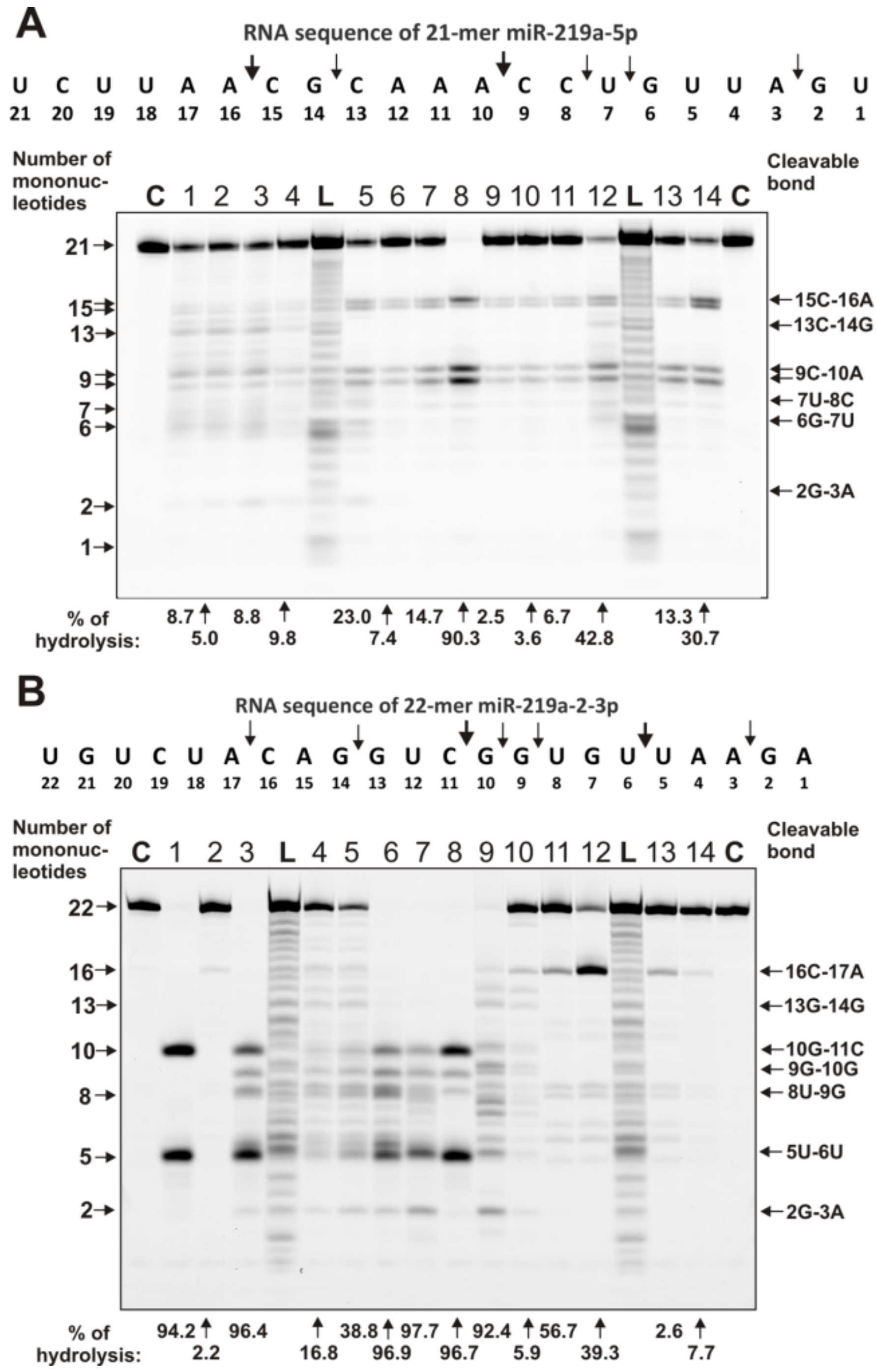
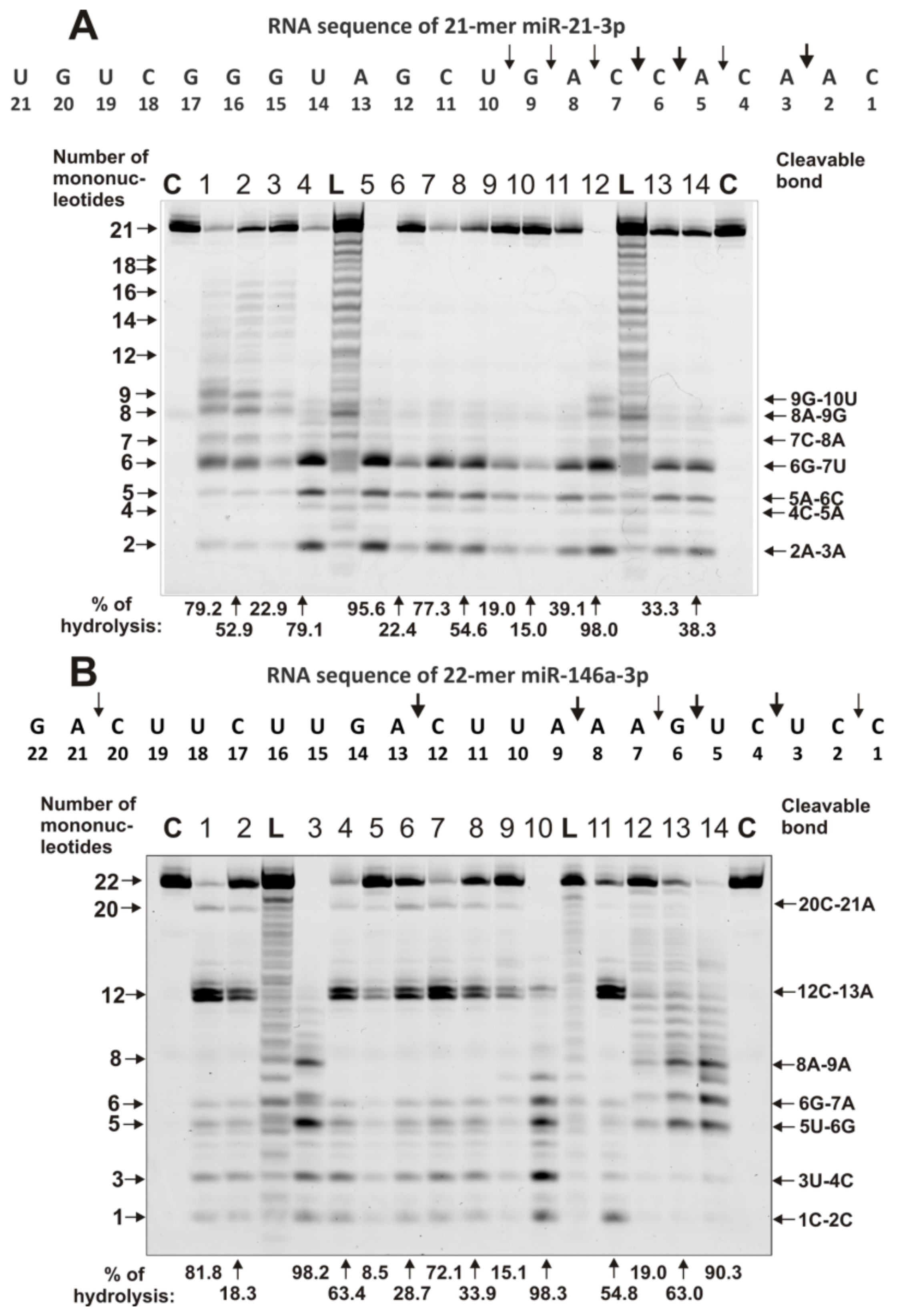
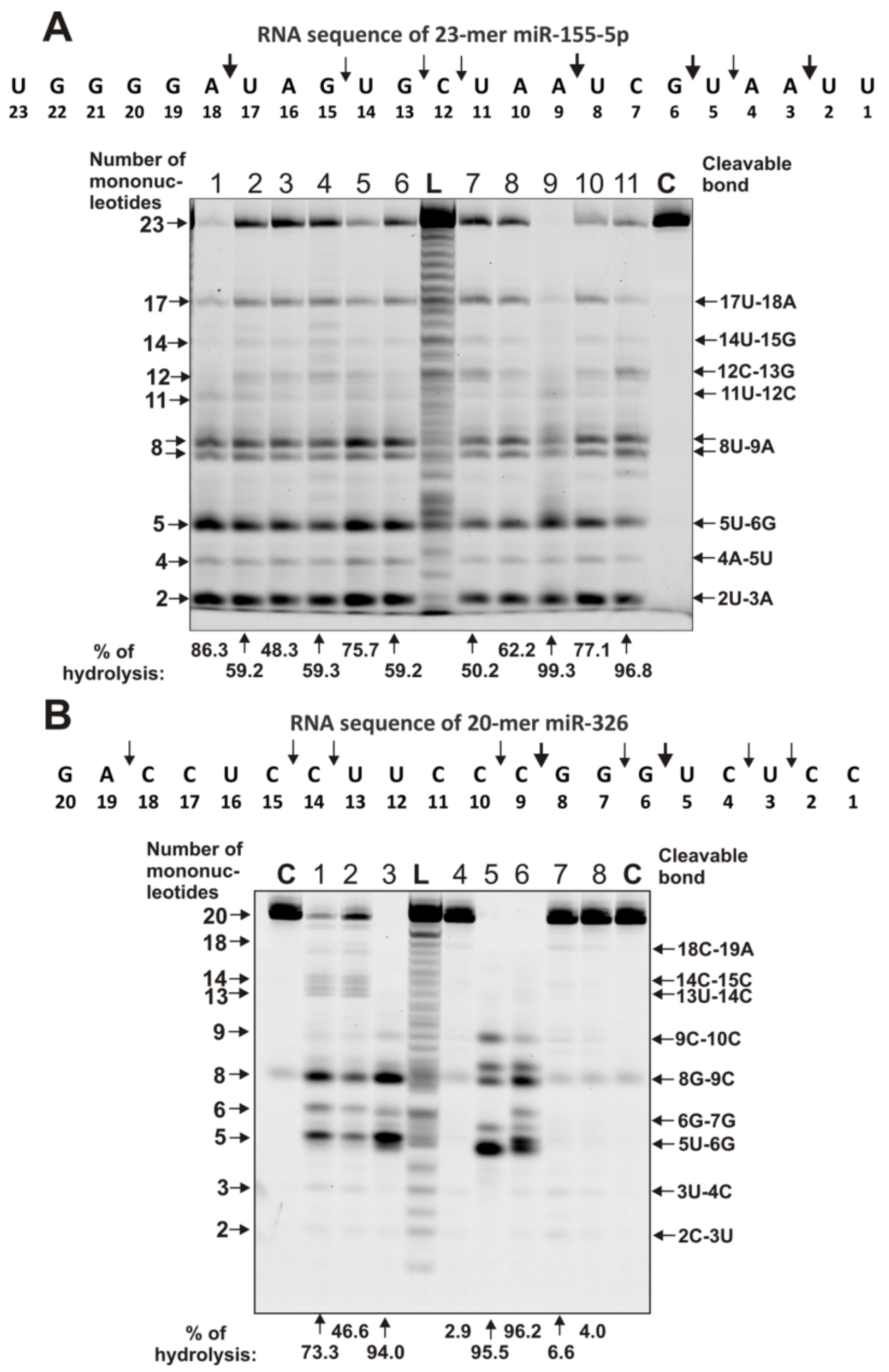
2.4. Estimation of Relative RNase Activity
2.5. Comparison of Relative Activities in the Hydrolysis of RNAs with Abzymes from the Blood of Different Patients
2.6. Correlation Coefficients
2.7. The Activity of Antibodies of Patients with a Different Course of the Disease
2.8. The Activity of Antibodies of Patients Treated with Different Drugs
3. Discussion
4. Materials and methods
4.1. Chemicals, Donors, and Patients
4.2. IgG Purification
4.3. Analysis of Homo-Oligonucleotides and miRNA Hydrolysis by IgGs
- miR-137 (5’-Flu-UUAUUGCUUAAGAAUACGCGUAG),
- miR-9-5p (5’-Flu-UCUUUGGUUAUCUAGCUGUAUGA),
- miR-219-2-3p (5’-Flu-AGAAUUGUGGCUGGACAUCUGU), and
- miR-219-5p (5’-Flu- UGAUUGUCCAAACGCAAUUCU)
- miR-21-3p (5′- Flu-CAACACCAGUCGAUGGGCUGU),
- miR-146a-3p (5′-Flu-CCUCUGAAAUUCAGUUCUUCAG),
- miR-155-5p (5′- Flu-UUAAUGCUAAUCGUGAUAGGGGU), and
- miR-326 (5′- Flu-CCUCUGGGCCCUUCCUCCAG).
4.4. Application of Strict Criteria
4.5. Determination of the Kinetic Parameters
4.6. Statistical Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Eschenko, N.D. (Ed.) Biochemistry of Psychiatric and Neurological Diseases. Selected Sections; Publishing House of St Petersburg State University: St Petersburg, Russia, 2004; pp. 1–200. [Google Scholar]
- Jenkins, T.A.; Harte, M.K.; Stenson, G.; Reynolds, G.P. Neonatal lipopolysaccharide induces pathological changes in parvalbumin immunoreactivity in the hippocampus of the rat. Behav. Brain Res. 2009, 205, 355–359. [Google Scholar] [CrossRef]
- O’Connor, K.C.; Bar-Or, A.; Hafler, D.A. The neuroimmunology of multiple sclerosis: Possible roles of T and B lymphocytes in immunopathogenesis. J. Clin. Immunol. 2001, 21, 81–92. [Google Scholar] [CrossRef]
- Libbey, J.E.; Cusick, M.F.; Fujinami, R.S. Role of pathogens in multiple sclerosis. Int. Rev. Immunol. 2014, 33, 266–283. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.; Lygner, P.E.; Bergstrom, T. Viral infections trigger multiple sclerosis relapses: A prospective seroepidemiological study. J. Neurol. 1993, 240, 417–422. [Google Scholar] [CrossRef]
- Keinan, E. (Ed.) Catalytic Antibodies; Wiley-VCH Verlag GmbH and Co. KgaA: Weinheim, Germany, 2005; pp. 1–586. [Google Scholar]
- Nevinsky, G.A.; Buneva, V.N. Natural catalytic antibodies–abzymes. In Catalytic Antibodies; Keinan, E., Ed.; VCH-Wiley Press: Weinheim, Germany, 2005; pp. 505–569. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in autoimmune diseases. In Autoimmune Diseases: Symptoms, Diagnosis and Treatment; Brenner, K.J., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2010; pp. 1–107. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in HIV-infected patients. In Understanding HIV/AIDS Management and Care—Pandemic Approaches the 21st Century; Kasenga, F.H., Ed.; InTech: Rijeka, Croatia, 2011; pp. 151–192. [Google Scholar]
- Nevinsky, G.A. Autoimmune processes in multiple sclerosis: Production of harmful catalytic antibodies associated with significant changes in the hematopoietic stem cell differentiation and proliferation. In Multiple Sclerosis; Conzalez-Quevedo, A., Ed.; InTech: Rijeka, Croatia, 2016; pp. 100–147. [Google Scholar]
- Nevinsky, G.A. Catalytic antibodies in norm and systemic lupus erythematosus. In Lupus; Khan, W.A., Ed.; InTech: Rijeka, Croatia, 2017; pp. 41–101. [Google Scholar]
- Planque, S.A.; Nishiyama, Y.; Hara, M.; Sonoda, S.; Murphy, S.K.; Watanabe, K.; Mitsuda, Y.; Brown, E.L.; Massey, R.J.; Primmer, S.R.; et al. Physiological IgM class catalytic antibodies selective for transthyretin amyloid. J. Biol. Chem. 2014, 289, 13243–13258. [Google Scholar] [CrossRef] [PubMed]
- Polosukhina, D.I.; Kanyshkova, T.G.; Doronin, B.M.; Tyshkevich, O.B.; Buneva, V.N.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Hydrolysis of myelin basic protein by polyclonal catalytic IgGs from the sera of patients with multiple sclerosis. J. Cell Mol. Med. 2004, 8, 359–368. [Google Scholar] [CrossRef]
- Polosukhina, D.I.; Kanyshkova, T.G.; Doronin, B.M.; Tyshkevich, O.B.; Buneva, V.N.; Boiko, A.N.; Gusev, E.I.; Nevinsky, G.A.; Favorova, O.O. Metal-dependent hydrolysis of myelin basic protein by IgGs from the sera of patients with multiple sclerosis. Immunol. Lett. 2006, 103, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Legostaeva, G.A.; Polosukhina, D.I.; Bezuglova, A.M.; Doronin, B.M.; Buneva, V.N.; Nevinsky, G.A. Affinity and catalytic heterogeneity of polyclonal myelin basic protein-hydrolyzing IgGs from sera of patients with multiple sclerosis. J. Cell Mol. Med. 2010, 14, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Bezuglova, A.V.; Konenkova, L.P.; Doronin, B.M.; Buneva, V.N.; Nevinsky, G.A. Affinity and catalytic heterogeneity and metal-dependence of polyclonal myelin basic protein-hydrolyzing IgGs from sera of patients with systemic lupus erythematosus. J. Mol. Recognit. 2011, 24, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Baranovskii, A.G.; Kanyshkova, T.G.; Mogelnitskii, A.S.; Naumov, V.A.; Buneva, V.N.; Gusev, E.I.; Boiko, A.N.; Zargarova, T.A.; Favorova, O.O.; Nevinsky, G.A. Polyclonal antibodies from blood and cerebrospinal fluid of patients with multiple sclerosis effectively hydrolyze DNA and RNA. Biochemistry (Moscow) 1998, 63, 1239–1248. [Google Scholar]
- Baranovskii, A.G.; Ershova, N.A.; Buneva, V.N.; Kanyshkova, T.G.; Mogelnitskii, A.S.; Doronin, B.M.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Catalytic heterogeneity of polyclonal DNA-hydrolyzing antibodies from the sera of patients with multiple sclerosis. Immunol. Lett. 2001, 76, 163–167. [Google Scholar] [CrossRef]
- Saveliev, A.N.; Ivanen, D.R.; Kulminskaya, A.A.; Ershova, N.A.; Kanyshkova, T.G.; Buneva, V.N.; Mogelnitskii, A.S.; Doronin, B.M.; Favorova, O.O.; Nevinsky, G.A.; et al. Amylolytic activity of IgM and IgG antibodies from patients with multiple sclerosis. Immunol. Lett. 2003, 86, 291–297. [Google Scholar] [CrossRef]
- Baranova, S.V.; Mikheeva, E.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies from the sera of multiple sclerosis patients efficiently hydrolyze five histones. Biomolecules 2019, 9, E741. [Google Scholar] [CrossRef] [PubMed]
- Tolmacheva, A.S.; Buneva, V.N.; Nevinsky, G.A. Substrate specificity of IgGs with peroxidase and oxidoreductase activities from sera of patients with systemic lupus erythematosus and multiple sclerosis. J. Mol. Recognit. 2019, 12, e2807. [Google Scholar] [CrossRef] [PubMed]
- Bezuglova, A.M.; Dmitrenok, P.S.; Konenkova, L.P.; Buneva, V.N.; Nevinsky, G.A. Multiple sites of the cleavage of 17- and 19-mer encephalytogenic oligopeptides corresponding to human myelin basic protein (MBP) by specific anti-MBP antibodies from patients with systemic lupus erythematosus. Peptides 2012, 37, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Bezuglova, A.M.; Konenkova, L.P.; Buneva, V.N.; Nevinsky, G.A. IgGs containing light chains of the λ- and κ- type and of all subclasses (IgG1-IgG4) from the sera of patients with systemic lupus erythematosus hydrolyze myelin basic protein. Int. Immunol. 2012, 24, 759–770. [Google Scholar] [CrossRef]
- Buneva, V.N.; Nevinsky, G.A. Exceptional Diversity of Catalytic Antibodies with Varying Activity in the Blood of Autoimmune and Viral Disease Patients. Mol. Biol. (Mosk) 2017, 51, 969–984. [Google Scholar] [CrossRef]
- Shuster, A.M.; Gololobov, G.V.; Kvashuk, O.A.; Bogomolova, A.E.; Smirnov, I.V.; Gabibov, A.G. DNA hydrolyzing autoantibodies. Science 1992, 256, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Kozyr, A.V.; Kolesnikov, A.V.; Aleksandrova, E.S.; Sashchenko, L.P.; Gnuchev, N.V.; Favorov, P.V.; Kotelnikov, M.A.; Iakhnina, E.I.; Astsaturov, I.A.; Prokaeva, T.B.; et al. Novel functional activities of anti-DNA autoantibodies from sera of patients with lymphoproliferative and autoimmune diseases. Appl. Biochem. Biotechnol. 1998, 75, 45–61. [Google Scholar] [CrossRef]
- Sinohara, H.; Matsuura, K. Does catalytic activity of Bence-Jones proteins contribute to the pathogenesis of multiple myeloma? Appl. Biochem. Biotechnol. 2000, 83, 85–94. [Google Scholar] [CrossRef]
- Nevinsky, G.A.; Buneva, V.N. Catalytic antibodies in healthy humans and patients with autoimmune and viral pathologies. J. Cell Mol. Med. 2003, 7, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Parkhomenko, T.A.; Doronin, V.B.; Castellazzi, M.; Padroni, M.; Pastore, M.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of DNA-hydrolyzing antibodies from the cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS ONE 2014, 9, e93001. [Google Scholar] [CrossRef]
- Doronin, V.B.; Parkhomenko, T.A.; Castellazzi, M.; Padroni, M.; Pastore, M.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of antibodies hydrolyzing myelin basic protein from the cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS ONE 2014, 9, e107807. [Google Scholar] [CrossRef] [PubMed]
- Doronin, V.B.; Parkhomenko, T.A.; Castellazzi, M.; Cesnik, E.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of Antibodies with Amylase Activity from Cerebrospinal Fluid and Serum of Patients with Multiple Sclerosis. PLoS ONE 2016, 11, e0154688. [Google Scholar] [CrossRef] [PubMed]
- Andrievskaya, O.A.; Buneva, V.N.; Naumov, V.A.; Nevinsky, G.A. Catalytic heterogenity of polyclonal RNA-hydrolyzing IgM from sera of patients with lupus erythematosus. Med. Sci. Monit. 2000, 6, 460–470. [Google Scholar] [PubMed]
- Andrievskaya, O.A.; Buneva, V.N.; Baranovskii, A.G.; Gal’vita, A.V.; Benzo, E.S.; Naumov, V.A.; Nevinsky, G.A. Catalytic diversity of polyclonal RNA-hydrolyzing IgG antibodies from the sera of patients with systemic lupus erythematosus. Immunol. Lett. 2002, 81, 191–198. [Google Scholar] [CrossRef]
- Boiko, A.N.; Favorova, O.O. Multiple sclerosis: Molecular and cellular mechanisms. Mol. Biol. 1995, 29, 727–749. [Google Scholar]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans Heterochronic Gene lin-4 Encodes Small RNAs with Antisense Complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Pasquinelli, A.E.; Reinhart, B.J.; Slack, F.; Martindale, M.Q.; Kuroda, M.I.; Maller, B.; Hayward, D.C.; Ball, E.E.; Degnan, B.; Müller, P.; et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 2000, 408, 86–88. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K.; Watkins, L.R.; Nelson, R.J.; Popovich, P.G. MicroRNAs: Roles in Regulating Neuroinflammation. Neuroscientist 2017, 24, 221–245. [Google Scholar] [CrossRef]
- Kacperska, M.; Walenczak, J.; Tomasik, B. Plasmatic microRNA as Potential Biomarkers of Multiple Sclerosis: Literature Review. Adv. Clin. Exp. Med. 2016, 25, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Baltimore, D. MicroRNAs as regulatory elements in immune system logic. Nat. Rev. Immunol. 2016, 16, 279–294. [Google Scholar] [CrossRef]
- Wang, H.; Peng, W.; Ouyang, X.; Li, W.; Dai, Y. Circulating microRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Transl. Res. 2012, 160, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Redis, R.S.; Calin, S.; Yang, Y.; You, M.J.; Calin, G.A. Cell-to-cell miRNA transfer: From body homeostasis to therapy. Pharmacol. Ther. 2012, 136, 169–174. [Google Scholar] [CrossRef]
- Keller, A.; Leidinger, P.; Bauer, A.; ElSharawy, A.; Haas, J.; Backes, C.; Wendschlag, A.; Giese, N.; Tjaden, C.; Ott, K.; et al. Toward the blood-borne miRNome of human diseases. Nat. Methods 2011, 8, 841–843. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Hydrolysis by catalytic IgGs of microRNA specific for patients with schizophrenia. IUBMB Life 2018, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, E.A.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Blood-derived RNA- and microRNA-hydrolyzing IgG antibodies in schizophrenia patients. Biochemistry (Mosc) 2018, 83, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, E.A.; Smirnova, L.P.; Parkhomenko, T.A.; Dmitrenok, P.S.; Krotenko, N.M.; Fattakhov, N.S.; Bokhan, N.A.; Semke, A.V.; Ivanova, S.A.; Buneva, V.N.; et al. DNA-hydrolyzing activity of IgG antibodies from the sera of patients with schizophrenia. Open Biol. 2015, 5, 150064. [Google Scholar] [CrossRef] [PubMed]
- Parshukova, D.; Sedykh, S.; Smirnova, L.; Buneva, V.; Ivanova, S.; Semke, A. Study of the level of IgG to myelin basic protein and their catalytic activity in schizophrenic patients. Eur. Neuropsychopharmacol. 2016, 26, S215–S216. [Google Scholar] [CrossRef]
- Parshukova, D.; Smirnova, L.P.; Ermakov, E.A.; Bokhan, N.A.; Semke, A.V.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Autoimmunity and immune system dysregulation in schizophrenia: IgGs from sera of patients hydrolyze myelin basic protein. J. Mol. Recognit. 2019, 32, e2759. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Lu, J.; Zhang, L.; Song, H.T.; Zhao, L.; Fan, H.M.; Zhong, A.F.; Niu, W.; Guo, Z.M.; Dai, Y.H.; et al. Aberrant microRNA expression in peripheral plasma and mononuclear cells as specific blood-based biomarkers in schizophrenia patients. J. Clin. Neurosci. 2015, 22, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Du, J.; Qi, Y.; Liang, G.; Wang, T.; Li, S.; Xie, S.; Zeshan, B.; Xiao, Z. Aberrant expression of serum miRNAs in schizophrenia. J. Psychiatr. Res. 2012, 46, 198–204. [Google Scholar] [CrossRef]
- Lai, C.Y.; Yu, S.L.; Hsieh, M.H.; Chen, C.H.; Chen, H.Y.; Wen, C.C.; Huang, Y.H.; Hsiao, P.C.; Hsiao, C.K.; Liu, C.M.; et al. MicroRNA expression aberration as potential peripheral blood biomarkers for schizophrenia. PLoS ONE 2011, 6, e21635. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Lee, S.Y.; Scarr, E.; Yu, Y.H.; Lin, Y.T.; Liu, C.M.; Hwang, T.J.; Hsieh, M.H.; Liu, C.C.; Chien, Y.L.; et al. Aberrant expression of microRNAs as biomarker for schizophrenia: From acute state to partial remission, and from peripheral blood to cortical tissue. Transl. Psychiatry 2016, 6, e717. [Google Scholar] [CrossRef]
- Perkins, D.O.; Jeffries, C.D.; Jarskog, L.F.; Thomson, J.M.; Woods, K.; Newman, M.A.; Parker, J.S.; Jin, J.; Hammond, S.M. MicroRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007, 8, R27. [Google Scholar] [CrossRef]
- Moreau, M.P.; Bruse, S.E.; David-Rus, R.; Buyske, S.; Brzustowicz, L.M. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol. Psychiatry 2011, 69, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Ripke, S.; O’Dushlaine, C.; Chambert, K.; Moran, J.L.; Kähler, A.K.; Akterin, S.; Bergen, S.E.; Collins, A.L.; Crowley, J.J.; Fromer, M.; et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat. Genet. 2013, 45, 1150–1159. [Google Scholar] [CrossRef]
- Ripke, S.; Sanders, A.R.; Kendler, K.S.; Levinson, D.F.; Sklar, P.; Holmans, P.A.; Lin, D.Y.; Duan, J.; Ophoff, R.A.; Andreassen, O.A.; et al. Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium (2011). Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011, 43, 969–976. [Google Scholar]
- Ma, X.; Zhou, J.; Zhong, Y.; Jiang, L.; Mu, P.; Li, Y.; Nagarkatti, P. Expression, regulation and function of microRNAs in multiple sclerosis. Int. J. Med. Sci. 2014, 11, 810. [Google Scholar] [CrossRef]
- Amoruso, A.; Blonda, M.; Gironi, M.; Grasso, R.; Di Francescantonio, V.; Scaroni, F.; Furlan, R.; Verderio, C.; Avolio, C. Immune and central nervous system-related miRNAs expression profiling in monocytes of multiple sclerosis patients. Sci. Rep. 2020, 10, 6125. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-San Martín, M.; Reverter, G.; Robles-Cedeño, R.; Buxò, M.; Ortega, F.J.; Gómez, I.; Tomàs-Roig, J.; Celarain, N.; Villar, L.M.; Perkal, H.; et al. Analysis of miRNA signatures in CSF identifies upregulation of miR-21 and miR-146a/b in patients with multiple sclerosis and active lesions. Neuroinflammation 2019, 16, 220. [Google Scholar] [CrossRef]
- Yin, J.; Lin, J.; Luo, X.; Chen, Y.; Li, Z.; Ma, G.; Li, K. MiR-137: A new player in schizophrenia. Int. J. Mol. Sci. 2014, 15, 3262–3271. [Google Scholar] [CrossRef]
- Olde Loohuis, N.F.; Ba, W.; Stoerchel, P.H.; Kos, A.; Jager, A.; Schratt, G.; Martens, G.J.; van Bokhoven, H.; Nadif Kasri, N.; Aschrafi, A. MicroRNA-137 Controls AMPA-Receptor-Mediated Transmission and mGluR-Dependent LTD. Cell Rep. 2015, 11, 1876–1884. [Google Scholar] [CrossRef]
- Siegert, S.; Seo, J.; Kwon, E.J.; Rudenko, A.; Cho, S.; Wang, W.; Flood, Z.; Martorell, A.J.; Ericsson, M.; Mungenast, A.E.; et al. The schizophrenia risk gene product miR-137 alters presynaptic plasticity. Nat. Neurosci. 2015, 18, 1008–1016. [Google Scholar] [CrossRef]
- Hauberg, M.E.; Holm-Nielsen, M.H.; Mattheisen, M.; Askou, A.L.; Grove, J.; Børglum, A.D.; Corydon, T.J. Schizophrenia risk variants affecting microRNA function and site-specific regulation of NT5C2 by miR-206. Eur. Neuropsychopharmacol. 2016, 26, 1522–1526. [Google Scholar] [CrossRef]
- Topol, A.; Zhu, S.; Hartley, B.J.; English, J.; Hauberg, M.E.; Tran, N.; Rittenhouse, C.A.; Simone, A.; Ruderfer, D.M.; Johnson, J.; et al. Dysregulation of miRNA-9 in a subset of schizophrenia patient-derived neural progenitor cells. Cell Rep. 2016, 15, 1024–1036. [Google Scholar] [CrossRef]
- Murai, K.; Sun, G.; Ye, P.; Tian, E.; Yang, S.; Cui, Q.; Sun, G.; Trinh, D.; Sun, O.; Hong, T.; et al. The TLX-miR-219 cascade regulates neural stem cell proliferation in neurodevelopment and schizophrenia iPSC model. Nat. Commun. 2016, 7, 10965. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.D.; Li, L.; Chan, W.Y. MicroRNAs: Key regulators in the central nervous system and their implication in neurological diseases. Int. J. Mol. Sci. 2016, 17, 842. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, E.A.; Kabirova, E.M.; Sizikov, A.E.; Buneva, V.N.; Nevinsky, G.A. IgGs-Abzymes from the Sera of Patients with Systemic Lupus Erythematosus Hydrolyzed miRNAs. J. Inflamm. Res. 2020, 13, 681–699. [Google Scholar] [CrossRef]
- Vlassov, A.; Florentz, C.; Helm, M.; Naumov, V.; Buneva, V.; Nevinsky, G.; Giegé, R. Characterization and selectivity of catalytic antibodies from human serum with RNase activity. Nucl. Acid Res. 1998, 26, 5243–5250. [Google Scholar] [CrossRef]
- Vlasov, A.V.; Baranovskii, A.G.; Kanyshkova, T.G.; Prints, A.V.; Zabara, V.G.; Naumov, V.A.; Breusov, A.A.; Giege, R.; Buneva, V.; Nevinskii, G.A. Substrate specificity of serum DNA- and RNA-hydrolyzing antibodies of patients with polyarthritis and autoimmune thyroiditis. Mol. Biol. 1998, 32, 559–569. [Google Scholar]
- Vlassov, A.V.; Helm, M.; Florentz, C.; Naumov, V.; Breusov, A.A.; Buneva, V.N.; Giege, R.; Nevinsky, G.A. Variability of substrate specificity of serum antibodies obtained from patients with different autoimmune and viral deseases in reaction of tRNA hydrolysis. Russ. J. Immunol. 1999, 4, 25–32. [Google Scholar]
- Vlasov, A.V.; Helm, M.; Naumov, V.A.; Breusov, A.A.; Buneva, V.N.; Florentz, C.; Giege, R.; Nevinskii, G.A. (Features of tRNA hydrolysis by autoantibodies from blood serum of patients with certain autoimmune and virus diseases. Mol. Biol. 1999, 33, 866–872. [Google Scholar]
- Paul, S.; Volle, D.J.; Beach, C.M.; Johnson, D.R.; Powell, M.J.; Massey, R.J. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science 1989, 244, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, F.J.; Palsson-McDermott, E.; Hennessy, E.J.; Martin, C.; O’leary, J.J.; Ruan, Q.; Johnson, D.S.; Chen, Y.; O’neill, L.A. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 2010, 11, 141–147. [Google Scholar] [CrossRef]
- Wang, H.; Moyano, A.L.; Ma, Z.; Deng, Y.; Lin, Y.; Zhao, C.; Zhang, L.; Jiang, M.; He, X.; Ma, Z.; et al. miR-219 cooperates with miR-338 in myelination and promotes myelin repair in the CNS. Dev. Cell 2017, 40, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Duffy, C.P.; McCoy, C.E. The Role of MicroRNAs in Repair Processes in Multiple Sclerosis. Cells 2020, 9, 1711. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.M.; Krüger, C.; Park, B.; Derkow, K.; Rosenberger, K.; Baumgart, J.; Trimbuch, T.; Eom, G.; Hinz, M.; Kaul, D.; et al. An unconventional role for miRNA: Let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012, 15, 827–835. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurological impairment in multiple sclerosis: An expanded disability scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Fersht, A. Enzyme Structure and Mechanism, 2nd ed.; W H Freeman Co.: New York, NY, USA, 1985. [Google Scholar]


| Groups of Patients and Healthy Donors | miR-137 | miR-9-5p | miR-219-2-3p | miR-219-5p | miR-21-3p | miR-146a-3p | miR-155-5p | miR-326 |
|---|---|---|---|---|---|---|---|---|
| Clinically isolated syndrome MS (CISMS, 8 patients) | ||||||||
| Mean ± SD for individual RNAs ** | 65.4 ± 27.2 | 40.7 ± 35.8 | 26.9 ± 30.2 | 25.6 ± 29.6 | 46.8 ± 28.6 | 50.6 ± 32.6 | 69.4 ± 17.3 | 33.6 ± 28.7 |
| Median (IQR) for individual RNAs *** | 64.9 (36.9) | 24.0 (62.0) | 12.5 (44.2) | 14.0 (31.6) | 38.7 (39.8) | 48.6 (53.4) | 68.5 (27.1) | 24.6 (27.9) |
| Relapsing-remitting MS (RRMS. 9 patients) | ||||||||
| Mean ± SD for individual RNAs | 33.3 ± 31.4 | 54.9 ± 39.7 | 55.3 ± 39.7 | 55.2 ± 32.2 | 59.8 ± 26.4 | 65.0 ± 33.4 | 83.0 ± 13.6 | 45.0 ± 33.4 |
| Median (IQR) for individual RNAs | 21.6 (11.9) | 41.2 (75.8) | 38.8 (76.7) | 67.8 (50.8) | 68.6 (38.7) | 63.0 (49.8) | 80.0 (22.8) | 36.9 (55.4) |
| Primary progressive MS (PPMS, 1 patient) | ||||||||
| Mean for individual RNAs | 50.4 | 98.1 | 96.4 | 93.2 | 98.7 | 89.6 | 97.9 | 94.0 |
| Secondary progressive MS (SPMS, 5 patients) | ||||||||
| Mean ± SD for individual RNAs | 39.8 ± 51.7 | 42.9 ± 50.2 | 43.7 ± 48.1 | 52.0 ± 44.9 | 45.1 ± 46.1 | 44.5 ± 47.5 | 49.2 ± 39.4 | 33.3 ± 31.4 |
| Median (IQR) for individual RNAs | 3.4 (91.9) | 12.2 (91.6) | 20.8 (90.4) | 50.8 (86.5) | 14.0 (81.6) | 14.7 (85.5) | 24.2 (66.1) | 6.6 (91.5) |
| Conditionally healthy donors (14 volunteers) | ||||||||
| Mean ± SD for individual RNAs | 1.2 ± 2.3 | 3.9 ± 2.7 | 1.7 ± 2.7 | 1.6 ± 2.4 | 1.5 ± 1.8 | 2.6 ± 3.0 | 2.8 ± 2.9 | 3.9 ± 3.3 |
| Median (IQR) for individual RNAs ** | 0 (2.3) | 4.2 (3.6) | 0 (4.6) | 0.4 (3.3) | 1.0 (3.0) | 2.5 (4.5) | 3.4 (6.5) | 0 (2.7) |
| Group MS Patients and Healthy Humans | Different microRNAs | |||||||
|---|---|---|---|---|---|---|---|---|
| miR-137 | miR-9-5p | miR-219-2-3p | miR-219-5p | miR-21-3p | miR-146a-3p | miR-155-5p | miR-326 | |
| Conventional Units of Relative Activity from 1 (Maximum Activity) to 8 (Minimum Activity)* | ||||||||
| CISMS, 8 patients | 2 | 5 | 7 | 8 | 4 | 3 | 1 | 6 |
| RRMS. 9 patients | 8 | 6 | 5 | 4 | 3 | 2 | 1 | 7 |
| PPMS. 1 patient | 8 | 2 | 4 | 6 | 1 | 7 | 3 | 5 |
| SPMS, 5 patients | 7 | 6 | 5 | 1 | 3 | 4 | 2 | 8 |
| Conditionally healthy donors (14 volunteers) | 8 | 1 | 5 | 6 | 7 | 4 | 3 | 2 |
| Average values | 6.6 | 4 | 5.2 | 5.0 | 3.6 | 4 | 2 | 5.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermakov, E.A.; Kabirova, E.M.; Buneva, V.N.; Nevinsky, G.A. IgGs-Abzymes from the Sera of Patients with Multiple Sclerosis Recognize and Hydrolyze miRNAs. Int. J. Mol. Sci. 2021, 22, 2812. https://doi.org/10.3390/ijms22062812
Ermakov EA, Kabirova EM, Buneva VN, Nevinsky GA. IgGs-Abzymes from the Sera of Patients with Multiple Sclerosis Recognize and Hydrolyze miRNAs. International Journal of Molecular Sciences. 2021; 22(6):2812. https://doi.org/10.3390/ijms22062812
Chicago/Turabian StyleErmakov, Evgeny A., Evelina M. Kabirova, Valentina N. Buneva, and Georgy A. Nevinsky. 2021. "IgGs-Abzymes from the Sera of Patients with Multiple Sclerosis Recognize and Hydrolyze miRNAs" International Journal of Molecular Sciences 22, no. 6: 2812. https://doi.org/10.3390/ijms22062812
APA StyleErmakov, E. A., Kabirova, E. M., Buneva, V. N., & Nevinsky, G. A. (2021). IgGs-Abzymes from the Sera of Patients with Multiple Sclerosis Recognize and Hydrolyze miRNAs. International Journal of Molecular Sciences, 22(6), 2812. https://doi.org/10.3390/ijms22062812







