Effect of Freshly Isolated Bone Marrow Mononuclear Cells and Cultured Bone Marrow Stromal Cells in Graft Cell Repopulation and Tendon-Bone Healing after Allograft Anterior Cruciate Ligament Reconstruction
Abstract
1. Introduction
2. Results
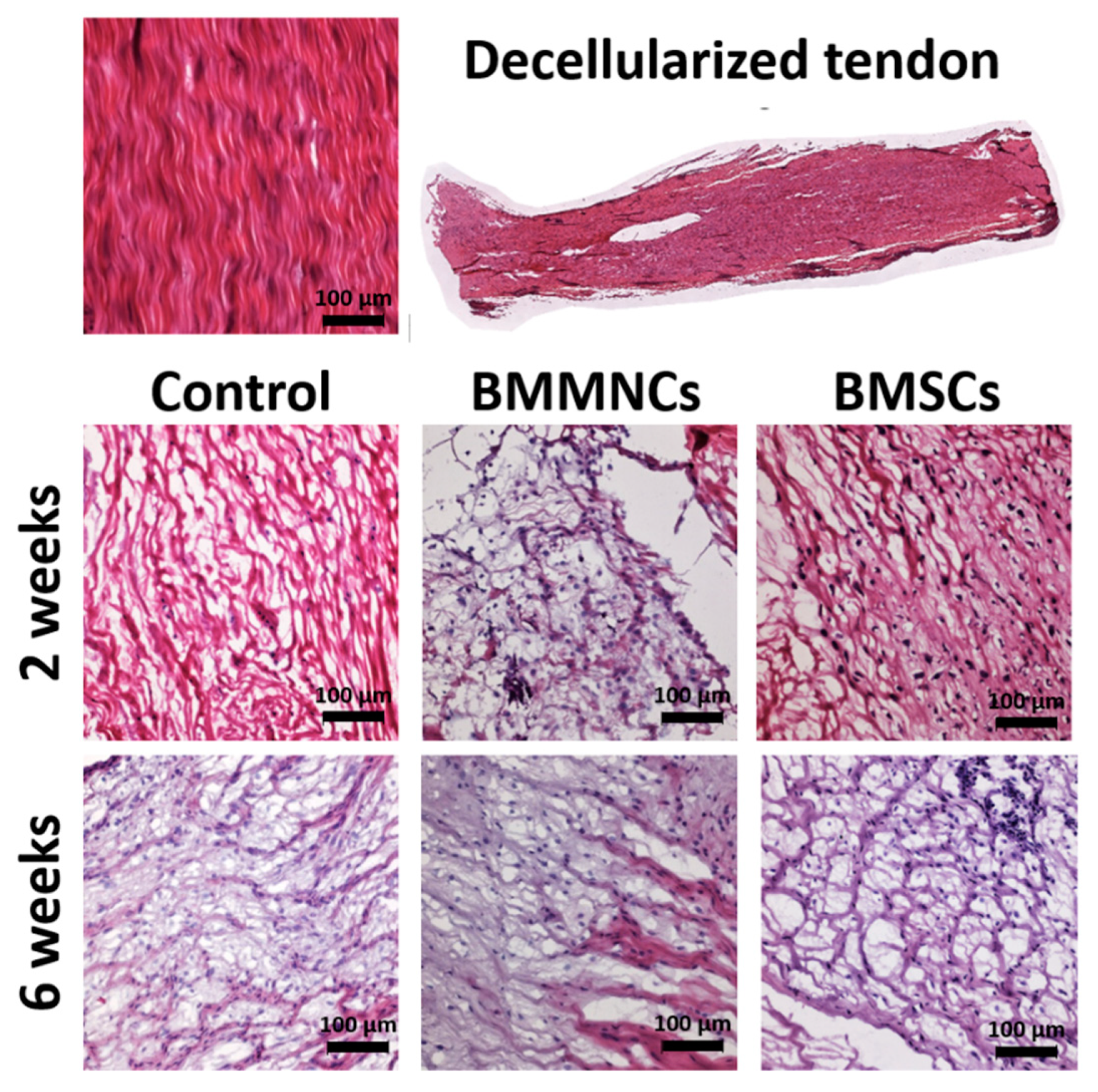
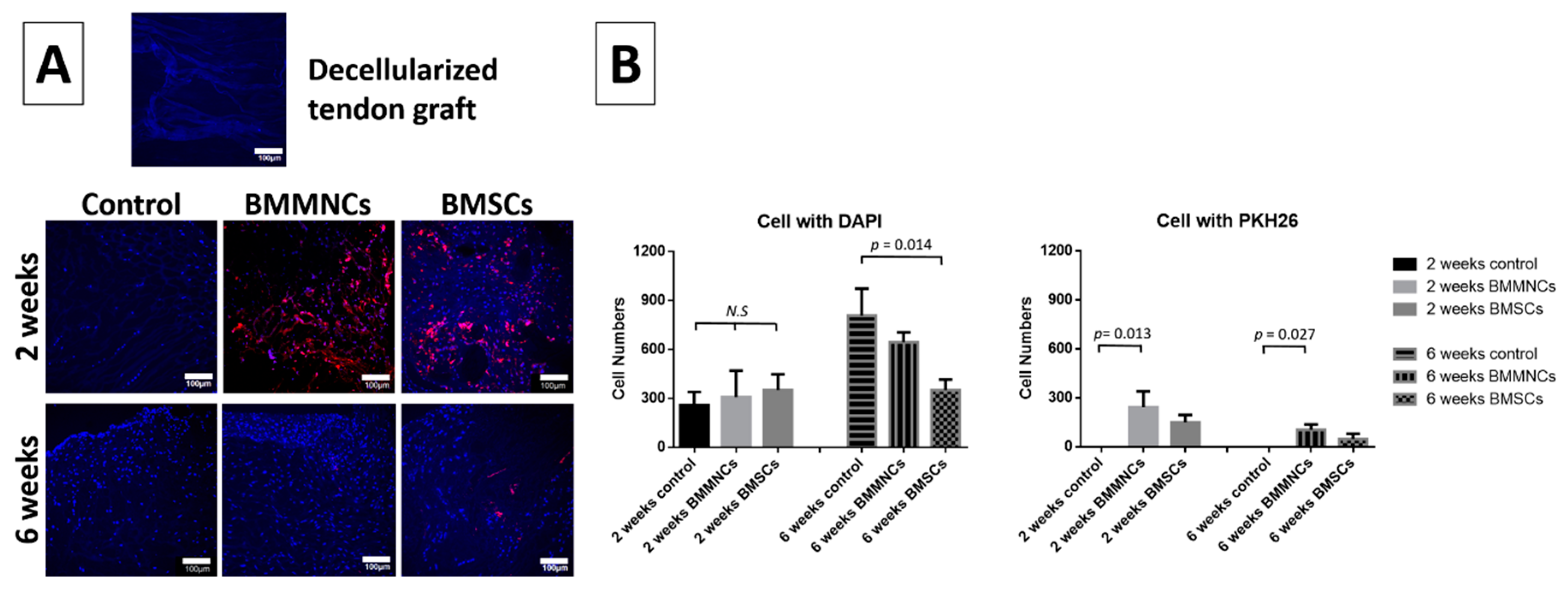
2.1. Analysis of Cell Repopulation in the Intra-Articular Graft
2.1.1. Cell Repopulation within Allograft
2.1.2. Higher Total Cell Population within Allograft in the Control Group Than in the Bone Marrow Cells-Seeded Groups
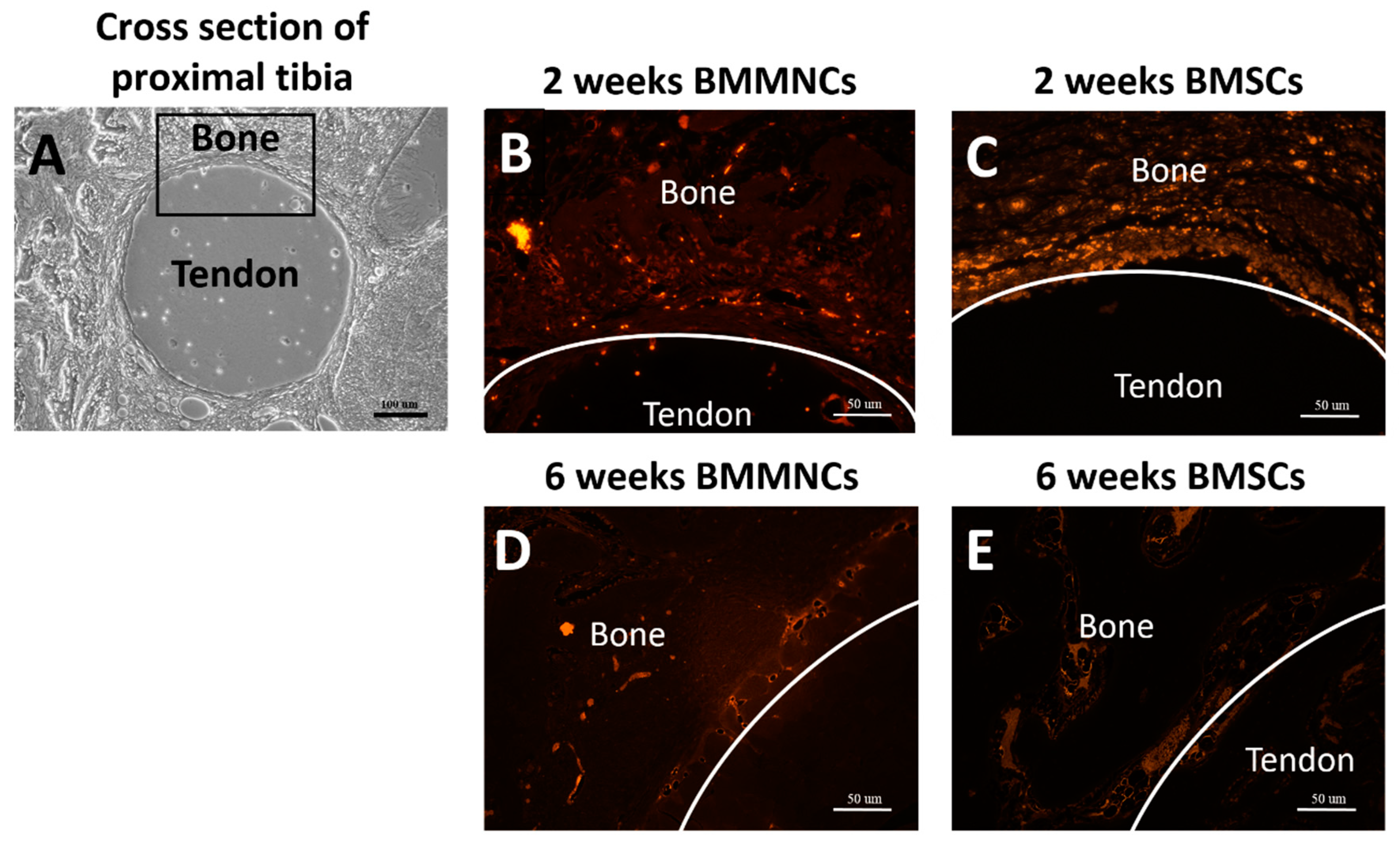
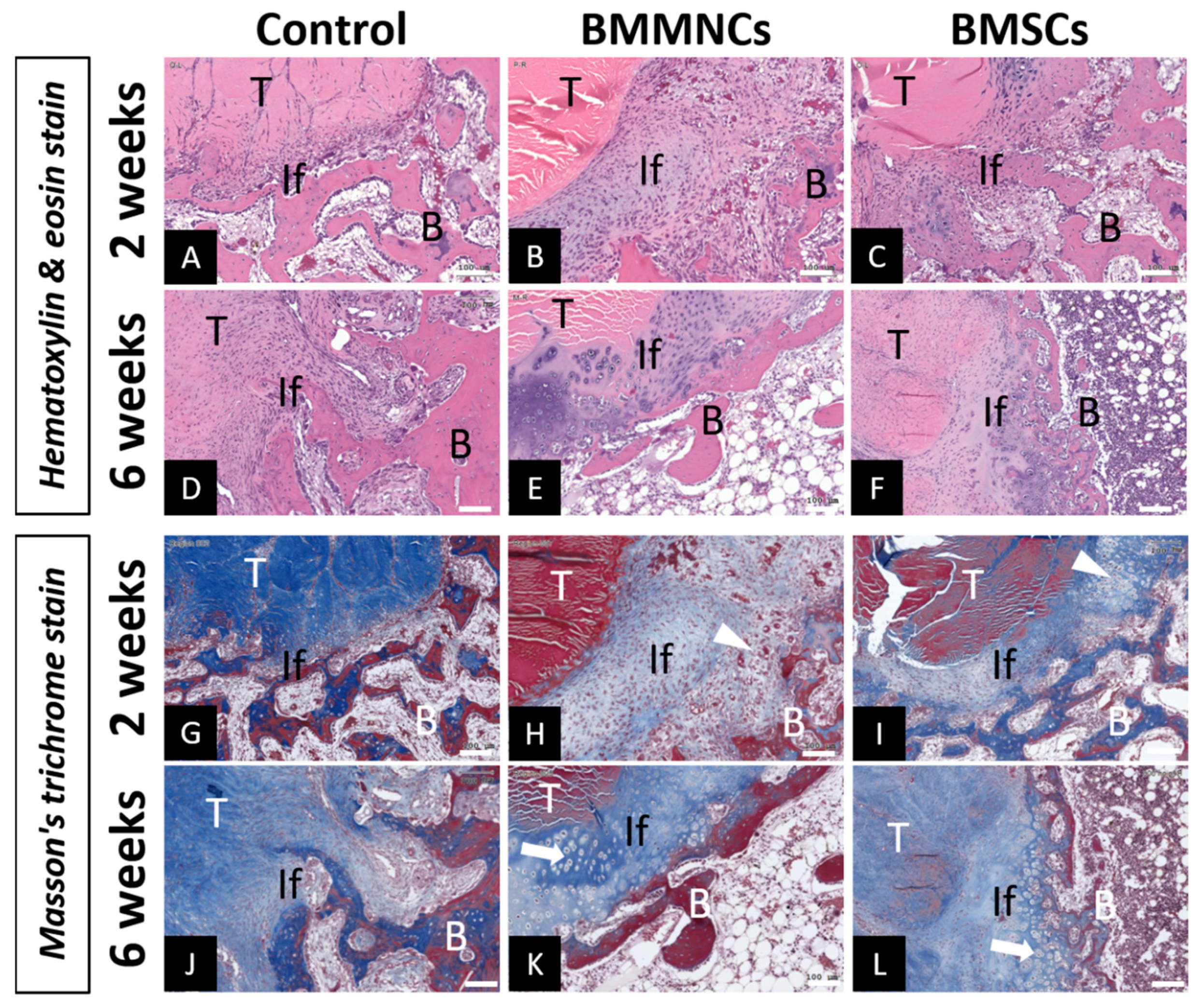
2.2. Analysis of Tendon-Bone Tunnel Interfacial Healing in the Proximal Tibia
2.2.1. The Presence of Intra-Articular-Injected Bone Marrow Cells in the Interface between the Tendon-Bone Tunnel
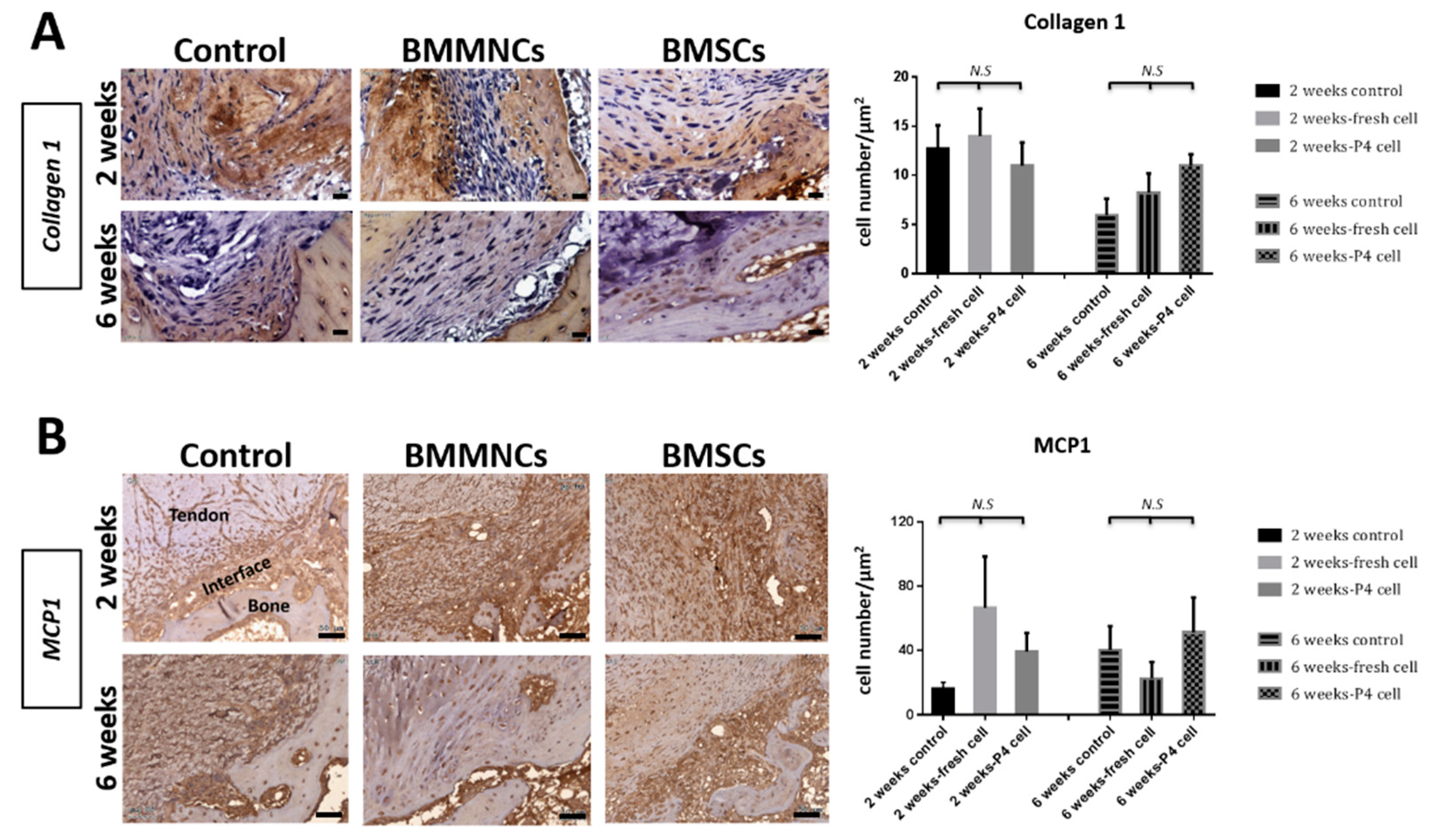
2.2.2. Interfacial Fibrocartilage Formation in the Tendon-Bone Tunnel in Bone Marrow Cells-Injected Groups
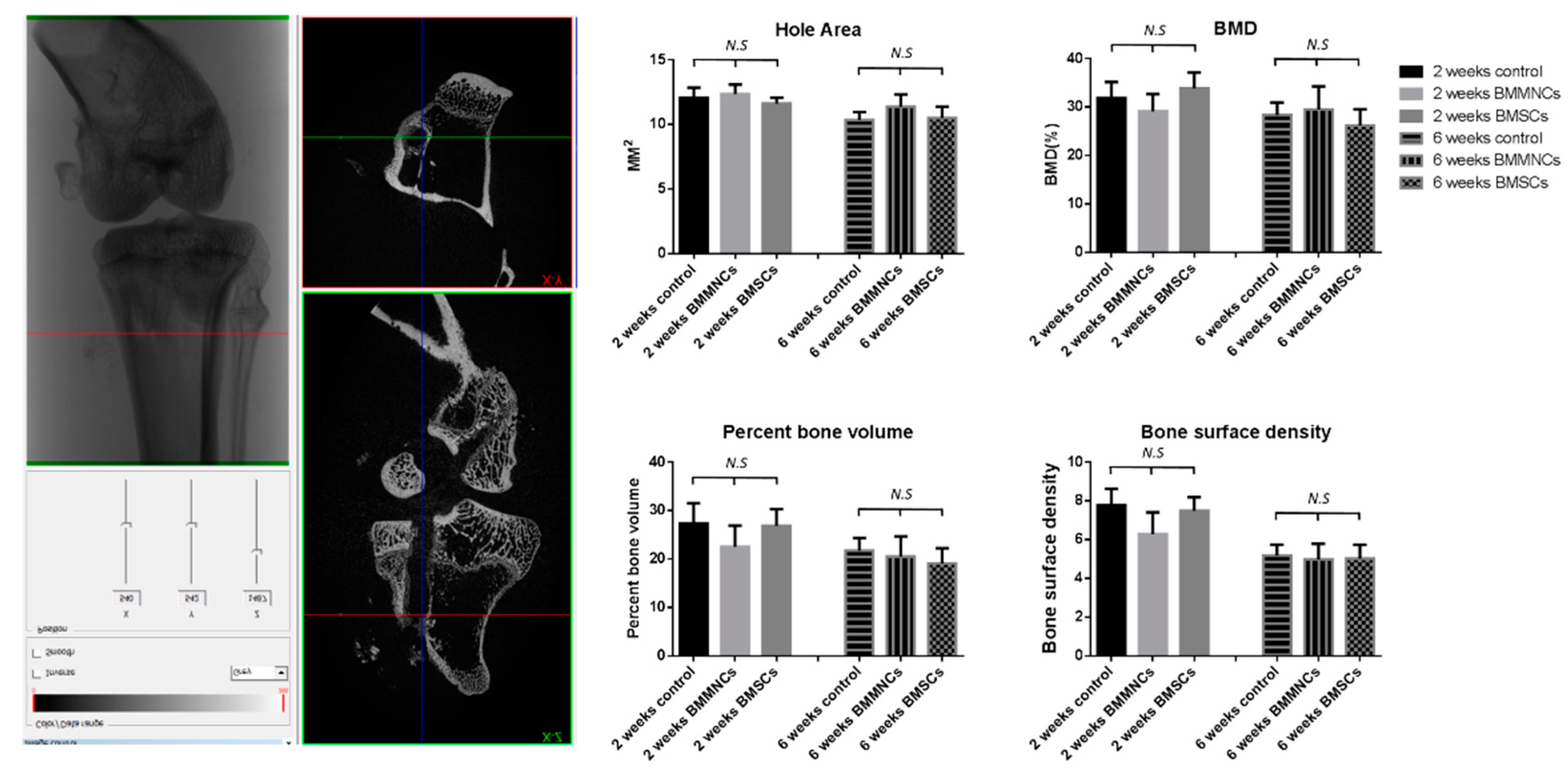
2.2.3. Micro-CT Revealed No Significant Differences between the Control and Bone Marrow Cells-Seeded Groups
2.2.4. Upregulated MCP1 Expression in the Bone Marrow Cells-Seeded Groups
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Harvesting Bone Marrow Stromal Cells
4.3. Preparation of Decellularized Tendon Graft
4.4. Harvesting Fresh Bone Marrow Cells
4.5. PKH26 Labelled Cells
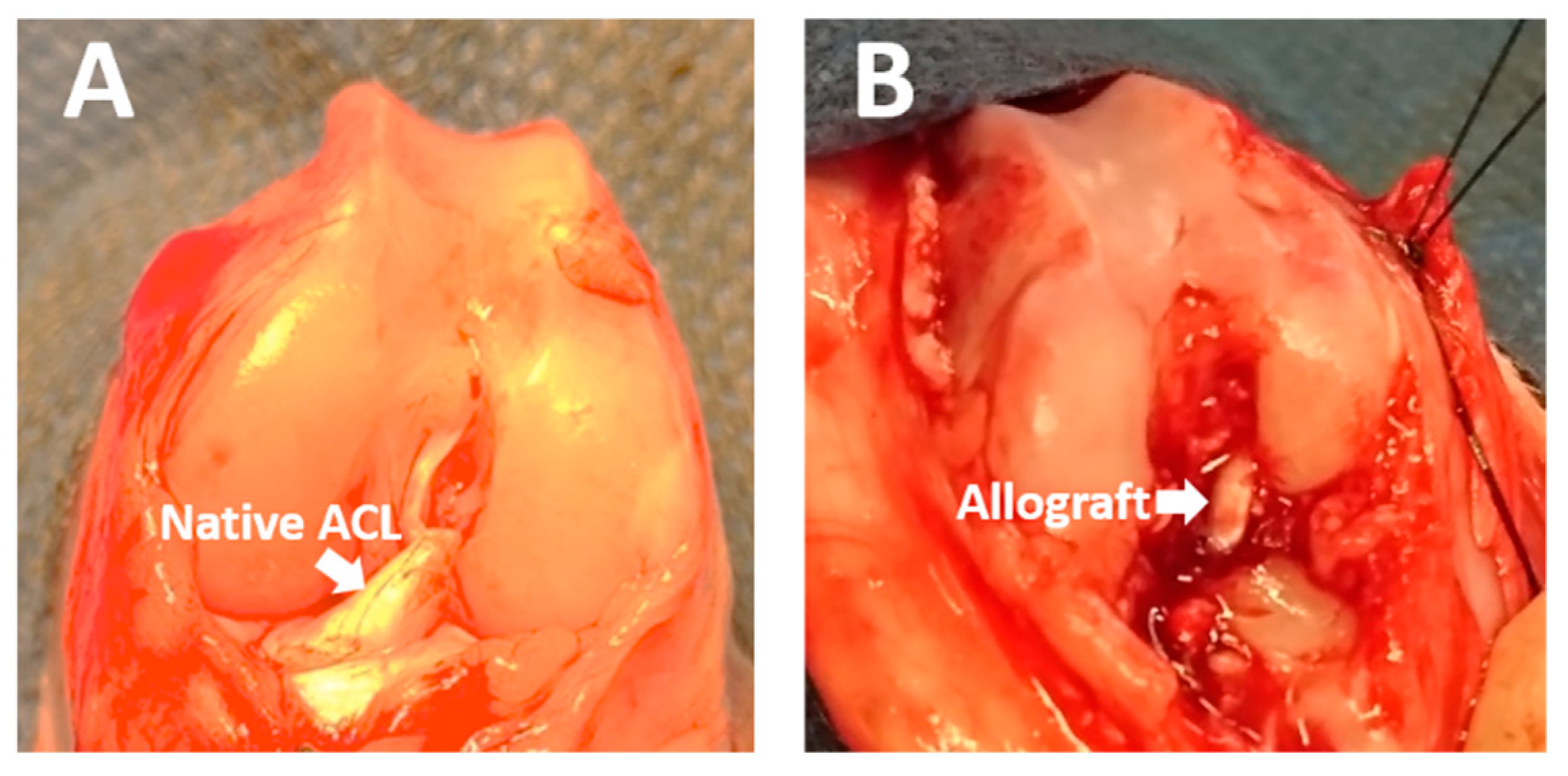
4.6. ACL Allograft Reconstruction
4.7. Analysis of Cell Repopulation in the Intra-Articular Graft
4.7.1. Histological Study
4.7.2. Total Cell Count
4.8. Analysis of Tendon-Bone Tunnel Interfacial Healing in the Proximal Tibia
4.8.1. Histological Study
4.8.2. Micro–Computed Tomography (Micro-CT)
4.8.3. Immunohistochemical Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janssen, R.P.; Scheffler, S.U. Intra-articular remodeling of hamstring tendon grafts after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2102–2108. [Google Scholar] [CrossRef]
- Sato, Y.; Akagi, R.; Akatsu, Y.; Matsuura, Y.; Takahashi, S.; Yamaguchi, S. The effect of femoral bone tunnel configuration on tendon-bone healing in an anterior cruciate ligament reconstruction: An animal study. Bone Joint Res. 2018, 7, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, H.; Yoshikawa, T.; Ju, Y.J.; Yasuda, K. Revascularization in the tendon graft following anterior cruciate ligament reconstruction of the knee: Its mechanisms and regulation. Chang. Gung. Med. J. 2009, 32, 133–139. [Google Scholar] [CrossRef]
- Hu, J.; Qu, J.; Xu, D.; Zhou, J.; Lu, H. Allograft versus autograft for anterior cruciate ligament reconstruction: An up-to-date meta-analysis of prospective studies. Int. Orthop. 2013, 37, 311–320. [Google Scholar] [CrossRef]
- Lamblin, C.J.; Waterman, B.R.; Lubowitz, J.H. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy 2013, 29, 1113–1122. [Google Scholar] [CrossRef]
- Tulloch, S.J.; Devitt, B.M.; Porter, T.; Hartwig, T.; Klemm, H.; Hookway, S.; Norsworthy, C.J. Primary ACL reconstruction using the LARS device is associated with a high failure rate at minimum of 6-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 3626–3632. [Google Scholar] [CrossRef]
- Tiefenboeck, T.M.; Thurmaier, E.; Tiefenboeck, M.M.; Ostermann, R.C.; Joestl, J.; Winnisch, M. Clinical and functional outcome after anterior cruciate ligament reconstruction using the LARS™ system at a minimum follow-up of 10 years. Knee 2015, 22, 565–568. [Google Scholar] [CrossRef]
- Granan, L.P.; Inacio, M.C.; Maletis, G.B.; Funahashi, T.T.; Engebretsen, L. Intraoperative findings and procedures in culturally and geographically different patient and surgeon populations: An anterior cruciate ligament reconstruction registry comparison between Norway and the USA. Acta Orthop. 2012, 83, 577–582. [Google Scholar] [CrossRef]
- Scheffler, S.U.; Schmidt, T.; Gangey, I.; Dustmann, M.; Unterhauser, F.; Weiler, A. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: Delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy 2008, 24, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tao, H.; Cho, S.; Chen, S.; Yao, Z.; Chen, S. Difference in graft maturity of the reconstructed anterior cruciate ligament 2 years postoperatively: A comparison between autografts and allografts in young men using clinical and 3.0-T magnetic resonance imaging evaluation. Am. J. Sports Med. 2012, 40, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.J.; Zhang, Y.J.; Zhang, Y.; Qing, Q.; Jiang, Y.L.; Yang, J.L.; Luo, J.-C.; Qin, T.-W. The utilization of decellularized tendon slices to provide an inductive microenvironment for the proliferation and tenogenic differentiation of stem cells. Biomaterials 2015, 52, 539–550. [Google Scholar] [CrossRef]
- Lu, C.C.; Zhang, T.; Amadio, P.C.; An, K.N.; Moran, S.L.; Gingery, A. Lateral slit delivery of bone marrow stromal cells enhances regeneration in the decellularized allograft flexor tendon. J. Orthop. Translat. 2019, 19, 58–67. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Z.B.; Song, Y.P.; Han, Z.C. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012, 16, 652034. [Google Scholar] [CrossRef] [PubMed]
- Blatt, A.; Minha, S.; Moravsky, G.; Vered, Z.; Krakover, R. Intracoronary administration of autologous bone marrow mononuclear cells in patients with chronic ischemic symptomatic cardiomyopathy: 5 years follow-up. Isr. Med. Assoc. J. 2010, 12, 738–741. [Google Scholar]
- Hisatome, T.; Yasunaga, Y.; Yanada, S.; Tabata, Y.; Ikada, Y.; Ochi, M. Neovascularization and bone regeneration by implantation of autologous bone marrow mononuclear cells. Biomaterials 2005, 26, 4550–4556. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Schaible, A.; Relja, B.; Jugold, M.; Bonig, H.; Marzi, I. Cell-based therapy by implanted human bone marrow-derived mononuclear cells improved bone healing of large bone defects in rats. Tissue Eng. Part A 2015, 21, 1565–1578. [Google Scholar] [CrossRef] [PubMed]
- Henrich, D.; Verboket, R.; Schaible, A.; Kontradowitz, K.; Oppermann, E.; Brune, J.C. Characterization of bone marrow mononuclear cells on biomaterials for bone tissue engineering in vitro. Biomed. Res. Int. 2015, 23, 762407. [Google Scholar]
- Hibino, N.; Nalbandian, A.; Devine, L.; Martinez, R.S.; McGillicuddy, E.; Yi, T.; Karandish, S. Comparison of human bone marrow mononuclear cell isolation methods for creating tissue-engineered vascular grafts: Novel filter system versus traditional density centrifugation method. Tissue Eng. Part C Methods 2011, 17, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Kamihata, H.; Matsubara, H.; Nishiue, T.; Fujiyama, S.; Tsutsumi, Y.; Ozono, R.; Masaki, H.; Mori, Y. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 2001, 104, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Xu, L.L.; Warren, M.K.; Rose, W.L.; Gong, W.; Wang, J.M. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J. Leukoc. Biol. 1996, 60, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Cvetanovich, G.L.; Mascarenhas, R.; Saccomanno, M.F.; Verma, N.N.; Cole, B.J.; Bush-Joseph, C.A.; Bach, B.R. Hamstring autograft versus soft-tissue allograft in anterior cruciate ligament reconstruction: A systematic review and meta-analysis of randomized controlled trials. Arthroscopy 2014, 30, 1616–1624. [Google Scholar] [CrossRef]
- Bottoni, C.R.; Smith, E.L.; Shaha, J.; Shaha, S.S.; Raybin, S.G.; Tokish, J.M.; Rowles, D.J. Autograft Versus Allograft Anterior Cruciate Ligament Reconstruction: A Prospective, Randomized Clinical Study with a Minimum 10-Year Follow-up. Am. J. Sports Med. 2015, 43, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Soejima, T.; Noguchi, K.; Tabuchi, K.; Noyama, M.; Nakamura, K.; Tabuchi, K. Tendon-to-bone healing using autologous bone marrow-derived mesenchymal stem cells in ACL reconstruction without a tibial bone tunnel-A histological study. Muscles Ligaments Tendons J. 2014, 4, 201–206. [Google Scholar] [CrossRef]
- Proffen, B.L.; Vavken, P.; Haslauer, C.M.; Fleming, B.C.; Harris, C.E.; Machan, J.T.; Murray, M.M. Addition of autologous mesenchymal stem cells to whole blood for bioenhanced ACL repair has no benefit in the porcine model. Am. J. Sports Med. 2015, 43, 320–330. [Google Scholar] [CrossRef]
- Guo, R.; Gao, L.; Xu, B. Current Evidence of Adult Stem Cells to Enhance Anterior Cruciate Ligament Treatment: A Systematic Review of Animal Trials. Arthroscopy 2018, 34, 331–340.e2. [Google Scholar] [CrossRef] [PubMed]
- Dustmann, M.; Schmidt, T.; Gangey, I.; Unterhauser, F.N.; Weiler, A.; Scheffler, S.U. The extracellular remodeling of free-soft-tissue autografts and allografts for reconstruction of the anterior cruciate ligament: A comparison study in a sheep model. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 360–369. [Google Scholar] [CrossRef]
- Park, M.J.; Lee, M.C.; Seong, S.C. A comparative study of the healing of tendon autograft and tendon-bone autograft using patellar tendon in rabbits. Int. Orthop. 2001, 25, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Soon, M.Y.; Hassan, A.; Hui, J.H.; Goh, J.C.; Lee, E.H. An analysis of soft tissue allograft anterior cruciate ligament reconstruction in a rabbit model: A short-term study of the use of mesenchymal stem cells to enhance tendon osteointegration. Am. J. Sports Med. 2007, 35, 962–971. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. 2015, 33, 832–839. [Google Scholar] [CrossRef]
- Lu, J.; Chamberlain, C.S.; Ji, M.L.; Saether, E.E.; Leiferman, E.M.; Li, W.J.; Vanderby, R. Tendon-to-Bone Healing in a Rat Extra-articular Bone Tunnel Model: A Comparison of Fresh Autologous Bone Marrow and Bone Marrow-Derived Mesenchymal Stem Cells. Am. J. Sports Med. 2019, 47, 2729–2736. [Google Scholar] [CrossRef]
- Fallouh, L.; Nakagawa, K.; Sasho, T.; Arai, M.; Kitahara, S.; Wada, Y.; Moriya, H.; Takahashi, K. Effects of autologous platelet-rich plasma on cell viability and collagen synthesis in injured human anterior cruciate ligament. J. Bone Joint Surg. Am. 2010, 92, 2909–2916. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, S.; Wu, H.; Xie, G.; Huangfu, X.; He, Y.; Zhao, Z. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J. Surg. Res. 2013, 183, 214–222. [Google Scholar] [CrossRef]
- Khatab, S.; van Buul, G.M.; Kops, N.; Bastiaansen-Jenniskens, Y.M.; Bos, P.K.; Verhaar, J.A.; Van Osch, G.J. Intra-articular Injections of Platelet-Rich Plasma Releasate Reduce Pain and Synovial Inflammation in a Mouse Model of Osteoarthritis. Am. J. Sports Med. 2018, 46, 977–986. [Google Scholar] [CrossRef]
- Mariani, E.; Roffi, A.; Cattini, L.; Pulsatelli, L.; Assirelli, E.; Krishnakumar, G.S.; Kon, E. Release kinetic of pro- and anti-inflammatory biomolecules from platelet-rich plasma and functional study on osteoarthritis synovial fibroblasts. Cytotherapy 2020, 22, 344–353. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Q.; Jiang, D. Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. J. Transl. Med. 2019, 17, 211. [Google Scholar] [CrossRef]
- Shi, Y.; Kang, X.; Wang, Y.; Bian, X.; He, G.; Zhou, M.; Tang, K. Exosomes Derived from Bone Marrow Stromal Cells (BMSCs) Enhance Tendon-Bone Healing by Regulating Macrophage Polarization. Med. Sci. Monit. 2020, 26, e923328. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, B.; Qi, Y.J.; Ni, Q.B.; Pan, Z.Q.; Wang, H.; Chen, L.-B. Enhancement of tendon-to-bone healing after anterior cruciate ligament reconstruction using bone marrow-derived mesenchymal stem cells genetically modified with bFGF/BMP2. Sci. Rep. 2016, 6, 25940. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.J.; Zhang, Y.; Chen, X.H.; Luo, J.C.; Li, X.Q.; Yang, Z.M.; Qin, T.-W. Preparation and characterization of decellularized tendon slices for tendon tissue engineering. J. Biomed. Mater. Res. A 2012, 100, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Ozasa, Y.; Amadio, P.C.; Thoreson, A.R.; An, K.N.; Zhao, C. Repopulation of intrasynovial flexor tendon allograft with bone marrow stromal cells: An ex vivo model. Tissue Eng. Part A 2014, 20, 566–574. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-C.; Ho, C.-J.; Huang, H.-T.; Lin, S.-Y.; Chou, S.-H.; Chou, P.-H.; Ho, M.-L.; Tien, Y.-C. Effect of Freshly Isolated Bone Marrow Mononuclear Cells and Cultured Bone Marrow Stromal Cells in Graft Cell Repopulation and Tendon-Bone Healing after Allograft Anterior Cruciate Ligament Reconstruction. Int. J. Mol. Sci. 2021, 22, 2791. https://doi.org/10.3390/ijms22062791
Lu C-C, Ho C-J, Huang H-T, Lin S-Y, Chou S-H, Chou P-H, Ho M-L, Tien Y-C. Effect of Freshly Isolated Bone Marrow Mononuclear Cells and Cultured Bone Marrow Stromal Cells in Graft Cell Repopulation and Tendon-Bone Healing after Allograft Anterior Cruciate Ligament Reconstruction. International Journal of Molecular Sciences. 2021; 22(6):2791. https://doi.org/10.3390/ijms22062791
Chicago/Turabian StyleLu, Cheng-Chang, Cheng-Jung Ho, Hsuan-Ti Huang, Sung-Yen Lin, Shih-Hsiang Chou, Pei-Hsi Chou, Mei-Ling Ho, and Yin-Chun Tien. 2021. "Effect of Freshly Isolated Bone Marrow Mononuclear Cells and Cultured Bone Marrow Stromal Cells in Graft Cell Repopulation and Tendon-Bone Healing after Allograft Anterior Cruciate Ligament Reconstruction" International Journal of Molecular Sciences 22, no. 6: 2791. https://doi.org/10.3390/ijms22062791
APA StyleLu, C.-C., Ho, C.-J., Huang, H.-T., Lin, S.-Y., Chou, S.-H., Chou, P.-H., Ho, M.-L., & Tien, Y.-C. (2021). Effect of Freshly Isolated Bone Marrow Mononuclear Cells and Cultured Bone Marrow Stromal Cells in Graft Cell Repopulation and Tendon-Bone Healing after Allograft Anterior Cruciate Ligament Reconstruction. International Journal of Molecular Sciences, 22(6), 2791. https://doi.org/10.3390/ijms22062791





