Preclinical Evaluation of 99mTc-ZHER2:41071, a Second-Generation Affibody-Based HER2-Visualizing Imaging Probe with a Low Renal Uptake
Abstract
1. Introduction
2. Results
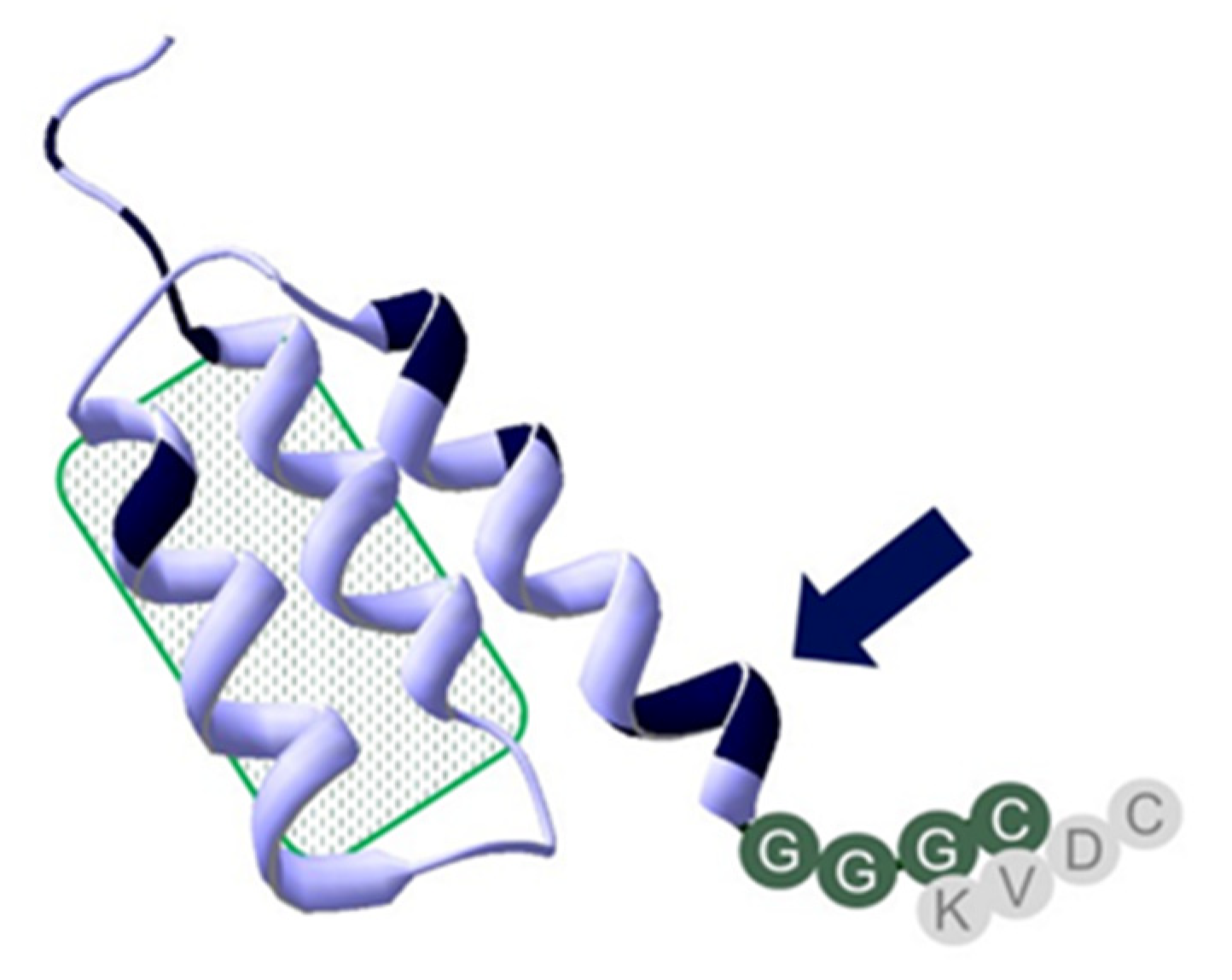
2.1. Production, Purification, and Characterization of Novel Anti-HER2 Affibody Molecules
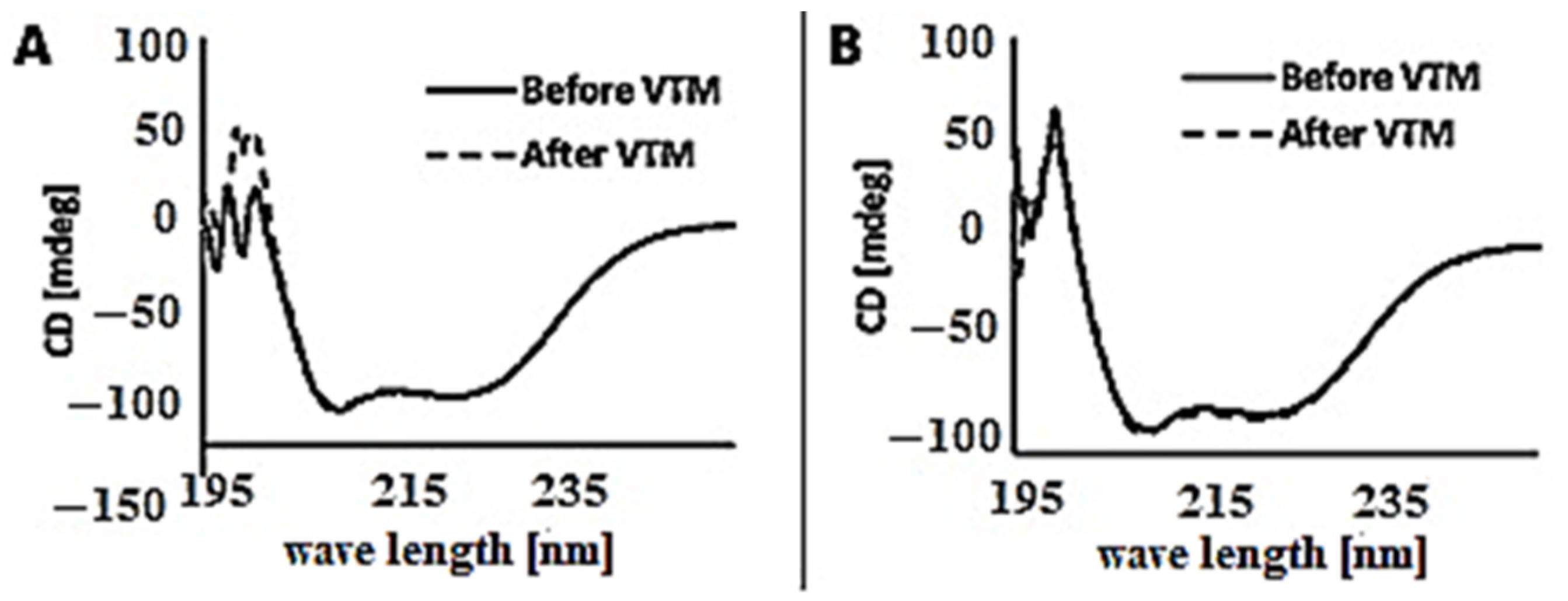
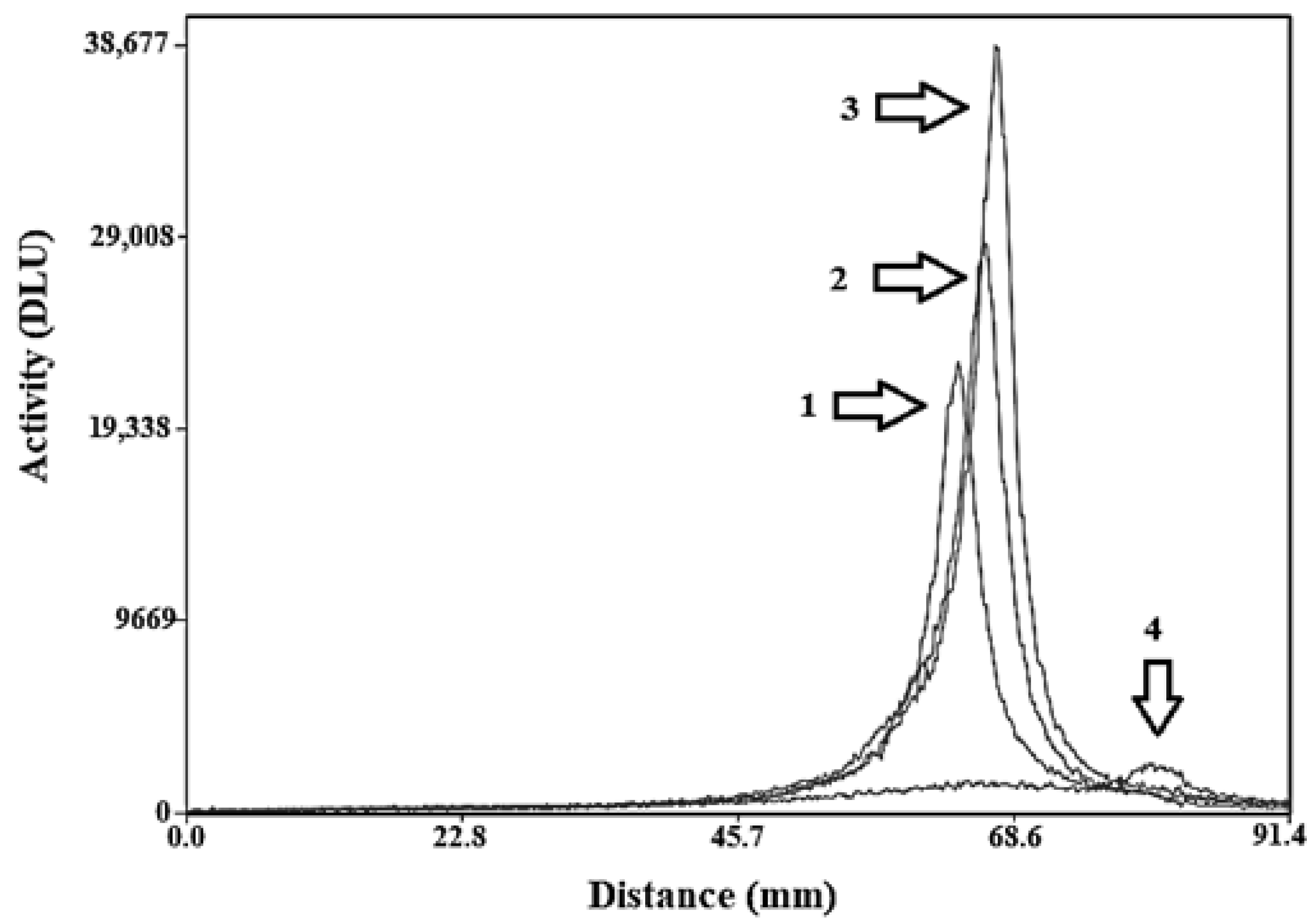
2.2. Radiolabelling and In Vitro Stability
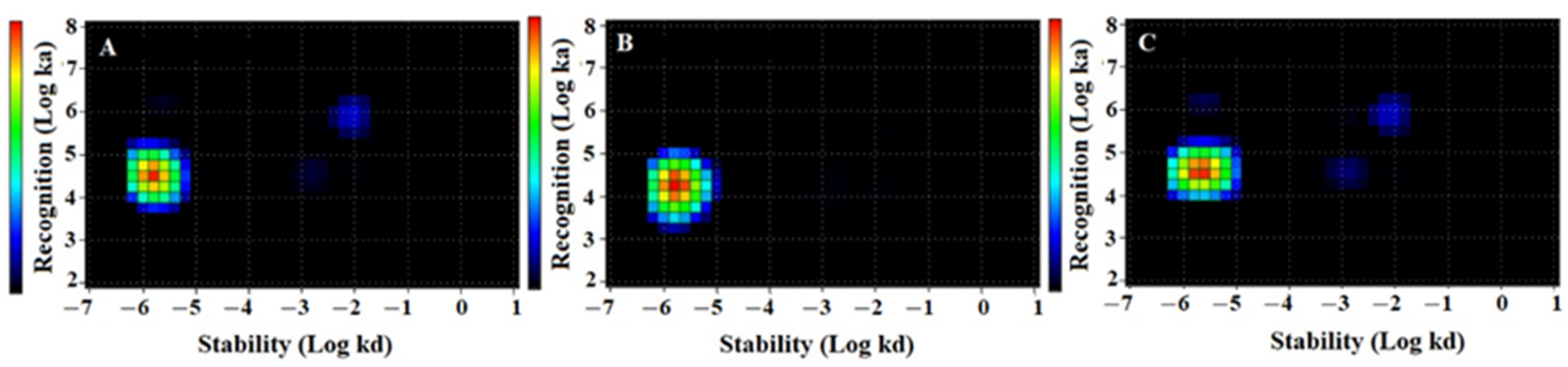
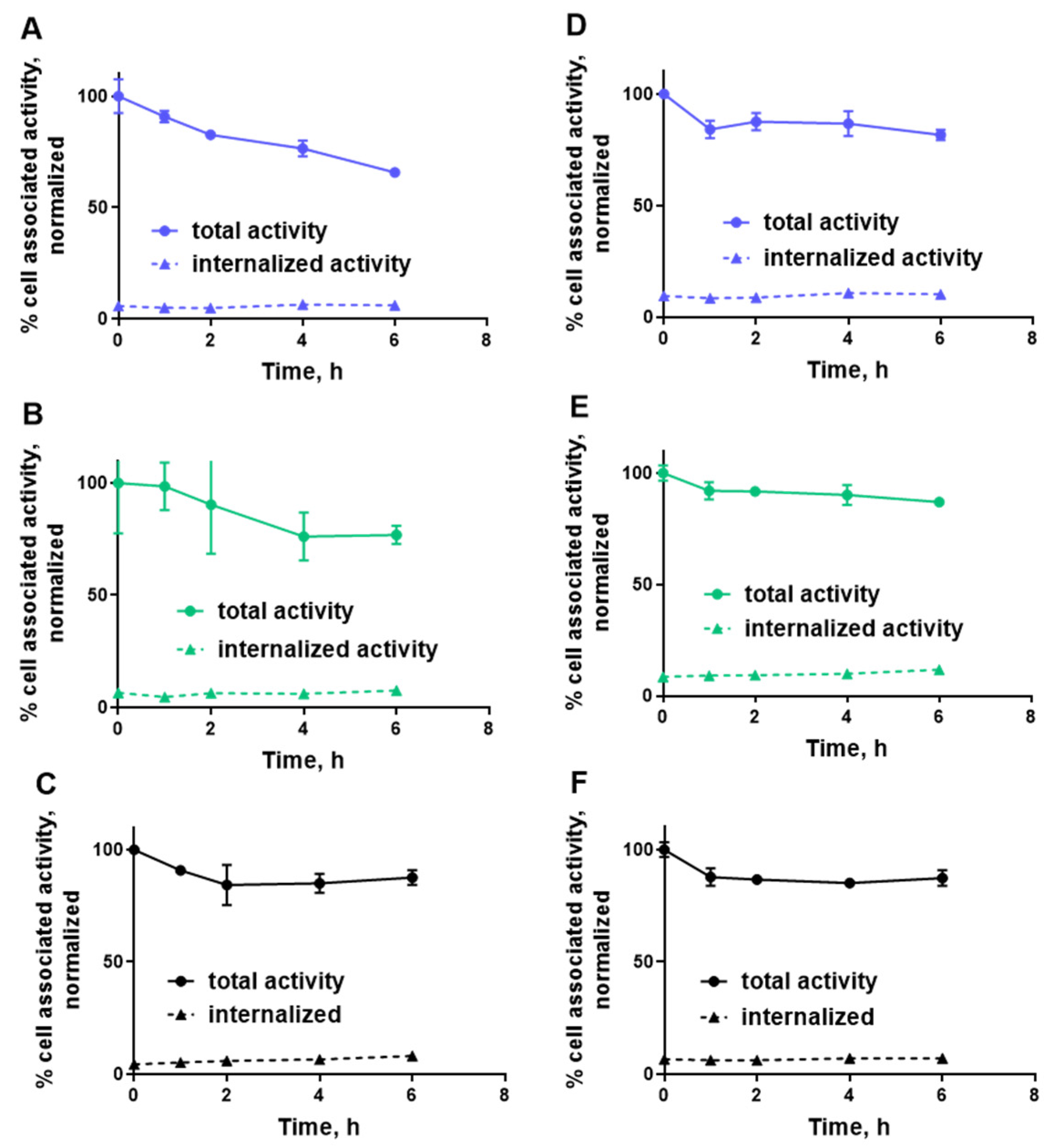
2.3. In Vitro Studies
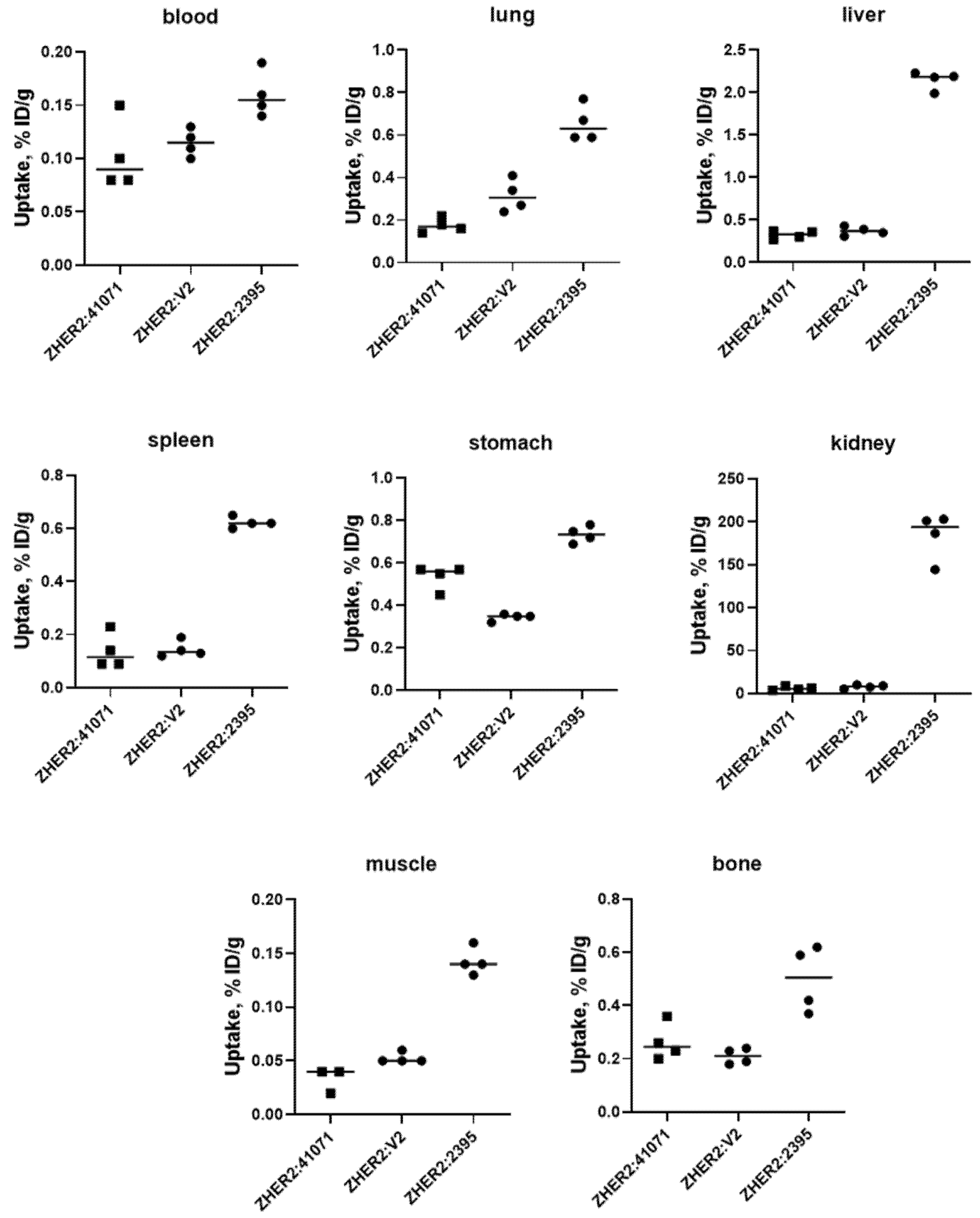
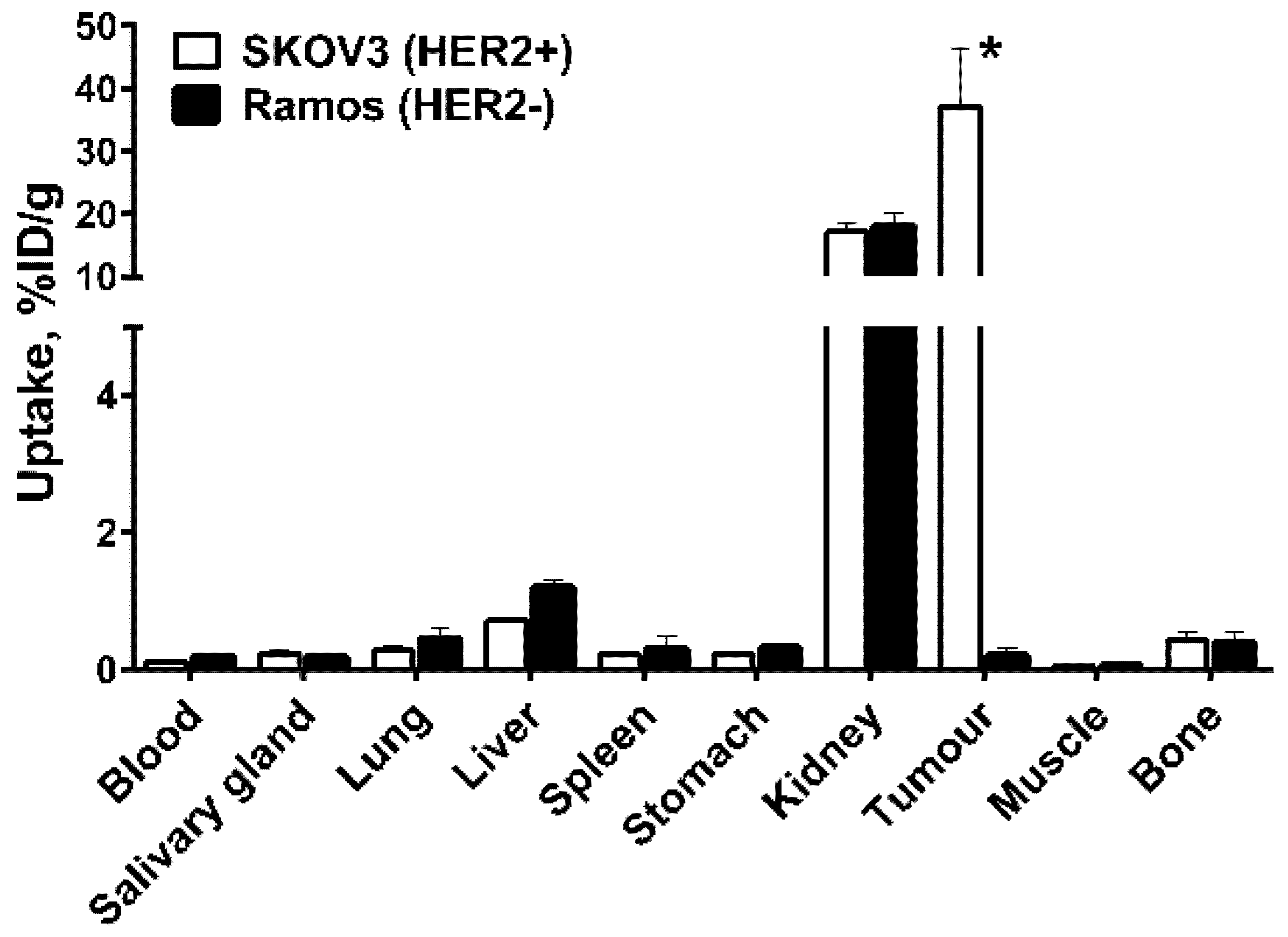
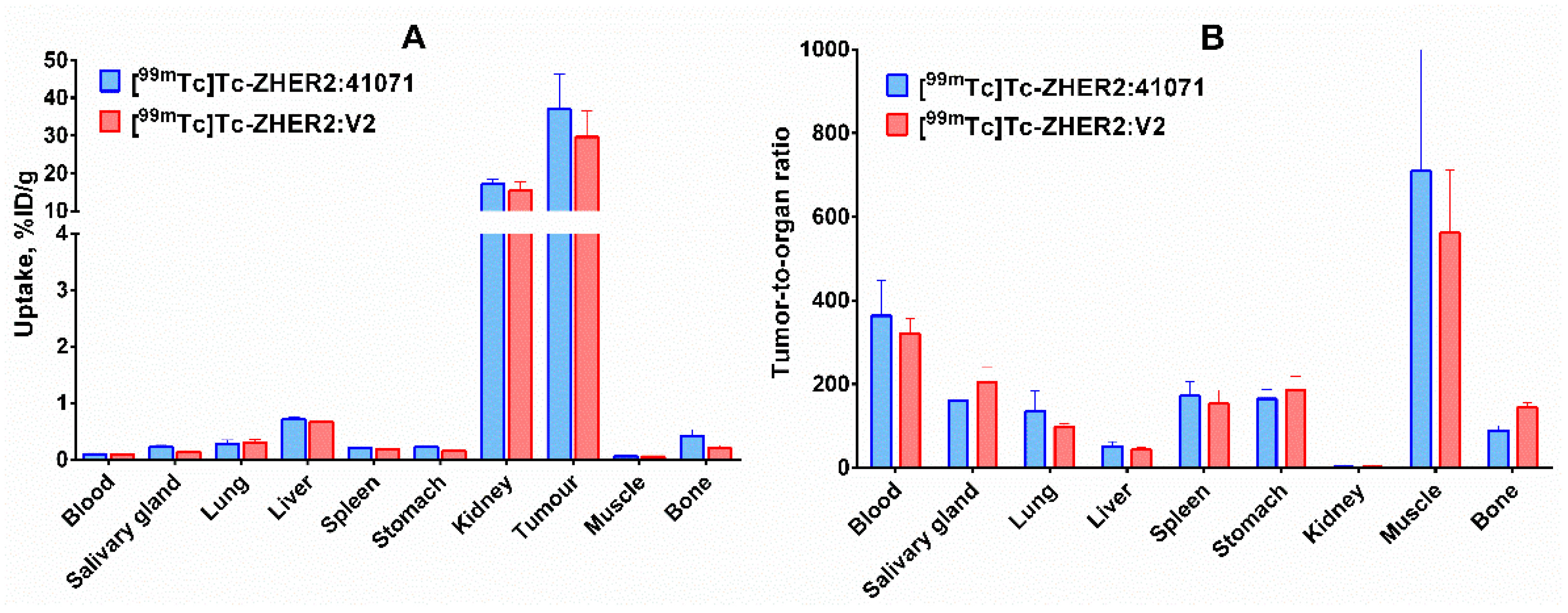
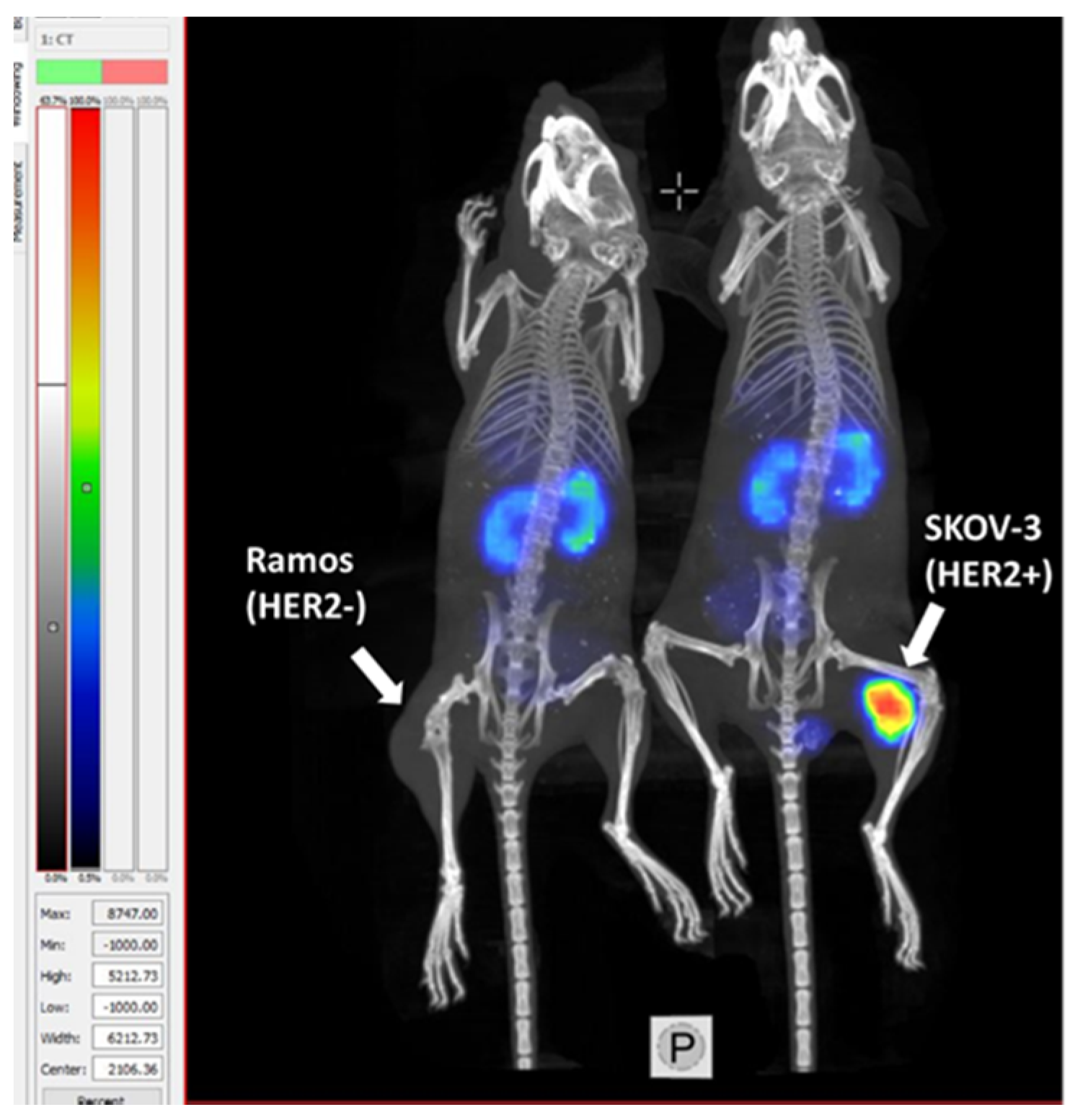
2.4. In Vivo Studies
3. Discussion
4. Materials and Methods
4.1. Production, Purification, and Characterization of Novel Anti-HER2 Affibody Molecules
4.2. Radiolabelling and In Vitro Stability
4.3. In Vitro Studies
4.4. In Vivo Studies
4.5. Dosimetry Evaluation for Humans
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neuoncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Hynes, N.E.; Lane, H.A. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer 2005, 5, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Seol, H.; Lee, H.J.; Choi, Y.; Lee, H.E.; Kim, Y.J.; Kang, E.; Kim, S.W.; Park, S.Y. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: Its clinicopathological significance. Mod. Pathol. 2012, 25, 938–948. [Google Scholar] [CrossRef]
- Niikura, N.; Tomotaki, A.; Miyata, H.; Iwamoto, T.; Kawai, M.; Anan, K.; Hayashi, N.; Aogi, K.; Ishida, T.; Masuoka, H.; et al. Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann. Oncol. 2016, 27, 480–487. [Google Scholar] [CrossRef]
- Behr, T.M.; Behe, M.; Wormann, B. Trastuzumab and breast cancer. N. Engl. J. Med. 2001, 345, 995–996. [Google Scholar]
- Perik, P.J.; Lub-De Hooge, M.N.; Gietema, J.A.; van der Graaf, W.T.A.; de Korte, M.A.; Jonkman, A.; Kosterink, J.G.W.; van Veldhuisen, D.J.; Sleijfer, D.T.; Jager, P.L.; et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2006, 24, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, G.; Flamen, P.; De Vries, E.G.; Jhaveri, K.; Wimana, Z. Imaging Diagnostic and Therapeutic Targets: Human Epidermal Growth Factor Receptor 2. J. Nucl. Med. 2016, 57 (Suppl. 1), 81S–88S. [Google Scholar] [CrossRef]
- Garousi, J.; Orlova, A.; Frejd, F.Y.; Tolmachev, V. Imaging using radiolabelled targeted proteins: Radioimmunodetection and beyond. EJNMMI Radiopharm. Chem. 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Krasniqi, A.; D’Huyvetter, M.; Devoogdt, N.; Frejd, F.Y.; Sörensen, J.; Orlova, A.; Keyaerts, M.; Tolmachev, V. Same-Day Imaging Using Small Proteins: Clinical Experience and Translational Prospects in Oncology. J. Nucl. Med. 2018, 59, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Orlova, A. Affibody Molecules as Targeting Vectors for PET Imaging. Cancers 2020, 12, 651. [Google Scholar] [CrossRef]
- Ståhl, S.; Gräslund, T.; Karlström, A.E.; Frejd, F.Y.; Nygren, P.Å.; Löfblom, J.; Tolmachev, V.; Orlova, A. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef] [PubMed]
- Orlova, A.; Magnusson, M.; Eriksson, T.; Nilsson, M.; Larsson, B.; Höiden-Guthenberg, I.; Widstrom, C.; Carlsson, J.; Tolmachev, V.; Stahl, S.; et al. Tumor imaging using a picomolar affinity HER-2 binding affibody molecules. Cancer Res. 2006, 66, 4339–4348. [Google Scholar] [CrossRef]
- Ahlgren, S.; Wållberg, H.; Tran, T.A.; Widström, C.; Hjertman, M.; Abrahmsén, L.; Berndorff, D.; Dinkelborg, L.M.; Cyr, J.E.; Feldwisch, J.; et al. Targeting of HER2-expressing tumors with a site-specifically 99mTc-labeled recombinant Affibody molecule, ZHER2:2395, with C-terminally engineered cysteine. J. Nucl. Med. 2009, 50, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Wållberg, H.; Orlova, A.; Altai, M.; Hosseinimehr, S.J.; Widström, C.; Malmberg, J.; Ståhl, S.; Tolmachev, V. Molecular design and optimization of 99mTc-labeled recombinant affibody molecules improves their biodistribution and imaging properties. J. Nucl. Med. 2011, 52, 461–469. [Google Scholar] [CrossRef]
- Baum, R.P.; Prasad, V.; Müller, D.; Schuchardt, C.; Orlova, A.; Wennborg, A.; Tolmachev, V.; Feldwisch, J. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J. Nucl. Med. 2010, 51, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Feldwisch, J.; Tolmachev, V.; Lendel, C.; Herne, N.; Sjöberg, A.; Larsson, B.; Rosik, D.; Lindqvist, E.; Fant, G.; Höidén-Guthenberg, I.; et al. Design of an optimized scaffold for affibody molecules. J. Mol. Biol. 2010, 398, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring HER2-Receptor Expression In Metastatic Breast Cancer Using [68Ga]ABY-025 Affibody PET/CT. Theranostics 2016, 6, 262–271. [Google Scholar]
- Sandström, M.; Lindskog, K.; Velikyan, I.; Wennborg, A.; Feldwisch, J.; Sandberg, D.; Tolmachev, V.; Orlova, A.; Sörensen, J.; Carlsson, J. Biodistribution and Radiation Dosimetry of the Anti-HER2 Affibody Molecule 68Ga-ABY-025 in Breast Cancer Patients. J. Nucl. Med. 2016, 57, 867–871. [Google Scholar] [CrossRef]
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J.; et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef]
- Duatti, A. Review on 99mTc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 2020, 92, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Hofström, C.; Altai, M.; Honarvar, H.; Strand, J.; Malmberg, J.; Hosseinimehr, S.J.; Orlova, A.; Gräslund, T.; Tolmachev, V. HAHAHA, HEHEHE, HIHIHI, or HKHKHK: Influence of position and composition of histidine containing tags on biodistribution of [(99m)Tc(CO)3](+)-labeled affibody molecules. J. Med. Chem. 2013, 56, 4966–4974. [Google Scholar] [CrossRef] [PubMed]
- Engfeldt, T.; Tran, T.; Orlova, A.; Widström, C.; Karlstroöm, E.A.; Tolmachev, V. 99mTc-chelator engineering to improve tumour targeting properties of a HER2-specific Affibody molecule. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Engfeldt, T.; Orlova, A.; Sandström, M.; Feldwisch, J.; Abrahmsén, L.; Wennborg, A.; Tolmachev, V.; Karlström, A.E. 99mTc-maEEE-ZHER2:342, an Affibodymolecule-based tracer for detection of HER2-expression in malignant tumors. Bioconjug. Chem. 2007, 18, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, T.; Tran, T.; Orlova, A.; Widström, C.; Feldwisch, J.; Abrahmsén, L.; Wennborg, A.; Karlström, A.E.; Tolmachev, V. Development and preclinical characterisationof 99mTc-labelled Affibody molecules with reduced renal uptake. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2245–2255. [Google Scholar] [CrossRef]
- Tran, T.A.; Rosik, D.; Abrahmsén, L.; Sandström, M.; Sjöberg, A.; Wållberg, H.; Ahlgren, S.; Orlova, A.; Tolmachev, V. Design, synthesis and biological evaluation of a HER2-specific affibody molecule for molecular imaging. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1864–1873. [Google Scholar] [CrossRef]
- Ahlgren, S.; Andersson, K.; Tolmachev, V. Kit formulation for 99mTc-labeling of recombinant anti-HER2 affibody molecules with a C-terminally engineered cysteine. Nucl. Med. Biol. 2010, 37, 539–546. [Google Scholar] [CrossRef]
- Altai, M.; Wållberg, H.; Orlova, A.; Rosestedt, M.; Hosseinimehr, S.J.; Tolmachev, V.; Ståhl, S. Order of amino acids in C-terminal cysteine-containing peptide-based chelators influences cellular processing and biodistribution of 99mTc-labeled recombinant Affibody molecules. Amino Acids 2012, 5, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Cyr, J.E.; Koppitz, M.; Srinivasan, A.; Dinkelborg, L.M. Improved peptide chelators for Tc/Re(V); stabilizing side chains [abstract]. J. Labelled Comp. Radiopharm. 2007, 50, S9. [Google Scholar]
- Lindberg, H.; Hofström, C.; Altai, M.; Honorvar, H.; Wållberg, H.; Orlova, A.; Ståhl, S.; Gräslund, T.; Tolmachev, V. Evaluation of a HER2-targeting affibody molecule combining an N-terminal HEHEHE-tag with a GGGC chelator for 99mTc-labelling at the C terminus. Tumour Biol. 2012, 33, 641–651. [Google Scholar] [CrossRef]
- Oroujeni, M.; Andersson, K.G.; Steinhardt, X.; Altai, M.; Orlova, A.; Mitran, B.; Vorobyeva, A.; Garousi, J.; Tolmachev, V.; Löfblom, J. Influence of composition of cysteine-containing peptide-based chelators on biodistribution of 99mTc-labeled anti-EGFR affibody molecules. Amino Acids 2018, 50, 981–994. [Google Scholar] [CrossRef]
- Rosenberg, A.S.; Sauna, Z.E. Immunogenicity assessment during the development of protein therapeutics. J. Pharm. Pharmacol. 2018, 70, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Gaykema, S.B.; de Jong, J.R.; Perik, P.J.; Brouwers, A.H.; Schröder, C.P.; Oude Munnink, T.H.; Bongaerts, A.H.; de Vries, E.G.; Lub-de Hooge, M.N. (111)In-trastuzumab scintigraphy in HER2-positive metastatic breast cancer patients remains feasible during trastuzumab treatment. Mol. Imaging 2014, 13. [Google Scholar] [CrossRef]
- Bragina, O.; von Witting, E.; Garousi, J.; Zelchan, R.; Sandström, M.; Medvedeva, A.; Orlova, A.; Doroshenko, A.; Vorobyeva, A.; Lindbo, S.; et al. Phase I study of 99mTc-ADAPT6, a scaffold protein-based probe for visualization of HER2 expression in breast cancer. J. Nucl. Med. 2020, 120. [Google Scholar] [CrossRef]
- Eckelman, W.C.; Kilbourn, M.R.; Mathis, C.A. Specific to nonspecific binding in radiopharmaceutical studies: It’s not so simple as it seems! Nucl. Med. Biol. 2009, 36, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Lub-de Hooge, M.N.; Kosterink, J.G.; Perik, P.J.; Nijnuis, H.; Tran, L.; Bart, J.; Suurmeijer, A.J.; de Jong, S.; Jager, P.L.; de Vries, E.G. Preclinical characterisation of 111In-DTPA-trastuzumab. Br. J. Pharmacol. 2004, 143, 99–106. [Google Scholar] [CrossRef]
- McLarty, K.; Cornelissen, B.; Scollard, D.A.; Done, S.J.; Chun, K.; Reilly, R.M. Associations between the uptake of 111In-DTPA-trastuzumab, HER2 density and response to trastuzumab (Herceptin) in athymic mice bearing subcutaneous human tumour xenografts. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Wong, K.J.; Baidoo, K.E.; Nayak, T.K.; Regino, C.A.; Garmestani, K.; Brechbiel, M.W. Targeting HER2: A report on the in vitro and in vivo pre-clinical data supporting trastuzumab as a radioimmunoconjugate for clinical trials. MAbs 2010, 2, 550–564. [Google Scholar] [CrossRef]
- McLarty, K.; Cornelissen, B.; Cai, Z.; Scollard, D.A.; Costantini, D.L.; Done, S.J.; Reilly, R.M. Micro-SPECT/CT with 111In-DTPA-pertuzumab sensitively detects trastuzumab-mediated HER2 downregulation and tumor response in athymic mice bearing MDA-MB-361 human breast cancer xenografts. J. Nucl. Med. 2009, 50, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Scollard, D.A.; McLarty, K.; Smith, S.; Reilly, R.M. A comparison of 111In- or 64Cu-DOTA-trastuzumab Fab fragments for imaging subcutaneous HER2-positive tumor xenografts in athymic mice using microSPECT/CT or microPET/CT. EJNMMI Res. 2011, 1, 15. [Google Scholar] [CrossRef]
- Vaneycken, I.; Devoogdt, N.; Van Gassen, N.; Vincke, C.; Xavier, C.; Wernery, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 2011, 25, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Xavier, C.; De Vos, J.; Caveliers, V.; Lahoutte, T.; Muyldermans, S.; Devoogdt, N. Site-specific labeling of cysteine-tagged camelid single-domain antibody-fragments for use in molecular imaging. Bioconjug. Chem. 2014, 25, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.; Sosabowski, J.; Livanos, M.; Leyton, J.; Vigor, K.; Bhavsar, G.; Nagy-Davidescu, G.; Rashid, M.; Miranda, E.; Yeung, J.; et al. Development of the designed ankyrin repeat protein (DARPin) G3 for HER2 molecular imaging. Eur. J. Nucl. Med. Mol. Imaging 2015, 2, 288–301. [Google Scholar] [CrossRef]
- Vorobyeva, A.; Schulga, A.; Konovalova, E.; Güler, R.; Löfblom, J.; Sandström, M.; Garousi, J.; Chernov, V.; Bragina, O.; Orlova, A.; et al. Optimal composition and position of histidine-containing tags improves biodistribution of (99m)Tc-labeled DARPin G3. Sci. Rep. 2019, 9, 9405. [Google Scholar] [CrossRef] [PubMed]
- Björke, H.; Andersson, K. Automated, high-resolution cellular retention and uptake studies in vitro. Appl. Radiat. Isot. 2006, 64, 901–905. [Google Scholar] [CrossRef]
- Altschuh, D.; Björkelund, H.; Strandgård, J.; Choulier, L.; Malmqvist, M.; Andersson, K. Deciphering complex protein interaction kinetics using Interaction Map. Biochem. Biophys. Commun. 2012, 428, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Wållberg, H.; Orlova, A. Slow internalization of anti-HER2 synthetic affibody monomer 111In-DOTA-ZHER2:342-pep2: Implications for development of labelled tracers. Cancer Biother. Radiopharm. 2008, 23, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Stabin, M.G. Fundamentals of Nuclear Medicine Dosimetry; Springer: New York, NY, USA, 2008; pp. 83–86. [Google Scholar]

| Compound | Radio-Labelling Yield, % | Stability in PBS, % | |
|---|---|---|---|
| 1 h | 4 h | ||
| ZHER2:2395 | 98.6 ± 1.4 | 96.2 ± 0.8 | 97.1 ± 0.4 |
| ZHER2:41071 | 95.6 ± 2.7 | 98.7 ± 0.7 | 97.5 ± 0.9 |
| ZHER2:V2 | 96.3 ± 1.9 | 98.1 ± 0.2 | 97.2 ± 0.3 |
| Label | ka (1/M×s) × 104 | kd (1/s) × 10−6 | KD (pM) |
|---|---|---|---|
| [99mTc]Tc-ZHER2:2395 | 2.9 ± 0.3 | 1.50 ± 0.05 | 53 ± 4 |
| [99mTc]Tc-ZHER2:41071 | 3.7 ± 0.1 | 2.17 ± 0.05 | 58 ± 2 |
| [99mTc]Tc-ZHER2:V2 | 2.4 ± 0.8 | 2.0 ± 0.1 | 90 ± 22 |
| Site | Uptake, %ID/g | |||
|---|---|---|---|---|
| 1 h | 4 h | 8 h | 24 h | |
| Blood | 1.6 ± 0.2 | 0.102 ± 0.004 | 0.08 ± 0.03 | 0.06 ± 0.02 |
| Salivary gland | 0.8 ± 0.1 | 0.23 ± 0.05 | 0.07 ± 0.01 | 0.06 ± 0.02 |
| Lung | 2.7 ± 0.6 | 0.28 ± 0.07 | 0.14 ± 0.01 | 0.11 ± 0.02 |
| Liver | 2.0 ± 0.4 | 0.71 ± 0.04 | 0.48 ± 0.08 | 0.42 ± 0.05 |
| Spleen | 0.9 ± 0.1 | 0.21 ± 0.02 | 0.17 ± 0.06 | 0.18 ± 0.02 |
| Stomach | 1.4 ± 0.3 | 0.22 ± 0.03 | 0.13 ± 0.03 | 0.11 ± 0.03 |
| Kidney | 40 ± 1 | 17 ± 1 | 9 ± 1 | 5.3 ± 0.3 |
| Tumour | 24 ± 7 | 37 ± 9 | 23 ± 6 | 13 ± 2 |
| Muscle | 0.5 ± 0.1 | 0.06 ± 0.02 | 0.04 ± 0.02 | 0.03 ± 0.02 |
| Bone | 1.4 ± 0.4 | 0.4 ± 0.1 | 0.21 ± 0.04 | 0.27 ± 0.02 |
| Uterus | 1.8 ± 0.5 | 0.20 ± 0.08 | 0.08 ± 0.04 | 0.10 ± 0.03 |
| Brain | 0.06 ± 0.01 | 0.0110 ± 0.0005 | 0.009 ± 0.002 | 0.009 ± 0.003 |
| Pancreas | 0.48 ± 0.01 | 0.054 ± 0.001 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| Small Intestine | 1.2 ± 0.3 | 0.13 ± 0.07 | 0.12 ± 0.03 | 0.08 ± 0.02 |
| Site | Tumour-to-Organ Ratio | |||
|---|---|---|---|---|
| 1 h | 4 h | 8 h | 24 h | |
| Blood | 16 ± 6 | 363 ± 84 | 284 ± 85 | 250 ± 9 |
| Salivary gland | 29 ± 6 | 161 ± 27 | 325 ± 118 | 220 ± 53 |
| Lung | 9 ± 2 | 136 ± 49 | 170 ± 51 | 127 ± 20 |
| Liver | 12 ± 1 | 52 ± 11 | 50 ± 20 | 32 ± 3 |
| Spleen | 27 ± 7 | 172 ± 34 | 148 ± 66 | 75 ± 10 |
| Stomach | 18 ± 5 | 165 ± 23 | 177 ± 38 | 138 ± 14 |
| Kidney | 0.6 ± 0.2 | 2.1 ± 0.5 | 2.8 ± 0.9 | 2.4 ± 0.4 |
| Muscle | 52 ± 8 | 709 ± 300 | 527 ± 95 | 538 ± 324 |
| Bone | 18 ± 1 | 89 ± 12 | 115 ± 46 | 48 ± 9 |
| Uterus | 14 ± 4 | 232 ± 178 | 315 ± 233 | 137 ± 37 |
| Brain | 423 ± 155 | 3397 ± 947 | 2629 ± 947 | 1548 ± 684 |
| Pancreas | 51 ± 15 | 690 ± 175 | 477 ± 193 | 276 ± 40 |
| Small Intestine | 21 ± 4 | 288 ± 103 | 209 ± 127 | 166 ± 49 |
| Organ | Absorbed Dose (mGy/MBq) | Organ | Absorbed Dose (mGy/MBq) |
|---|---|---|---|
| Adrenals | 0.0009 | Muscle | 0.00034 |
| Brain | 0.00016 | Ovaries | 0.00042 |
| Breasts | 0.00029 | Pancreas | 0.00073 |
| Gallbladder wall | 0.0007 | Red marrow | 0.00062 |
| Lower large intestine wall | 0.0004 | Osteogenic cells | 0.0026 |
| Small intestine | 0.00049 | Skin | 0.00021 |
| Stomach wall | 0.00052 | Spleen | 0.00078 |
| Upper large intestine wall | 0.0005 | Thymus | 0.00049 |
| Heart wall | 0.00160 | Thyroid | 0.00029 |
| Kidney | 0.0069 | Urinary bladder wall | 0.00037 |
| Liver | 0.00088 | Uterus | 0.00051 |
| Lungs | 0.0007 | Total body | 0.00048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oroujeni, M.; Rinne, S.S.; Vorobyeva, A.; Loftenius, A.; Feldwisch, J.; Jonasson, P.; Chernov, V.; Orlova, A.; Frejd, F.Y.; Tolmachev, V. Preclinical Evaluation of 99mTc-ZHER2:41071, a Second-Generation Affibody-Based HER2-Visualizing Imaging Probe with a Low Renal Uptake. Int. J. Mol. Sci. 2021, 22, 2770. https://doi.org/10.3390/ijms22052770
Oroujeni M, Rinne SS, Vorobyeva A, Loftenius A, Feldwisch J, Jonasson P, Chernov V, Orlova A, Frejd FY, Tolmachev V. Preclinical Evaluation of 99mTc-ZHER2:41071, a Second-Generation Affibody-Based HER2-Visualizing Imaging Probe with a Low Renal Uptake. International Journal of Molecular Sciences. 2021; 22(5):2770. https://doi.org/10.3390/ijms22052770
Chicago/Turabian StyleOroujeni, Maryam, Sara S. Rinne, Anzhelika Vorobyeva, Annika Loftenius, Joachim Feldwisch, Per Jonasson, Vladimir Chernov, Anna Orlova, Fredrik Y. Frejd, and Vladimir Tolmachev. 2021. "Preclinical Evaluation of 99mTc-ZHER2:41071, a Second-Generation Affibody-Based HER2-Visualizing Imaging Probe with a Low Renal Uptake" International Journal of Molecular Sciences 22, no. 5: 2770. https://doi.org/10.3390/ijms22052770
APA StyleOroujeni, M., Rinne, S. S., Vorobyeva, A., Loftenius, A., Feldwisch, J., Jonasson, P., Chernov, V., Orlova, A., Frejd, F. Y., & Tolmachev, V. (2021). Preclinical Evaluation of 99mTc-ZHER2:41071, a Second-Generation Affibody-Based HER2-Visualizing Imaging Probe with a Low Renal Uptake. International Journal of Molecular Sciences, 22(5), 2770. https://doi.org/10.3390/ijms22052770









