Lung Surfactant Decreases Biochemical Alterations and Oxidative Stress Induced by a Sub-Toxic Concentration of Carbon Nanoparticles in Alveolar Epithelial and Microglial Cells
Abstract
1. Introduction
2. Results
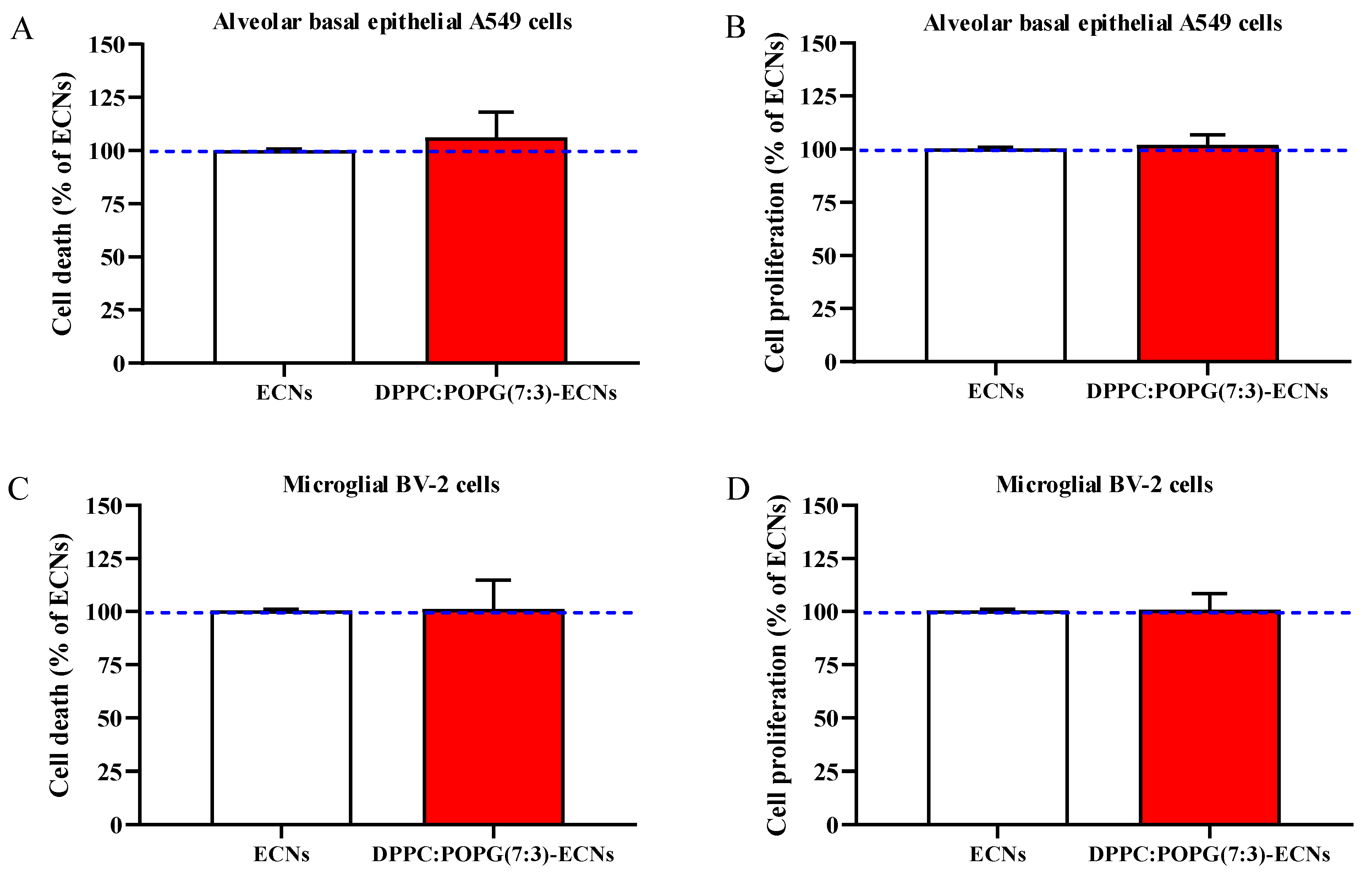
2.1. The Presence of LS Does Not Affect Cell Death and Proliferation
2.2. The Presence of LS Decreases the Production of ROS Induced by a Sub-Toxic Concentration of ECNs
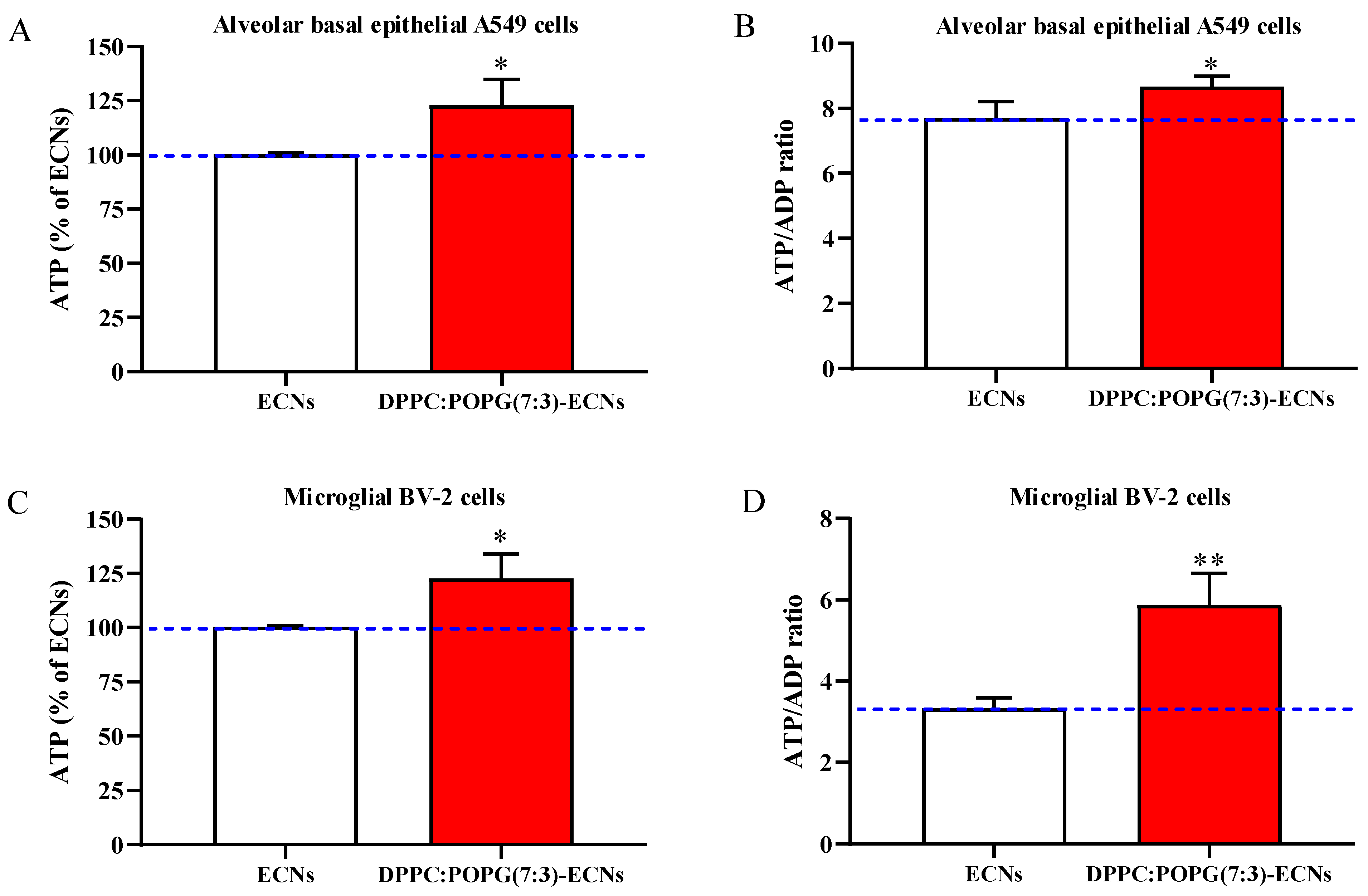
2.3. LS Ameliorates Energy State and Mitochondrial Activity of Alveolar Epithelial and Microglial Cells Challenged with ECNs
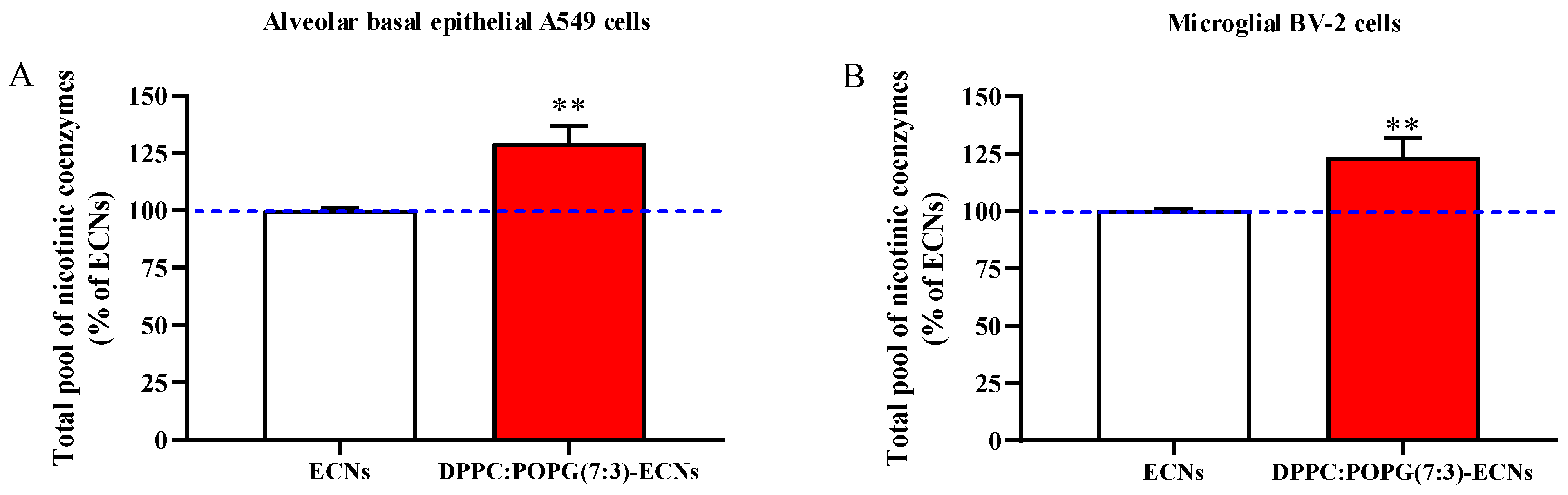
2.4. LS Increases the Cellular Pool of Nicotinic Coenzymes
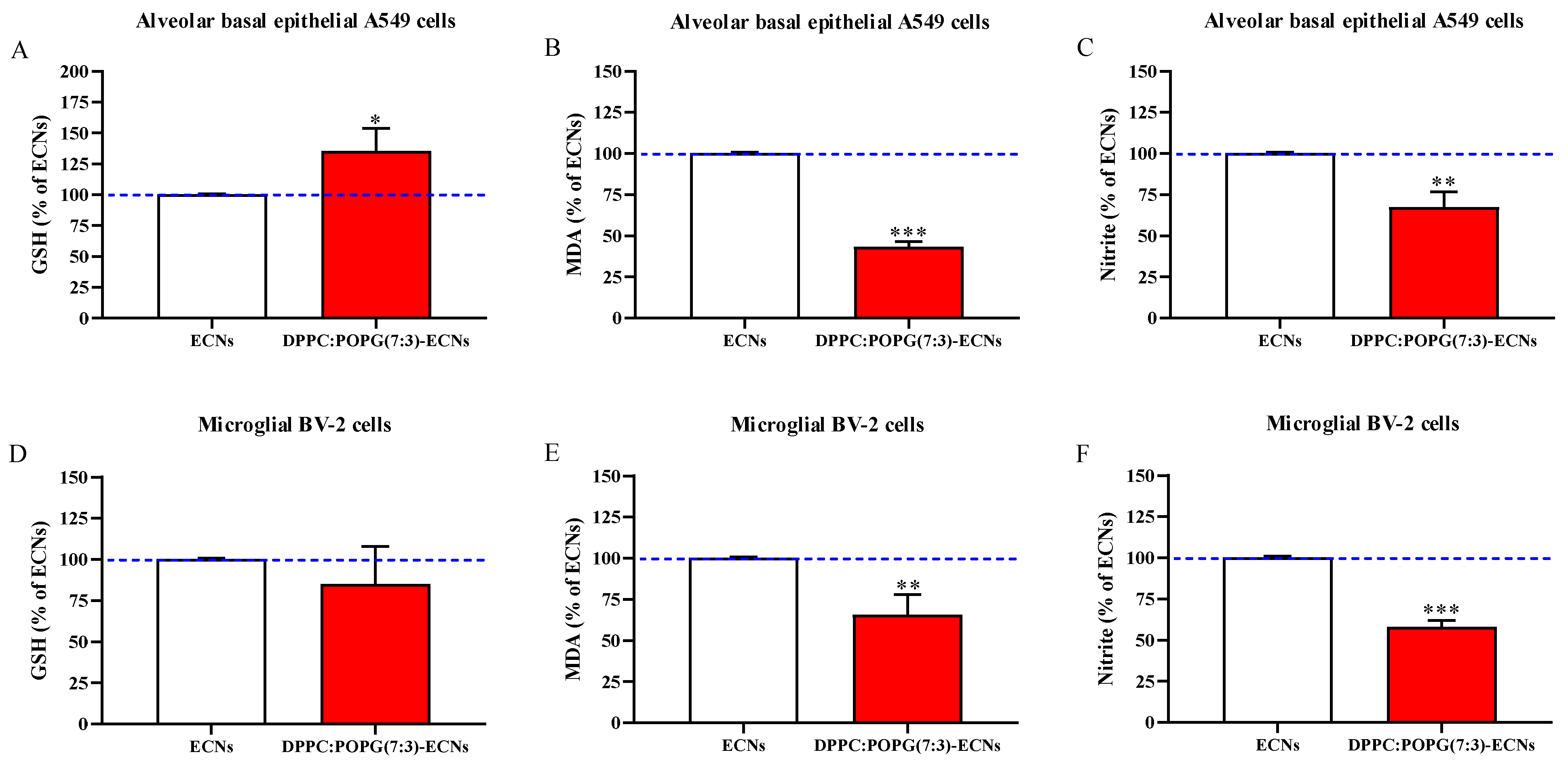
2.5. LS Protects GSH Levels and Decreases Oxidative/Nitrosative Stress
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of Nanoparticle Suspensions
4.3. Propagation and Maintenance of Cells
4.4. Analysis of Cell Viability and Proliferation
4.5. Intracellular ROS Levels Determination
4.6. Analysis of Metabolites
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Building Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Sandhu, A. Who invented nano? Nat. Nanotechnol. 2006, 1, 87. [Google Scholar] [CrossRef]
- Grillo, R.; Rosa, A.H.; Fraceto, L.F. Engineered nanoparticles and organic matter: A review of the state-of-the-art. Chemosphere 2015, 119, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Denora, N.; Lopedota, A.; Perrone, M.; Laquintana, V.; Iacobazzi, R.M.; Milella, A.; Fanizza, E.; Depalo, N.; Cutrignelli, A.; Lopalco, A.; et al. Spray-dried mucoadhesives for intravesical drug delivery using N-acetylcysteine- and glutathione-glycol chitosan conjugates. Acta Biomater. 2016, 43, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Lopedota, A.; Cutrignelli, A.; Laquintana, V.; Denora, N.; Iacobazzi, R.M.; Perrone, M.; Fanizza, E.; Mastrodonato, M.; Mentino, D.; Lopalco, A.; et al. Spray Dried Chitosan Microparticles for Intravesical Delivery of Celecoxib: Preparation and Characterization. Pharm. Res. 2016, 33, 2195–2208. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P.C.; Cormode, D.P.; Koo, H. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 2016, 101, 272–284. [Google Scholar] [CrossRef]
- Wang, D.; Luo, L.; Zheng, S.; Niu, Y.; Bo, R.; Huang, Y.; Xing, J.; Li, Z.; Liu, Z. Cubosome nanoparticles potentiate immune properties of immunostimulants. Int. J. Nanomed. 2016, 11, 3571–3583. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Serpooshan, V.; Laurent, S. Engineered nanoparticles for biomolecular imaging. Nanoscale 2011, 3, 3007–3026. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef]
- Barnard, A.S. Predicting the impact of structural diversity on the performance of nanodiamond drug carriers. Nanoscale 2018, 10, 8893–8910. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2011, 7, 11–23. [Google Scholar] [CrossRef]
- Huang, H.; Pierstorff, E.; Osawa, E.; Ho, D. Active Nanodiamond Hydrogels for Chemotherapeutic Delivery. Nano Lett. 2007, 7, 3305–3314. [Google Scholar] [CrossRef]
- Wu, X.; Bruschi, M.; Waag, T.; Schweeberg, S.; Tian, Y.; Meinhardt, T.; Stigler, R.; Larsson, K.; Funk, M.; Steinmüller-Nethl, D.; et al. Functionalization of bone implants with nanodiamond particles and angiopoietin-1 to improve vascularization and bone regeneration. J. Mater. Chem. B 2017, 5, 6629–6636. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Robinson, E.M.; Zhang, X.-Q.; Chow, E.K.; Lin, Y.; Osawa, E.; Xi, J.; Ho, D. Triggered release of therapeutic antibodies from nanodiamond complexes. Nanoscale 2011, 3, 2844–2848. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Robinson, E.; Mania-Farnell, B.; Vanin, E.F.; Shim, K.-W.; Takao, T.; Allender, E.V.; Mayanil, C.S.; Soares, M.B.; Ho, D.; et al. Convection-enhanced delivery of nanodiamond drug delivery platforms for intracranial tumor treatment. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Heyder, J. Deposition of Inhaled Particles in the Human Respiratory Tract and Consequences for Regional Targeting in Respiratory Drug Delivery. Proc. Am. Thorac. Soc. 2004, 1, 315–320. [Google Scholar] [CrossRef]
- Han, S.; Mallampalli, R.K. The Role of Surfactant in Lung Disease and Host Defense against Pulmonary Infections. Ann. Am. Thorac. Soc. 2015, 12, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.Y.; Veldhuizen, R.A.; Neumann, A.W.; Petersen, N.O.; Possmayer, F. Current perspectives in pulmonary surfactant—Inhibition, enhancement and evaluation. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 1947–1977. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.; Rijo, P.; Reis, C.P. Nanotechnological strategies for nerve growth factor delivery: Therapeutic implications in Alzheimer’s disease. Pharmacol. Res. 2017, 120, 68–87. [Google Scholar] [CrossRef]
- Gomes, A.; Sengupta, J.; Datta, P.; Ghosh, S.; Gomes, A.; Antony, G.; Jayeeta, S.; Poulami, D.; Sourav, G.; Aparna, G. Physiological Interactions of Nanoparticles in Energy Metabolism, Immune Function and Their Biosafety: A Review. J. Nanosci. Nanotechnol. 2016, 16, 92–116. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Fresta, C.G.; Chakraborty, A.; Wijesinghe, M.B.; Amorini, A.M.; Lazzarino, G.; Lazzarino, G.; Tavazzi, B.; Lunte, S.M.; Caraci, F.; Dhar, P.; et al. Non-toxic engineered carbon nanodiamond concentrations induce oxidative/nitrosative stress, imbalance of energy metabolism, and mitochondrial dysfunction in microglial and alveolar basal epithelial cells. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Uboldi, C.; Bonacchi, D.; Lorenzi, G.; Hermanns, M.I.; Pohl, C.; Baldi, G.; Unger, R.E.; Kirkpatrick, C.J. Gold nanoparticles induce cytotoxicity in the alveolar type-II cell lines A549 and NCIH441. Part. Fibre Toxicol. 2009, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chairuangkitti, P.; Lawanprasert, S.; Roytrakul, S.; Aueviriyavit, S.; Phummiratch, D.; Kulthong, K.; Chanvorachote, P.; Maniratanachote, R. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol. Vitr. 2013, 27, 330–338. [Google Scholar] [CrossRef]
- Escamilla-Rivera, V.; Uribe-Ramirez, M.; Gonzalez-Pozos, S.; Velumani, S.; Arreola-Mendoza, L.; De Vizcaya-Ruiz, A. Cytotoxicity of semiconductor nanoparticles in A549 cells is attributable to their intrinsic oxidant activity. J. Nanopart. Res. 2016, 18, 85. [Google Scholar] [CrossRef]
- Henn, A.; Lund, S.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The suitability of bv2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. Altex 2009, 26, 83–94. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Musso, N.; Giambirtone, M.; Grasso, M.; Spampinato, S.F.; Merlo, S.; Drago, F.; Lazzarino, G.; Sortino, M.A.; et al. Carnosine Prevents Aβ-Induced Oxidative Stress and Inflammation in Microglial Cells: A Key Role of TGF-β1. Cells 2019, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.D.; Harry, G.J. Features of Microglia and Neuroinflammation Relevant to Environmental Exposure and Neurotoxicity. Int. J. Environ. Res. Public Health 2011, 8, 2980–3018. [Google Scholar] [CrossRef]
- Plante, A.F.; Stone, M.M.; McGill, W.B. The Metabolic Physiology of Soil Microorganisms. Soil Microbiol. Ecol. Biochem. 2015, 245–272. [Google Scholar] [CrossRef]
- Maldonado, E.N.; Lemasters, J.J. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 2014, 19, 78–84. [Google Scholar] [CrossRef]
- Tavazzi, B.; Signoretti, S.; Lazzarino, G.; Amorini, A.M.; Delfini, R.; Cimatti, M.; Marmarou, A.; Vagnozzi, R. Cerebral Oxidative Stress and Depression of Energy Metabolism Correlate with Severity of Diffuse Brain Injury in Rats. Neurosurgery 2005, 56, 582–589. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Kiseleva, R.; Vertegel, A.; Ray, S.K. Carbon Nanomaterials for Drug Delivery and Cancer Therapy. J. Nanosci. Nanotechnol. 2015, 15, 5501–5511. [Google Scholar] [CrossRef]
- Meliţă, E.D.; Purcel, G.; Grumezescu, A.M. Carbon nanotubes for cancer therapy and neurodegenerative diseases. Rom. J. Morphol. Embryol. 2015, 56, 349–356. [Google Scholar] [PubMed]
- Yang, Z.; Zhang, Y.; Yang, Y.; Sun, L.; Han, D.; Li, H.; Wang, C. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 427–441. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.; Derreumaux, P.; Wei, G. Carbon nanotube inhibits the formation of β-sheet-rich oligomers of the alzheimer’s amyloid-β(16-22) peptide. Biophys. J. 2011, 101, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Ratoi, M.; Hoet, P.H.M.; Crossley, A.; Dobson, P. Impact of lung surfactant on wettability and cytotoxicity of nanoparticles. RSC Adv. 2014, 4, 20573–20581. [Google Scholar] [CrossRef]
- Kasper, J.Y.; Feiden, L.; Hermanns, M.I.; Bantz, C.; Maskos, M.; Unger, R.E.; Kirkpatrick, C.J. Pulmonary surfactant augments cytotoxicity of silica nanoparticles: Studies on an in vitro air–blood barrier model. Beilstein J. Nanotechnol. 2015, 6, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Capote, K.; Faulkner, J.; Possmayer, F.; Nag, K. Alteration of alveolar surfactant function by reactive oxygen species. Lung Biol. Health Dis. 2005, 201, 425. [Google Scholar]
- Rodríguez-Capote, K.; Manzanares, D.; Haines, T.; Possmayer, F. Reactive Oxygen Species Inactivation of Surfactant Involves Structural and Functional Alterations to Surfactant Proteins SP-B and SP-C. Biophys. J. 2006, 90, 2808–2821. [Google Scholar] [CrossRef]
- Crowther, J.E.; Kutala, V.K.; Kuppusamy, P.; Ferguson, J.S.; Beharka, A.A.; Zweier, J.L.; McCormack, F.X.; Schlesinger, L.S. Pulmonary Surfactant Protein A Inhibits Macrophage Reactive Oxygen Intermediate Production in Response to Stimuli by Reducing NADPH Oxidase Activity. J. Immunol. 2004, 172, 6866–6874. [Google Scholar] [CrossRef] [PubMed]
- Bohovych, I.; Chan, S.S.; Khalimonchuk, O. Mitochondrial Protein Quality Control: The Mechanisms Guarding Mitochondrial Health. Antioxid. Redox Signal. 2015, 22, 977–994. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Bukowski, M.J.; Wider, J.M.; Reynolds, C.A.; Calo, L.; Lepore, B.; Tousignant, R.; Jones, M.; Przyklenk, K.; Sanderson, T.H. Mitochondrial dynamics following global cerebral ischemia. Mol. Cell. Neurosci. 2016, 76, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, V.; Lazzarino, G.; Amorini, A.M.; Signoretti, S.; Hill, L.J.; Porto, E.; Tavazzi, B.; Lazzarino, G.; Belli, A. Fusion or Fission: The Destiny of Mitochondria In Traumatic Brain Injury of Different Severities. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGAVERN, D.B. Microglia Development and Function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef] [PubMed]
- Lauro, C.; Limatola, C. Metabolic Reprograming of Microglia in the Regulation of the Innate Inflammatory Response. Front. Immunol. 2020, 11, 493. [Google Scholar] [CrossRef]
- Humphrey, S.M.; Cartner, L.A.; Holliss, D.G. Critical early metabolic changes associated with myocardial recovery or failure after total ischaemia in the rat heart. Basic Res. Cardiol. 1987, 82, 304–316. [Google Scholar] [CrossRef]
- Amorini, A.M.; Lazzarino, G.; Di Pietro, V.; Signoretti, S.; Lazzarino, G.; Belli, A.; Tavazzi, B. Metabolic, enzymatic and gene involvement in cerebral glucose dysmetabolism after traumatic brain injury. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 679–687. [Google Scholar] [CrossRef]
- Ferreira, I.L.; Resende, R.; Ferreiro, E.; Rego, A.C.; Pereira, C.F. Multiple defects in energy metabolism in alzheimer’s disease. Curr. Drug Targets 2010, 11, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rapun, R.; De Matteis, L.; Ambrosone, A.; Garcia-Embid, S.; Gutierrez, L.; de la Fuente, J.M. Targeted nanoparticles for the treatment of alzheimer’s disease. Curr. Pharm. Des. 2017, 23, 1927–1952. [Google Scholar] [CrossRef]
- Bejarano, J.; Navarro-Marquez, M.; Morales-Zavala, F.; Morales, J.O.; Garcia-Carvajal, I.; Araya-Fuentes, E.; Flores, Y.; Verdejo, H.E.; Castro, P.F.; Lavandero, S.; et al. Nanoparticles for diagnosis and therapy of atherosclerosis and myocardial infarction: Evolution toward prospective theranostic approaches. Theranostics 2018, 8, 4710–4732. [Google Scholar] [CrossRef]
- Fyfe, I. Nanoparticles improve outcomes of traumatic brain injury in mice. Nat. Rev. Neurol. 2020, 16, 129. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free. Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; McBean, G.J.; Cindric, M.; Egea, J.; López, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox Control of Microglial Function: Molecular Mechanisms and Functional Significance. Antioxid. Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef]
- Zuberek, M.; Grzelak, A. Nanoparticles-Caused Oxidative Imbalance. Adv. Exp. Med. Biol. 2018, 1048, 85–98. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Garcia-Hevia, L.; Valiente, R.; Gonzalez, J.; Fernandez-Luna, J.L.; Villegas, J.C.; Fanarraga, M.L. Anti-cancer cytotoxic effects of multiwalled carbon nanotubes. Curr. Pharm. Des. 2015, 21, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, J.; Samadder, A.; Paul, A.; Khuda-Bukhsh, A.R. Efficacy of PLGA-loaded apigenin nanoparticles in Benzo[a]pyrene and ultraviolet-B induced skin cancer of mice: Mitochondria mediated apoptotic signalling cascades. Food Chem. Toxicol. 2013, 62, 670–680. [Google Scholar] [CrossRef]
- Russell-Schulz, B.; Booth, V.; Morrow, M.R. Perturbation of DPPC/POPG bilayers by the N-terminal helix of lung surfactant protein SP-B: A 2H NMR study. Eur. Biophys. J. 2009, 38, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Farver, R.S.; Mills, F.D.; Antharam, V.C.; Chebukati, J.N.; Fanucci, G.E.; Long, J.R. Lipid Polymorphism Induced by Surfactant Peptide SP-B1-25. Biophys. J. 2010, 99, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Mucci, N.J.; Tan, M.L.; Steckley, A.; Zhang, T.; Forrest, M.L.; Dhar, P. Phospholipid Composition Modulates Carbon Nanodiamond-Induced Alterations in Phospholipid Domain Formation. Langmuir 2015, 31, 5093–5104. [Google Scholar] [CrossRef]
- Caruso, G.; Distefano, D.A.; Parlascino, P.; Fresta, C.G.; Lazzarino, G.; Lunte, S.M.; Nicoletti, V.G. Receptor-mediated toxicity of human amylin fragment aggregated by short- and long-term incubations with copper ions. Mol. Cell. Biochem. 2016, 425, 85–93. [Google Scholar] [CrossRef]
- Musso, N.; Caruso, G.; Bongiorno, D.; Grasso, M.; Bivona, D.; Campanile, F.; Caraci, F.; Stefani, S. Different Modulatory Effects of Four Methicillin-Resistant Staphylococcus aureus Clones on MG-63 Osteoblast-Like Cells. Biomolecules 2021, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Fresta, C.G.; Hogard, M.L.; Caruso, G.; Costa, E.E.M.; Lazzarino, G.; Lunte, S.M. Monitoring carnosine uptake by RAW 264.7 macrophage cells using microchip electrophoresis with fluorescence detection. Anal. Methods 2016, 9, 402–408. [Google Scholar] [CrossRef]
- Mainz, E.R.; Gunasekara, D.B.; Caruso, G.; Jensen, D.T.; Hulvey, M.K.; Da Silva, J.A.F.; Metto, E.C.; Culbertson, A.H.; Culbertson, C.T.; Lunte, S.M. Monitoring intracellular nitric oxide production using microchip electrophoresis and laser-induced fluorescence detection. Anal. Methods 2012, 4, 414–420. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Fidilio, A.; O’Donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages. Antioxidants 2019, 8, 281. [Google Scholar] [CrossRef]
- De Campos, R.P.; Siegel, J.M.; Fresta, C.G.; Caruso, G.; da Silva, J.A.; Lunte, S.M. Indirect detection of superoxide in raw 264.7 macrophage cells using microchip electrophoresis coupled to laser-induced fluorescence. Anal. Bioanal. Chem. 2015, 407, 7003–7012. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Martinez-Becerra, F.; Antonio, L.; Johnson, R.T.; De Campos, R.P.S.; Siegel, J.M.; Wijesinghe, M.B.; Lazzarino, G.; Lunte, S.M. Carnosine modulates nitric oxide in stimulated murine RAW 264.7 macrophages. Mol. Cell. Biochem. 2017, 431, 197–210. [Google Scholar] [CrossRef]
- Lazzarino, G.; Amorini, A.M.; Fazzina, G.; Vagnozzi, R.; Signoretti, S.; Donzelli, S.; Di Stasio, E.; Giardina, B.; Tavazzi, B. Single-sample preparation for simultaneous cellular redox and energy state determination. Anal. Biochem. 2003, 322, 51–59. [Google Scholar] [CrossRef]
- Romitelli, F.; Santini, S.A.; Chierici, E.; Pitocco, D.; Tavazzi, B.; Amorini, A.M.; Lazzarino, G.; Di Stasio, E. Comparison of nitrite/nitrate concentration in human plasma and serum samples measured by the enzymatic batch Griess assay, ion-pairing HPLC and ion-trap GC–MS: The importance of a correct removal of proteins in the Griess assay. J. Chromatogr. B 2007, 851, 257–267. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, G.; Fresta, C.G.; Costantino, A.; Lazzarino, G.; Amorini, A.M.; Lazzarino, G.; Tavazzi, B.; Lunte, S.M.; Dhar, P.; Gulisano, M.; et al. Lung Surfactant Decreases Biochemical Alterations and Oxidative Stress Induced by a Sub-Toxic Concentration of Carbon Nanoparticles in Alveolar Epithelial and Microglial Cells. Int. J. Mol. Sci. 2021, 22, 2694. https://doi.org/10.3390/ijms22052694
Caruso G, Fresta CG, Costantino A, Lazzarino G, Amorini AM, Lazzarino G, Tavazzi B, Lunte SM, Dhar P, Gulisano M, et al. Lung Surfactant Decreases Biochemical Alterations and Oxidative Stress Induced by a Sub-Toxic Concentration of Carbon Nanoparticles in Alveolar Epithelial and Microglial Cells. International Journal of Molecular Sciences. 2021; 22(5):2694. https://doi.org/10.3390/ijms22052694
Chicago/Turabian StyleCaruso, Giuseppe, Claudia G. Fresta, Angelita Costantino, Giacomo Lazzarino, Angela M. Amorini, Giuseppe Lazzarino, Barbara Tavazzi, Susan M. Lunte, Prajnaparamita Dhar, Massimo Gulisano, and et al. 2021. "Lung Surfactant Decreases Biochemical Alterations and Oxidative Stress Induced by a Sub-Toxic Concentration of Carbon Nanoparticles in Alveolar Epithelial and Microglial Cells" International Journal of Molecular Sciences 22, no. 5: 2694. https://doi.org/10.3390/ijms22052694
APA StyleCaruso, G., Fresta, C. G., Costantino, A., Lazzarino, G., Amorini, A. M., Lazzarino, G., Tavazzi, B., Lunte, S. M., Dhar, P., Gulisano, M., & Caraci, F. (2021). Lung Surfactant Decreases Biochemical Alterations and Oxidative Stress Induced by a Sub-Toxic Concentration of Carbon Nanoparticles in Alveolar Epithelial and Microglial Cells. International Journal of Molecular Sciences, 22(5), 2694. https://doi.org/10.3390/ijms22052694












