A2A Receptor Dysregulation in Dystonia DYT1 Knock-Out Mice
Abstract
1. Introduction
2. Results
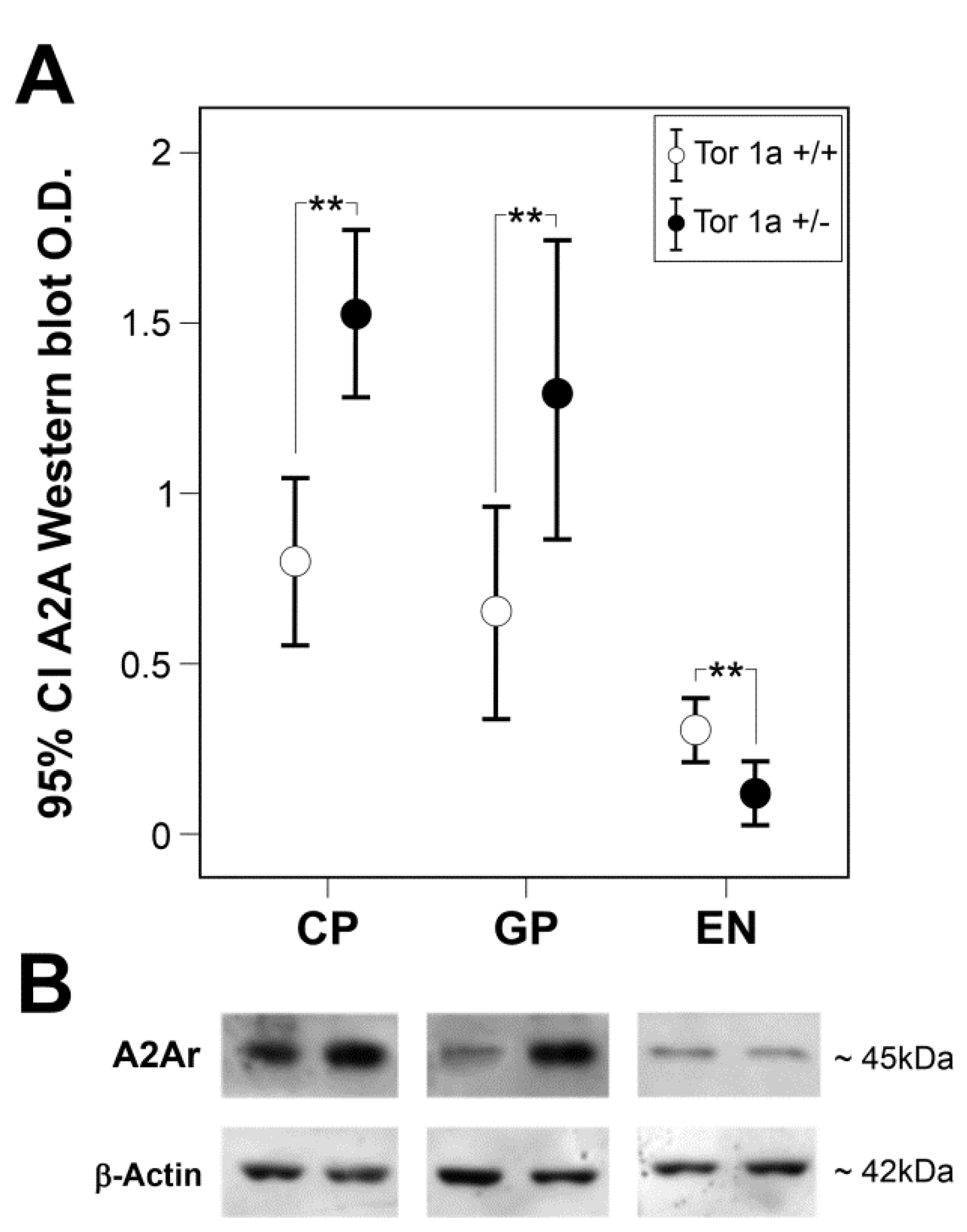
2.1. Expression of A2A Receptors
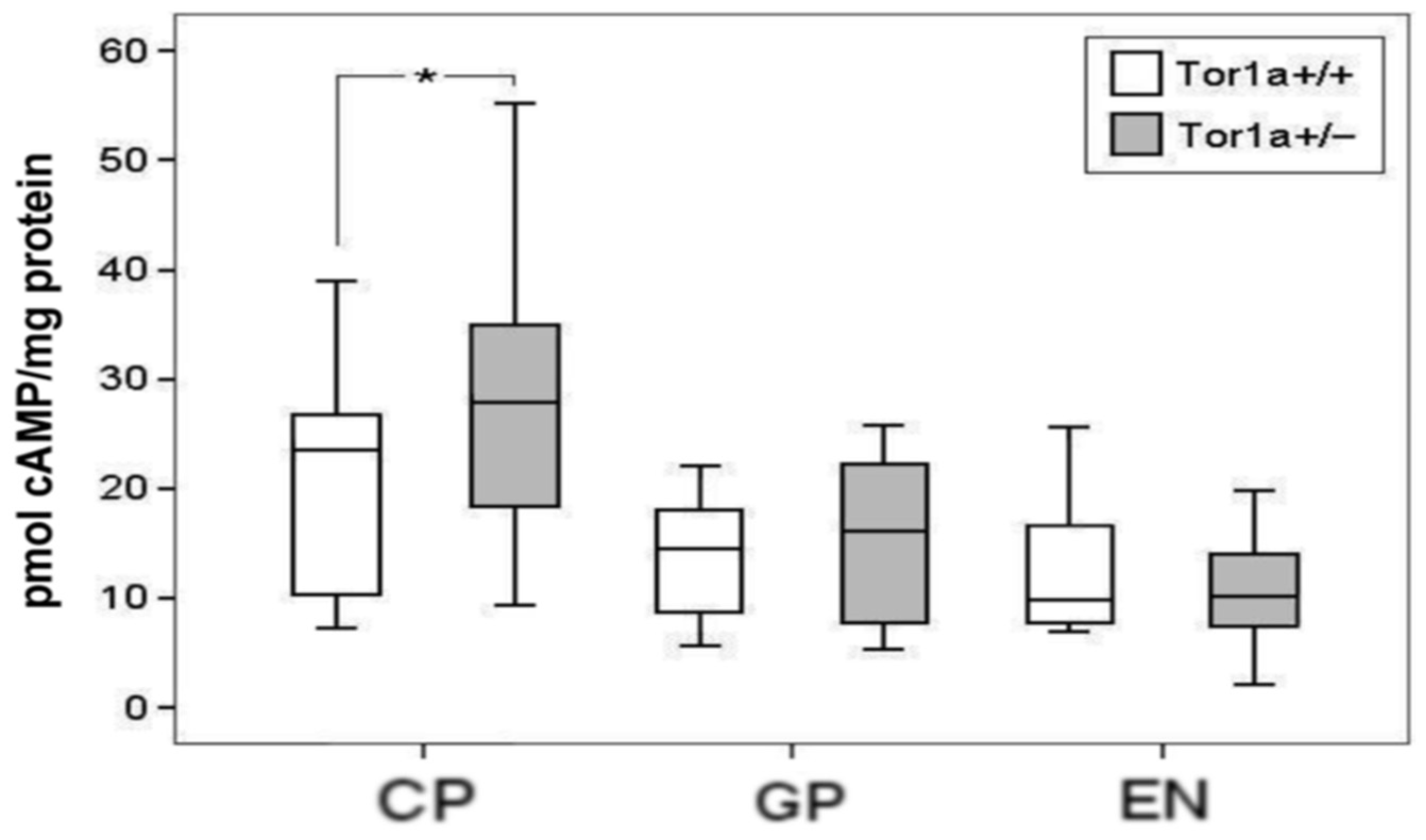
2.2. Cyclic AMP Levels
2.3. Expression of A2A mRNA
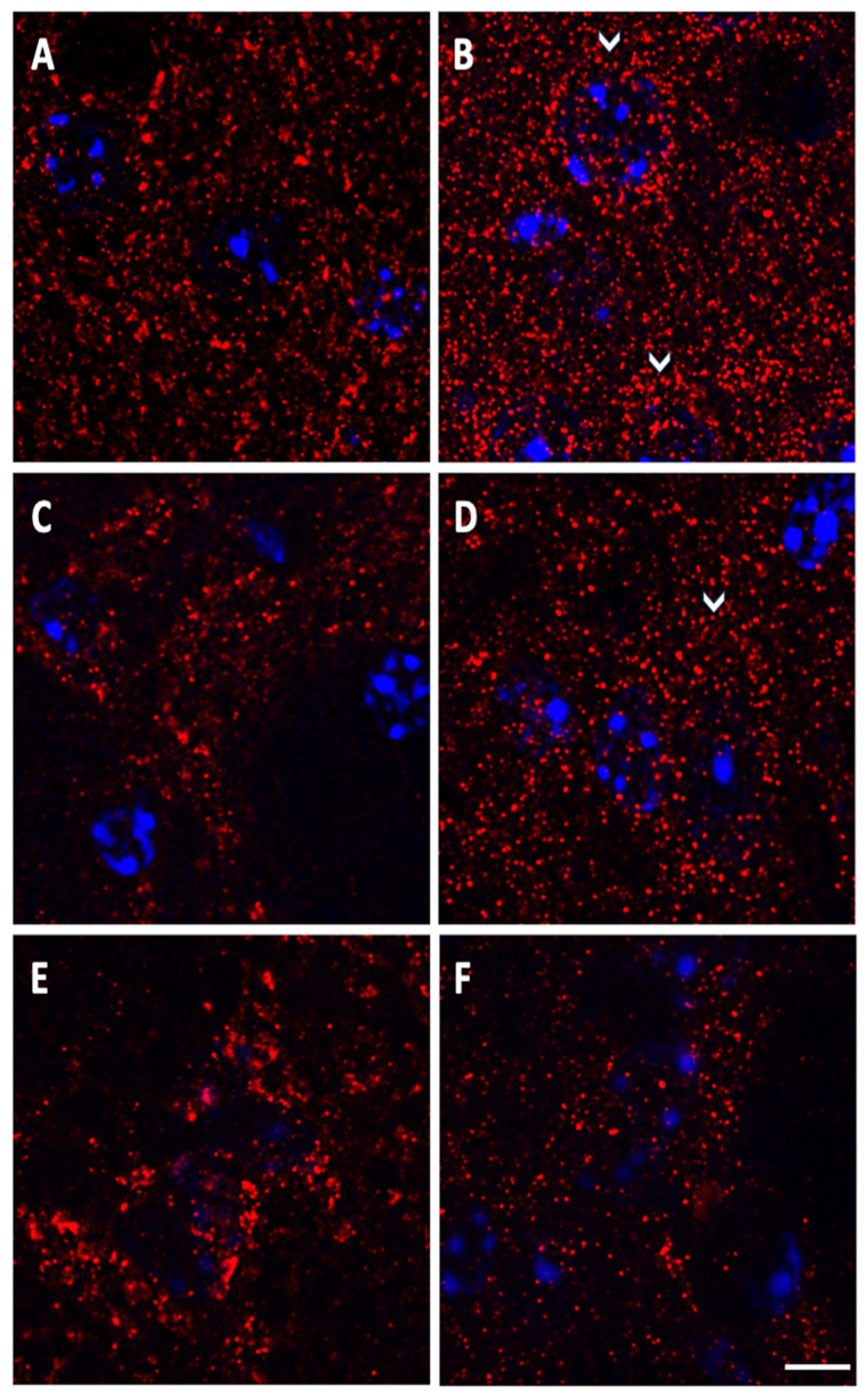
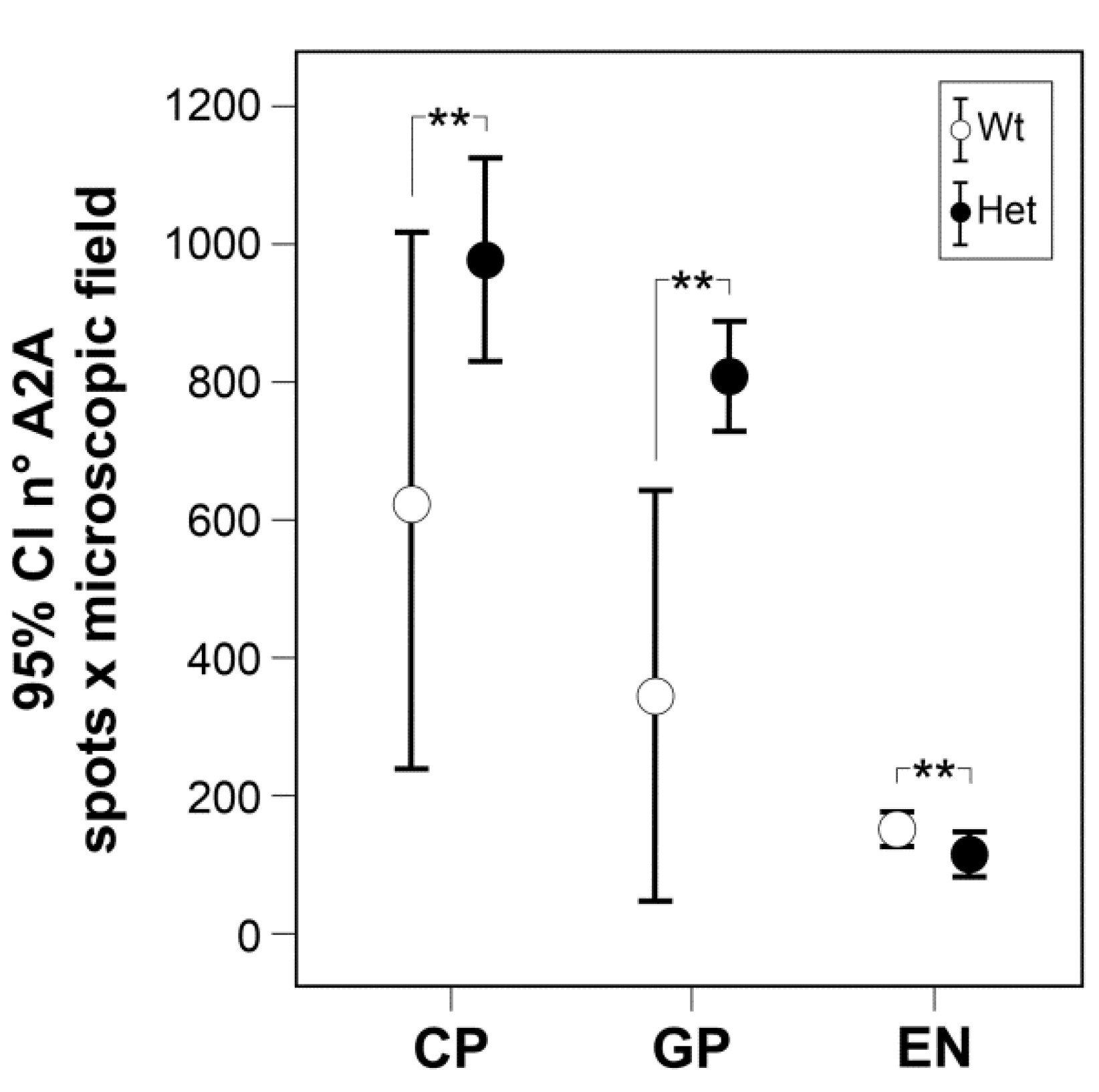
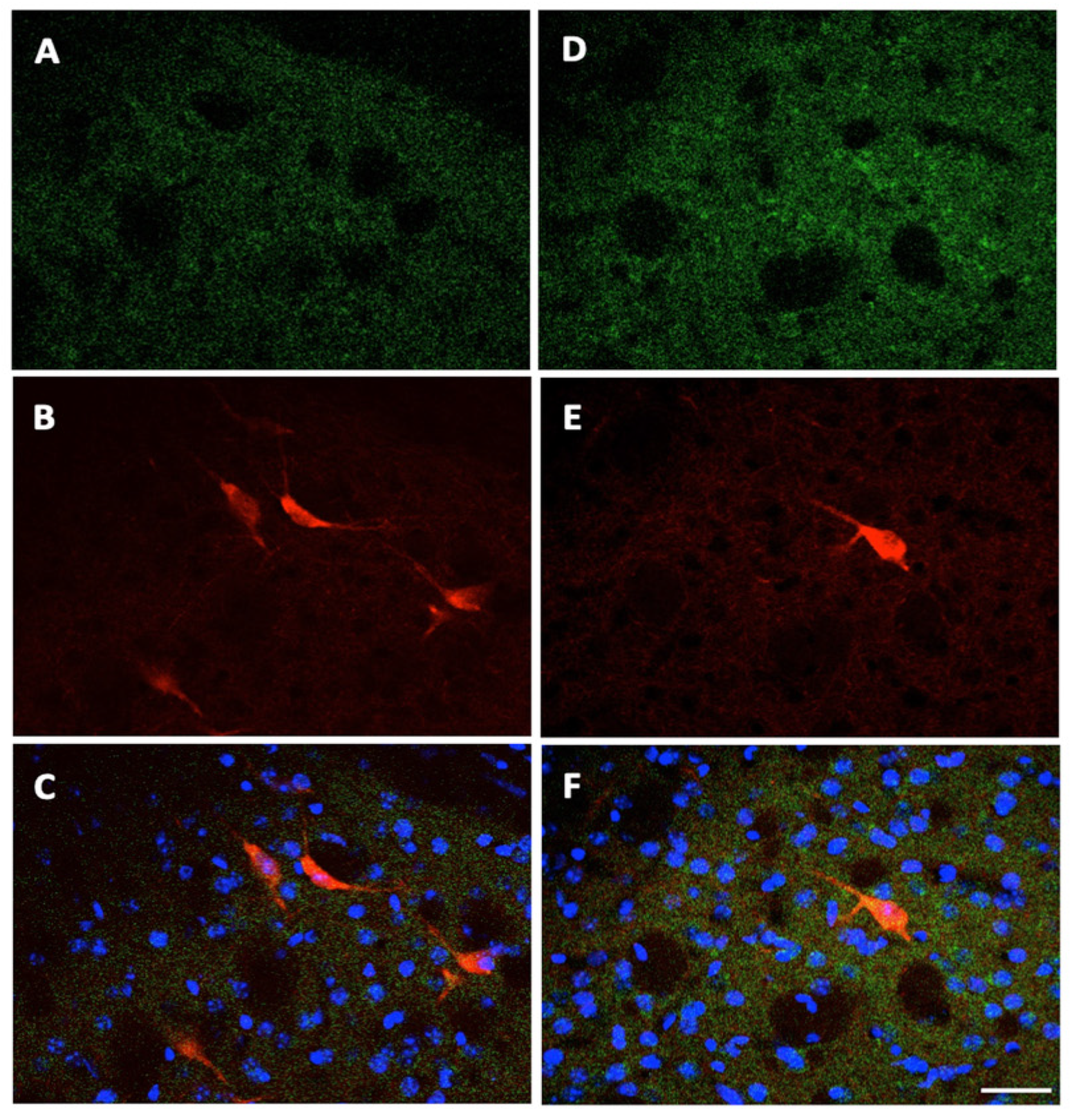
2.4. A2A Immunofluorescence in Confocal Microscopy
2.5. Colocalization of A2A in Cholinergic Neurons
3. Discussion
3.1. Morphological Characteristics of A2A Receptor Aggregates
3.2. Distribution of A2A Aggregates
3.3. Changes in Second Messenger cAMP
3.4. Changes in A2A mRNA
4. Materials and Methods
4.1. Animals
4.2. Quantitative Analysis of A2A Protein
4.3. cAMP Measurement
4.4. RNA Extraction and Real–Time PCR
4.5. A2A Immunofluorescence in Confocal Microscopy
4.6. A2A Localization in Striatal Cholinergic Interneurons
4.7. Experimental Design and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsden, C.D.; Sheehy, M.P. Writer’s cramp. Trends Neurosci. 1990, 13, 148–153. [Google Scholar] [CrossRef]
- Berardelli, A.; Rothwell, J.C.; Hallett, M.; Thompson, P.D.; Manfredi, M.; Marsden, C.D. The pathophysiology of primary dystonia. Brain 1998, 121, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Bhatia, K.; Bressman, S.B.; Delong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Lanska, D.J. Chapter 33: The History of Movement Disorders. In The History of Neurology. Handbook of Clinical Neurology; Aminoff, M., Boller, F., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 95, pp. 501–546. [Google Scholar]
- Standaert, D.G. Update on the pathology of dystonia. Neurobiol. Dis. 2011, 42, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Ozelius, L.J.; Hewett, J.W.; Page, C.E.; Bressman, S.B.; Kramer, P.L.; Shalish, C.; de Leon, D.; Brin, M.F.; Raymond, D.; Corey, D.P.; et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat. Genet. 1997, 17, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Ma, Y.; Okulski, J.; Dhawan, V.; Chaly, T.; Carbon, M.; Bressman, S.B.; Eidelberg, D. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology 2005, 64, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Martella, G.; Tscherter, A.; Bonsi, P.; Sharma, N.; Bernardi, G.; Standaert, D.G. Altered responses to dopaminergic D2 receptor activation and N-type calcium currents in striatal cholinergic interneurons in a mouse model of DYT1 dystonia. Neurobiol. Dis. 2006, 24, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.D.; Spealman, R.D.; Zhang, J. Dopaminergic signaling in dendritic spines. Biochem. Pharmacol. 2008, 75, 2055–2069. [Google Scholar] [CrossRef] [PubMed]
- Carbon, M.; Niethammer, M.; Peng, S.; Raymond, D.; Dhawan, V.; Chaly, T.; Ma, Y.; Bressman, S.; Eidelberg, D. Abnormal striatal and thalamic dopamine neurotransmission: Genotype-related features of dystonia. Neurology 2009, 72, 2097–2103. [Google Scholar] [CrossRef]
- Napolitano, F.; Pasqualetti, M.; Usiello, A.; Santini, E.; Pacini, G.; Sciamanna, G.; Errico, F.; Tassone, A.; Di Dato, V.; Martella, G.; et al. Dopamine D2 receptor dysfunction is rescued by adenosine A2A receptor antagonism in a model of DYT1 dystonia. Neurobiol. Dis. 2010, 38, 434–445. [Google Scholar] [CrossRef]
- Sciamanna, G.; Tassone, A.; Martella, G.; Mandolesi, G.; Puglisi, F.; Cuomo, D.; Madeo, G.; Ponterio, G.; Standaert, D.G.; Bonsi, P.; et al. Developmental profile of the aberrant dopamine D2 receptors response in striatal cholinergic interneurons in DYT1 dystonia. PLoS ONE 2011, 6, e24261. [Google Scholar] [CrossRef] [PubMed]
- Sciamanna, G.; Hollis, R.; Ball, C.; Martella, G.; Tassone, A.; Marshall, A.; Parsons, D.; Li, X.; Yokoi, F.; Zhang, L.; et al. Cholinergic dysregulation produced by selective inactivation of the dystonia-associated protein torsinA. Neurobiol. Dis. 2012, 47, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, F.; Dang, M.T.; Li, J.; Standaert, D.G.; Li, Y. Motor deficits and decreased striatal dopamine receptor 2 binding activity in the striatum-specific Dyt1 conditional knock- out mice. PLoS ONE 2011, 6, e24539. [Google Scholar] [CrossRef] [PubMed]
- Martella, G.; Maltese, M.; Nistico, R.; Schirinzi, T.; Madeo, G.; Sciamanna, G.; Ponterio, G.; Tassone, A.; Mandolesi, G.; Vanni, V.; et al. Regional specificity of synaptic plasticity deficits in a knock-in mouse model of DYT1 dystonia. Neurobiol. Dis. 2014, 65, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Scarduzio, M.; Zimmerman, C.N.; Jaunarais, K.L.; Wang, Q.; Standaert, D.G.; McMahon, L.L. Strength of cholinergic tone dictates the polarity of dopamine D2 receptor modulation of striatal cholinergic interneuron excitability in DYT1 dystonia. Exp. Neurol. 2017, 295, 162–175. [Google Scholar] [CrossRef]
- Bonsi, P.; Ponterio, G.; Vanni, V.; Tassone, A.; Sciamanna, G.; Migliarini, S.; Martella, G.; Meringolo, M.; Dehay, B.; Doudnikoff, E.; et al. RGS 9-2 rescues dopamine D2 receptor levels and signaling in DYT1 dystonia mouse models. EMBO Mol. Med. 2019, 11, e9283. [Google Scholar] [CrossRef]
- D’Angelo, V.; Paldino, E.; Cardarelli, S.; Sorge, R.; Fusco, F.R.; Biagioni, S.; Mercuri, N.B.; Giorgi, M.; Sancesario, G. Dystonia: Sparse synapses for D2 receptors in the striatum of a DYT1 knock-out mouse model. Int J. Mol. Sci. 2020, 21, 1073. [Google Scholar] [CrossRef] [PubMed]
- Maltese, M.; Martella, G.; Imbriani, P.; Schuermans, J.; Billion, K.; Sciamanna, G.; Farook, F.; Ponterio, G.; Tassone, A.; Santoro, M.; et al. Abnormal striatal plasticity in a DYT11/SGCE myoclonus dystonia mouse model is reversed by adenosine A2A receptor inhibition. Neurobiol. Dis. 2017, 108, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Yu-Taeger, L.; Ott, T.; Bonsi, P.; Tomczak, C.; Wassouf, Z.; Martella, G.; Sciamanna, G.; Imbriani, P.; Ponterio, G.; Tassone, A.; et al. Impaired dopamine- and adenosine-mediated signaling and plasticity in a novel rodent model for DYT25 dystonia. Neurobiol. Dis. 2020, 134, 104634. [Google Scholar] [CrossRef] [PubMed]
- Preston, Z.; Lee, K.; Widdowson, L.; Freeman, T.; Dixon, A.K.; Richardson, P.J. Adenosine receptor expression and function in rat striatal cholinergic interneurons. Brit. J. Pharm. 2000, 130, 886–890. [Google Scholar] [CrossRef]
- Tozzi, A.; de Iure, A.; Di Filippo, M.; Tantucci, M.; Costa, C.; Borsini, F.; Ghiglieri, V.; Giampà, C.; Fusco, F.R.; Picconi, B.; et al. The Distinct Role of Medium Spiny Neurons and Cholinergic Interneurons in the D2/A2A Receptor Interaction in the Striatum: Implications for Parkinson’s Disease. J. Neurosci. 2011, 31, 1850–1862. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, S.; Jacobs, O.; Vanderhaeghen, J.J. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: An in-situ hybridization histochemistry study. J. Neurochem. 1991, 57, 1062–1067. [Google Scholar] [CrossRef]
- Fink, J.S.; Weaver, D.R.; Rivkees, S.A.; Peterfreund, R.A.; Pollack, A.E.; Adler, E.M.; Reppert, S.M. Molecular cloning of the rat A2 adenosine receptor: Selective co-expression with D2 dopamine receptors in rat striatum. Mol. Brain Res. 1992, 14, 186–195. [Google Scholar] [CrossRef]
- Schiffmann, S.N.; Fisone, G.; Moresco, R.; Cunha, R.A.; Ferré, S. Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol. 2007, 83, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Marcellino, D.; Borroto-Escuela, D.O.; Guescini, M.; Fernández-Dueñas, V.; Tanganelli, S.; Rivera, A.; Ciruela, F.; Agnati, L.F. Adenosine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 2010, 16, e18–e42. [Google Scholar] [CrossRef] [PubMed]
- Rosin, D.L.; Robeva, A.; Woodard, R.L.; Guyenet, P.G.; Linden, J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J. Comp. Neurol. 1998, 401, 163–186. [Google Scholar] [CrossRef]
- Hettinger, B.D.; Lee, A.; Linden, J.; Rosin, D.L. Ultrastructural Localization of Adenosine A2A Receptors Suggests Multiple Cellular Sites for Modulation of GABAergic Neurons in Rat Striatum. J. Comp. Neurol. 2001, 431, 331–346. [Google Scholar] [CrossRef]
- Bogenpohl, J.W.; Ritter, S.L.; Hall, R.A.; Smith, Y. Adenosine A2A Receptor in the Monkey Basal Ganglia: Ultrastructural Localization and Colocalization with the Metabotropic Glutamate Receptor 5 in the Striatum. J. Comp. Neurol. 2012, 520, 570–589. [Google Scholar] [CrossRef]
- Santuy, A.; Rodríguez, J.R.; De Felipe, J.; Merchán-Pérez, A. Study of the size and shape of synapses in the juvenile rat somatosensory cortex with 3D electron microscopy. eNeuro 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, C.; Luján, R.; Uchigashima, M.; Simoes, A.P.; Lerner, T.N. Key Modulatory Role of Presynaptic Adenosine A2A Receptors in Cortical Neurotransmission to the Striatal Direct Pathway. Sci. World J. 2009, 9, 1321–1344. [Google Scholar] [CrossRef] [PubMed]
- Sciamanna, G.; Ponterio, G.; Vanni, V.; Laricchiuta, D.; Martella, G.; Bonsi, P.; Meringolo, M.; Tassone, A.; Mercuri, N.B.; Pisani, A. Optogenetic Activation of Striatopallidal Neurons Reveals Altered HCN Gating in DYT1 Dystonia. Cell Rep. 2020, 31, 107644. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, L.; Beggiato, S.; Tomasini, M.C.; Fuxe, K.; Antonelli, T.; Tanganelli, S. A2A/D2 receptor heteromerization in a model of Parkinson’s disease. Focus on striatal aminoacidergic signaling. Brain Res. 2012, 1476, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Shindou, T. Modulation of GABAergic transmission in the striatopallidal system by adenosine A2A receptors: A potential mechanism for the antiparkinsonian effects of A2A antagonists. Neurology 2003, 61, S44–S448. [Google Scholar] [CrossRef]
- Montes, J.; Pena, J.M.; De Felipe, J.; Herreras, O.; Merchan-Perez, A. The Influence of Synaptic Size on AMPA Receptor Activation: A Monte Carlo Model. PLoS ONE 2015, 10, e0130924. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, F.; Dang, M.T.; Liu, J.; Gandre, J.R.; Kwon, K.; Yuen, R.; Li, Y. Decreased dopamine receptor 1 activity and impaired motor-skill transfer in Dyt1ΔGAG heterozygous knock-in mice. Behav. Brain Res. 2015, 279, 202–210. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, V.; Castelli, V.; Giorgi, M.; Cardarelli, S.; Saverioni, I.; Palumbo, F.; Bonsi, P.; Pisani, A.; Giampà, C.; Sorge, R.; et al. Phosphodiesterase-10A Inverse Changes in Striatopallidal and Striatoentopeduncular Pathways of a Transgenic Mouse Model of DYT1 Dystonia. J. Neurosci. 2017, 37, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Adamowicz, W.; Eldren, W.D.; Jacowski, A.B.; Kleiman, R.J.; Morton, D.G.; Stephenson, D.T.; Strick, C.A.; Williams, R.D.; Menniti, F.S. Cellular and Subcellular localization of PDE10A, a striatum-enriched phosphodiesterase. Neuroscience 2006, 139, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, A.; Fusco, F.R. Inhibition of phosphodiesterases as a strategy to achieve neuroprotection in Huntington’s disease. CNS Neurosci. Ther. 2018, 24, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Eskow Jaunarais, K.L.; Bonsi, P.; Chesselet, M.F.; Standaert, D.G.; Pisani, A. Striatal cholinergic dysfunction as a unifying theme in the pathophysiology of dystonia. Prog. Neurobiol. 2015, 127–128, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D.; O’Dowd, D.K. cAMP-Dependent Plasticity at Excitatory Cholinergic Synapses in Drosophila Neurons: Alterations in the Memory Mutant Dunce. J. Neurosci. 2000, 20, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Threlfell, S.; West, A.R. Review: Modulation of striatal neuron activity by cyclic nucleotide signaling and phosphodiesterase inhibition. Basal Ganglia 2013, 3, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D. Global and local missions of cAMP signaling in neural plasticity, learning, and memory. Front Pharmacol. 2015, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Bonsi, P.; Cuomo, D.; Martella, G.; Madeo, G.; Schirinzi, T.; Puglisi, F.; Ponterio, G.; Pisani, A. Centrality of Striatal Cholinergic Transmission in Basal Ganglia Function. Front. Neuroanat. 2011, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.K.; Gubiz, A.K.; Sirinathsinghji, D.J.S.; Richardson, P.J.; Freeman, T.C. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996, 18, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.J.; Mash, D.C.; Jenner, P. Adenosine A2A receptor mRNA expression in Parkinson’s disease. Neurosci. Lett. 2000, 291, 54–58. [Google Scholar] [CrossRef]
- Goodchild, R.E.; Kim, C.E.; Dauer, W.T. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron 2005, 48, 923–932. [Google Scholar] [CrossRef]
- Giorgi, M.; D’Angelo, V.; Esposito, Z.; Nuccetelli, V.; Sorge, R.; Martorana, A.; Stefani, A.; Bernardi, G.; Sancesario, G. Lowered cAMP and cGMP signalling in the brain during levodopa-induced dyskinesias in hemiparkinsonian rats: New aspects in the pathogenetic mechanisms. Eur. J. Neurosci. 2008, 28, 941–950. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angelo, V.; Giorgi, M.; Paldino, E.; Cardarelli, S.; Fusco, F.R.; Saverioni, I.; Sorge, R.; Martella, G.; Biagioni, S.; Mercuri, N.B.; et al. A2A Receptor Dysregulation in Dystonia DYT1 Knock-Out Mice. Int. J. Mol. Sci. 2021, 22, 2691. https://doi.org/10.3390/ijms22052691
D’Angelo V, Giorgi M, Paldino E, Cardarelli S, Fusco FR, Saverioni I, Sorge R, Martella G, Biagioni S, Mercuri NB, et al. A2A Receptor Dysregulation in Dystonia DYT1 Knock-Out Mice. International Journal of Molecular Sciences. 2021; 22(5):2691. https://doi.org/10.3390/ijms22052691
Chicago/Turabian StyleD’Angelo, Vincenza, Mauro Giorgi, Emanuela Paldino, Silvia Cardarelli, Francesca R. Fusco, Ilaria Saverioni, Roberto Sorge, Giuseppina Martella, Stefano Biagioni, Nicola B. Mercuri, and et al. 2021. "A2A Receptor Dysregulation in Dystonia DYT1 Knock-Out Mice" International Journal of Molecular Sciences 22, no. 5: 2691. https://doi.org/10.3390/ijms22052691
APA StyleD’Angelo, V., Giorgi, M., Paldino, E., Cardarelli, S., Fusco, F. R., Saverioni, I., Sorge, R., Martella, G., Biagioni, S., Mercuri, N. B., Pisani, A., & Sancesario, G. (2021). A2A Receptor Dysregulation in Dystonia DYT1 Knock-Out Mice. International Journal of Molecular Sciences, 22(5), 2691. https://doi.org/10.3390/ijms22052691









