Targeting the Extracellular Matrix in Abdominal Aortic Aneurysms Using Molecular Imaging Insights
Abstract
1. Introduction
2. The Role of the Extracellular Matrix in AAA
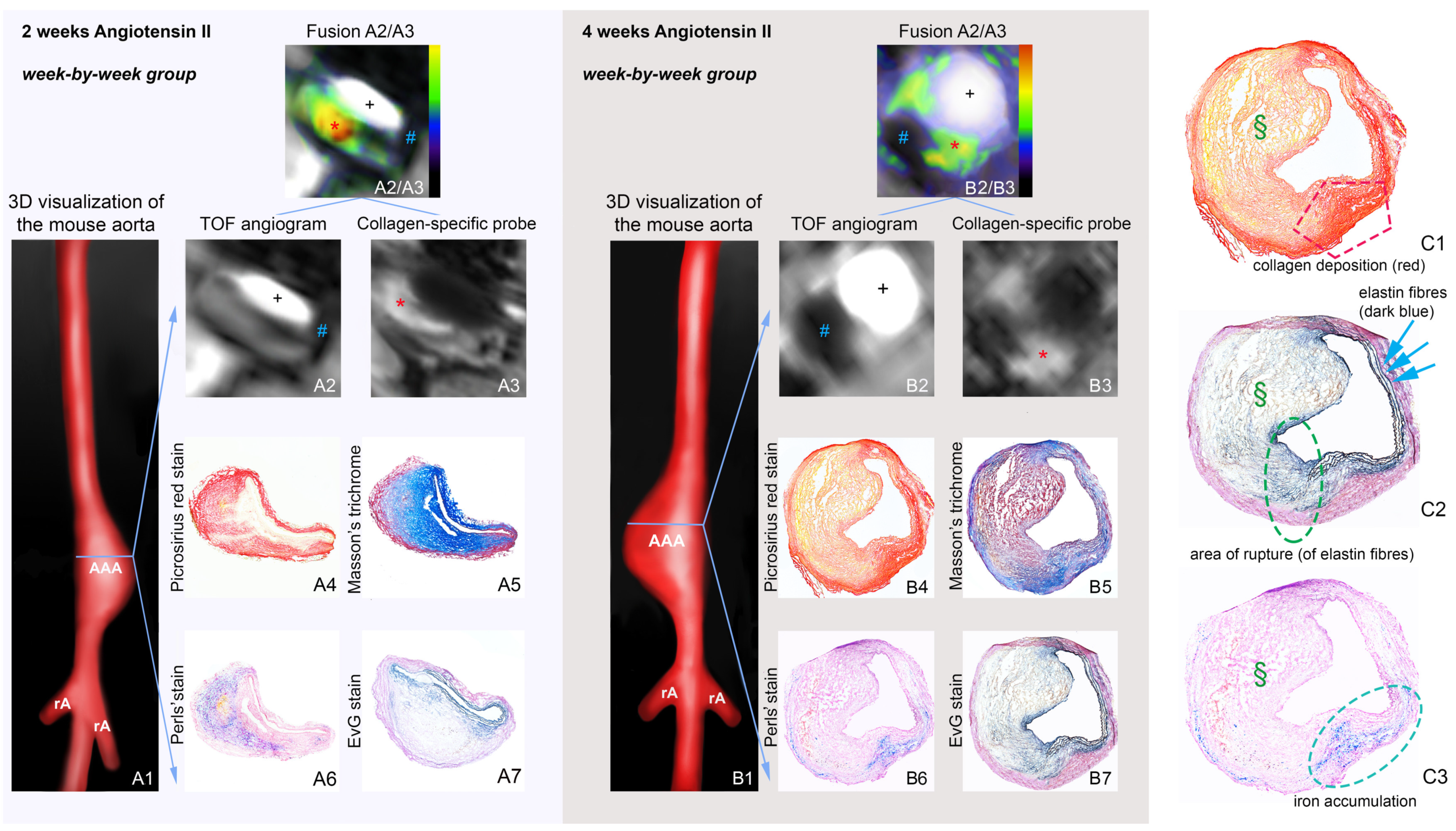
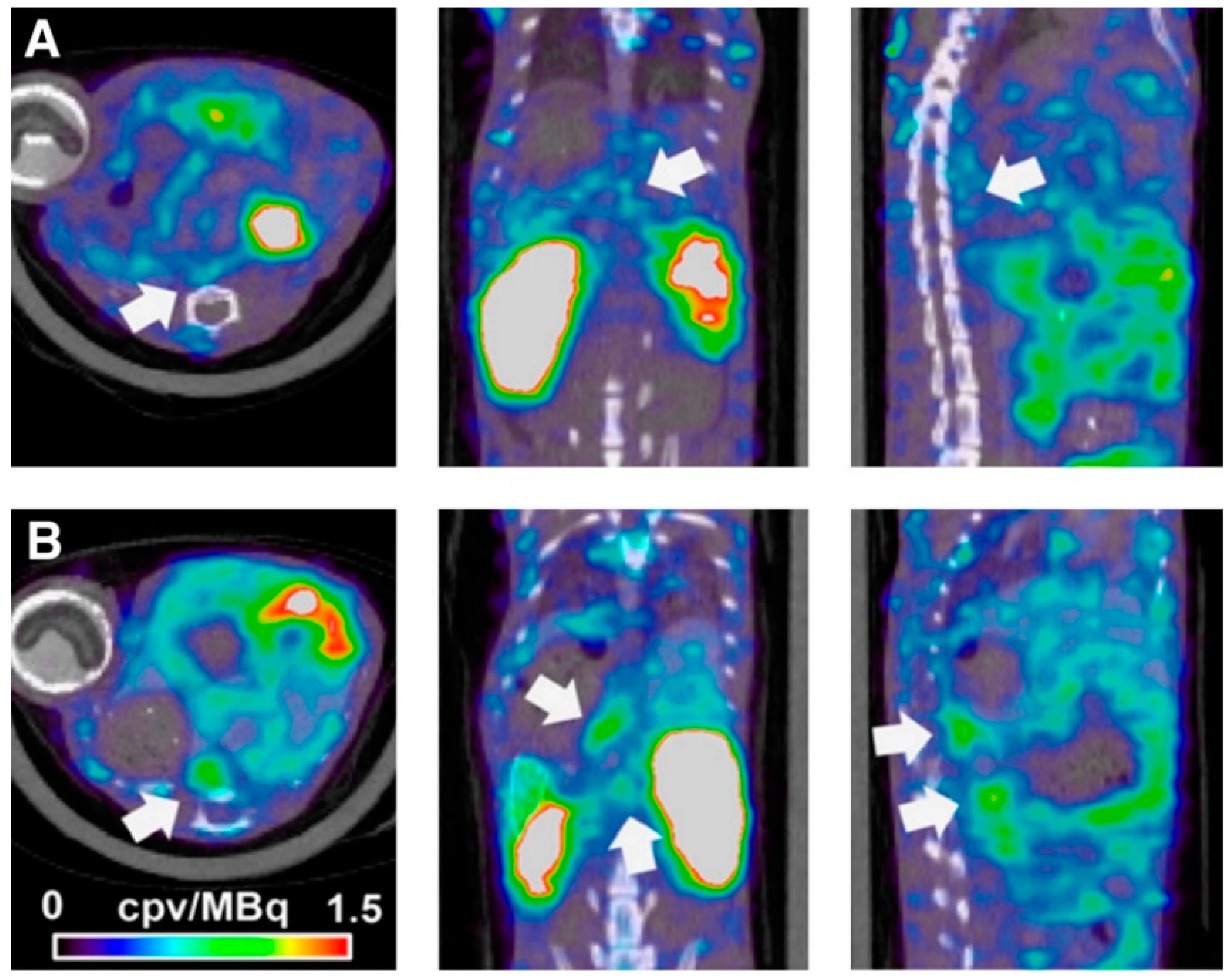
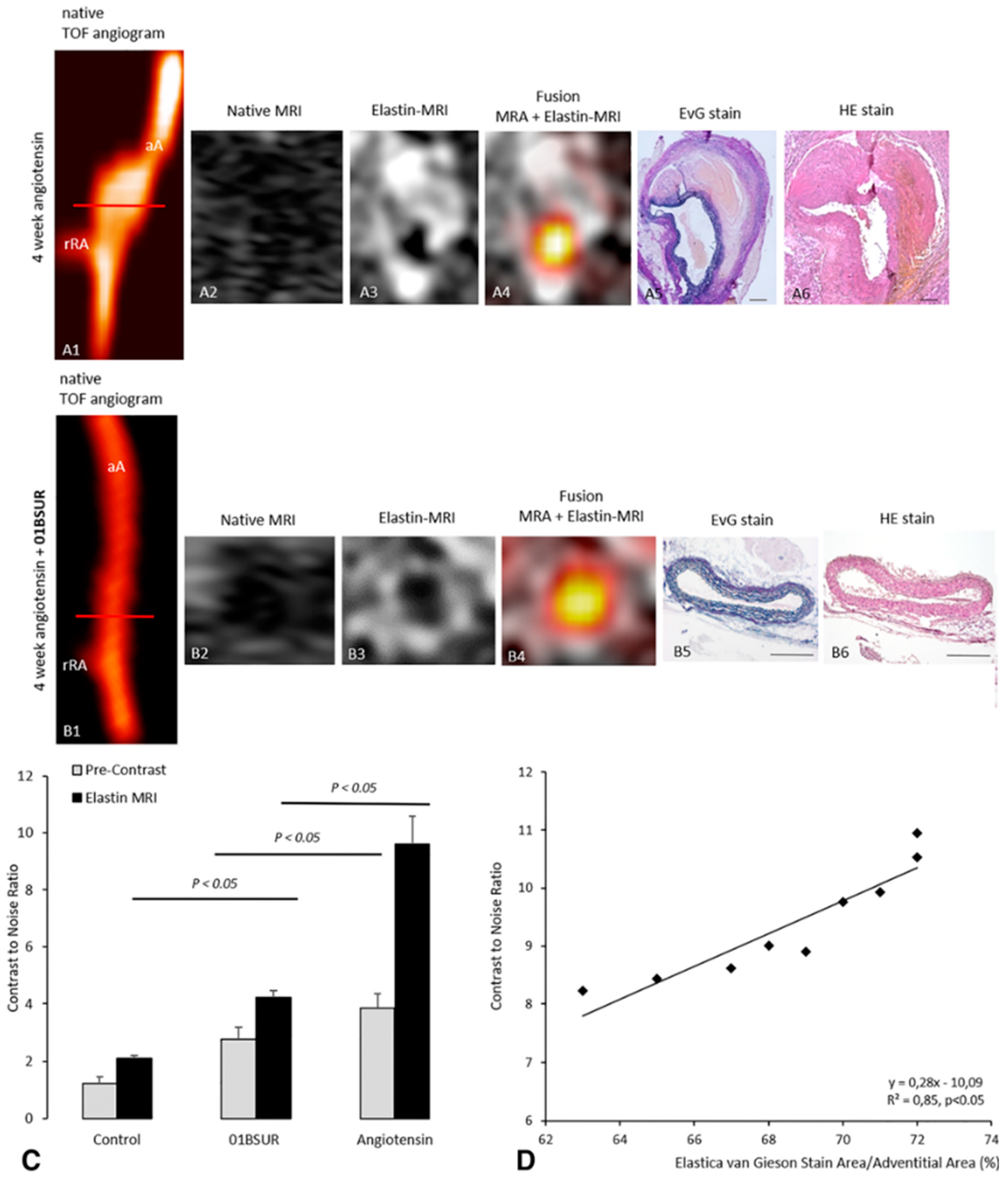
2.1. Molecular Magnetic Resonance Imaging for Identification of Potential Diagnostic Targets and Monitoring of Therapeutic Success
2.2. Pharmacologic Treatment Strategies Targeted to the ECM
2.2.1. MMPs as Pharmacologic Treatment Targets in AAA
Statins for Reducing MMP Levels
Doxycycline as a General MMP Inhibitor
Other MMP-Targeting Drugs
2.2.2. ADAMs/ADAMTS Inhibition
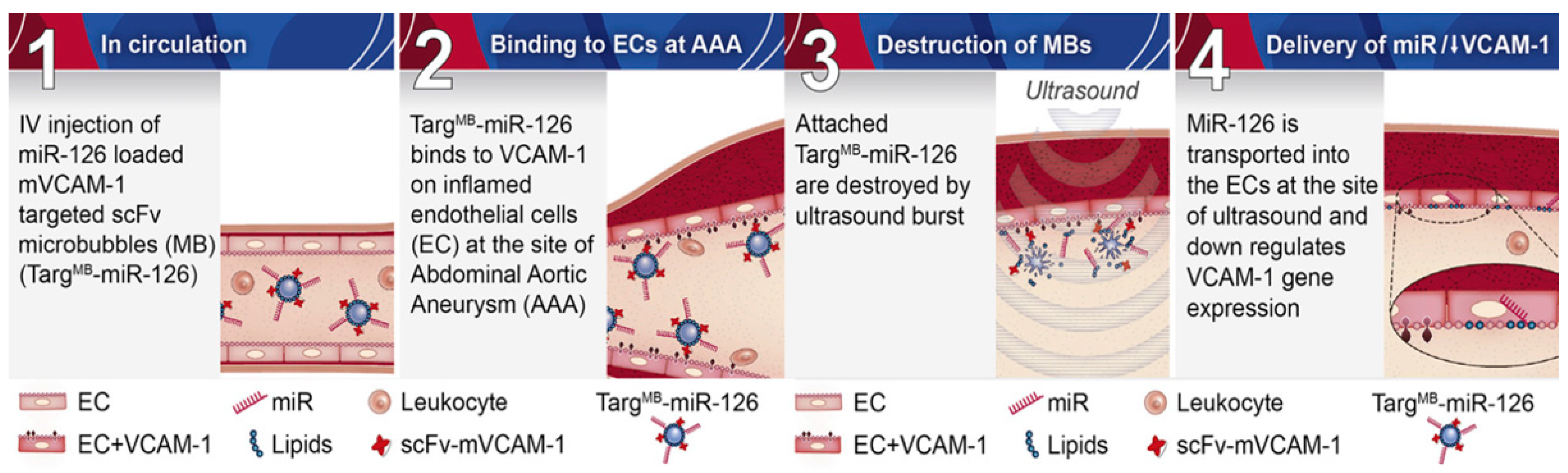
2.2.3. MikroRNA Inhibition
2.2.4. Interleukins
3. Discussion and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | Abdominal aortic aneurysm |
| ApoE | Apolipoprotein E |
| CCR2 | Chemokine receptor type 2 |
| ECM | Extracellular matrix |
| FDG | Fluorine-18-2-deoxyglucose |
| GZMB | Granzyme B |
| IL | Interleukin |
| MMP | Matrix metalloproteinases |
| MRI | Magnetic Resonance Imaging |
| PET | Positron emission tomography |
| PGG | Pentagalloyl glucose |
| PPE | Porcine pancreatic elastase |
| ROS | Reactive oxygen species |
| TGF | Transforming growth factor |
| TIMP | Tissue inhibitors of metalloproteases |
| USPIO | Ultrasmall superparamagnetic particles of iron oxide |
| VEGF | Vascular endothelial growth factor |
| VSMC | Vascular smooth muscle cells |
References
- Toczek, J.; Meadows, J.L.; Sadeghi, M.M. Novel Molecular Imaging Approaches to Abdominal Aortic Aneurysm Risk Stratification. Circ. Cardiovasc. Imaging 2016, 9, e003023. [Google Scholar] [CrossRef]
- Hallett, J.W. Management of Abdominal Aortic Aneurysms. Mayo Clin. Proc. 2000, 75, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Sakalihasan, N.; Limet, R.; Defawe, O. Abdominal aortic aneurysm. Lancet 2005, 365, 1577–1589. [Google Scholar] [CrossRef]
- Weissleder, R.; Mahmood, U. Molecular Imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef]
- Makowski, M.R.; Botnar, R.M. MR Imaging of the Arterial Vessel Wall: Molecular Imaging from Bench to Bedside. Radiology 2013, 269, 34–51. [Google Scholar] [CrossRef]
- Makowski, M.R.; Wiethoff, A.J.; Jansen, C.H.; Botnar, R.M. Molecular Imaging with Targeted Contrast Agents. Top. Magn. Reson. Imaging 2009, 20, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Pisani, L.J.; Dalal, A.R.; Emrich, F.; Dake, B.A.; Arakawa, M.; Onthank, D.C.; Cesati, R.R.; Robinson, S.P.; Milanesi, M.; et al. Assessment of Elastin Deficit in a Marfan Mouse Aneurysm Model Using an Elastin-Specific Magnetic Resonance Imaging Contrast Agent. Circ. Cardiovasc. Imaging 2014, 7, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.T. Could medical intervention work for aortic aneurysms? Am. J. Surg. 2004, 188, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.T. Invited commentary: Abdominal aortic aneurysm regression by medical treatment: Possibility or pipe dream? J. Vasc. Surg. 2006, 43, 1068–1069. [Google Scholar] [CrossRef]
- Sonbol, H.S. Extracellular matrix remodeling in human disease. J. Microsc. Ultrastruct. 2018, 6, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Keeling, W.B.; Armstrong, P.A.; Stone, P.A.; Bandyk, D.F.; Shames, M.L. An Overview of Matrix Metalloproteinases in the Pathogenesis and Treatment of Abdominal Aortic Aneurysms. Vasc. Endovasc. Surg. 2005, 39, 457–464. [Google Scholar] [CrossRef]
- Didangelos, A.; Yin, X.; Mandal, K.; Saje, A.; Smith, A.; Xu, Q.; Jahangiri, M.; Mayr, M. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: A proteomics approach. Mol. Cell Proteom. 2011, 10, M111.008128. [Google Scholar] [CrossRef]
- Yagi, H.; Nishigori, M.; Murakami, Y.; Osaki, T.; Muto, S.; Iba, Y.; Minatoya, K.; Ikeda, Y.; Ishibashi-Ueda, H.; Morisaki, T. Discovery of novel biomarkers for atherosclerotic aortic aneurysm through proteomics-based assessment of disease progression. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Kudo, A. Periostin in fibrillogenesis for tissue regeneration: Periostin actions inside and outside the cell. Cell. Mol. Life Sci. 2011, 68, 3201–3207. [Google Scholar] [CrossRef]
- Hakuno, D.; Kimura, N.; Yoshioka, M.; Mukai, M.; Kimura, T.; Okada, Y.; Yozu, R.; Shukunami, C.; Hiraki, Y.; Kudo, A. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J. Clin. Investig. 2010, 120, 2292–2306. [Google Scholar] [CrossRef]
- Yamashita, O.; Yoshimura, K.; Nagasawa, A.; Ueda, K.; Morikage, N.; Ikeda, Y.; Hamano, K. Periostin Links Mechanical Strain to Inflammation in Abdominal Aortic Aneurysm. PLoS ONE 2013, 8, e79753. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, A.; Quarto, C.; Bancone, C.; Castaldo, C.; Di Meglio, F.; Nurzynska, D.; De Santo, L.S.; De Feo, M.; Scardone, M.; Montagnani, S.; et al. Spatiotemporal patterns of smooth muscle cell changes in ascending aortic dilatation with bicuspid and tricuspid aortic valve stenosis: Focus on cell–matrix signaling. J. Thorac. Cardiovasc. Surg. 2008, 135, 8–18. [Google Scholar] [CrossRef]
- Brangsch, J.; Reimann, C.; Collettini, F.; Buchert, R.; Botnar, R.M.; Makowski, M.R. Molecular Imaging of Abdominal Aortic Aneurysms. Trends Mol. Med. 2017, 23, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Salarian, M.; Ibhagui, O.Y.; Yang, J.J. Molecular imaging of extracellular matrix proteins with targeted probes using magnetic resonance imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1622. [Google Scholar] [CrossRef] [PubMed]
- Brangsch, J.; Reimann, C.; Kaufmann, J.O.; Adams, L.C.; Onthank, D.C.; Thöne-Reineke, C.; Robinson, S.P.; Buchholz, R.; Karst, U.; Botnar, R.M.; et al. Concurrent Molecular Magnetic Resonance Imaging of Inflammatory Activity and Extracellular Matrix Degradation for the Prediction of Aneurysm Rupture. Circ. Cardiovasc. Imaging 2019, 12, e008707. [Google Scholar] [CrossRef]
- Adams, L.C.; Brangsch, J.; Reimann, C.; Kaufmann, J.O.; Buchholz, R.; Karst, U.; Botnar, R.M.; Hamm, B.; Makowski, M.R. Simultaneous molecular MRI of extracellular matrix collagen and inflammatory activity to predict abdominal aortic aneurysm rupture. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Botnar, R.M.; Brangsch, J.; Reimann, C.; Janssen, C.H.P.; Razavi, R.; Hamm, B.; Makowski, M.R. In Vivo Molecular Characterization of Abdominal Aortic Aneurysms Using Fibrin-Specific Magnetic Resonance Imaging. J. Am. Hear Assoc. 2018, 7, e007909. [Google Scholar] [CrossRef]
- Klink, A.; Heynens, J.; Herranz, B.; Lobatto, M.E.; Arias, T.; Sanders, H.M.H.F.; Strijkers, G.J.; Merkx, M.; Nicolay, K.; Fuster, V.; et al. In Vivo Characterization of a New Abdominal Aortic Aneurysm Mouse Model With Conventional and Molecular Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2011, 58, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Botnar, R.M.; Wiethoff, A.J.; Ebersberger, U.; Lacerda, S.; Blume, U.; Warley, A.; Jansen, C.H.; Onthank, D.C.; Cesati, R.R.; Razavi, R.; et al. In Vivo Assessment of Aortic Aneurysm Wall Integrity Using Elastin-Specific Molecular Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2014, 7, 679–689. [Google Scholar] [CrossRef]
- Investigators, M.R.S. Aortic Wall Inflammation Predicts Abdominal Aortic Aneurysm Expansion, Rupture, and Need for Surgical Repair. Circulation 2017, 136, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Conlisk, N.; Forsythe, R.O.; Hollis, L.; Doyle, B.J.; McBride, O.M.; Robson, J.M.; Wang, C.; Gray, C.D.; Semple, S.I.; MacGillivray, T.; et al. Exploring the Biological and Mechanical Properties of Abdominal Aortic Aneurysms Using USPIO MRI and Peak Tissue Stress: A Combined Clinical and Finite Element Study. J. Cardiovasc. Transl. Res. 2017, 10, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Makowski, M.R.; Wiethoff, A.; Ebersberger, U.; Blume, U.; Warley, A.; Jansen, C.; Onthank, D.C.; Cesati, R.R.; Razavi, R.; Marber, M.; et al. Molecular assessment of aortic aneurysm wall integrity using an elastin-specific MR imaging probe. J. Cardiovasc. Magn. Reson. 2013, 15, O4. [Google Scholar] [CrossRef]
- Brangsch, J.; Reimann, C.; Kaufmann, J.O.; Adams, L.C.; Onthank, D.; Thöne-Reineke, C.; Robinson, S.; Wilke, M.; Weller, M.; Buchholz, R.; et al. Molecular MR-Imaging for Noninvasive Quantification of the Anti-Inflammatory Effect of Targeting Interleukin-1β in a Mouse Model of Aortic Aneurysm. Mol. Imaging 2020, 19. [Google Scholar] [CrossRef]
- Lavin, B.; Lacerda, S.; Andia, M.E.; Lorrio, S.; Bakewell, R.; Smith, A.; Rashid, I.; Botnar, R.M.; Phinikaridou, A. Tropoelastin: An in vivo imaging marker of dysfunctional matrix turnover during abdominal aortic dilation. Cardiovasc. Res. 2019, 116, 995–1005. [Google Scholar] [CrossRef]
- Bazeli, R.; Coutard, M.; Duport, B.D.; Lancelot, E.; Corot, C.; Laissy, J.-P.; Letourneur, D.; Michel, J.-B.; Serfaty, J.-M. In Vivo Evaluation of a New Magnetic Resonance Imaging Contrast Agent (P947) to Target Matrix Metalloproteinases in Expanding Experimental Abdominal Aortic Aneurysms. Investig. Radiol. 2010, 45, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Golestani, R.; Razavian, M.; Nie, L.; Zhang, J.; Jung, J.-J.; Ye, Y.; De Roo, M.; Hilgerink, K.; Liu, C.; Robinson, S.P.; et al. Imaging vessel wall biology to predict outcome in abdominal aortic aneurysm. Circ. Cardiovasc. Imaging 2015, 8, e002471. [Google Scholar] [CrossRef] [PubMed]
- Toczek, J.; Ye, Y.; Gona, K.; Kim, H.-Y.; Han, J.; Razavian, M.; Golestani, R.; Zhang, J.; Wu, T.L.; Jung, J.-J.; et al. Preclinical Evaluation of RYM1, a Matrix Metalloproteinase–Targeted Tracer for Imaging Aneurysm. J. Nucl. Med. 2017, 58, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cheng, K.; Cheng, Z. Evaluation of a smart activatable MRI nanoprobe to target matrix metalloproteinases in the early-stages of abdominal aortic aneurysms. Nanomed. Nanotechnol. Biol. Med. 2020, 26, 102177. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.M.; Krassilnikova, S.; Zhang, J.; Gharaei, A.A.; Fassaei, H.R.; Esmailzadeh, L.; Kooshkabadi, A.; Edwards, S.; Yalamanchili, P.; Harris, T.D.; et al. Detection of injury-induced vascular remodeling by targeting activated alphavbeta3 integrin in vivo. Circulation 2004, 110, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Meoli, D.F.; Sadeghi, M.M.; Krassilnikova, S.; Bourke, B.N.; Giordano, F.J.; Dione, D.P.; Su, H.; Edwards, D.S.; Liu, S.; Harris, T.D.; et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J. Clin. Investig. 2004, 113, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Razavian, M.; Marfatia, R.; Mongue-Din, H.; Tavakoli, S.; Sinusas, A.J.; Zhang, J.; Nie, L.; Sadeghi, M.M. Integrin-Targeted Imaging of Inflammation in Vascular Remodeling. Arter. Thromb. Vasc. Biol. 2011, 31, 2820–2826. [Google Scholar] [CrossRef]
- English, S.J.; Sastriques, S.E.; Detering, L.; Sultan, D.; Luehmann, H.; Arif, B.; Heo, G.S.; Zhang, X.; Laforest, R.; Zheng, J.; et al. CCR2 Positron Emission Tomography for the Assessment of Abdominal Aortic Aneurysm Inflammation and Rupture Prediction. Circ. Cardiovasc. Imaging 2020, 13, e009889. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Nasu, T.; Osaki, T.; Hitomi, S. Thrombospondin-1 contributes to slower aortic aneurysm growth by inhibiting maladaptive remodeling of extracellular matrix. Clin. Sci. 2017, 131, 1283–1285. [Google Scholar] [CrossRef]
- Qin, Y.; Cao, X.; Guo, J.; Zhang, Y.; Pan, L.; Zhang, H.; Li, H.; Tang, C.; Du, J.; Shi, G.-P. Deficiency of cathepsin S attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Cardiovasc. Res. 2012, 96, 401–410. [Google Scholar] [CrossRef]
- Ang, L.; Boivin, W.; Williams, S.; Zhao, H.; Abraham, T.; Carmine-Simmen, K.; McManus, B.; Bleackley, R.; Granville, D. Serpina3n attenuates granzyme B-mediated decorin cleavage and rupture in a murine model of aortic aneurysm. Cell Death Dis. 2011, 2, e209. [Google Scholar] [CrossRef]
- Ennis, T.; Jin, J.; Bartlett, S.; Arif, B.; Grapperhaus, K.; Curci, J.A. Effect of Novel Limited-Spectrum MMP Inhibitor XL784 in Abdominal Aortic Aneurysms. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Ijaz, T.; Sun, H.; LeJeune, W.; Vargas, G.; Shilagard, T.; Recinos III, A.; Milewicz, D.M.; Brasier, A.R.; Tilton, R.G. IL-6 Regulates Extracellular Matrix Remodeling Associated With Aortic Dilation in a Fibrillin-1 Hypomorphic mgR/mgR Mouse Model of Severe M arfan Syndrome. J. Am. Heart Assoc. 2014, 3, e000476. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Aoki, H.; Ikeda, Y.; Fujii, K.; Akiyama, N.; Furutani, A.; Hoshii, Y.; Tanaka, N.; Ricci, R.; Ishihara, T.; et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat. Med. 2005, 11, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Zampetaki, A.; Attia, R.; Mayr, U.; Gomes, R.S.; Phinikaridou, A.; Yin, X.; Langley, S.R.; Willeit, P.; Lu, R.; Fanshawe, B.; et al. Role of miR-195 in Aortic Aneurysmal Disease. Circ. Res. 2014, 115, 857–866. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, T.; Sorenson, C.M.; Sheibani, N.; Liu, B. Myeloid-Derived TSP1 (Thrombospondin-1) Contributes to Abdominal Aortic Aneurysm Through Suppressing Tissue Inhibitor of Metalloproteinases-1. Arter. Thromb. Vasc. Biol. 2020, 40, 350. [Google Scholar] [CrossRef] [PubMed]
- Klaus, V.; Schmies, F.; Reeps, C.; Trenner, M.; Geisbüsch, S.; Lohoefer, F.; Eckstein, H.-H.; Pelisek, J. Cathepsin S is associated with degradation of collagen I in abdominal aortic aneurysm. Vasa 2018, 47, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.K.; Schiffler, M.A.; Gavardinas, K.; Kim, E.J.; Matthews, D.P.; Staszak, M.A.; Coffey, D.S.; Shaw, B.W.; Cassidy, K.C.; Brier, R.A.; et al. Discovery of Cathepsin S Inhibitor LY3000328 for the Treatment of Abdominal Aortic Aneurysm. ACS Med. Chem. Lett. 2014, 5, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Chang, J.Y.; Wang, K.C.; Lee, F.T.; Wu, H.L.; Cheng, T.L. Pharmacological Inhibition of Cathepsin S Suppresses Abdominal Aortic Aneurysm in Mice. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 990–999. [Google Scholar] [CrossRef]
- Li, W.; Qiu, X.; Jiang, H.; Zhi, Y.; Fu, J.; Liu, J. Ulinastatin inhibits the inflammation of LPS-induced acute lung injury in mice via regulation of AMPK/NF-κB pathway. Int Immunopharmacol 2015, 29, 560–567. [Google Scholar] [CrossRef]
- Aziz, F.; Kuivaniemi, H. Role of Matrix Metalloproteinase Inhibitors in Preventing Abdominal Aortic Aneurysm. Ann. Vasc. Surg. 2007, 21, 392–401. [Google Scholar] [CrossRef]
- Yamada, S.; Wang, K.-Y.; Tanimoto, A.; Fan, J.; Shimajiri, S.; Kitajima, S.; Morimoto, M.; Tsutsui, M.; Watanabe, T.; Yasumoto, K.; et al. Matrix Metalloproteinase 12 Accelerates the Initiation of Atherosclerosis and Stimulates the Progression of Fatty Streaks to Fibrous Plaques in Transgenic Rabbits. Am. J. Pathol. 2008, 172, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Loffek, S.; Schilling, O.; Franzke, C. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2010, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Gineitis, A.; Wågberg, F.; Ängquist, K.-A. Activity of Matrix Metalloproteinase-2 and -9 in Abdominal Aortic Aneurysms. Relation to Size and Rupture. Eur. J. Vasc. Endovasc. Surg. 2000, 20, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Prall, A.K.; Longo, G.; Mayhan, W.G.; Waltke, E.A.; Fleckten, B.; Thompson, R.W.; Baxter, B. Doxycycline in patients with abdominal aortic aneurysms and in mice: Comparison of serum levels and effect on aneurysm growth in mice. J. Vasc. Surg. 2002, 35, 923–929. [Google Scholar] [CrossRef]
- Thompson, R.W.; Parks, W.C. Role of Matrix Metalloproteinases in Abdominal Aortic Aneurysms. Ann. N. Y. Acad. Sci. 1996, 800, 157–174. [Google Scholar] [CrossRef]
- Mao, D.; Lee, J.K.; VanVickle, S.J.; Thompson, R.W. Expression of Collagenase-3 (MMP-13) in Human Abdominal Aortic Aneurysms and Vascular Smooth Muscle Cells in Culture. Biochem. Biophys. Res. Commun. 1999, 261, 904–910. [Google Scholar] [CrossRef]
- McMillan, W.D.; Tamarina, N.A.; Cipollone, M.; Johnson, D.A.; Parker, M.A.; Pearce, W.H. Size matters: The relationship between MMP-9 expression and aortic diameter. Circulation 1997, 96, 2228–2232. [Google Scholar] [CrossRef]
- McMillan, W.D.; Patterson, B.K.; Keen, R.R.; Shively, V.P.; Cipollone, M.; Pearce, W.H. In Situ Localization and Quantification of mRNA for 92-kD Type IV Collagenase and Its Inhibitor in Aneurysmal, Occlusive, and Normal Aorta. Arter. Thromb. Vasc. Biol. 1995, 15, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgi, G.; D’Averio, R.; Mauriello, A.; Bondio, M.; Pontillo, M.; Castelvecchio, S.; Trimarchi, S.; Tolva, V.; Nano, G.; Rampoldi, V.; et al. Plasma levels of metalloproteinases-3 and -9 as markers of successful abdominal aortic aneurysm exclusion after endovascular graft treatment. Circulation 2001, 104, 288–295. [Google Scholar] [CrossRef]
- Gona, K.; Toczek, J.; Ye, Y.; Sanzida, N.; Golbazi, A.; Boodagh, P.; Salarian, M.; Jung, J.-J.; Rajendran, S.; Kukreja, G.; et al. Hydroxamate-Based Selective Macrophage Elastase (MMP-12) Inhibitors and Radiotracers for Molecular Imaging. J. Med. Chem. 2020, 63, 15037–15049. [Google Scholar] [CrossRef]
- Katsuki, S.; Koga, J.-i.; Matoba, T.; Umezu, R.; Nakashiro, S.; Nakano, K.; Tsutsui, H.; Egashira, K. Nanoparticle-Mediated Delivery of Pitavastatin to Monocytes/Macrophages Inhibits Angiotensin II-Induced Abdominal Aortic Aneurysm Formation in Apoe-/-Mice. J. Atheroscler. Thromb. 2021, 54379. [Google Scholar]
- Curci, J.A.; Petrinec, D.; Liao, S.; Golub, L.M.; Thompson, R.W. Pharmacologic suppression of experimental abdominal aortic aneurysms: A comparison of doxycycline and four chemically modified tetracyclines. J. Vasc. Surg. 1998, 28, 1082–1093. [Google Scholar] [CrossRef]
- Yu, M.; Chen, C.; Cao, Y.; Qi, R. Inhibitory effects of doxycycline on the onset and progression of abdominal aortic aneurysm and its related mechanisms. Eur. J. Pharmacol. 2017, 811, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, J.H. The pathophysiologic basis of abdominal aortic aneurysm progression: A critical appraisal. Expert Rev. Cardiovasc. Ther. 2015, 13, 839–851. [Google Scholar] [CrossRef]
- Meijer, C.A.; Stijnen, T.; Wasser, M.N.; Hamming, J.F.; van Bockel, J.H.; Lindeman, J.H.; Pharmaceutical Aneurysm Stabilisation Trial Study, G. Doxycycline for stabilization of abdominal aortic aneurysms: A randomized trial. Ann. Intern Med. 2013, 159, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.T.; Matsumura, J.; Curci, J.A.; McBride, R.; Larson, L.; Blackwelder, W.; Lam, D.; Wijesinha, M.; Terrin, M. Effect of Doxycycline on Aneurysm Growth Among Patients With Small Infrarenal Abdominal Aortic Aneurysms: A Randomized Clinical Trial. JAMA 2020, 323, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Vyavahare, N.R. Nanoparticle-based targeted delivery of pentagalloyl glucose reverses elastase-induced abdominal aortic aneurysm and restores aorta to the healthy state in mice. PLoS ONE 2020, 15, e0227165. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-H.; Wang, J.-C.; Liao, W.-I.; Po-Hsun, H.; Lin, C.-Y.; Liao, M.-T.; Huang, P.-H.; Lin, S.-J. Fucoidan attenuates angiotensin II-induced abdominal aortic aneurysms through the inhibition of c-Jun N-terminal kinase and nuclear factor κB activation. J. Vasc. Surg. 2018, 68, 72S–81S. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Li, L.; Khalil, R.A. MMPs and ADAMs/ADAMTS inhibition therapy of abdominal aortic aneurysm. Life Sci. 2020, 253, 117659. [Google Scholar] [CrossRef]
- Kawai, T.; Takayanagi, T.; Forrester, S.J.; Preston, K.J.; Obama, T.; Tsuji, T.; Kobayashi, T.; Boyer, M.J.; Cooper, H.A.; Kwok, H.F.; et al. Vascular ADAM17 (a Disintegrin and Metalloproteinase Domain 17) Is Required for Angiotensin II/β-Aminopropionitrile-Induced Abdominal Aortic Aneurysm. Hypertension 2017, 70, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Hughes, M.; Krishnamoorthy, S.; Zou, S.; Zhang, L.; Wu, D.; Zhang, C.; Curci, J.A.; Coselli, J.S.; Milewicz, D.M.; et al. Critical Role of ADAMTS-4 in the Development of Sporadic Aortic Aneurysm and Dissection in Mice. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Fava, M.; Barallobre-Barreiro, J.; Mayr, U.; Lu, R.; Didangelos, A.; Baig, F.; Lynch, M.; Catibog, N.; Joshi, A.; Barwari, T. Role of ADAMTS-5 in aortic dilatation and extracellular matrix remodeling. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.E.; Nelson, E.L.; Hozik, B.; Porto, S.C.; Rogers-DeCotes, A.; Fosang, A.; Kern, C.B. Adamts5−/−Mice Exhibit Altered Aggrecan Proteolytic Profiles That Correlate With Ascending Aortic Anomalies. Arter. Thromb. Vasc. Biol. 2019, 39, 2067–2081. [Google Scholar] [CrossRef] [PubMed]
- Vorkapic, E.; Folkesson, M.; Magnell, K.; Bohlooly, Y.M.; Lanne, T.; Wågsäter, D. ADAMTS-1 in abdominal aortic aneurysm. PLoS ONE 2017, 12, e0178729. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Zorio, E.; Medina, P.; Rueda, J.; Millán, J.M.; Arnau, M.A.; Beneyto, M.; Marin, F.; Gimeno, J.R.; Osca, J.; Salvador, A.; et al. Insights Into the Role of microRNAs in Cardiac Diseases: From Biological Signalling to Therapeutic Targets. Cardiovasc. Hematol. Agents Med. Chem. 2009, 7, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef]
- Chen, K.C.; Wang, Y.S.; Hu, C.Y.; Chang, W.C.; Liao, Y.C.; Dai, C.Y.; Juo, S.H. OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: A novel mechanism for cardiovascular diseases. FASEB J. 2011, 25, 1718–1728. [Google Scholar] [CrossRef]
- Maegdefessel, L.; Azuma, J.; Toh, R.; Merk, D.R.; Deng, A.; Chin, J.T.; Raaz, U.; Schoelmerich, A.M.; Raiesdana, A.; Leeper, N.J.; et al. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J. Clin. Investig. 2012, 122, 497–506. [Google Scholar] [CrossRef]
- Wang, X.; Searle, A.K.; Hohmann, J.D.; Liu, A.L.; Abraham, M.-K.; Palasubramaniam, J.; Lim, B.; Yao, Y.; Wallert, M.; Yu, E.; et al. Dual-Targeted Theranostic Delivery of miRs Arrests Abdominal Aortic Aneurysm Development. Mol. Ther. 2018, 26, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Chernogubova, E.; Busch, A.; Kath, P.; Winter, H.; Winski, G.; Eckstein, H.-H.; Dacken, B.; Svart, J.; Boon, R.; Dimmeler, S. Targeting Micrornas to Block Abdominal Aortic Aneurysm Progression in a Novel Yucatan Ldlr-KOMini-pig Model. Arterioscler. Thromb. Vasc. Biol. 2019, 39, A127. [Google Scholar]
- Dale, M.A.; Ruhlman, M.K.; Baxter, B.T. Inflammatory cell phenotypes in AAAs: Their role and potential as targets for therapy. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1746–1755. [Google Scholar] [CrossRef]
- Longo, G.M.; Xiong, W.; Greiner, T.C.; Zhao, Y.; Fiotti, N.; Baxter, B.T. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Investig. 2002, 110, 625–632. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhou, Y.-Z.; Wu, Y.; Wu, Q.-Y.; Liao, X.-B.; Fu, X.-M.; Zhou, X.-M. Diverse roles of macrophage polarization in aortic aneurysm: Destruction and repair. J. Transl. Med. 2018, 16, 354. [Google Scholar] [CrossRef]
- Raffort, J.; Lareyre, F.; Clément, M.; Hassen-Khodja, R.; Chinetti, G.; Mallat, Z. Monocytes and macrophages in abdominal aortic aneurysm. Nat. Rev. Cardiol. 2017, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Hu, Y.; Akk, A.; Ye, K.; Bacon, J.; Pham, C.T.N. Interleukin-12 and -23 blockade mitigates elastase-induced abdominal aortic aneurysm. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Isoda, K.; Akita, K.; Kitamura, K.; Sato-Okabayashi, Y.; Kadoguchi, T.; Isobe, S.; Ohtomo, F.; Sano, M.; Shimada, K.; Iwakura, Y.; et al. Inhibition of interleukin-1 suppresses angiotensin II-induced aortic inflammation and aneurysm formation. Int. J. Cardiol. 2018, 270, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, J.H.; Matsumura, J.S. Pharmacologic Management of Aneurysms. Circ. Res. 2019, 124, 631–646. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, L.; Brangsch, J.; Hamm, B.; Makowski, M.R.; Keller, S. Targeting the Extracellular Matrix in Abdominal Aortic Aneurysms Using Molecular Imaging Insights. Int. J. Mol. Sci. 2021, 22, 2685. https://doi.org/10.3390/ijms22052685
Adams L, Brangsch J, Hamm B, Makowski MR, Keller S. Targeting the Extracellular Matrix in Abdominal Aortic Aneurysms Using Molecular Imaging Insights. International Journal of Molecular Sciences. 2021; 22(5):2685. https://doi.org/10.3390/ijms22052685
Chicago/Turabian StyleAdams, Lisa, Julia Brangsch, Bernd Hamm, Marcus R. Makowski, and Sarah Keller. 2021. "Targeting the Extracellular Matrix in Abdominal Aortic Aneurysms Using Molecular Imaging Insights" International Journal of Molecular Sciences 22, no. 5: 2685. https://doi.org/10.3390/ijms22052685
APA StyleAdams, L., Brangsch, J., Hamm, B., Makowski, M. R., & Keller, S. (2021). Targeting the Extracellular Matrix in Abdominal Aortic Aneurysms Using Molecular Imaging Insights. International Journal of Molecular Sciences, 22(5), 2685. https://doi.org/10.3390/ijms22052685





