Magic Peptide: Unique Properties of the LRR11 Peptide in the Activation of Leukotriene Synthesis in Human Neutrophils
Abstract
1. Introduction
2. Results
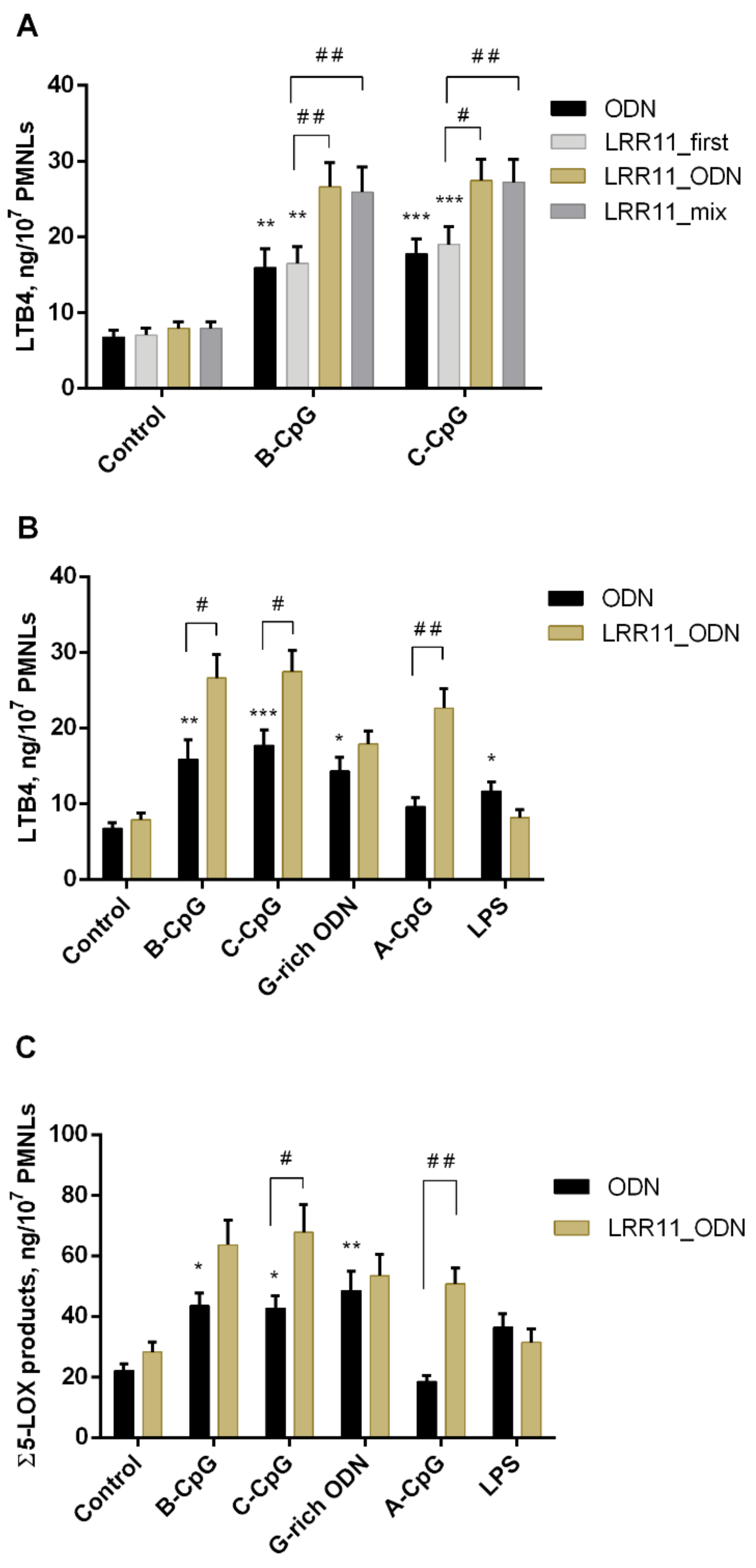
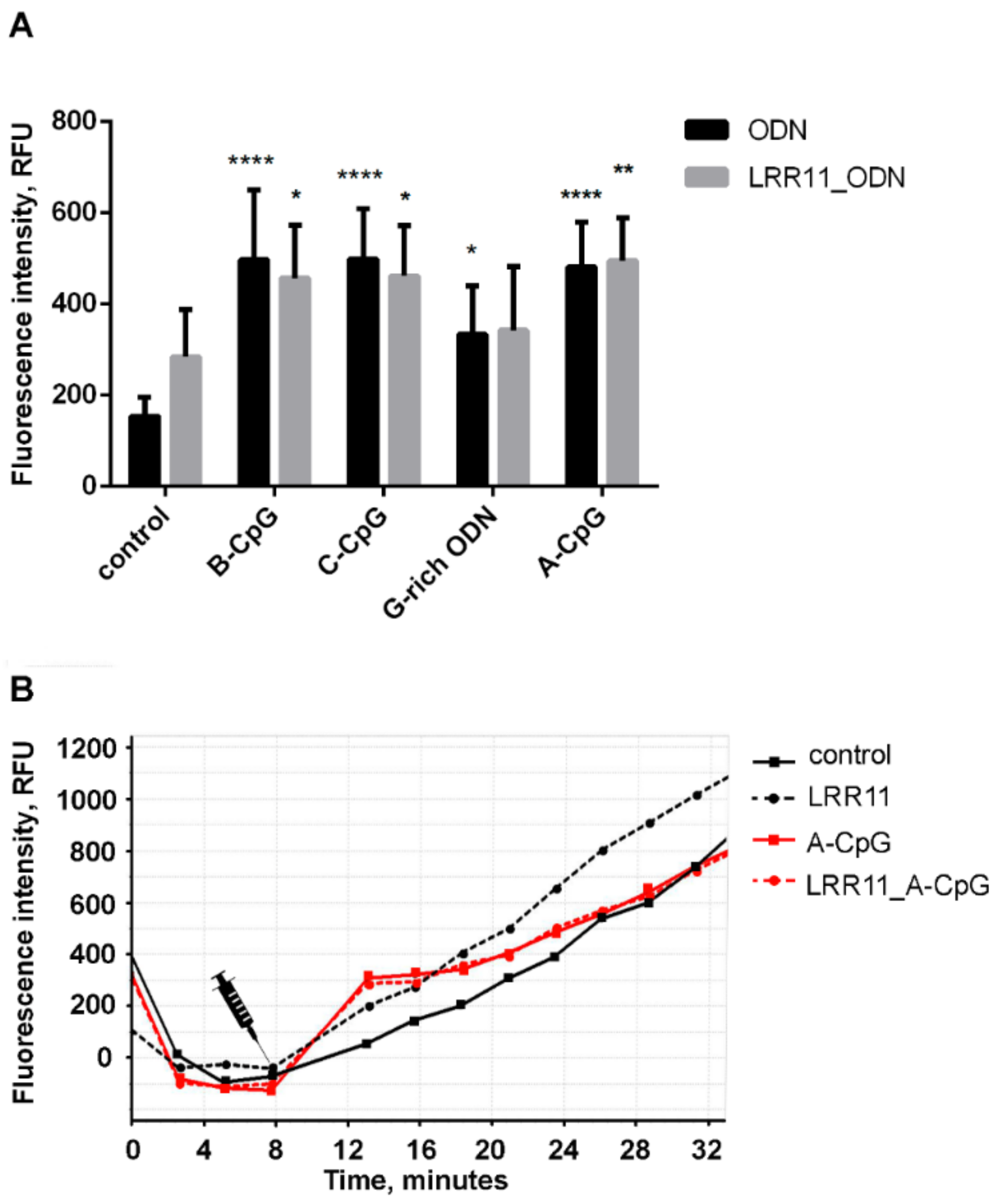
2.1. Effect of ODNs and LRR11 Peptide on 5-LOX Metabolite Synthesis
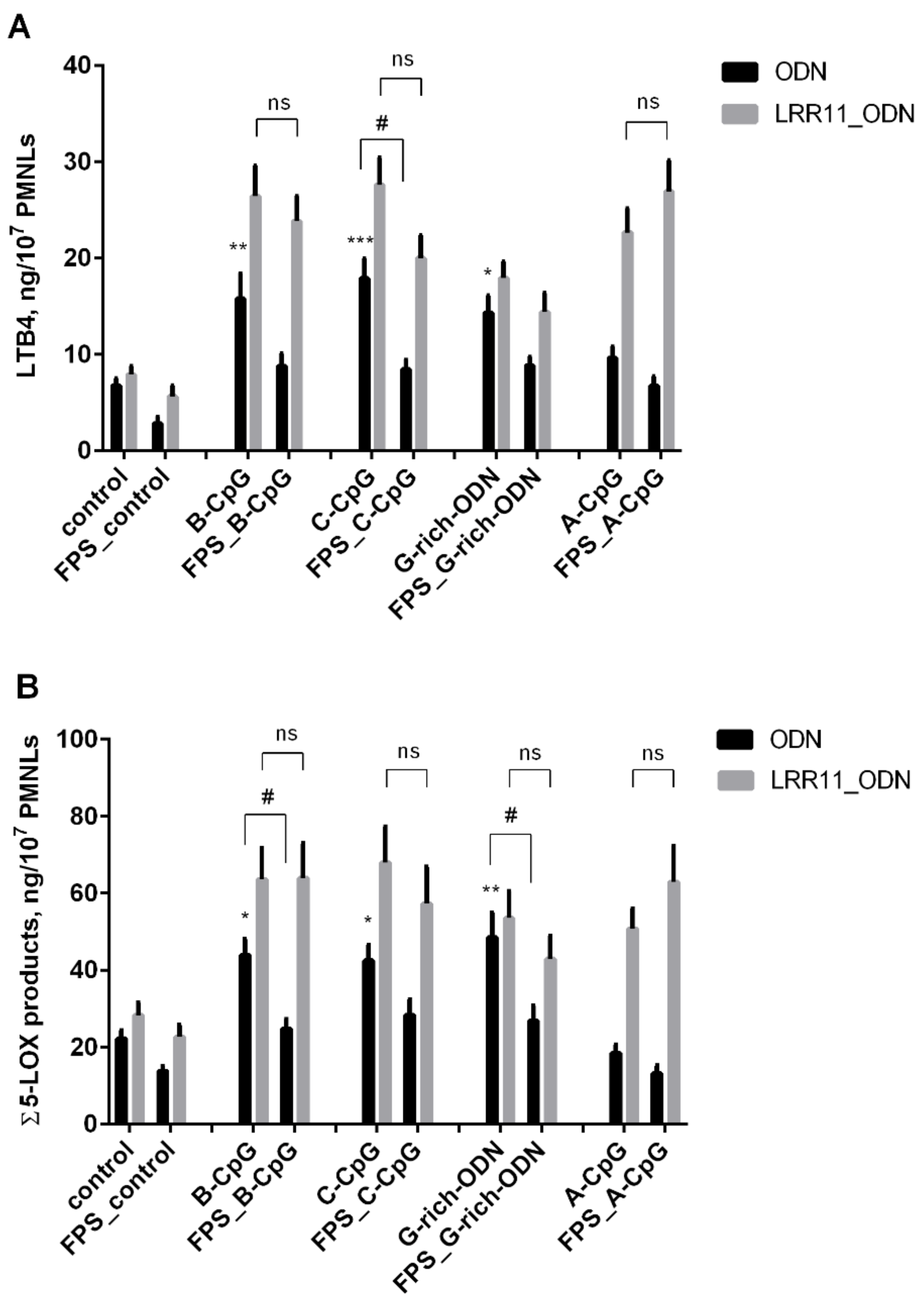
2.2. RAGE Inhibitor FPS-ZM1 Attenuates the Potency of ODNs to Increase 5-LOX Products Synthesis in PMNLs, But LRR11 Peptide Abolishes the Effects of FPS-ZM1
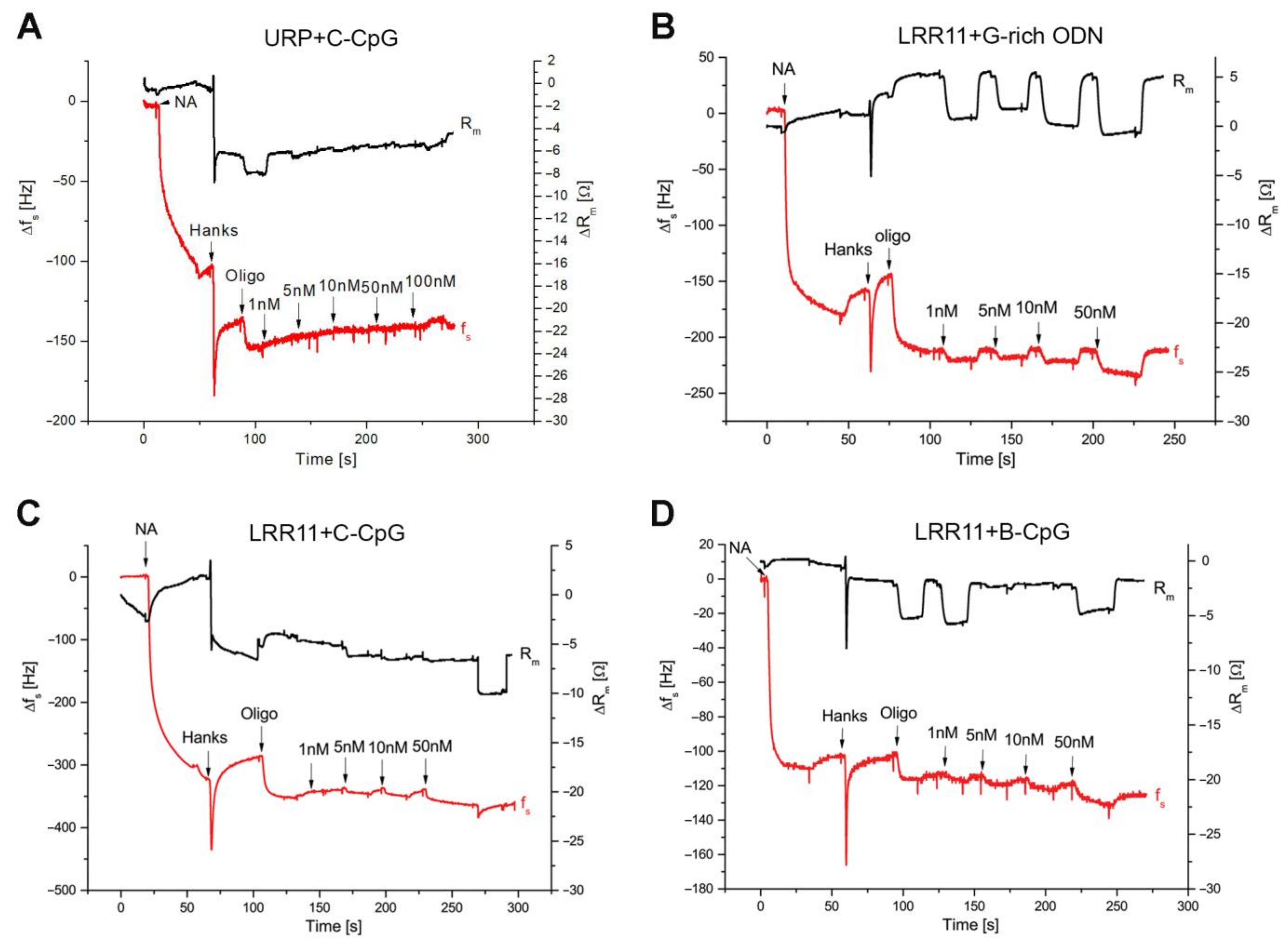
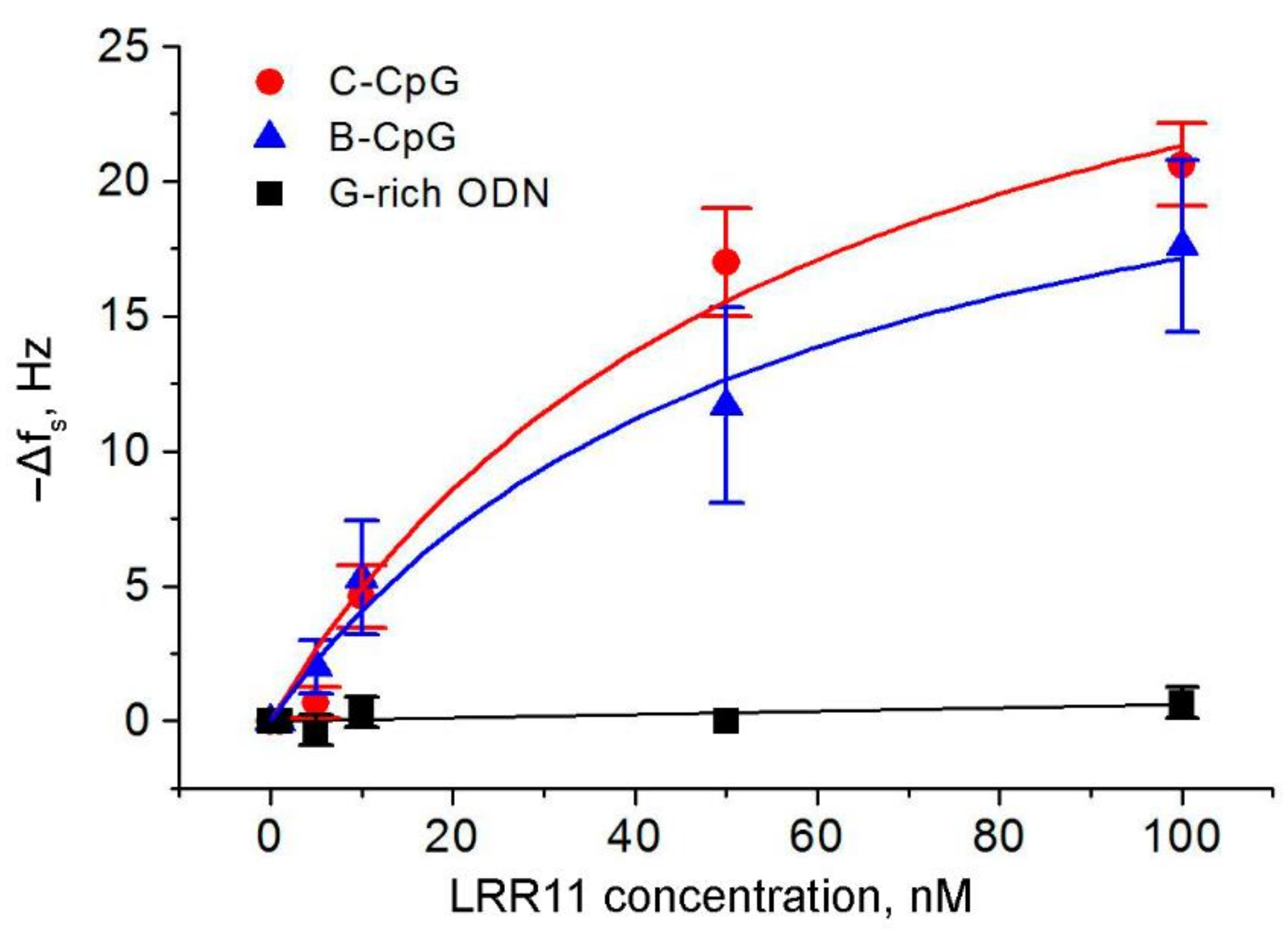
2.3. The Ability of the LRR11 Peptide to Form a Complex with ODNs of Different Sequences
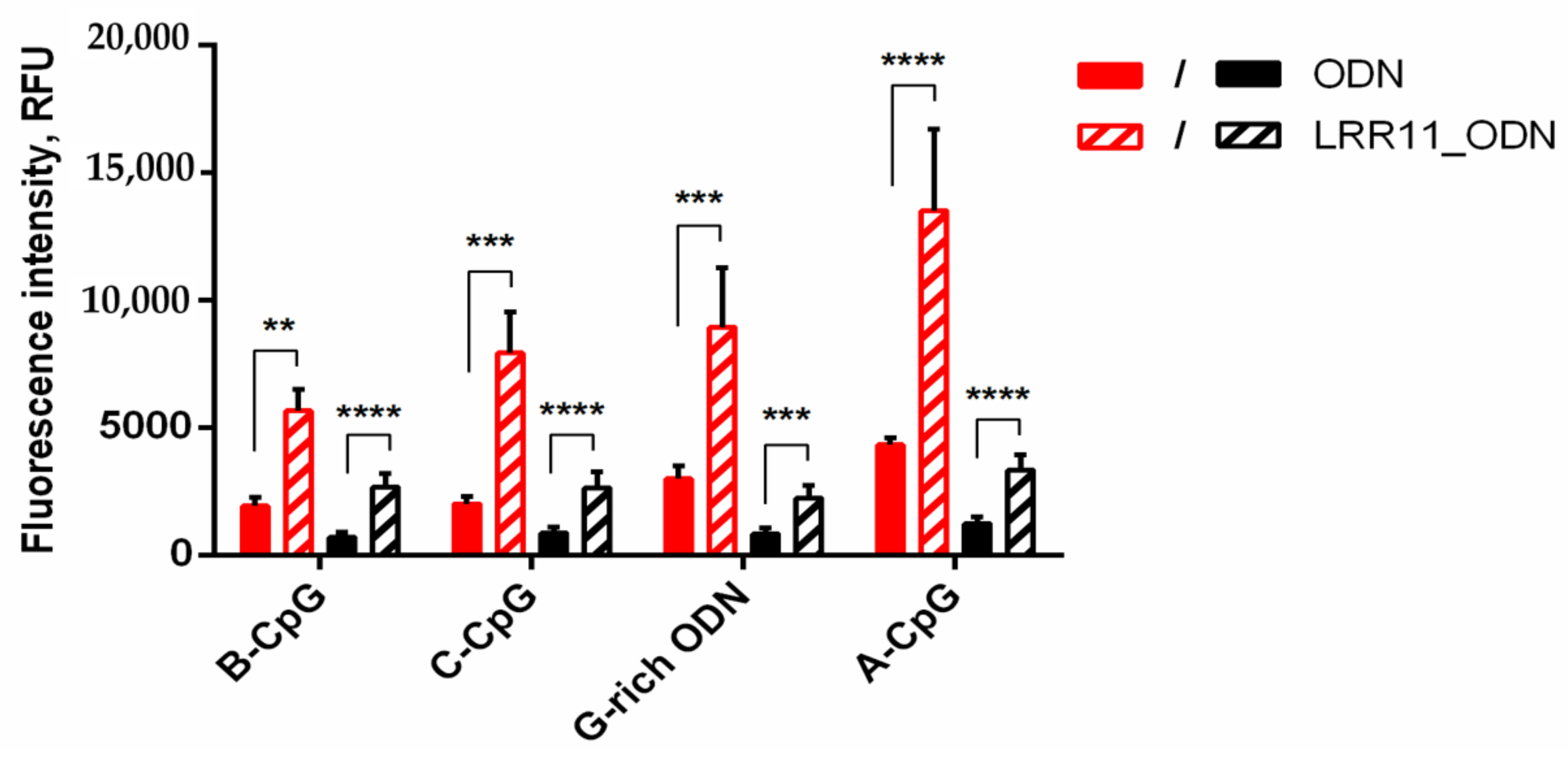
2.4. The LRR11 Peptide Promotes the Uptake of Synthetic ODNs by Neutrophils
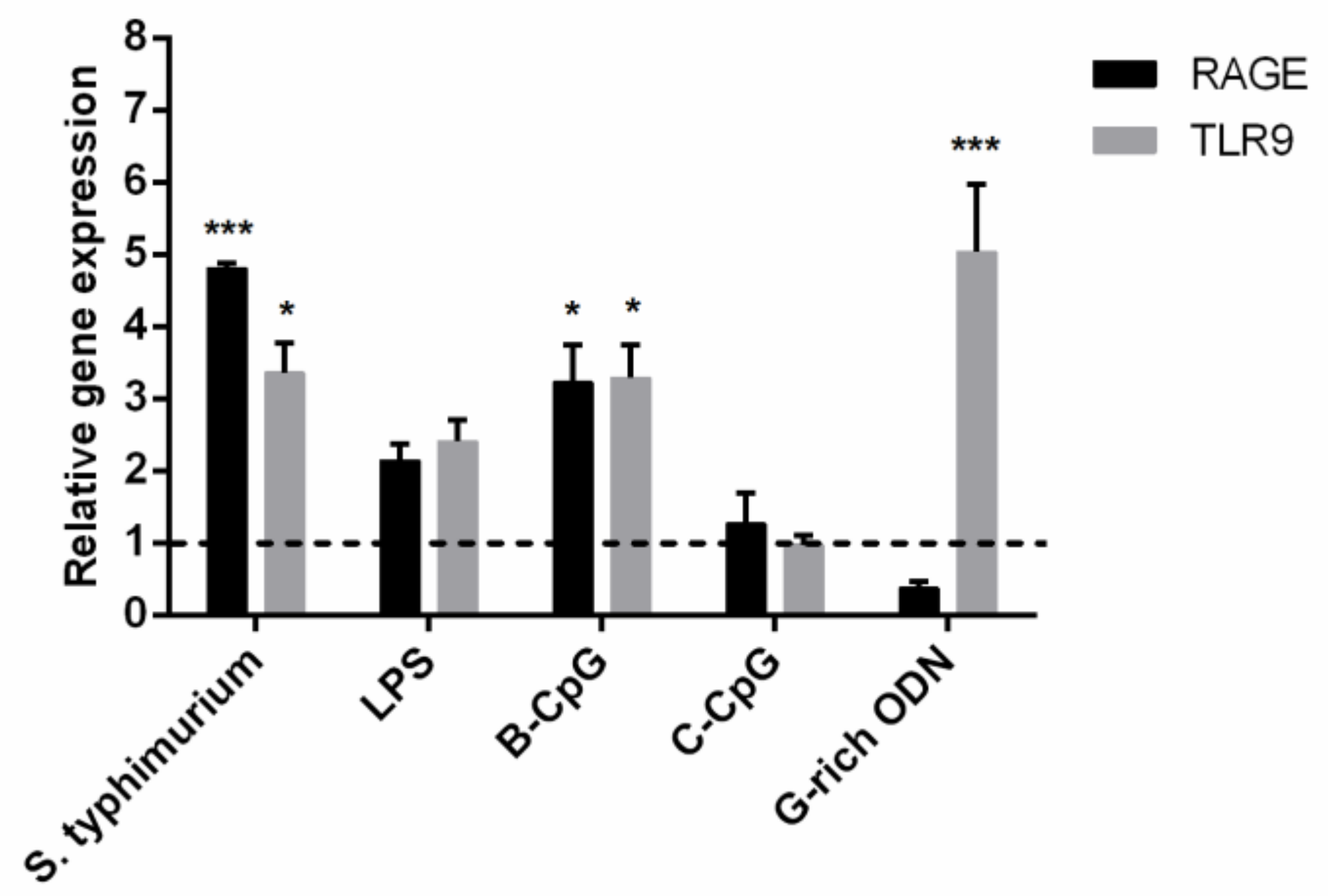
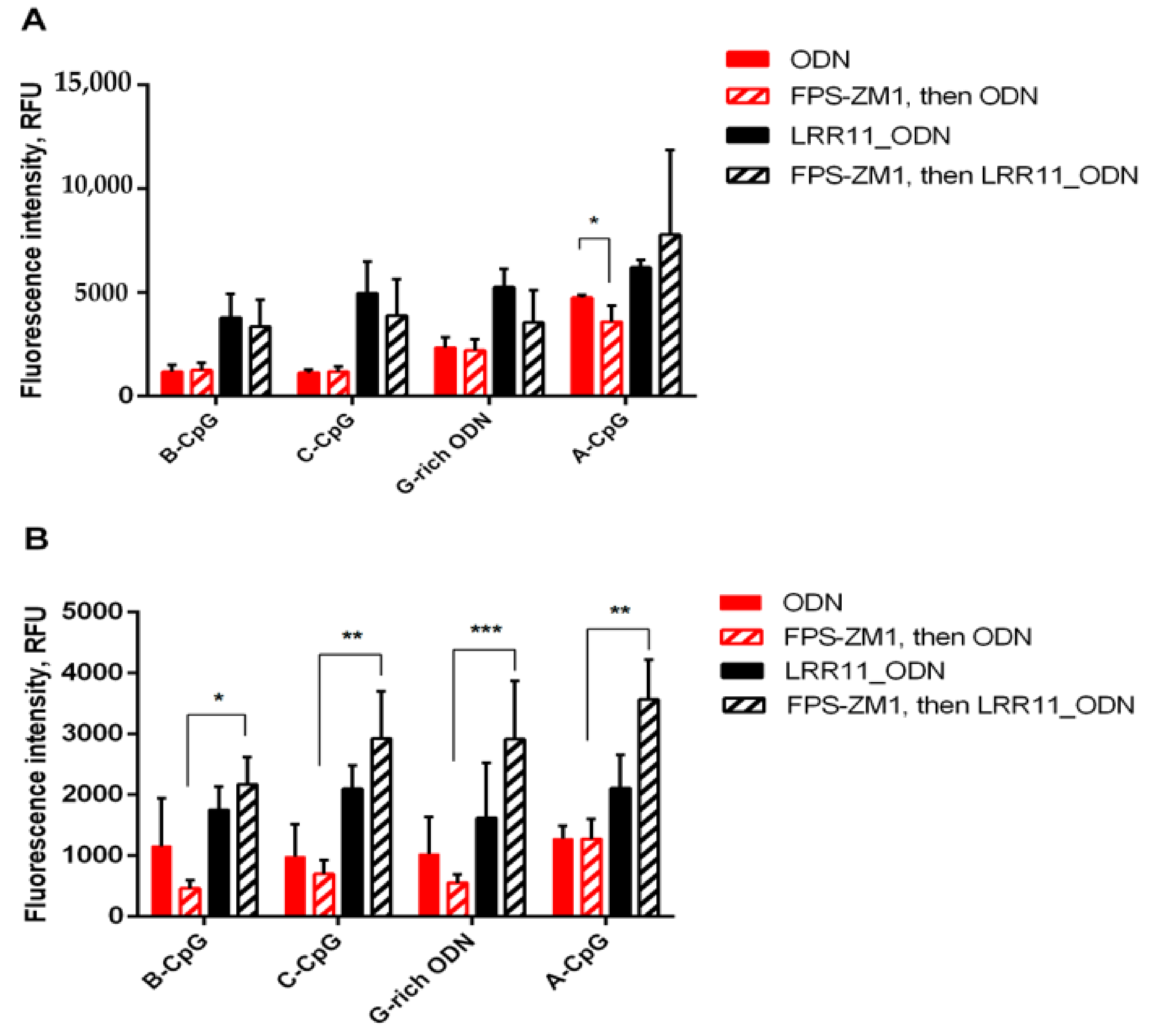
2.5. RAGE Antagonist Inhibits ODN Internalization, But the Addition of LRR11 Peptide Neutralizes This Effect
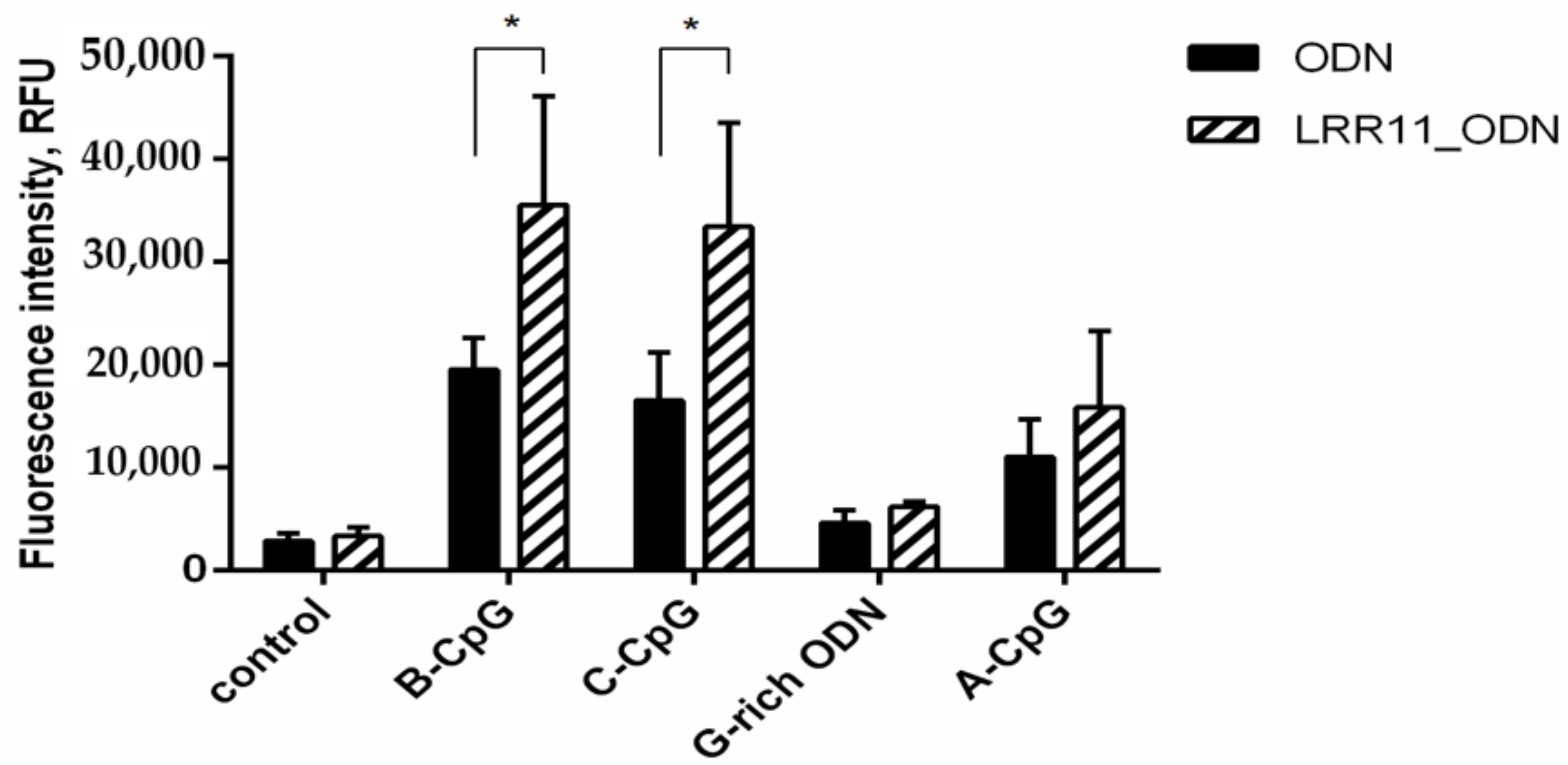
2.6. LRR11 Peptide Promotes an Increase in the Production of Reactive Oxygen Species Induced by Oligonucleotides
2.7. LRR11 Peptide Stimulates an Increase in the Intracellular Concentration of Free Ca2+ in Neutrophils
3. Discussion
4. Materials and Methods
4.1. Oligonucleotides and Peptides
4.2. Primers, RNA Isolation and cDNA Synthesis
4.3. Isolation of Neutrophils
4.4. Preparation of Bacteria
4.5. Synthesis of 5-LOX Metabolites in Human Neutrophils
4.6. HPLC Analysis of Water/Methanol Extracts
4.7. Assessment of Calcium Ion Influx
4.8. Uptake Assay with FAM-Labeled ODNs
4.9. Reactive Oxygen Species Formation Assay
4.10. Detection of LRR11-ODN Interaction by TSM Method
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viryasova, G.M.; Dolinnaya, N.G.; Golenkina, E.A.; Gaponova, T.V.; Viryasov, M.B.; Romanova, Y.M.; Sud’ina, G.F. G-quadruplex-forming oligodeoxyribonucleotides activate leukotriene synthesis in human neutrophils. J. Biomol. Struct. Dyn. 2019, 37, 3649–3659. [Google Scholar] [CrossRef]
- Golenkina, E.A.; Viryasova, G.M.; Dolinnaya, N.G.; Bannikova, V.A.; Gaponova, T.V.; Romanova, Y.M.; Sud’ina, G.F. The Potential of Telomeric G-quadruplexes Containing Modified Oligoguanosine Overhangs in Activation of Bacterial Phagocytosis and Leukotriene Synthesis in Human Neutrophils. Biomolecules 2020, 10, 249. [Google Scholar] [CrossRef]
- Viryasova, G.M.; Golenkina, E.A.; Galkina, S.I.; Gaponova, T.V.; Romanova, Y.M.; Sud’ina, G.F. Effects of phosphodiester and phosphorothioate ODN2216 on leukotriene synthesis in human neutrophils and neutrophil apoptosis. Biochimie 2016, 125, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Sekheri, M.; El Kebir, D.; Edner, N.; Filep, J.G. 15-Epi-LXA4 and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation. Proc. Natl. Acad. Sci. USA 2020, 117, 7971–7980. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, L.; Yu, H.; Wang, R.; Gou, Y.; Zhang, M.; Kang, C.; Liu, T.; Lan, Y.; Wang, X.; et al. Membrane TLR9 Positive Neutrophil Mediated MPLA Protects Against Fatal Bacterial Sepsis. Theranostics 2019, 9, 6269–6283. [Google Scholar] [CrossRef] [PubMed]
- Lentini, G.; Fama, A.; Biondo, C.; Mohammadi, N.; Galbo, R.; Mancuso, G.; Iannello, D.; Zummo, S.; Giardina, M.; De Gaetano, G.V.; et al. Neutrophils Enhance Their Own Influx to Sites of Bacterial Infection via Endosomal TLR-Dependent Cxcl2 Production. J. Immunol. 2020, 204, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Ohto, U.; Shibata, T.; Tanji, H.; Ishida, H.; Krayukhina, E.; Uchiyama, S.; Miyake, K.; Shimizu, T. Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9. Nature 2015, 520, 702–705. [Google Scholar] [CrossRef]
- Pan, X.; Yue, J.; Ding, G.; Li, B.; Liu, X.; Zheng, X.; Yu, M.; Li, J.; Jiang, W.; Wu, C.; et al. Leucine-rich repeat 11 of Toll-like receptor 9 can tightly bind to CpG-containing oligodeoxynucleotides, and the positively charged residues are critical for the high affinity. J. Biol. Chem. 2012, 287, 30596–30609. [Google Scholar] [CrossRef]
- Pan, X.; Li, B.; Kuang, M.; Liu, X.; Cen, Y.; Qin, R.; Ding, G.; Zheng, J.; Zhou, H. Synthetic Human TLR9-LRR11 Peptide Attenuates TLR9 Signaling by Binding to and thus Decreasing Internalization of CpG Oligodeoxynucleotides. Int. J. Mol. Sci. 2016, 17, 242. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37–51. [Google Scholar] [CrossRef]
- Sierra, J.M.; Fuste, E.; Rabanal, F.; Vinuesa, T.; Vinas, M. An overview of antimicrobial peptides and the latest advances in their development. Expert Opin. Biol. 2017, 17, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, D.M.; Davidson, D.J.; Scott, M.G.; Hancock, R.E. Immunomodulatory activities of small host defense peptides. Antimicrob. Agents Chemother. 2005, 49, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef] [PubMed]
- van der Does, A.M.; Hiemstra, P.S.; Mookherjee, N. Antimicrobial Host Defence Peptides: Immunomodulatory Functions and Translational Prospects. Adv. Exp. Med. Biol. 2019, 1117, 149–171. [Google Scholar] [CrossRef]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Pütsep, K.; Carlsson, G.; Boman, H.G.; Andersson, M. Deficiency of antibacterial peptides in patients with morbus Kostmann: An observation study. Lancet 2002, 360, 1144–1149. [Google Scholar] [CrossRef]
- Zheng, Y.; Niyonsaba, F.; Ushio, H.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br. J. Derm. 2007, 157, 1124–1131. [Google Scholar] [CrossRef]
- Wan, M.; Godson, C.; Guiry, P.J.; Agerberth, B.; Haeggstrom, J.Z. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolvin E1. FASEB J. 2011, 25, 1697–1705. [Google Scholar] [CrossRef]
- Lindau, D.; Mussard, J.; Wagner, B.J.; Ribon, M.; Ronnefarth, V.M.; Quettier, M.; Jelcic, I.; Boissier, M.C.; Rammensee, H.G.; Decker, P. Primary blood neutrophils express a functional cell surface Toll-like receptor 9. Eur. J. Immunol. 2013, 43, 2101–2113. [Google Scholar] [CrossRef]
- Heeg, K.; Dalpke, A.; Peter, M.; Zimmermann, S. Structural requirements for uptake and recognition of CpG oligonucleotides. Int. J. Med. Microbiol. 2008, 298, 33–38. [Google Scholar] [CrossRef]
- Dalpke, A.H.; Zimmermann, S.; Albrecht, I.; Heeg, K. Phosphodiester CpG oligonucleotides as adjuvants: Polyguanosine runs enhance cellular uptake and improve immunostimulative activity of phosphodiester CpG oligonucleotides in vitro and in vivo. Immunology 2002, 106, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Zagryazhskaya, A.N.; Lindner, S.C.; Grishina, Z.V.; Galkina, S.I.; Steinhilber, D.; Sud’ina, G.F. Nitric oxide mediates distinct effects of various LPS chemotypes on phagocytosis and leukotriene synthesis in human neutrophils. Int. J. Biochem. Cell Biol. 2010, 42, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Golenkina, E.A.; Galkina, S.I.; Romanova, J.M.; Lazarenko, M.I.; Sud’ina, G.F. Involvement of red blood cells in the regulation of leukotriene synthesis in polymorphonuclear leucocytes upon interaction with Salmonella Typhimurium. APMIS 2011, 119, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, J.; Weeratna, R.; Payette, P.; Jurk, M.; Schetter, C.; Laucht, M.; Wader, T.; Tluk, S.; Liu, M.; Davis, H.L.; et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur. J. Immunol. 2004, 34, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Kuznik, A.; Bencina, M.; Svajger, U.; Jeras, M.; Rozman, B.; Jerala, R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 2011, 186, 4794–4804. [Google Scholar] [CrossRef]
- Sirois, C.M.; Jin, T.; Miller, A.L.; Bertheloot, D.; Nakamura, H.; Horvath, G.L.; Mian, A.; Jiang, J.; Schrum, J.; Bossaller, L.; et al. RAGE is a nucleic acid receptor that promotes inflammatory responses to DNA. J. Exp. Med. 2013, 210, 2447–2463. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, I.; Romero, J.; Rodriguez, B.L.; Perez-Castro, R.; Rojas, A. The immunobiology of the receptor of advanced glycation end-products: Trends and challenges. Immunobiology 2013, 218, 790–797. [Google Scholar] [CrossRef]
- Lin, H.J.; Hsu, F.Y.; Chen, W.W.; Lee, C.H.; Lin, Y.J.; Chen, Y.Y.; Chen, C.J.; Huang, M.Z.; Kao, M.C.; Chen, Y.A.; et al. Helicobacter pylori Activates HMGB1 Expression and Recruits RAGE into Lipid Rafts to Promote Inflammation in Gastric Epithelial Cells. Front. Immunol. 2016, 7, 341. [Google Scholar] [CrossRef]
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue, B.; Love, R.; Perry, S.; Paquette, N.; Deane, R.J.; et al. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Investig. 2012, 122, 1377–1392. [Google Scholar] [CrossRef]
- Tassew, N.; Thompson, M. Kinetic characterization of TAR RNA–Tat peptide and neomycin interactions by acoustic wave biosensor. Biophys. Chem. 2003, 106, 241–252. [Google Scholar] [CrossRef]
- Ellis, J.S.; Thompson, M. Acoustic coupling at multiple interfaces and the liquid phase response of the thickness shear-mode acoustic wave sensor. Chem. Commun. (Camb.) 2004, 11, 1310–1311. [Google Scholar] [CrossRef]
- Viglasky, V.; Hianik, T. Potential uses of G-quadruplex-forming aptamers. Gen. Physiol. Biophys. 2013, 32, 149–172. [Google Scholar] [CrossRef] [PubMed]
- Hianik, T.; Ostatna, V.; Sonlajtnerova, M.; Grman, I. Influence of ionic strength, pH and aptamer configuration for binding affinity to thrombin. Bioelectrochemistry 2007, 70, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ryazanova, A.Y.; Kubareva, E.A.; Grman, I.; Lavrova, N.V.; Ryazanova, E.M.; Oretskaya, T.S.; Hianik, T. The study of the interaction of (cytosine-5)-DNA methyltransferase SsoII with DNA by acoustic method. Analyst 2011, 136, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. Verwendung Von Schwingquarzen Zur Wagung Dunner Schichten Und Zur Mikrowagung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Coffey, M.J.; Phare, S.M.; Peters-Golden, M. Interaction between nitric oxide, reactive oxygen intermediates, and peroxynitrite in the regulation of 5-lipoxygenase metabolism. Biochim. Et. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2002, 1584, 81–90. [Google Scholar] [CrossRef]
- Woods, J.W.; Coffey, M.J.; Brock, T.G.; Singer, I.I.; Peters-Golden, M. 5-Lipoxygenase is located in the euchromatin of the nucleus in resting human alveolar macrophages and translocates to the nuclear envelope upon cell activation. J. Clin. Investig. 1995, 95, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Lehrer, R. Cationic peptides: A new source of antibiotics. Trends Biotechnol. 1998, 16, 82–88. [Google Scholar] [CrossRef]
- Scott, M.G.; Vreugdenhil, A.C.; Buurman, W.A.; Hancock, R.E.; Gold, M.R. Cutting edge: Cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J. Immunol. 2000, 164, 549–553. [Google Scholar] [CrossRef]
- Sandgren, S.; Wittrup, A.; Cheng, F.; Jönsson, M.; Eklund, E.; Busch, S.; Belting, M. The human antimicrobial peptide LL-37 transfers extracellular DNA plasmid to the nuclear compartment of mammalian cells via lipid rafts and proteoglycan-dependent endocytosis. J. Biol. Chem. 2004, 279, 17951–17956. [Google Scholar] [CrossRef]
- Tuomela, J.M.; Sandholm, J.A.; Kaakinen, M.; Hayden, K.L.; Haapasaari, K.-M.; Jukkola-Vuorinen, A.; Kauppila, J.H.; Lehenkari, P.P.; Harris, K.W.; Graves, D.E.; et al. Telomeric G-quadruplex-forming DNA fragments induce TLR9-mediated and LL-37-regulated invasion in breast cancer cells in vitro. Breast Cancer Res. Treat. 2016, 155, 261–271. [Google Scholar] [CrossRef]
- Zhang, X.; Oglecka, K.; Sandgren, S.; Belting, M.; Esbjorner, E.K.; Norden, B.; Graslund, A. Dual functions of the human antimicrobial peptide LL-37-target membrane perturbation and host cell cargo delivery. Biochim. Biophys. Acta 2010, 1798, 2201–2208. [Google Scholar] [CrossRef]
- Hurtado, P.; Peh, C.A. LL-37 promotes rapid sensing of CpG oligodeoxynucleotides by B lymphocytes and plasmacytoid dendritic cells. J. Immunol. 2010, 184, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, O.E.; Follin, P.; Johnsen, A.H.; Calafat, J.; Tjabringa, G.S.; Hiemstra, P.S.; Borregaard, N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 2001, 97, 3951–3959. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms: My Perspective. Adv. Exp. Med. Biol. 2019, 1117, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Galkina, S.I.; Fedorova, N.V.; Ksenofontov, A.L.; Stadnichuk, V.I.; Baratova, L.A.; Sud’Ina, G.F. Neutrophils as a source of branched-chain, aromatic and positively charged free amino acids. Cell. Adh. Migr. 2019, 13, 98–105. [Google Scholar] [CrossRef]
- Aichinger, M.C.; Ginzler, M.; Weghuber, J.; Zimmermann, L.; Riedl, K.; Schutz, G.; Nagy, E.; von Gabain, A.; Schweyen, R.; Henics, T. Adjuvating the adjuvant: Facilitated delivery of an immunomodulatory oligonucleotide to TLR9 by a cationic antimicrobial peptide in dendritic cells. Vaccine 2011, 29, 426–436. [Google Scholar] [CrossRef]
- Chuang, C.M.; Monie, A.; Wu, A.; Mao, C.P.; Hung, C.F. Treatment with LL-37 peptide enhances antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer. Hum. Gene 2009, 20, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.C.; Liu, S.J. A TLR9 agonist enhances the anti-tumor immunity of peptide and lipopeptide vaccines via different mechanisms. Sci. Rep. 2015, 5, 12578. [Google Scholar] [CrossRef]
- Koch, M.; Chitayat, S.; Dattilo, B.M.; Schiefner, A.; Diez, J.; Chazin, W.J.; Fritz, G. Structural basis for ligand recognition and activation of RAGE. Structure 2010, 18, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Ruan, B.H.; Li, X.; Winkler, A.R.; Cunningham, K.M.; Kuai, J.; Greco, R.M.; Nocka, K.H.; Fitz, L.J.; Wright, J.F.; Pittman, D.D.; et al. Complement C3a, CpG oligos, and DNA/C3a complex stimulate IFN-alpha production in a receptor for advanced glycation end product-dependent manner. J. Immunol. 2010, 185, 4213–4222. [Google Scholar] [CrossRef]
- Evankovich, J.; Lear, T.; McKelvey, A.; Dunn, S.; Londino, J.; Liu, Y.; Chen, B.B.; Mallampalli, R.K. Receptor for advanced glycation end products is targeted by FBXO10 for ubiquitination and degradation. FASEB J. 2017, 31, 3894–3903. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Kinoshita, R.; Putranto, E.W.; Ruma, I.M.W.; Sumardika, I.W.; Youyi, C.; Tomonobu, N.; Yamamoto, K.I.; Murata, H. Signal Diversity of Receptor for Advanced Glycation End Products. Acta Med. Okayama 2017, 71, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xiang, A.; Peng, T.; Doran, A.C.; Tracey, K.J.; Barnes, B.J.; Tabas, I.; Son, M.; Diamond, B. HMGB1-C1q complexes regulate macrophage function by switching between leukotriene and specialized proresolving mediator biosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 23254–23263. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.J.; Baek, S.E.; Kim, E.J.; Park, S.Y.; Kim, C.D. HMGB1 enhances AGE-mediated VSMC proliferation via an increase in 5-LO-linked RAGE expression. Vasc. Pharm. 2019, 118–119, 106559. [Google Scholar] [CrossRef]
- Ichiki, T.; Koga, T.; Okuno, T.; Saeki, K.; Yamamoto, Y.; Yamamoto, H.; Sakaguchi, M.; Yokomizo, T. Modulation of leukotriene B4 receptor 1 signaling by receptor for advanced glycation end products (RAGE). FASEB J. 2016, 30, 1811–1822. [Google Scholar] [CrossRef]
- Aleksandrov, D.A.; Zagryagskaya, A.N.; Pushkareva, M.A.; Bachschmid, M.; Peters-Golden, M.; Werz, O.; Steinhilber, D.; Sud’ina, G.F. Cholesterol and its anionic derivatives inhibit 5-lipoxygenase activation in polymorphonuclear leukocytes and MonoMac6 cells. FEBS J. 2006, 273, 548–557. [Google Scholar] [CrossRef]
- Ngo, T.T.; Lenhoff, H.M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal. Biochem. 1980, 105, 389–397. [Google Scholar] [CrossRef]
| Designation | ODN Primary Structure a | Reference |
|---|---|---|
| A-CpG b | 5′- ggGGACGACGTCGTGgggggg-3′ | [24] ODN 2336 |
| B-CpG b | 5′-tcgtcgttttgtcgttttgtcgtt-3′ | [24] ODN 2006 |
| C-CpG b | 5′-tcgtcgttttcggcgcgcgccg-3′ | [24] ODN 2395 |
| G-rich ODN | 5′-ggTTAGGGTTAGGGTTAGGGTTAGGGggggg-3′ | [2] g2-G4-g5 |
| PMNLs+ | Σ 5-LOX Metabolites (ng/107 PMNLs) |
|---|---|
| 1 µM A23187 | 416 ± 37 |
| 150 nM FPS-ZM1; +1 µM A23187 | 438 ± 42 |
| ODN | Molecular Weight, Da | −(Δfs)max, Hz | KD, nM | KA, nM−1 |
|---|---|---|---|---|
| C-CpG | 7115.4 | 33.9 ± 6.4 | 59.0 ± 24.0 | 0.017 ± 0.007 |
| B-CpG | 7733.8 | 26.6 ± 3.8 | 55.1 ± 17.4 | 0.018 ± 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viryasova, G.M.; Golenkina, E.A.; Hianik, T.; Soshnikova, N.V.; Dolinnaya, N.G.; Gaponova, T.V.; Romanova, Y.M.; Sud’ina, G.F. Magic Peptide: Unique Properties of the LRR11 Peptide in the Activation of Leukotriene Synthesis in Human Neutrophils. Int. J. Mol. Sci. 2021, 22, 2671. https://doi.org/10.3390/ijms22052671
Viryasova GM, Golenkina EA, Hianik T, Soshnikova NV, Dolinnaya NG, Gaponova TV, Romanova YM, Sud’ina GF. Magic Peptide: Unique Properties of the LRR11 Peptide in the Activation of Leukotriene Synthesis in Human Neutrophils. International Journal of Molecular Sciences. 2021; 22(5):2671. https://doi.org/10.3390/ijms22052671
Chicago/Turabian StyleViryasova, Galina M., Ekaterina A. Golenkina, Tibor Hianik, Nataliya V. Soshnikova, Nina G. Dolinnaya, Tatjana V. Gaponova, Yulia M. Romanova, and Galina F. Sud’ina. 2021. "Magic Peptide: Unique Properties of the LRR11 Peptide in the Activation of Leukotriene Synthesis in Human Neutrophils" International Journal of Molecular Sciences 22, no. 5: 2671. https://doi.org/10.3390/ijms22052671
APA StyleViryasova, G. M., Golenkina, E. A., Hianik, T., Soshnikova, N. V., Dolinnaya, N. G., Gaponova, T. V., Romanova, Y. M., & Sud’ina, G. F. (2021). Magic Peptide: Unique Properties of the LRR11 Peptide in the Activation of Leukotriene Synthesis in Human Neutrophils. International Journal of Molecular Sciences, 22(5), 2671. https://doi.org/10.3390/ijms22052671








