Functional Dysregulations in CA1 Hippocampal Networks of a 3-Hit Mouse Model of Schizophrenia
Abstract
1. Introduction
2. Results
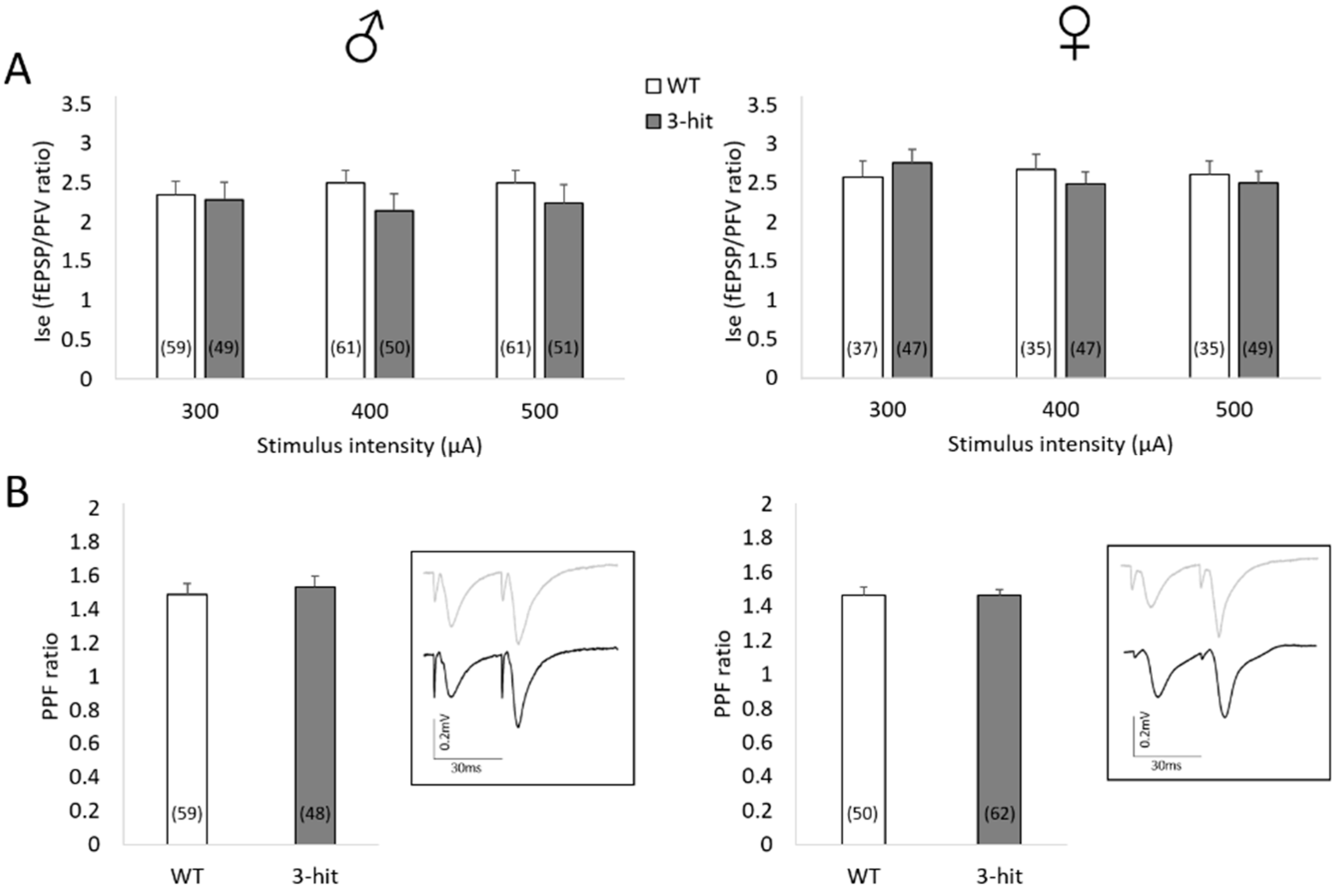
2.1. Basal Synaptic Transmission
2.2. Paired-Pulse Facilitation
2.3. Functional Plasticity
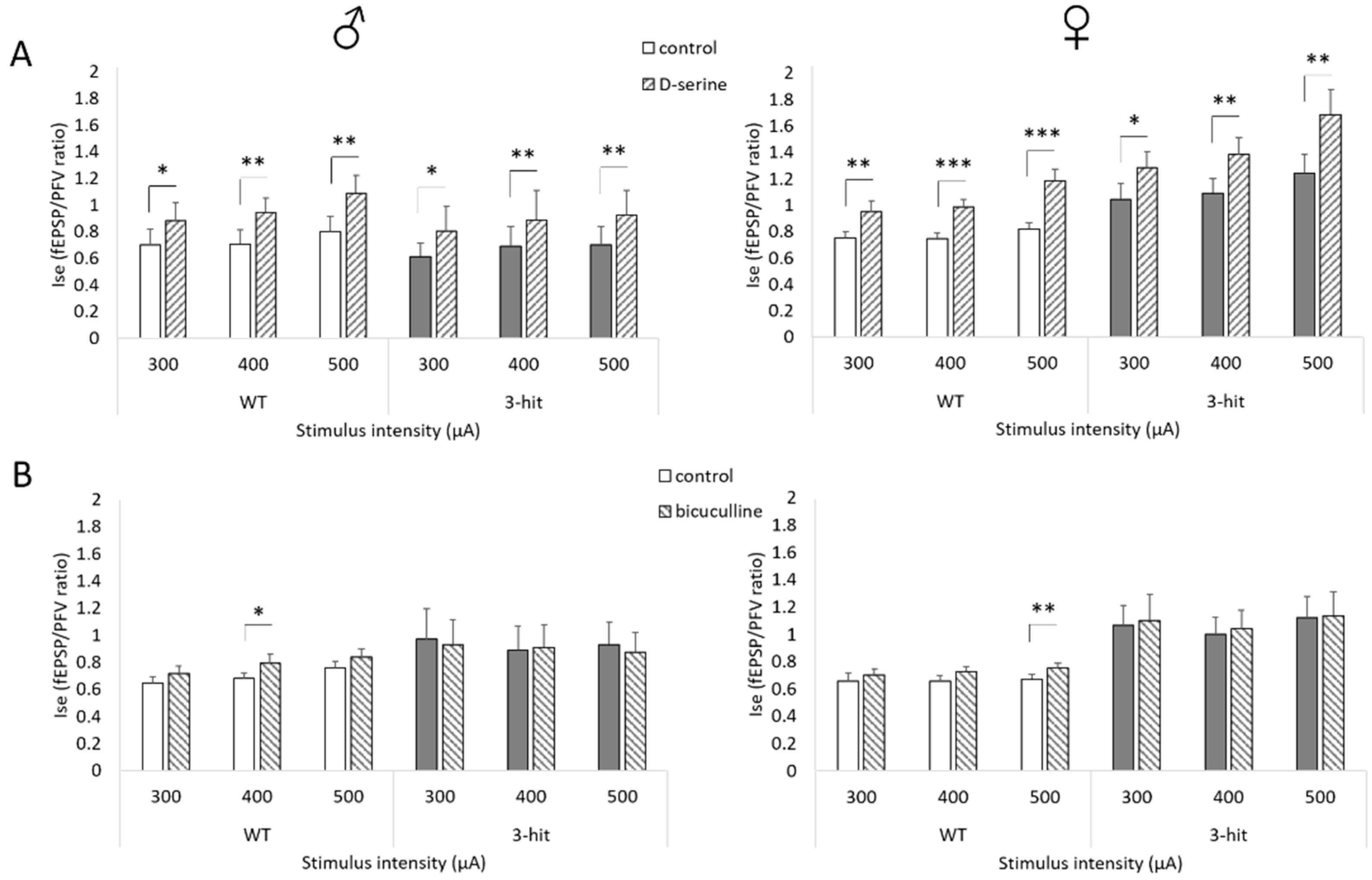
2.4. NMDAr Activation
3. Discussion
4. Materials and Methods
4.1. Genetic Susceptibility
4.2. Maternal Separation (MS)
4.3. Δ-9-Tetrahydrocannabinol Treatment
4.4. Ex Vivo Electrophysiology
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Addington, J.; Addington, D.; Maticka-Tyndale, E. Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophr. Res. 1991, 5, 123–134. [Google Scholar] [CrossRef]
- Murphy, B.P.; Chung, Y.-C.; Park, T.-W.; McGorry, P.D. Pharmacological treatment of primary negative symptoms in schizophrenia: A systematic review. Schizophr. Res. 2006, 88, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Krogmann, A.; Peters, L.; Von Hardenberg, L.; Bödeker, K.; Nöhles, V.B.; Correll, C.U. Keeping up with the therapeutic advances in schizophrenia: A review of novel and emerging pharmacological entities. CNS Spectr. 2019, 24, 38–69. [Google Scholar] [CrossRef] [PubMed]
- Bouet, V.; Boulouard, M.; Freret, T. Animal models of schizophrenia: An update. In Advances in Schizophrenia Research; Normandie University: Caen, France, 2016; Available online: www.openaccessebooks.com (accessed on 21 September 2020).
- Ellenbroek, B.A.; Riva, M.A. Early maternal deprivation as an animal model for schizophrenia. Clin. Neurosci. Res. 2003, 3, 297–302. [Google Scholar] [CrossRef]
- Jones, C.A.; Watson, D.J.G.; Fone, K.C.F. Animal models of schizophrenia. Br. J. Pharmacol. 2011, 164, 1162–1194. [Google Scholar] [CrossRef]
- Millan, M.J.; Andrieux, A.; Bartzokis, G.; Cadenhead, K.; Dazzan, P.; Fusar-Poli, P.D.P.; Gallinat, J.; Giedd, J.; Grayson, D.R.; Heinrichs, M.; et al. Altering the course of schizophrenia: Progress and perspectives. Nat. Rev. Drug Discov. 2016, 15, 485–515. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, M.; Southcott, S.; Lister, J.; Tamminga, C.A. Animal Models of Schizophrenia; Elsevier: Amsterdam, The Neatherlands, 2012; Volume 105, pp. 411–444. [Google Scholar]
- Moran, P.; Stokes, J.; Marr, J.; Bock, G.; Desbonnet, L.; Waddington, J.; O’Tuathaigh, C. Gene × environment interactions in schizophrenia: Evidence from genetic mouse models. Neural Plast. 2016, 2016, 1–23. [Google Scholar] [CrossRef]
- Ayhan, Y.; McFarland, R.; Pletnikov, M.V. Animal models of gene–environment interaction in schizophrenia: A dimensional perspective. Prog. Neurobiol. 2016, 136, 1–27. [Google Scholar] [CrossRef]
- Winship, I.R.; Dursun, S.M.; Baker, G.B.; Balista, P.A.; Kandratavicius, L.; Maia-De-Oliveira, J.P.; Hallak, J.; Howland, J.G. An overview of animal models related to schizophrenia. Can. J. Psychiatry 2018, 64, 5–17. [Google Scholar] [CrossRef]
- Bouet, V.; Percelay, S.; Leroux, E.; Diarra, B.; Léger, M.; Delcroix, N.; Andrieux, A.; Dollfus, S.; Freret, T.; Boulouard, M. A new 3-hit mouse model of schizophrenia built on genetic, early and late factors. Schizophr. Res. 2020. [Google Scholar] [CrossRef]
- Volle, J.; Brocard, J.; Saoud, M.; Gory-Faure, S.; Brunelin, J.; Andrieux, A.; Suaud-Chagny, M.-F. Reduced expression of STOP/MAP6 in mice leads to cognitive deficits. Schizophr. Bull. 2012, 39, 969–978. [Google Scholar] [CrossRef]
- Ellenbroek, B.A.; van den Kroonenberg, P.T.; Cools, A.R. The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophr. Res. 1998, 30, 251–260. [Google Scholar] [CrossRef]
- O’Tuathaigh, C.M.P.; Hryniewiecka, M.; Behan, A.T.; Tighe, O.; Coughlan, C.; Desbonnet, L.; Cannon, M.; Karayiorgou, M.; Gogos, J.A.; Cotter, D.R.; et al. Chronic adolescent exposure to Δ-9-tetrahydrocannabinol in COMT mutant mice: Impact on psychosis-related and other phenotypes. Neuropsychopharmacology 2010, 35, 2262–2273. [Google Scholar] [CrossRef]
- Leger, M.; Neill, J.C. A systematic review comparing sex differences in cognitive function in schizophrenia and in rodent models for schizophrenia, implications for improved therapeutic strategies. Neurosci. Biobehav. Rev. 2016, 68, 979–1000. [Google Scholar] [CrossRef]
- Dunn, A.L.; Michie, P.T.; Hodgson, D.M.; Harms, L. Adolescent cannabinoid exposure interacts with other risk factors in schizophrenia: A review of the evidence from animal models. Neurosci. Biobehav. Rev. 2020, 116, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Wolosker, H. D-serine regulation of NMDA receptor activity. Sci. STKE 2006, 2006, pe41. [Google Scholar] [CrossRef] [PubMed]
- Junjaud, G.; Rouaud, E.; Turpin, F.; Mothet, J.-P.; Billard, J.-M. Age-related effects of the neuromodulator d-serine on neurotransmission and synaptic potentiation in the CA1 hippocampal area of the rat. J. Neurochem. 2006, 98, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Li, Y.; Cai, G.-Q.; Niu, R.-F.; Fang, Q.; Wu, K.; Chen, Z.; Lin, L.-N.; Xu, L.; Fei, J.; et al. GABA transporter-1 Activity modulates hippocampal theta oscillation and theta burst stimulation-induced long-term potentiation. J. Neurosci. 2009, 29, 15836–15845. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Krystal, J.H.; Howes, O.D. Dopamine and glutamate in schizophrenia: Biology, symptoms and treatment. World Psychiatry 2020, 19, 15–33. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Wong, A.H.C. GABAergic inhibitory neurons as therapeutic targets for cognitive impairment in schizophrenia. Acta Pharmacol. Sin. 2018, 39, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Lee, C.C. Expression of behavioral phenotypes in genetic and environmental mouse models of schizophrenia. Front. Behav. Neurosci. 2020, 14, 1–12. [Google Scholar] [CrossRef]
- Tsuang, M. Schizophrenia: Genes and environment. Biol. Psychiatry 2000, 47, 210–220. [Google Scholar] [CrossRef]
- Ripke, S.; Neale, B.M.; Corvin, A.; Walters, J.T.R.; Farh, K.-H.; Holmans, P.A.; Lee, P.; Sullvian-Bulik, B.; Collier, D.A.; Huang, H.; et al. Biological insights from 108 schizophrenia-associated genetic loci. Nat. Cell Biol. 2014, 511, 421–427. [Google Scholar] [CrossRef]
- Deloulme, J.-C.; Gory-Fauré, S.; Mauconduit, F.; Chauvet, S.; Jonckheere, J.; Boulan, B.; Mire, E.; Xue, J.; Jany, M.; Maucler, C.; et al. Microtubule-associated protein 6 mediates neuronal connectivity through Semaphorin 3E-dependent signalling for axonal growth. Nat. Commun. 2015, 6, 7246. [Google Scholar] [CrossRef] [PubMed]
- Merenlender-Wagner, A.; Pikman, R.; Giladi, E.; Andrieux, A.; Gozes, I. NAP (davunetide) enhances cognitive behavior in the STOP heterozygous mouse—A microtubule-deficient model of schizophrenia. Peptides 2010, 31, 1368–1373. [Google Scholar] [CrossRef]
- Merenlender-Wagner, A.; Shemer, Z.; Touloumi, O.; Lagoudaki, R.; Giladi, E.; Andrieux, A.; Grigoriadis, N.C.; Gozes, I. New horizons in schizophrenia treatment: Autophagy protection is coupled with behavioral improvements in a mouse model of schizophrenia. Autophagy 2014, 10, 2324–2332. [Google Scholar] [CrossRef]
- Andrieux, A.; Salin, P.A.; Vernet, M.; Kujala, P.; Baratier, J.; Gory-Fauré, S.; Bosc, C.; Pointu, H.; Proietto, D.; Schweitzer, A.; et al. The suppression of brain cold-stable microtubules in mice induces synaptic defects associated with neuroleptic-sensitive behavioral disorders. Genes Dev. 2002, 16, 2350–2364. [Google Scholar] [CrossRef]
- Bouvrais-Veret, C.; Weiss, S.; Hanoun, N.; Andrieux, A.; Schweitzer, A.; Job, D.; Hamon, M.; Giros, B.; Martres, M.-P. Microtubule-associated STOP protein deletion triggers restricted changes in dopaminergic neurotransmission. J. Neurochem. 2007, 104, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Brenner, E.; Sonnewald, U.; Schweitzer, A.; Andrieux, A.; Nehlig, A. Hypoglutamatergic activity in the STOP knockout mouse: A potential model for chronic untreated schizophrenia. J. Neurosci. Res. 2007, 85, 3487–3493. [Google Scholar] [CrossRef]
- Anglin, D.M.; Cohen, P.R.; Chen, H. Duration of early maternal separation and prediction of schizotypal symptoms from early adolescence to midlife. Schizophr. Res. 2008, 103, 143–150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mäki, P.; Veijola, J.; Joukamaa, M.; Läärä, E.; Hakko, H.; Jones, P.B.; Isohanni, M. Maternal separation at birth and schizophrenia--a long-term follow-up of the Finnish Christmas Seal Home Children. Schizophr. Res. 2003, 60, 13–19. [Google Scholar] [CrossRef]
- Bouet, V.; Lecrux, B.; Tran, G.; Freret, T. Effect of pre- versus post-weaning environmental disturbances on social behaviour in mice. Neurosci. Lett. 2011, 488, 221–224. [Google Scholar] [CrossRef]
- Vetulani, J. Early maternal separation: A rodent model of depression and a prevailing human condition. Pharmacol. Rep. 2013, 65, 1451–1461. [Google Scholar] [CrossRef]
- Di Forti, M.; Quattrone, D.; Freeman, T.P.; Tripoli, G.; Gayer-Anderson, C.; Quigley, H.; Rodriguez, V.; Jongsma, H.E.; Ferraro, L.; La Cascia, C.; et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): A multicentre case-control study. Lancet Psychiatry 2019, 6, 427–436. [Google Scholar] [CrossRef]
- Renard, J.; Krebs, M.-O.; Le Pen, G.; Jay, T.M. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front. Neurosci. 2014, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Rubino, T.; Parolaro, D. The Impact of exposure to cannabinoids in adolescence: Insights from animal models. Biol. Psychiatry 2016, 79, 578–585. [Google Scholar] [CrossRef]
- Murphy, M.; Mills, S.; Winstone, J.; Leishman, E.; Wager-Miller, J.; Bradshaw, H.; Mackie, K. Chronic adolescent Δ9-Tetrahydrocannabinol treatment of male mice leads to long-term cognitive and behavioral dysfunction, which are prevented by concurrent cannabidiol treatment. Cannabis Cannabinoid Res. 2017, 2, 235–246. [Google Scholar] [CrossRef]
- Zamberletti, E.; Rubino, T. Impact of the endocannabinoid system manipulation on neurodevelopmental processes relevant to schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020. [Google Scholar] [CrossRef]
- Hasan, A.; Nitsche, M.A.; Rein, B.; Schneider-Axmann, T.; Guse, B.; Gruber, O.; Falkai, P.; Wobrock, T. Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behav. Brain Res. 2011, 224, 15–22. [Google Scholar] [CrossRef]
- Frantseva, M.V.; Fitzgerald, P.B.; Chen, R.; Möller, B.; Daigle, M.; Daskalakis, Z.J. Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill leaning. Cereb. Cortex 2007, 18, 990–996. [Google Scholar] [CrossRef]
- Roberts, L.; Greene, J. Post-weaning social isolation of rats leads to a diminution of LTP in the CA1 to subiculum pathway. Brain Res. 2003, 991, 271–273. [Google Scholar] [CrossRef]
- Glen, W.B.; Horowitz, B.; Carlson, G.C.; Cannon, T.D.; Talbot, K.; Jentsch, J.D.; Lavin, A. Dysbindin-1 loss compromises NMDAR-dependent synaptic plasticity and contextual fear conditioning. Hippocampus 2013, 24, 204–213. [Google Scholar] [CrossRef]
- Manahan-Vaughan, D.; Von Haebler, D.; Winter, C.; Juckel, G.; Heinemann, U. A single application of MK801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus 2008, 18, 125–134. [Google Scholar] [CrossRef]
- Booth, C.A.; Brown, J.T.; Randall, A.D. Neurophysiological modification of CA1 pyramidal neurons in a transgenic mouse expressing a truncated form of disrupted-in-schizophrenia 1. Eur. J. Neurosci. 2014, 39, 1074–1090. [Google Scholar] [CrossRef]
- Sanderson, T.M.; Cotel, M.-C.; O’Neill, M.J.; Tricklebank, M.D.; Collingridge, G.L.; Sher, E. Alterations in hippocampal excitability, synaptic transmission and synaptic plasticity in a neurodevelopmental model of schizophrenia. Neuropharmacology 2012, 62, 1349–1358. [Google Scholar] [CrossRef]
- Larson, J.; Munkácsy, E. Theta-burst LTP. Brain Res. 2015, 1621, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Drew, L.J.; Stark, K.L.; Fénelon, K.; Karayiorgou, M.; MacDermott, A.B.; Gogos, J.A. Evidence for altered hippocampal function in a mouse model of the human 22q11.2 microdeletion. Mol. Cell. Neurosci. 2011, 47, 293–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nomura, T.; Oyamada, Y.; Fernandes, H.B.; Remmers, C.L.; Xu, J.; Meltzer, H.Y.; Contractor, A. Subchronic phencyclidine treatment in adult mice increases GABAergic transmission and LTP threshold in the hippocampus. Neuropharmacology 2016, 100, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, K.; Velli, A.; Kyriazidis, E.; Stavroulaki, V.; Vouvoutsis, V.; Chatzaki, E.; Aivaliotis, M.; Sidiropoulou, K. Development of the MAM model of schizophrenia in mice: Sex similarities and differences of hippocampal and prefrontal cortical function. Neuropharmacology 2019, 144, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Hauser, J.; Rudolph, U.; Keist, R.; Möhler, H.; Feldon, J.; Yee, B.K. Hippocampal α5 subunit-containing GABAA receptors modulate the expression of prepulse inhibition. Mol. Psychiatry 2004, 10, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-N.; Bast, T.; Feldon, J. Prepulse inhibition in rats with temporary inhibition/inactivation of ventral or dorsal hippocampus. Pharmacol. Biochem. Behav. 2002, 73, 929–940. [Google Scholar] [CrossRef]
- Fradley, R.L.; O’Meara, G.F.; Newman, R.J.; Andrieux, A.; Job, D.; Reynolds, D.S. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behav. Brain Res. 2005, 163, 257–264. [Google Scholar] [CrossRef]
- Jain, A.; Huang, G.Z.; Woolley, C.S. Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. J. Neurosci. 2019, 39, 1552–1565. [Google Scholar] [CrossRef]
- Brandt, N.; Vierk, R.; Fester, L.; Anstötz, M.; Zhou, L.; Heilmann, L.F.; Kind, S.; Steffen, P.; Rune, G.M. Sex-specific difference of hippocampal synaptic plasticity in response to sex neurosteroids. Cereb. Cortex 2020, 30, 2627–2641. [Google Scholar] [CrossRef]
- Wang, W.; Le, A.A.; Hou, B.; Lauterborn, J.C.; Cox, C.D.; Levin, E.R.; Lynch, G.; Gall, C.M. Memory-Related synaptic plasticity is sexually dimorphic in rodent hippocampus. J. Neurosci. 2018, 38, 7935–7951. [Google Scholar] [CrossRef] [PubMed]
- Derks, N.A.V.; Krugers, H.J.; Hoogenraad, C.C.; Joëls, M.; Sarabdjitsingh, R.A. Effects of early life stress on synaptic plasticity in the developing hippocampus of male and female rats. PLoS ONE 2016, 11, e0164551. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J. Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: Long-term potentiation, long-term depression, short-term potentiation and scaling. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160260. [Google Scholar] [CrossRef]
- Nguyen, R.; Morrissey, M.D.; Mahadevan, V.; Cajanding, J.D.; Woodin, M.A.; Yeomans, J.S.; Takehara-Nishiuchi, K.; Kim, J.C. Parvalbumin and GAD65 interneuron inhibition in the ventral hippocampus induces distinct behavioral deficits relevant to schizophrenia. J. Neurosci. 2014, 34, 14948–14960. [Google Scholar] [CrossRef] [PubMed]
- Glover, M.E.; Clinton, S.M. Of rodents and humans: A comparative review of the neurobehavioral effects of early life SSRI exposure in preclinical and clinical research. Int. J. Dev. Neurosci. 2016, 51, 50–72. [Google Scholar] [CrossRef] [PubMed]
- Yonelinas, A.P. The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behav. Brain Res. 2013, 254, 34–44. [Google Scholar] [CrossRef]
- Neves, G.; Cooke, S.F.; Bliss, T.V.P. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat. Rev. Neurosci. 2008, 9, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Bures, J.; Fenton, A.A.; Kaminsky, Y.; Zinyuk, L. Place cells and place navigation. Proc. Natl. Acad. Sci. USA 1997, 94, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.F.; Tso, I.F. GABA abnormalities in schizophrenia: A methodological review of in vivo studies. Schizophr. Res. 2015, 167, 84–90. [Google Scholar] [CrossRef]
- Nakazawa, K.; Zsiros, V.; Jiang, Z.; Nakao, K.; Kolata, S.; Zhang, S.; Belforte, J.E. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 2012, 62, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Potier, B.; Poindessous-Jazat, F.; Dutar, P.; Billard, J.M. NMDA receptor activation in the aged rat hippocampus. Exp. Gerontol. 2000, 35, 1185–1199. [Google Scholar] [CrossRef]
- Anderson, W.W.; Collingridge, G.L. The LTP Program: A data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J. Neurosci. Methods 2001, 108, 71–83. [Google Scholar] [CrossRef]
- Anderson, W.W.; Collingridge, G.L. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J. Neurosci. Methods 2007, 162, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Lecouflet, P.; Roux, C.M.; Potier, B.; Leger, M.; Brunet, E.; Billard, J.-M.; Schumann-Bard, P.; Freret, T. Interplay between 5-HT4 receptors and GABAergic system within CA1 hippocampal synaptic plasticity. Cereb. Cortex 2021, 31, 694–701. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Percelay, S.; Billard, J.-M.; Freret, T.; Andrieux, A.; Boulouard, M.; Bouet, V. Functional Dysregulations in CA1 Hippocampal Networks of a 3-Hit Mouse Model of Schizophrenia. Int. J. Mol. Sci. 2021, 22, 2644. https://doi.org/10.3390/ijms22052644
Percelay S, Billard J-M, Freret T, Andrieux A, Boulouard M, Bouet V. Functional Dysregulations in CA1 Hippocampal Networks of a 3-Hit Mouse Model of Schizophrenia. International Journal of Molecular Sciences. 2021; 22(5):2644. https://doi.org/10.3390/ijms22052644
Chicago/Turabian StylePercelay, Solenn, Jean-Marie Billard, Thomas Freret, Annie Andrieux, Michel Boulouard, and Valentine Bouet. 2021. "Functional Dysregulations in CA1 Hippocampal Networks of a 3-Hit Mouse Model of Schizophrenia" International Journal of Molecular Sciences 22, no. 5: 2644. https://doi.org/10.3390/ijms22052644
APA StylePercelay, S., Billard, J.-M., Freret, T., Andrieux, A., Boulouard, M., & Bouet, V. (2021). Functional Dysregulations in CA1 Hippocampal Networks of a 3-Hit Mouse Model of Schizophrenia. International Journal of Molecular Sciences, 22(5), 2644. https://doi.org/10.3390/ijms22052644





