Momordica cochinchinensis Aril Ameliorates Diet-Induced Metabolic Dysfunction and Non-Alcoholic Fatty Liver by Modulating Gut Microbiota
Abstract
1. Introduction
2. Results
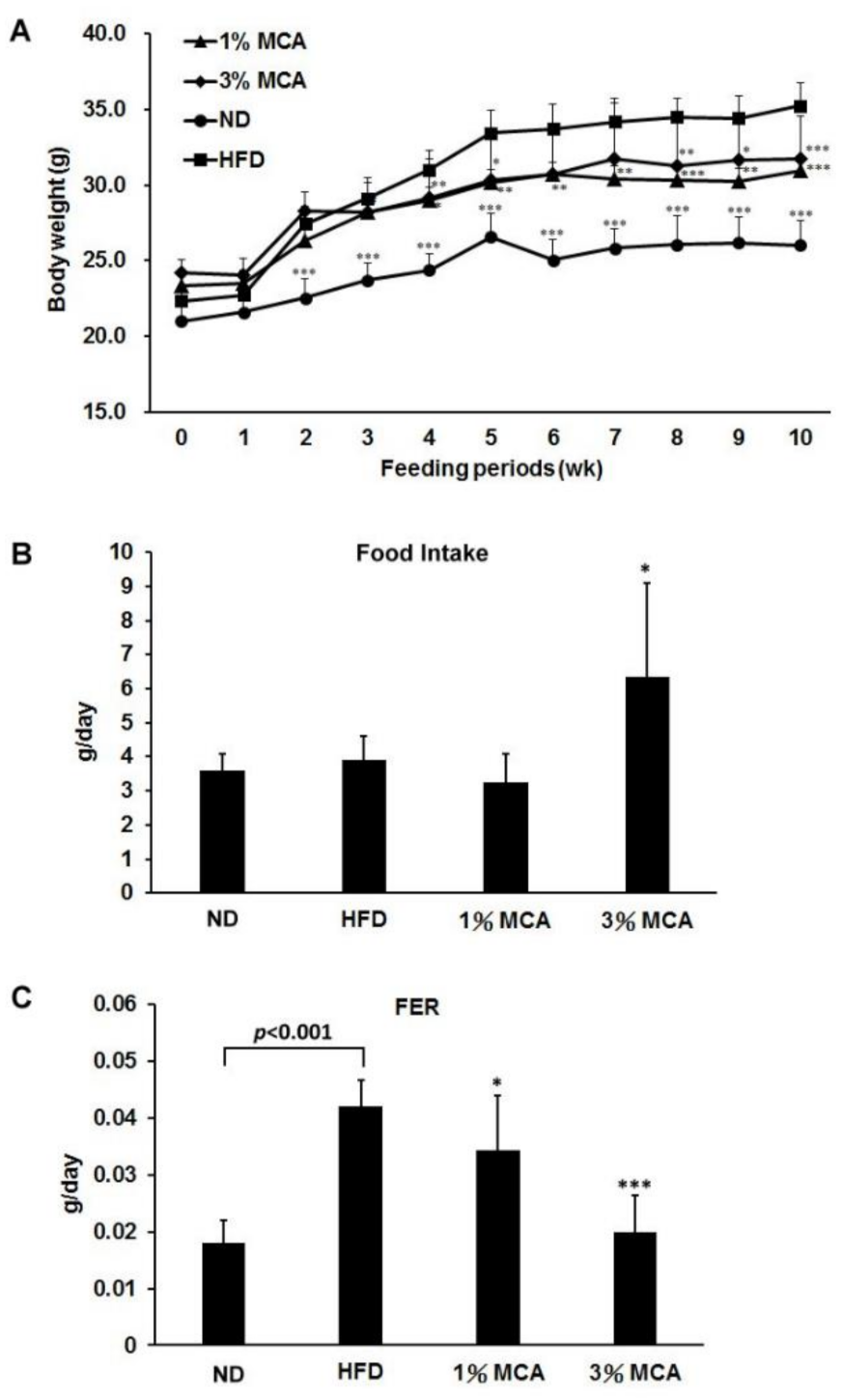
2.1. Effects of Momordica cochinchinensis Aril (MCA) on Body Weight and Food Efficiency in High-Fat Diet (HFD)-Fed Mice
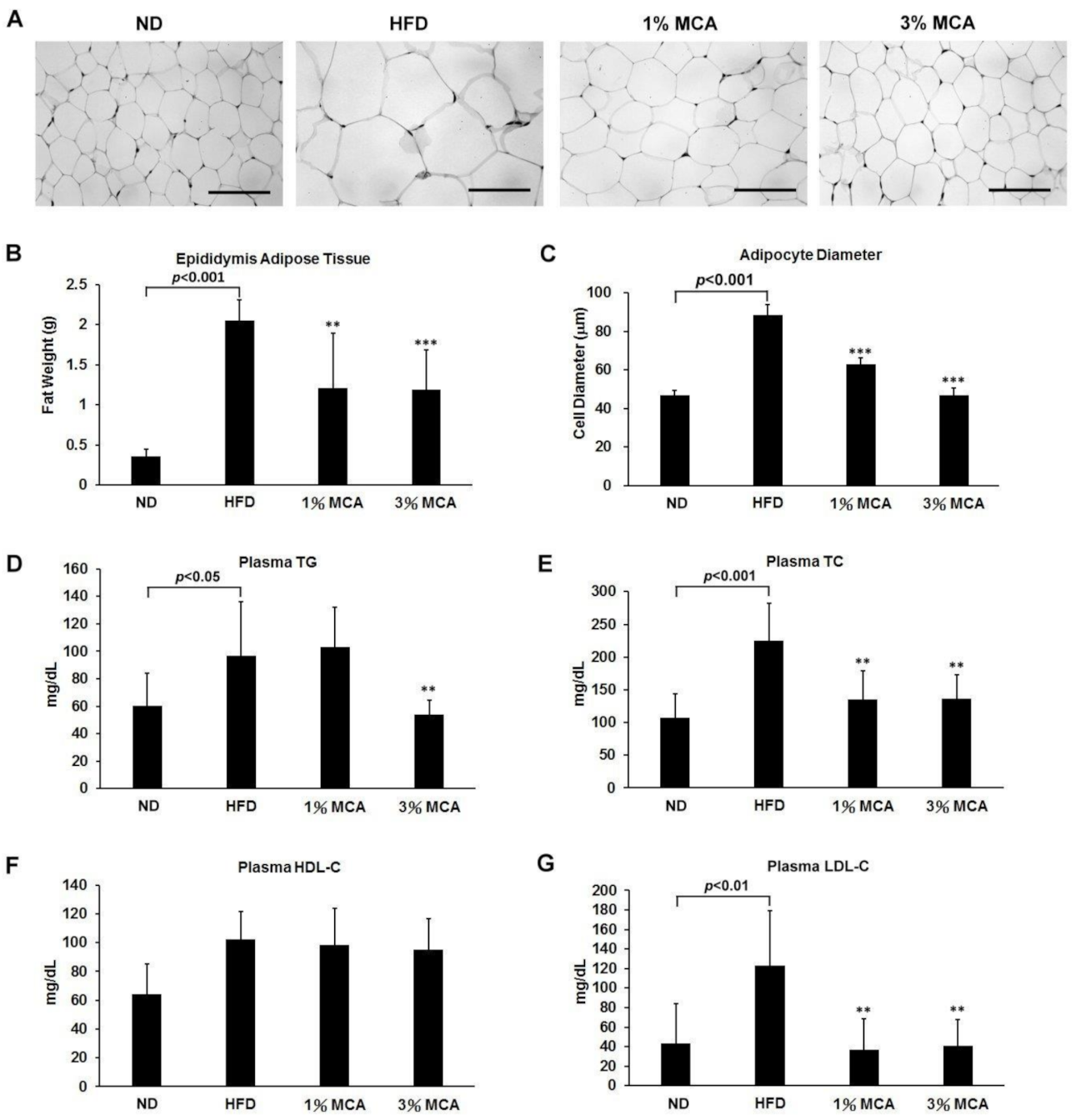
2.2. MCA Decreased Visceral Fat Deposition and Prevented HFD-Induced Hyperlipidemia
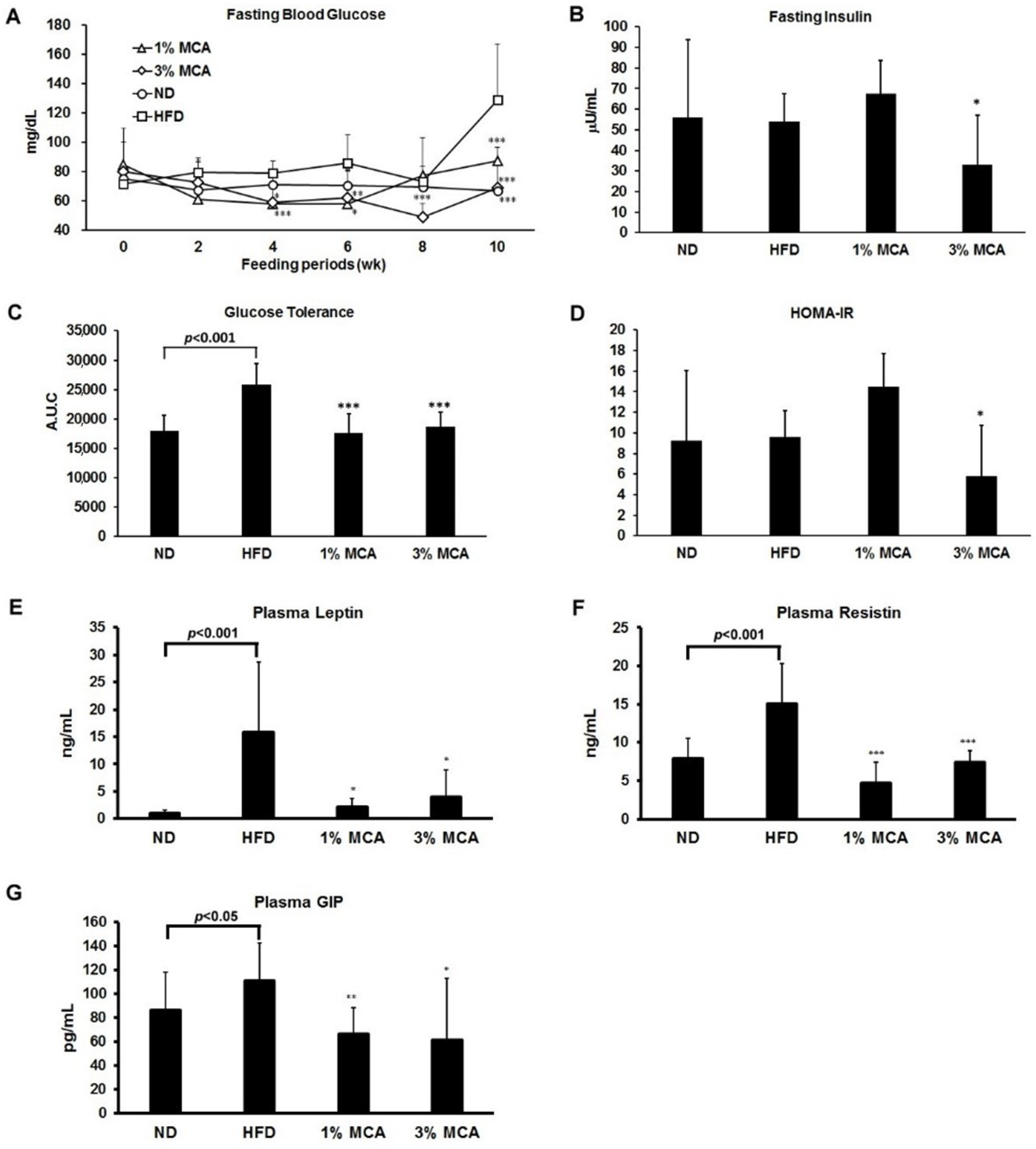
2.3. Effects of MCA on HFD-Induced Insulin Resistance and Plasma Adipocytokines and Glucose-Dependent Insulinotropic Polypeptide (GIP)
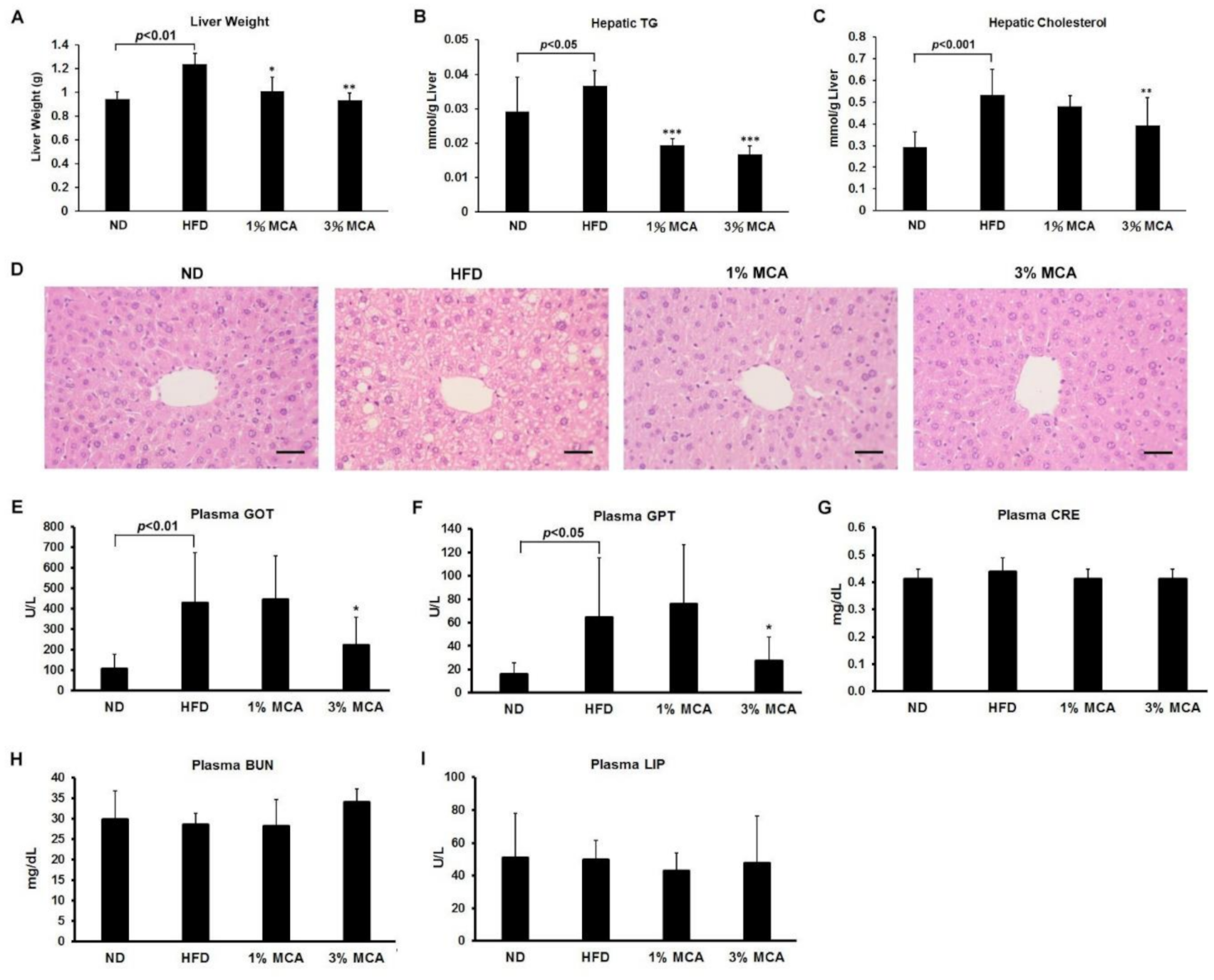
2.4. MCA Treatment Reduced HFD-Induced Fatty Liver and Inhibited Liver Damage
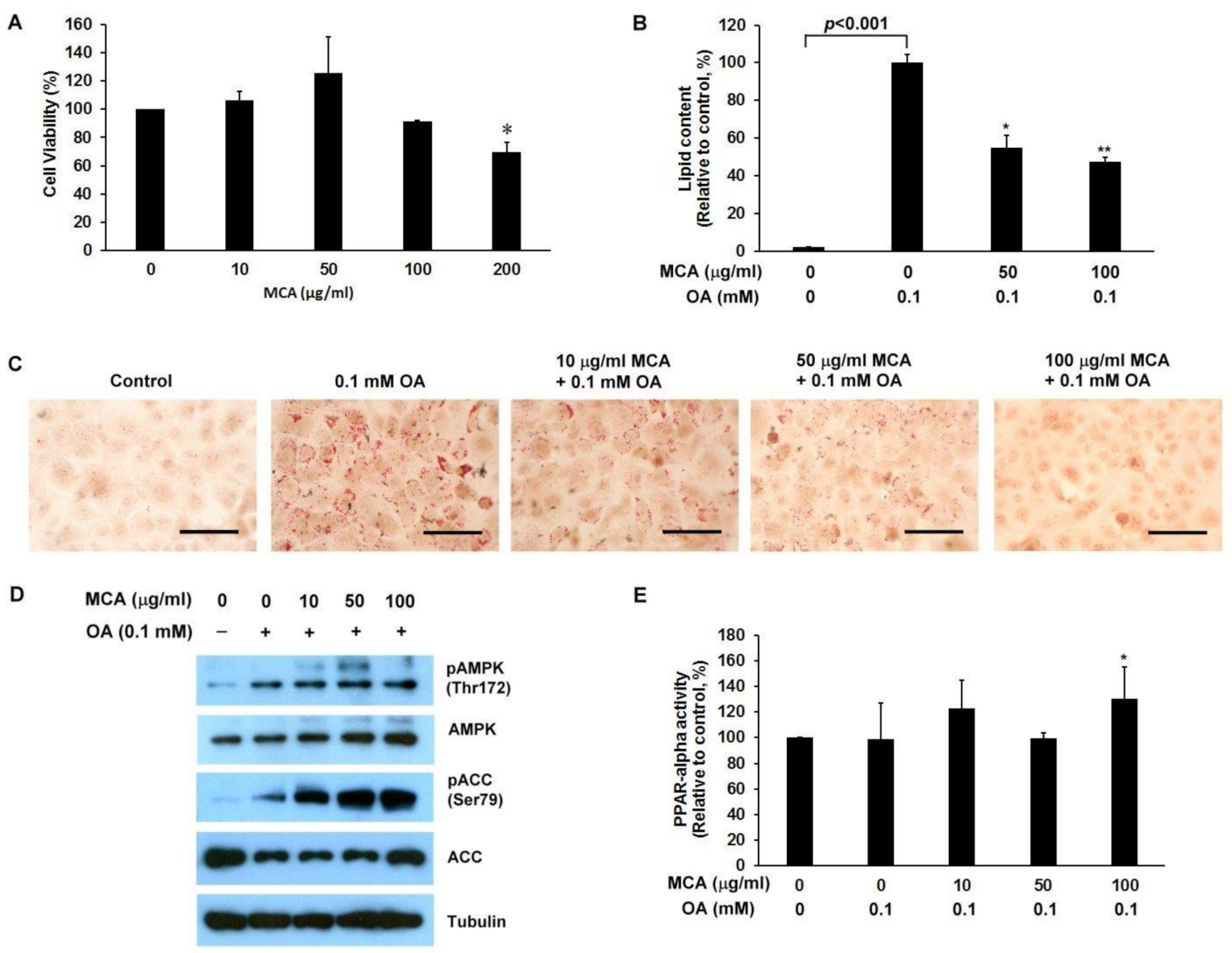
2.5. MCA Treatment Downregulates Lipid Accumulation and AMP-Activated Protein Kinase (AMPK)/ACC Phosphorylation, and Upregulates Peroxisome Proliferator-Activated Receptor-Alpha (PPAR-α) Activity in a Human Fatty Liver Cell Model
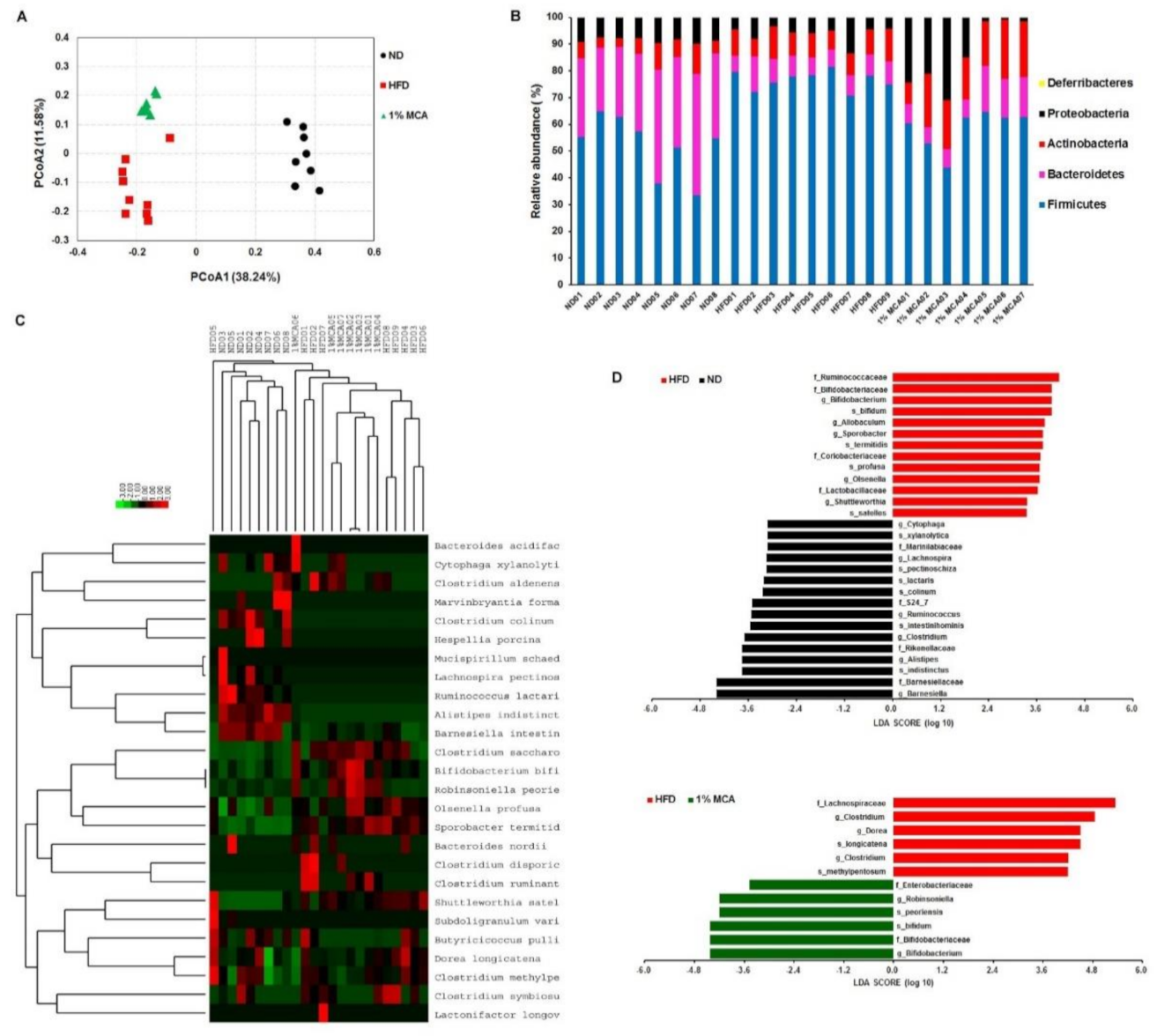
2.6. MCA Consumption Modulates Obesity-Driven Dysbiosis of the Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Preparation of Lyophilized Momordica cochinchinensis Aril (MCA)
4.2. Animals
4.3. Morphology of the Liver and Fat Tissues
4.4. Biochemical Analysis of Plasma
4.5. Blood Glucose, Plasma Insulin, and the Homeostasis Model Assessment of Insulin Resistance Index
4.6. Triglyceride and Cholesterol Analysis of Liver Tissue
4.7. Antibodies and Reagents
4.8. Cell Culture
4.9. Cell Viability Assay
4.10. Western Blot Analysis
4.11. PPAR-Alpha Activity Assay
4.12. Gut Microbiota Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kopelman, P.G. Obesity as a medical problem. Nat. Cell Biol. 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and societal implications of the diabetes epidemic. Nat. Cell Biol. 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Bray, G.A.; Paeratakul, S.; Popkin, B.M. Dietary fat and obesity: A review of animal, clinical and epidemiological studies. Physiol. Behav. 2004, 83, 549–555. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Nilsson, P.M. The metabolic syndrome: A glance at its history. J. Hypertens. 2006, 24, 621–626. [Google Scholar] [CrossRef]
- Hong, L.; Guo, Z.; Huang, K.; Wei, S.; Liu, B.; Meng, S.; Long, C. Ethnobotanical study on medicinal plants used by Maonan people in China. J. Ethnobiol. Ethnomedicine 2015, 11, 1–35. [Google Scholar] [CrossRef]
- Tien, P.G.; Kayama, F.; Konishi, F.; Tamemoto, H.; Kasono, K.; Hung, N.T.K.; Kuroki, M.; Ishikawa, S.-E.; Van, C.N.; Kawakami, M. Inhibition of tumor growth and angiogenesis by water extract of Gac fruit (Momordica cochinchinensis Spreng). Int. J. Oncol. 2005, 26, 881–889. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Y.; Liu, Y.; Yang, X.O.; Zhan, Y. Momordica cochinchinensis Spreng. seed extract suppresses breast cancer growth by inducing cell cycle arrest and apoptosis. Mol. Med. Rep. 2015, 12, 6300–6310. [Google Scholar] [CrossRef] [PubMed]
- Kubola, J.; Siriamornpun, S. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chem. 2011, 127, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Agócs, A.; Nagy, V.; Szabó, Z.; Márk, L.; Ohmacht, R.; Deli, J. Comparative study on the carotenoid composition of the peel and the pulp of different citrus species. Innov. Food Sci. Emerg. Technol. 2007, 8, 390–394. [Google Scholar] [CrossRef]
- Vuong, L.T.; King, J.C. A method of preserving and testing the acceptability of gac fruit oil, a good source of beta-carotene and essential fatty acids. Food Nutr. Bull. 2003, 24, 224–230. [Google Scholar] [CrossRef]
- Jiang, J.; Fei, A.; Wang, Y.; Fang, Z.; Teng, Y.; Hao, X. Studied on the extraction, response surface analysis and antioxidant activity of flavonoids in the Momordia cochinchinensis seeds. MATEC Web Conf. 2018, 238, 04004. [Google Scholar] [CrossRef][Green Version]
- Liu, H.-K.; Hung, T.-M.; Huang, H.-C.; Lee, I.-J.; Chang, C.-C.; Cheng, J.-J.; Lin, L.-C.; Huang, C. Bai-Hu-Jia-Ren-Shen-Tang Decoction Reduces Fatty Liver by Activating AMP-Activated Protein Kinase In Vitro and In Vivo. Evid. Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.M. Fat Mass and Obesity Associated (FTO) Gene and Hepatic Glucose and Lipid Metabolism. Nutrient 2018, 10, 1600. [Google Scholar] [CrossRef] [PubMed]
- Canals, M.; Figueroa, D.; Alfaro, C.; Kawamoto, T.; Torres-Contreras, H.; Sabat, P.; Veloso, C. Effects of diet and water supply on energy intake and water loss in a mygalomorph spider in a fluctuating environment of the central Andes. J. Insect Physiol. 2011, 57, 1489–1494. [Google Scholar] [CrossRef]
- Huang, T.; Beaty, T.; Li, J.; Liu, H.; Zhao, W.; Wang, Y. Association between dietary fat intake and insulin resistance in Chinese child twins. Br. J. Nutr. 2017, 117, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Tatsumi, Y.; Soyano, F.; Miyamatsu, N.; Sonoda, N.; Godai, K.; Ohno, Y.; Noda, M.; Deura, K. Increase in Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) Had a Strong Impact on the Development of Type 2 Diabetes in Japanese Individuals with Impaired Insulin Secretion: The Saku Study. PLoS ONE 2014, 9, e105827. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.V.; Du, M.; Wang, S.; Bergen, W.G.; Fernyhough-Culver, M.; Basu, U.; Poulos, S.P.; Hausman, G.J. Adipose depots differ in cellularity, adipokines produced, gene expression, and cell systems. Adipocyte 2014, 3, 236–241. [Google Scholar] [CrossRef][Green Version]
- McIntosh, C.H.; Widenmaier, S.; Kim, S.J. Glucose-dependent insulinotropic polypeptide (Gastric Inhibitory Polypeptide; GIP). Vitam. Horm. 2009, 80, 409–471. [Google Scholar] [PubMed]
- Asrih, M.; Jornayvaz, F.R. Metabolic syndrome and nonalcoholic fatty liver disease: Is insulin resistance the link? Mol. Cell. Endocrinol. 2015, 418, 55–65. [Google Scholar] [CrossRef]
- Kakimoto, P.A.; Kowaltowski, A.J. Effects of high fat diets on rodent liver bioenergetics and oxidative imbalance. Redox Biol. 2016, 8, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Dufour, D.R.; Lott, J.A.; Nolte, F.S.; Gretch, D.R.; Koff, R.S.; Seeff, L.B. Diagnosis and Monitoring of Hepatic Injury. II. Recommendations for Use of Laboratory Tests in Screening, Diagnosis, and Monitoring. Clin. Chem. 2000, 46, 2050–2068. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef]
- Holmes, E.; Li, J.V.; Athanasiou, T.; Ashrafian, H.; Nicholson, J.K. Understanding the role of gut microbiome–host metabolic signal disruption in health and disease. Trends Microbiol. 2011, 19, 349–359. [Google Scholar] [CrossRef]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014, 8, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.G.; Wu, G.D. Diet and the Intestinal Microbiome: Associations, Functions, and Implications for Health and Disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D.; Parks, S.E.; Stathopoulos, C. Gac Fruit: Nutrient and Phytochemical Composition, and Options for Processing. Food Rev. Int. 2013, 29, 92–106. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Gac fruit (Momordica cochinchinensisSpreng.): A rich source of bioactive compounds and its potential health benefits. Int. J. Food Sci. Technol. 2014, 50, 567–577. [Google Scholar] [CrossRef]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Ota, T.; Takamura, T.; Kurita, S.; Matsuzawa, N.; Kita, Y.; Uno, M.; Akahori, H.; Misu, H.; Sakurai, M.; Zen, Y.; et al. Insulin Resistance Accelerates a Dietary Rat Model of Nonalcoholic Steatohepatitis. Gastroenterology 2007, 132, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; He, W.; Jia, Z.; Hao, S. Lycopene Improves Insulin Sensitivity through Inhibition of STAT3/Srebp-1c-Mediated Lipid Accumulation and Inflammation in Mice fed a High-Fat Diet. Exp. Clin. Endocrinol. Diabetes 2017, 125, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Amara, N.B.; Tourniaire, F.; Maraninchi, M.; Attia, N.; Amiot-Carlin, M.J.; Raccah, D.; Valero, R.; Landrier, J.F.; Darmon, P. Independent positive association of plasma beta-carotene concentrations with adiponectin among non-diabetic obese subjects. Eur. J. Nutr. 2015, 54, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, W.M.; Reynolds, T.B. Serum Lactic Dehydrogenase in the Differential Diagnosis of Acute Hepatocellular Injury. J. Clin. Gastroenterol. 1994, 19, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, C.E.; Everhart, J.E. Relation of elevated serum alanine aminotransferase activity with iron and antioxidant levels in the United States. Gastroenterology 2003, 124, 1821–1829. [Google Scholar] [CrossRef]
- García, O.P.; Long, K.Z.; Rosado, J.L. Impact of micronutrient deficiencies on obesity. Nutr. Rev. 2009, 67, 559–572. [Google Scholar] [CrossRef]
- Gunanti, I.R.; Marks, G.C.; Al-Mamun, A.; Long, K.Z. Low Serum Concentrations of Carotenoids and Vitamin E Are Associated with High Adiposity in Mexican-American Children. J. Nutr. 2014, 144, 489–495. [Google Scholar] [CrossRef]
- Siiteri, P.K. Adipose tissue as a source of hormones. Am. J. Clin. Nutr. 1987, 45, 277–282. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. Cell Biol. 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Goodman, A.L.; Kallstrom, G.; Faith, J.J.; Reyes, A.; Moore, A.; Dantas, G.; Gordon, J.I. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc. Natl. Acad. Sci. USA 2011, 108, 6252–6257. [Google Scholar] [CrossRef]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Yakout, S.; Alnaami, A.M.; Alokail, M.S.; McTernan, P.G. Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naïve T2DM patients: A randomized clinical trial. J. Transl. Med. 2017, 15, 1–9. [Google Scholar] [CrossRef]
- Nobili, V.; Putignani, L.; Mosca, A.; Del Chierico, F.; Vernocchi, P.; Alisi, A.; Stronati, L.; Cucchiara, S.; Toscano, M.; Drago, L. Bifidobacteria and lactobacilli in the gut microbiome of children with non-alcoholic fatty liver disease: Which strains act as health players? Arch. Med. Sci. 2018, 1, 81–87. [Google Scholar] [CrossRef]
- Matsuki, T.; Tajima, S.; Hara, T.; Yahagi, K.; Ogawa, E.; Kodama, H. Infant formula with galacto-oligosaccharides (OM55N) stimulates the growth of indigenous bifidobacteria in healthy term infants. Benef. Microbes 2016, 7, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Yahagi, K.; Mori, H.; Matsumoto, H.; Hara, T.; Tajima, S.; Ogawa, E.; Kodama, E.O.H.; Yamamoto, K.; Yamada, T.; et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat. Commun. 2016, 7, 11939. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.H.; Watashi, K.; Hijikata, M.; Kaneko, H.; Takada, Y.; Egawa, H.; Uemoto, S.; Shimotohno, K. Serum-derived hepatitis C virus infectivity in interferon regulatory factor-7-suppressed human primary hepatocytes. J. Hepatol. 2007, 46, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Saldanha, A.J. Java Treeview—Extensible visualization of microarray data. Bioinformatics 2004, 20, 3246–3248. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-C.; Chen, C.-J.; Lai, Y.-H.; Lin, Y.-C.; Chiou, W.-C.; Lu, H.-F.; Chen, Y.-F.; Chen, Y.-H.; Huang, C. Momordica cochinchinensis Aril Ameliorates Diet-Induced Metabolic Dysfunction and Non-Alcoholic Fatty Liver by Modulating Gut Microbiota. Int. J. Mol. Sci. 2021, 22, 2640. https://doi.org/10.3390/ijms22052640
Huang H-C, Chen C-J, Lai Y-H, Lin Y-C, Chiou W-C, Lu H-F, Chen Y-F, Chen Y-H, Huang C. Momordica cochinchinensis Aril Ameliorates Diet-Induced Metabolic Dysfunction and Non-Alcoholic Fatty Liver by Modulating Gut Microbiota. International Journal of Molecular Sciences. 2021; 22(5):2640. https://doi.org/10.3390/ijms22052640
Chicago/Turabian StyleHuang, Hsiu-Chen, Chiung-Ju Chen, Yu-Heng Lai, Yu-Chun Lin, Wei-Chung Chiou, Hsu-Feng Lu, Ying-Fang Chen, Yu-Hsin Chen, and Cheng Huang. 2021. "Momordica cochinchinensis Aril Ameliorates Diet-Induced Metabolic Dysfunction and Non-Alcoholic Fatty Liver by Modulating Gut Microbiota" International Journal of Molecular Sciences 22, no. 5: 2640. https://doi.org/10.3390/ijms22052640
APA StyleHuang, H.-C., Chen, C.-J., Lai, Y.-H., Lin, Y.-C., Chiou, W.-C., Lu, H.-F., Chen, Y.-F., Chen, Y.-H., & Huang, C. (2021). Momordica cochinchinensis Aril Ameliorates Diet-Induced Metabolic Dysfunction and Non-Alcoholic Fatty Liver by Modulating Gut Microbiota. International Journal of Molecular Sciences, 22(5), 2640. https://doi.org/10.3390/ijms22052640





