Nanomaterials and the Serosal Immune System in the Thoracic and Peritoneal Cavities
Abstract
1. Introduction/Background
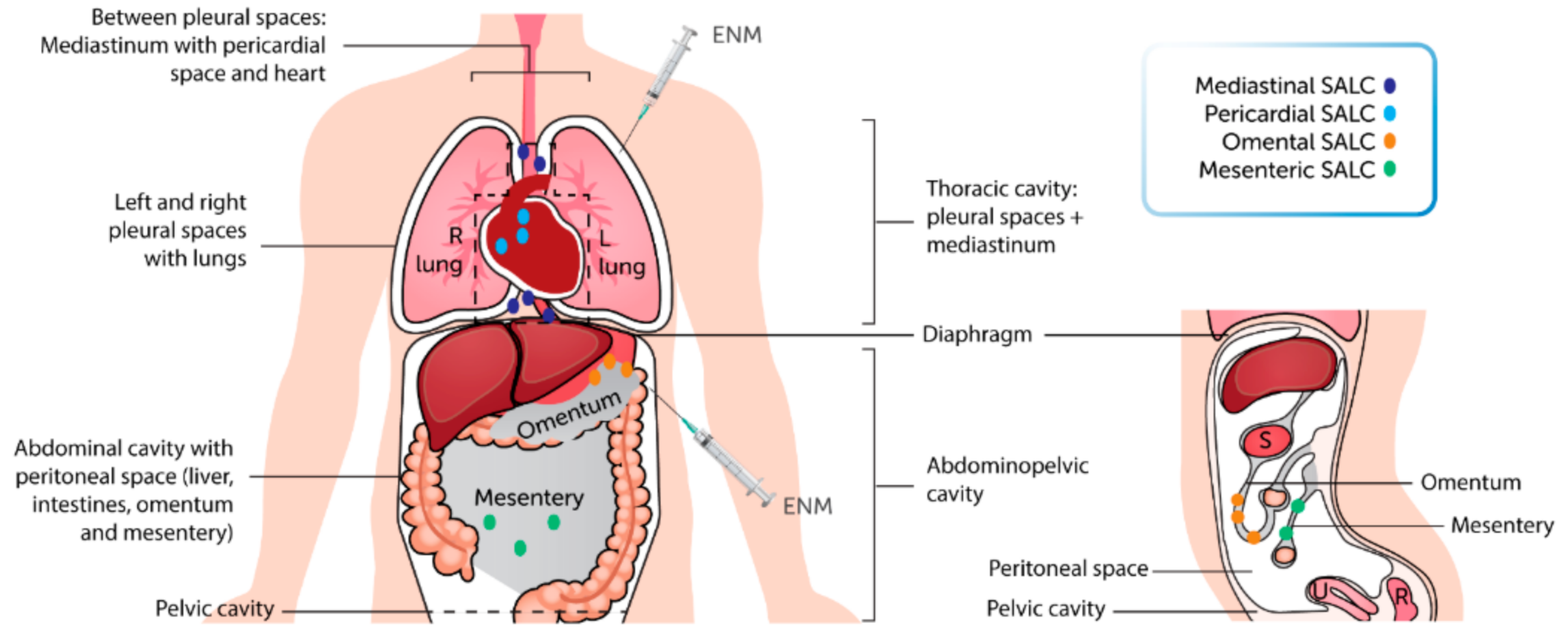
2. NM and the Thoracic Cavity
2.1. Translocation Route of Inhaled NM to the Thoracic Cavity
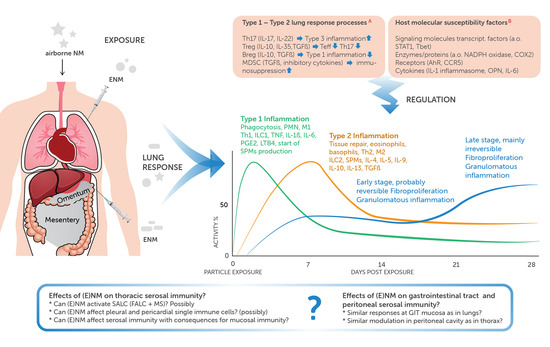
2.2. General Effects in the Thoracic Cavity
3. NM and the Peritoneal Cavity
4. Effects of NM on Serosal Lymphoid Clusters and Immune Cells
4.1. SALC
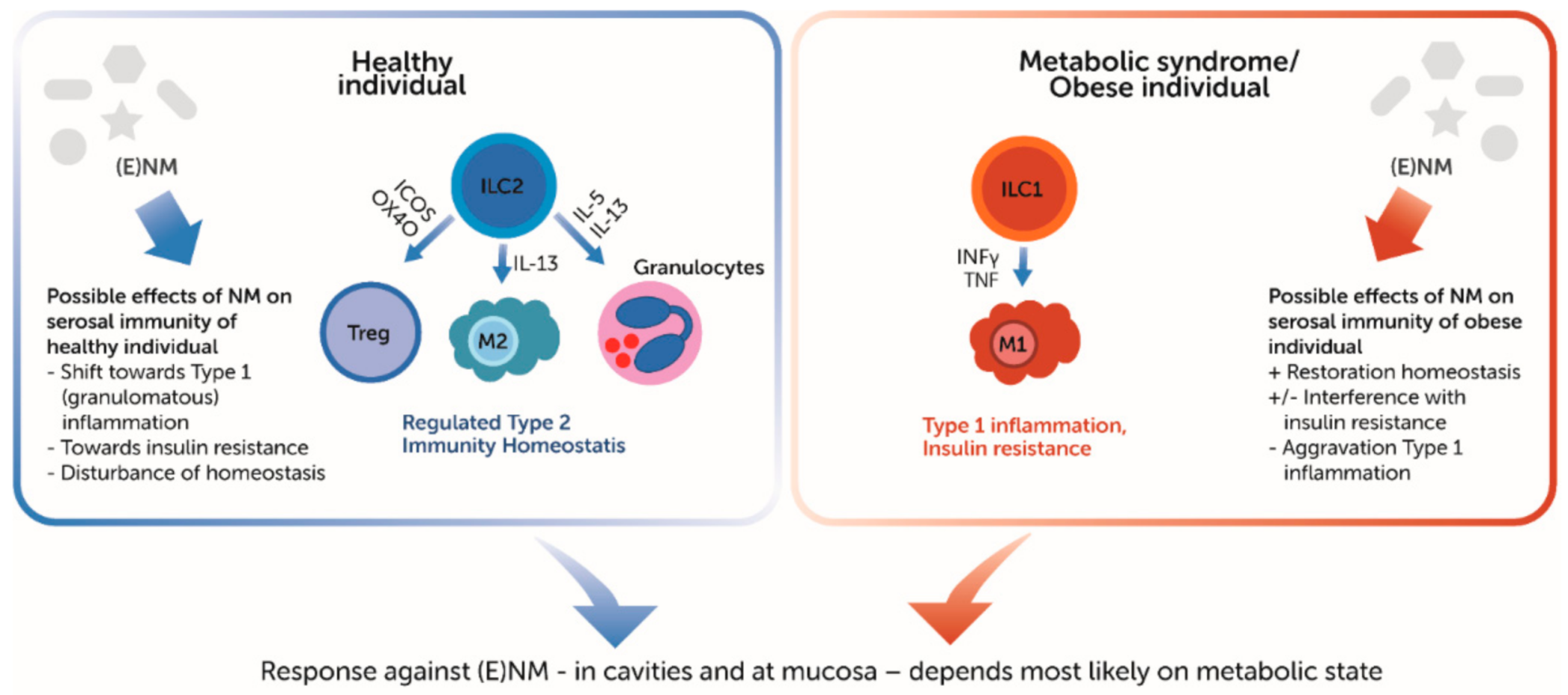
4.2. Single Immune Cells
5. Serosal–Mucosal Interaction
6. Discussion/Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMs | alveolar macrophages |
| BAT | brown adipose tissue |
| Breg | regulatory B cell |
| EMT | epithelium to mesenchymal transition |
| ENMs | engineered nanomaterials |
| FALC(s) | fat-associated lymphoid cluster(s) |
| GIT | gastrointestinal tract |
| ICOS | inducible costimulatory molecule |
| ILC | innate lymphoid cell |
| IFNγ | interferon gamma |
| LNs | lymph nodes |
| LTis | lymphoid tissue inducer cells |
| M1 | macrophage type 1 |
| M2 | macrophage type 2 |
| MDSC | myeloid-derived suppressor cell |
| M | microfold |
| MMT | mesothelium to mesenchymal transition |
| MSs | milky spots |
| NMs | nanomaterials |
| PMNs | polymorphonuclear cells (neutrophils) |
| PPs | Peyer’s patches |
| SALC(s) | serosa-associated lymphoid cluster(s) |
| SPM | specialized pro-resolving mediator |
| Teff | Teffector cell |
| Th | Thelper cell |
| TNF | tumor necrosis factor |
| Treg | regulatory T cell |
| WAT | white adipose tissue |
References
- Bernstein, D.M.; Rogers, R.A.; Sepulveda, R.; Kunzendorf, P.; Bellmann, B.; Ernst, H.; Creutzenberg, O.; Phillips, J.I. Evaluation of the fate and pathological response in the lung and pleura of brake dust alone and in combination with added chrysotile compared to crocidolite asbestos following short-term inhalation exposure. Toxicol. Appl. Pharmacol. 2015, 283, 20–34. [Google Scholar] [CrossRef]
- Bénézech, C.; Luu, N.T.; Walker, J.A.; Kruglov, A.A.; Loo, Y.; Nakamura, K.; Zhang, Y.; Nayar, S.; Jones, L.H.; Flores-Langarica, A.; et al. Inflammation-induced formation of fat-associated lymphoid clusters. Nat. Immunol. 2015, 16, 819–828. [Google Scholar] [CrossRef]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review and the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010, 7, 5. [Google Scholar] [CrossRef]
- Oberdörster, G. Safety assessment for nanotechnology and nanomedicine: Concepts of nanotoxicology. J. Intern. Med. 2010, 267, 89–105. [Google Scholar] [CrossRef]
- Landsiedel, R.; Sauer, U.G.; Ma-Hock, L.; Schnekenburger, J.; Wiemann, M. Pulmonary toxicity of nanomaterials: A critical comparison of published in vitro assays and in vivo inhalation or instillation studies. Nanomedicine 2014, 9, 2557–2585. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef]
- Haque, S.; Whittaker, M.R.; McIntosh, M.P.; Pouton, C.W.; Kaminskas, L.M. Disposition and safety of inhaled biodegradable nanomedicines: Opportunities and challenges. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1703–1724. [Google Scholar] [CrossRef]
- Kooter, I.; Ilves, M.; Gröllers-Mulderij, M.; Duistermaat, E.; Tromp, P.C.; Kuper, F.; Kinaret, P.; Savolainen, K.; Greco, D.; Karisola, P.; et al. Molecular Signature of Asthma-Enhanced Sensitivity to CuO Nanoparticle Aerosols from 3D Cell Model. ACS Nano 2019, 13, 6932–6946. [Google Scholar] [CrossRef]
- Van de Sande, L.; Cosyns, S.; Willaert, W.; Ceelen, W. Albumin-based cancer therapeutics for intraperitoneal drug delivery: A review. Drug Deliv. 2020, 27, 40–53. [Google Scholar] [CrossRef]
- Jackson-Jones, L.H.; Bénézech, C. FALC stromal cells define a unique immunological niche for the surveillance of serous cavities. Curr. Opin. Immunol. 2020, 64, 42–49. [Google Scholar] [CrossRef]
- Rangel-Moreno, J.; Moyron-Quiroz, J.E.; Carragher, D.M.; Kusser, K.; Hartson, L.; Moquin, A.; Randall, T.D. Omental Milky Spots Develop in the Absence of Lymphoid Tissue-Inducer Cells and Support B and T Cell Responses to Peritoneal Antigens. Immunity 2009, 30, 731–743. [Google Scholar] [CrossRef]
- Cruz-Migoni, S.; Caamaño, J. Fat-associated lymphoid clusters in inflammation and immunity. Front. Immunol. 2016, 7, 7. [Google Scholar] [CrossRef]
- Perez-Shibayama, C.; Ludewig, B. Tuning up FALCs: Immunological shielding in the body cavities. Nat. Immunol. 2015, 16, 796–798. [Google Scholar] [CrossRef]
- Schäffler, A.; Schölmerich, J. Innate immunity and adipose tissue biology. Trends Immunol. 2010, 31, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, D.A.; Randall, T.D. Adaptive immunity and adipose tissue biology. Trends Immunol. 2010, 31, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Kuper, C.F.; van Bilsen, J.; Wijnands, M.V.W. The serosal immune system of the thorax in toxicology. Toxicol. Sci. 2018, 164, 31–38. [Google Scholar] [CrossRef]
- Elewa, Y.H.A.; Ichii, O.; Otsuka, S.; Hashimoto, Y.; Kon, Y. Characterization of mouse mediastinal fat-associated lymphoid clusters. Cell Tissue Res. 2014, 357, 731–741. [Google Scholar] [CrossRef]
- Elewa, Y.H.A.; Ichii, O.; Kon, Y. Sex-related differences in autoimmune-induced lung lesions in MRL/MpJ- fas lpr mice are mediated by the development of mediastinal fat-associated lymphoid clusters. Autoimmunity 2017, 50, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Koyasu, S.; Moro, K. Role of Innate Lymphocytes in Infection and Inflammation. Front. Immunol. 2012, 3, 13. [Google Scholar] [CrossRef]
- Saenz, S.A.; Noti, M.; Artis, D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010, 31, 407–413. [Google Scholar] [CrossRef]
- Elewa, Y.H.A.; Ichii, O.; Kon, Y. Comparative analysis of mediastinal fat-associated lymphoid cluster development and lung cellular infiltration in murine autoimmune disease models and the corresponding normal control strains. Immunology 2016, 147, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Jones, L.H.; Bénézech, C. Control of innate-like B cell location for compartmentalised IgM production. Curr. Opin. Immunol. 2018, 50, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Bénézech, C.; Jackson-Jones, L.H. ILC2 Orchestration of Local Immune Function in Adipose Tissue. Front. Immunol. 2019, 10, 171. [Google Scholar] [CrossRef]
- Grimaldi, A.; Moriondo, A.; Sciacca, L.; Guidali, M.L.; Tettamanti, G.; Negrini, D. Functional arrangement of rat diaphragmatic initial lymphatic network. Am. J. Physiol. Circ. Physiol. 2006, 291, H876–H885. [Google Scholar] [CrossRef]
- Shibata, S.; Yamaguchi, S.; Kaseda, M.; Ichihara, N.; Hayakawa, T.; Asari, M. The Time Course of Lymphatic Routes Emanating from the Peritoneal Cavity in Rats. Anat. Histol. Embryol. 2007, 36, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, S.; de Rooster, H.; Doom, M.; Van den Broeck, W. The Microscopic Structure of the Omentum in Healthy Dogs: The Mystery Unravelled. Anat. Histol. Embryol. 2016, 45, 209–218. [Google Scholar] [CrossRef]
- Parungo, C.P.; Soybel, D.I.; Colson, Y.L.; Kim, S.-W.; Ohnishi, S.; De Grand, A.M.; Laurence, R.G.; Soltesz, E.G.; Chen, F.Y.; Cohn, L.H.; et al. Lymphatic Drainage of the Peritoneal Space: A Pattern Dependent on Bowel Lymphatics. Ann. Surg. Oncol. 2007, 14, 286–298. [Google Scholar] [CrossRef]
- Scotland, R.S.; Stables, M.J.; Madalli, S.; Watson, P.; Gilroy, D.W. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 2011, 118, 5918–5927. [Google Scholar] [CrossRef]
- Shumko, J.Z.; Feinberg, R.N.; Shalvoy, R.M.; Defouw, D.O. Responses of Rat Pleural Mesothelia to Increased Intrathoracic Pressure. Exp. Lung Res. 1993, 19, 283–297. [Google Scholar] [CrossRef]
- Hermans, C.; Lesur, O.; Weynand, B.; Pieters, T.; Lambert, M.; Bernard, A. Clara Cell Protein (CC16) in Pleural Fluids. Am. J. Respir. Crit. Care Med. 1998, 157, 962–969. [Google Scholar] [CrossRef]
- Wang, Q.-X.; Ohtani, O.; Saitoh, M.; Ohtani, Y. Distribution and Ultrastructure of the Stomata Connecting the Pleural Cavity with Lymphatics in the Rat Costal Pleura. Cells Tissues Organs 1997, 158, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Wang, N.-S. Anatomy of the pleura. In Textbook of Pleural Diseases, 2nd ed.; Light, R.W., Lee, Y.C.G., Eds.; CRC Press: London, UK, 2008; Part I; pp. 13–25. [Google Scholar]
- Cortez, V.S.; Colonna, M. Diversity and function of group 1 innate lymphoid cells. Immunol. Lett. 2016, 179, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Weizman, O.-E.; Adams, N.M.; Schuster, I.S.; Krishna, C.; Pritykin, Y.; Lau, C.; Degli-Esposti, M.A.; Leslie, C.S.; Sun, J.C.; O’Sullivan, T.E. ILC1 Confer Early Host Protection at Initial Sites of Viral Infection. Cell 2017, 171, 795–808. [Google Scholar] [CrossRef]
- Gupta, O.T.; Gupta, R.K. Visceral Adipose Tissue Mesothelial Cells: Living on the Edge or Just Taking Up Space? Trends Endocrinol. Metab. 2015, 26, 515–523. [Google Scholar] [CrossRef]
- Katz, S.; Balogh, P.; Kiss, A.L. Mesothelial cells can detach from the mesentery and differentiate into macrophage-like cells. APMIS 2011, 119, 782–793. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Whitaker, D.; Papadimitriou, J.M. Stimulation of Mesothelial Cell Proliferation by Exudate Macrophages Enhances Serosal Wound Healing in a Murine Model. Am. J. Pathol. 2002, 160, 681–692. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Birnie, K.; Lansley, S.; Herrick, S.E.; Lim, C.-B.; PrÃale, C.M. Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol. 2015, 6, 113. [Google Scholar] [CrossRef]
- Jackson-Jones, L.H.; Smith, P.; Portman, J.R.; Magalhaes, M.S.; Mylonas, K.J.; Vermeren, M.M.; Nixon, M.; Henderson, B.E.P.; Dobie, R.; Vermeren, S.; et al. Stromal Cells Covering Omental Fat-Associated Lymphoid Clusters Trigger Formation of Neutrophil Aggregates to Capture Peritoneal Contaminants. Immunity 2020, 52, 700–715. [Google Scholar] [CrossRef]
- Padilla, J.; Jenkins, N.T.; Vieira-Potter, V.J.; Laughlin, M.H. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R543–R552. [Google Scholar] [CrossRef]
- AlZaim, I.; Hammoud, S.H.; Al-Koussa, H.; Ghazi, A.; Eid, A.H.; El-Yazbi, A.F. Adipose Tissue Immunomodulation: A Novel Therapeutic Approach in Cardiovascular and Metabolic Diseases. Front. Cardiovasc. Med. 2020, 7, 602088. [Google Scholar] [CrossRef]
- Baragetti, A.; Pisano, G.; Bertelli, C.; Garlaschelli, K.; Grigore, L.; Fracanzani, A.L.; Fargion, S.; Norata, G.D.; Catapano, A.L. Subclinical atherosclerosis is associated with Epicardial Fat Thickness and hepatic steatosis in the general population. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.R.; Patil, N.T.; King, S.I.; O’Keefe, E.; Chhabra, R.; Ansari, S.; Kennedy, K.F.; Dey, D.; O’Keefe, J.H.; Helzberg, J.H.; et al. Increased intrathoracic and hepatic visceral adipose tissue independently correlates with coronary artery calcification in asymptomatic patients. J. Nucl. Cardiol. 2014, 21, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Dalmas, E.; Clément, K.; Guerre-Millo, M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011, 32, 307–314. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Chawla, A. Type 2 responses at the interface between immunity and fat metabolism. Curr. Opin. Immunol. 2015, 36, 67–72. [Google Scholar] [CrossRef]
- Kissig, M.; Shapira, S.N.; Seale, P. SnapShot: Brown and Beige Adipose Thermogenesis. Cell 2016, 166, 258–258.e1. [Google Scholar] [CrossRef] [PubMed]
- Bénézech, C.; Mader, E.; Desanti, G.; Khan, M.; Nakamura, K.; White, A.; Ware, C.F.; Anderson, G.; Caamaño, J.H. Lymphotoxin-β Receptor Signaling through NF-κB2-RelB Pathway Reprograms Adipocyte Precursors as Lymph Node Stromal Cells. Immunity 2012, 37, 721–734. [Google Scholar] [CrossRef]
- Mercer, R.R.; Scabilloni, J.F.; Hubbs, A.F.; Wang, L.; Battelli, L.A.; McKinney, W.; Castranova, V.; Porter, D.W. Extrapulmonary transport of MWCNT following inhalation exposure. Part. Fibre Toxicol. 2013, 10, 38. [Google Scholar] [CrossRef]
- Xu, J.; Futakuchi, M.; Shimizu, H.; Alexander, D.B.; Yanagihara, K.; Fukamachi, K.; Suzui, M.; Kanno, J.; Hirose, A.; Ogata, A.; et al. Multi-walled carbon nanotubes translocate into the pleural cavity and induce visceral mesothelial proliferation in rats. Cancer Sci. 2012, 103, 2045–2050. [Google Scholar] [CrossRef]
- Mills, N.L.; Donaldson, K.; Hadoke, P.W.; Boon, N.A.; MacNee, W.; Cassee, F.R.; Sandström, T.; Blomberg, A.; Newby, D.E. Adverse cardiovascular effects of air pollution. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 36–44. [Google Scholar] [CrossRef]
- Mercer, R.R.; Hubbs, A.F.; Scabilloni, J.F.; Wang, L.; Battelli, L.A.; Friend, S.; Castranova, V.; Porter, D.W. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part. Fibre Toxicol. 2011, 8, 21. [Google Scholar] [CrossRef]
- Mercer, R.R.; Scabilloni, J.F.; Hubbs, A.F.; Battelli, L.A.; McKinney, W.; Friend, S.; Wolfarth, M.G.; Andrew, M.; Castranova, V.; Porter, D.W. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol. 2013, 10, 33. [Google Scholar] [CrossRef]
- Staal, Y.C.M.; van Triel, J.J.; Maarschalkerweerd, T.V.P.; Arts, J.H.E.; Duistermaat, E.; Muijser, H.; van de Sandt, J.J.M.; Kuper, C.F. Inhaled Multiwalled Carbon Nanotubes Modulate the Immune Response of Trimellitic Anhydride–induced Chemical Respiratory Allergy in Brown Norway Rats. Toxicol. Pathol. 2014, 42, 1130–1142. [Google Scholar] [CrossRef]
- Kuempel, E.D.; Jaurand, M.-C.; Møller, P.; Morimoto, Y.; Kobayashi, N.; Pinkerton, K.E.; Sargent, L.M.; Vermeulen, R.C.H.; Fubini, B.; Kane, A.B. Evaluating the mechanistic evidence and key data gaps in assessing the potential carcinogenicity of carbon nanotubes and nanofibers in humans. Crit. Rev. Toxicol. 2017, 47, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Ryman-Rasmussen, J.P.; Cesta, M.F.; Brody, A.R.; Shipley-Phillips, J.K.; Everitt, J.I.; Tewksbury, E.W.; Moss, O.R.; Wong, B.A.; Dodd, D.E.; Andersen, M.E.; et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat. Nanotechnol. 2009, 4, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Alexander, D.B.; Futakuchi, M.; Numano, T.; Fukamachi, K.; Suzui, M.; Omori, T.; Kanno, J.; Hirose, A.; Tsuda, H. Size- and shape-dependent pleural translocation, deposition, fibrogenesis, and mesothelial proliferation by multiwalled carbon nanotubes. Cancer Sci. 2014, 105, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, X.; Du, X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur. Respir. J. 2009, 34, 559–567. [Google Scholar] [CrossRef]
- Hillegass, J.M.; Miller, J.M.; MacPherson, M.B.; Westbom, C.M.; Sayan, M.; Thompson, J.K.; Macura, S.L.; Perkins, T.N.; Beuschel, S.L.; Alexeeva, V.; et al. Asbestos and erionite prime and activate the NLRP3 inflammasome that stimulates autocrine cytokine release in human mesothelial cells. Part. Fibre Toxicol. 2013, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Jean, D.; Jaurand, M.-C. Mesotheliomas in Genetically Engineered Mice Unravel Mechanism of Mesothelial Carcinogenesis. Int. J. Mol. Sci. 2018, 19, 2191. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.F.; Vargas, F.S.; Marchi, E.; Acencio, M.M.P.; Genofre, E.H.; Capelozzi, V.L.; Antonangelo, L. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur. Respir. J. 2010, 35, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Dakwar, G.R.; Shariati, M.; Willaert, W.; Ceelen, W.; De Smedt, S.C.; Remaut, K. Nanomedicine-based intraperitoneal therapy for the treatment of peritoneal carcinomatosis—Mission possible? Adv. Drug Deliv. Rev. 2017, 108, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, M.; Peterson, M.; Kloskowski, T.; McCabe, E.; Guiral, D.C.; Polom, K.; Pietkun, K.; Zegarska, B.; Pokrywczynska, M.; Drewa, T.; et al. Nanoparticle as a novel tool in hyperthermic intraperitoneal and pressurized intraperitoneal aerosol chemotheprapy to treat patients with peritoneal carcinomatosis. Oncotarget 2017, 8, 78208–78224. [Google Scholar] [CrossRef][Green Version]
- Shariati, M.; Zhang, H.; Van de Sande, L.; Descamps, B.; Vanhove, C.; Willaert, W.; Ceelen, W.; De Smedt, S.C.; Remaut, K. High Pressure Nebulization (PIPAC) Versus Injection for the Intraperitoneal Administration of mRNA Complexes. Pharm. Res. 2019, 36, 126. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, F.; Cocco, E.; Song, E.; Zhang, J.; Cui, J.; Mohideen, M.; Bellone, S.; Santin, A.D.; Saltzman, W.M. Improved i.p. drug delivery with bioadhesive nanoparticles. Proc. Natl. Acad. Sci. USA 2016, 113, 11453–11458. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.; Colvin, E.; Pham, N.; Kim, B.; Fuller, E.; Moon, E.; Barbey, R.; Yuen, S.; Rickman, B.; Bryce, N.; et al. Biodistribution and Clearance of Stable Superparamagnetic Maghemite Iron Oxide Nanoparticles in Mice Following Intraperitoneal Administration. Int. J. Mol. Sci. 2018, 19, 205. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian Cancer Development and Metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Fan, H.; Yin, L.; Zhang, J.; Dong, A.; Deng, L.; Tang, H. Thermosensitive hydrogel system assembled by PTX-loaded copolymer nanoparticles for sustained intraperitoneal chemotherapy of peritoneal carcinomatosis. Eur. J. Pharm. Biopharm. 2016, 104, 251–259. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, R.; Tong, A.; Li, X.; Gao, X.; Mei, L.; Zhang, X.; You, C.; Guo, G. Enhanced antitumor effects by docetaxel/LL37-loaded thermosensitive hydrogel nanoparticles in peritoneal carcinomatosis of colorectal cancer. Int. J. Nanomed. 2015, 10, 7291. [Google Scholar] [CrossRef]
- Wang, W.; Deng, L.; Xu, S.; Zhao, X.; Lv, N.; Zhang, G.; Gu, N.; Hu, R.; Zhang, J.; Liu, J.; et al. A reconstituted “two into one” thermosensitive hydrogel system assembled by drug-loaded amphiphilic copolymernanoparticles for the local delivery of paclitaxel. J. Mater. Chem. B 2013, 1, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Lee, B.-J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef]
- Pandey, R.K.; Prajapati, V.K. Molecular and immunological toxic effects of nanoparticles. Int. J. Biol. Macromol. 2018, 107, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Mitchev, K.; Dumortier, P.; De Vuyst, P. ‘Black Spots’ and Hyaline Pleural Plaques on the Parietal Pleura of 150 Urban Necropsy Cases. Am. J. Surg. Pathol. 2002, 26, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.; Dumortier, P.; Rey, F.; Viallat, J.R.; De Vuyst, P. Black spots concentrate oncogenic asbestos fibers in the parietal pleura. Thoracoscopic and mineralogic study. Am. J. Respir. Crit. Care Med. 1996, 153, 444–449. [Google Scholar] [CrossRef]
- Panasco, M.S.; Pelajo-Machado, M.; Lenzi, H.L. Omental and pleural milky spots: Different reactivity patterns in mice infected with Schistosoma mansoni reveals coelomic compartmentalisation. Mem. Inst. Oswaldo Cruz 2010, 105, 440–444. [Google Scholar] [CrossRef][Green Version]
- Jackson-Jones, L.H.; Duncan, S.M.; Magalhaes, M.S.; Campbell, S.M.; Maizels, R.M.; McSorley, H.J.; Allen, J.E.; Bénézech, C. Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat. Commun. 2016, 7, 12651. [Google Scholar] [CrossRef]
- Lehnert, B.E.; Tech, C. Quantitative evaluation of opsonin-independent phagocytosis by alveolar macrophages in monolayer using polystyrene microspheres. J. Immunol. Methods 1985, 78, 337–344. [Google Scholar] [CrossRef]
- Peão, M.N.D.; Águas, A.P.; Grande, N.R. Cellular Kinetics of Inflammation in the Pleural Space of Mice in Response to the Injection of Exogenous Particles. Exp. Lung Res. 1992, 18, 863–876. [Google Scholar] [CrossRef]
- Ma, Q. Polarization of Immune Cells in the Pathologic Response to Inhaled Particulates. Front. Immunol. 2020, 11, 1060. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Mucosal Immunity: Induction, Dissemination, and Effector Functions. Scand. J. Immunol. 2009, 70, 505–515. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef]
- van den Brink, W.; van Bilsen, J.; Salic, K.; Hoevenaars, F.P.M.; Verschuren, L.; Kleemann, R.; Bouwman, J.; Ronnett, G.V.; van Ommen, B.; Wopereis, S. Current and Future Nutritional Strategies to Modulate Inflammatory Dynamics in Metabolic Disorders. Front. Nutr. 2019, 6, 129. [Google Scholar] [CrossRef]
- Liu, M.; Silva-Sanchez, A.; Randall, T.D.; Meza-Perez, S. Specialized immune responses in the peritoneal cavity and omentum. J. Leukoc. Biol. 2020. [Google Scholar] [CrossRef]
- Li, J.; Cha, R.; Luo, H.; Hao, W.; Zhang, Y.; Jiang, X. Nanomaterials for the theranostics of obesity. Biomaterials 2019, 223, 119474. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.A.; Poland, C.A.; Duffin, R.; Al-Jamal, K.T.; Ali-Boucetta, H.; Nunes, A.; Byrne, F.; Prina-Mello, A.; Volkov, Y.; Li, S.; et al. Length-Dependent Retention of Carbon Nanotubes in the Pleural Space of Mice Initiates Sustained Inflammation and Progressive Fibrosis on the Parietal Pleura. Am. J. Pathol. 2011, 178, 2587–2600. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, E. Adipose tissue inflammation in obesity: A metabolic or immune response? Curr. Opin. Pharmacol. 2017, 37, 35–40. [Google Scholar] [CrossRef]
- Drasler, B.; Sayre, P.; Steinhäuser, K.G.; Petri-Fink, A.; Rothen-Rutishauser, B. In vitro approaches to assess the hazard of nanomaterials. NanoImpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V.; Borm, P.J.A.; Jimenez, L.A.; Gilmour, P.S.; Schins, R.P.F.; Knaapen, A.M.; Rahman, I.; Faux, S.P.; Brown, D.M.; et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic. Biol. Med. 2003, 34, 1369–1382. [Google Scholar] [CrossRef]
- Schins, R.P.F.; Knaapen, A.M. Genotoxicity of Poorly Soluble Particles. Inhal. Toxicol. 2007, 19, 189–198. [Google Scholar] [CrossRef]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 163. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, H.M.; Park, M.V.; Gosens, I.; De Jong, W.H.; Cassee, F.R. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part. Fibre Toxicol. 2014, 11, 18. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
| Serosa-Associated Lymphoid Clusters (SALCs) | |||
|---|---|---|---|
| Milky Spot (MS) Synonym: omFALC [12,17,18,19,20,21,22] | Fat-Associated Lymphoid Cluster (FALC) | Lymphoid Cluster, not Embedded in Adipose/Fat Tissue | |
| Ontogeny | Develop independent of ILC3/LTi cells and CCL19 and CCL21, but are defective/absent in Cxcl13−/− and Ltα−/− mice Observed at 35 weeks of gestation in humans [12,17,18,19,20,21,22] | FALCs develop independent of ILC3/LTi cells and LTβR signaling, but depend on TNF signalling on stromal cells, IL-4R signalling and invariant natural killer T cells [12,17,18,19,20,21,22] Mesenteric FALC are formed after birth | Possibly MS in translucent areas of the serosal membranes |
| Location | Especially in greater omentum Prominent at adipose tissue locations, but also in translucent areas of the serosal membranes [26] | In omentum, mesentery, pleural and pericardial serosal membranes | In omentum [26], and rodent retrocardiac pleural fold [16] |
| Function | Central role in innate B cell maintenance and activation [19] | Possibly identical function, if they are similar to MS in translucent areas | |
| Microanatomy | Always covered by mesothelium Often, but not always embedded in adipose tissue | Always covered by mesothelium Embedded in adipose tissue | Always covered by mesothelium Not embedded in adipose tissue |
| Types of Innate Lymphocytes (ILCs) | Types of Innate B Cells (IBCs) |
|---|---|
| Non-cytotoxic Tbet-dependent ILC1 (NK) | B1a cells CD5+, CD11b/Mac1+ Responses are T cell-independent Cells have high production of natural antibodies B1a antigens are often carbohydrate- and rarely protein-specific Antibody isotype is IgM and antibody avidity is low Cells develop in foetal liver and are self-renewing in situ |
| Secrete TNFalpha and INFgamma Are key to control early (viral) replication at initial sites of infection | |
| GATA3-dependent ILC2 | B1b cells CD19hiCD5loCD11bhi Responses are largely T cell-independent Natural antibody production poorly investigated B1b antigens are possibly carbohydrate- and protein-specific Antibody isotype is IgM and antibody avidity is low Cells develop in foetal liver and are self-renewing in situ Cells share many characteristics with marginal zone B Cells but differ in distribution and B cell receptor (BCR) signalling pathways |
| Secrete IL-5 and IL-13 and are regulators of Type 2 immune cells to maintain WAT homeostasis Are involved in browning of WAT, leading to beige adipose tissue | |
| RORgamma T-dependent ILC3, including LTi-cells | |
| Secrete IL-17A and IL-22 Can convert into ILC1 cells Required to initiate antibacterial actions of epithelial cells | |
| Id3-dependent ILCregs | |
| Secrete IL-10 Suppress ILC1 and ILC3 activity Regulatory role in intestinal homeostasis and innate immune defences, like Treg cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuper, C.F.; Pieters, R.H.H.; van Bilsen, J.H.M. Nanomaterials and the Serosal Immune System in the Thoracic and Peritoneal Cavities. Int. J. Mol. Sci. 2021, 22, 2610. https://doi.org/10.3390/ijms22052610
Kuper CF, Pieters RHH, van Bilsen JHM. Nanomaterials and the Serosal Immune System in the Thoracic and Peritoneal Cavities. International Journal of Molecular Sciences. 2021; 22(5):2610. https://doi.org/10.3390/ijms22052610
Chicago/Turabian StyleKuper, C. Frieke, Raymond H. H. Pieters, and Jolanda H. M. van Bilsen. 2021. "Nanomaterials and the Serosal Immune System in the Thoracic and Peritoneal Cavities" International Journal of Molecular Sciences 22, no. 5: 2610. https://doi.org/10.3390/ijms22052610
APA StyleKuper, C. F., Pieters, R. H. H., & van Bilsen, J. H. M. (2021). Nanomaterials and the Serosal Immune System in the Thoracic and Peritoneal Cavities. International Journal of Molecular Sciences, 22(5), 2610. https://doi.org/10.3390/ijms22052610