Functional and Transcriptional Adaptations of Blood Monocytes Recruited to the Cystic Fibrosis Airway Microenvironment In Vitro
Abstract
1. Introduction
2. Results
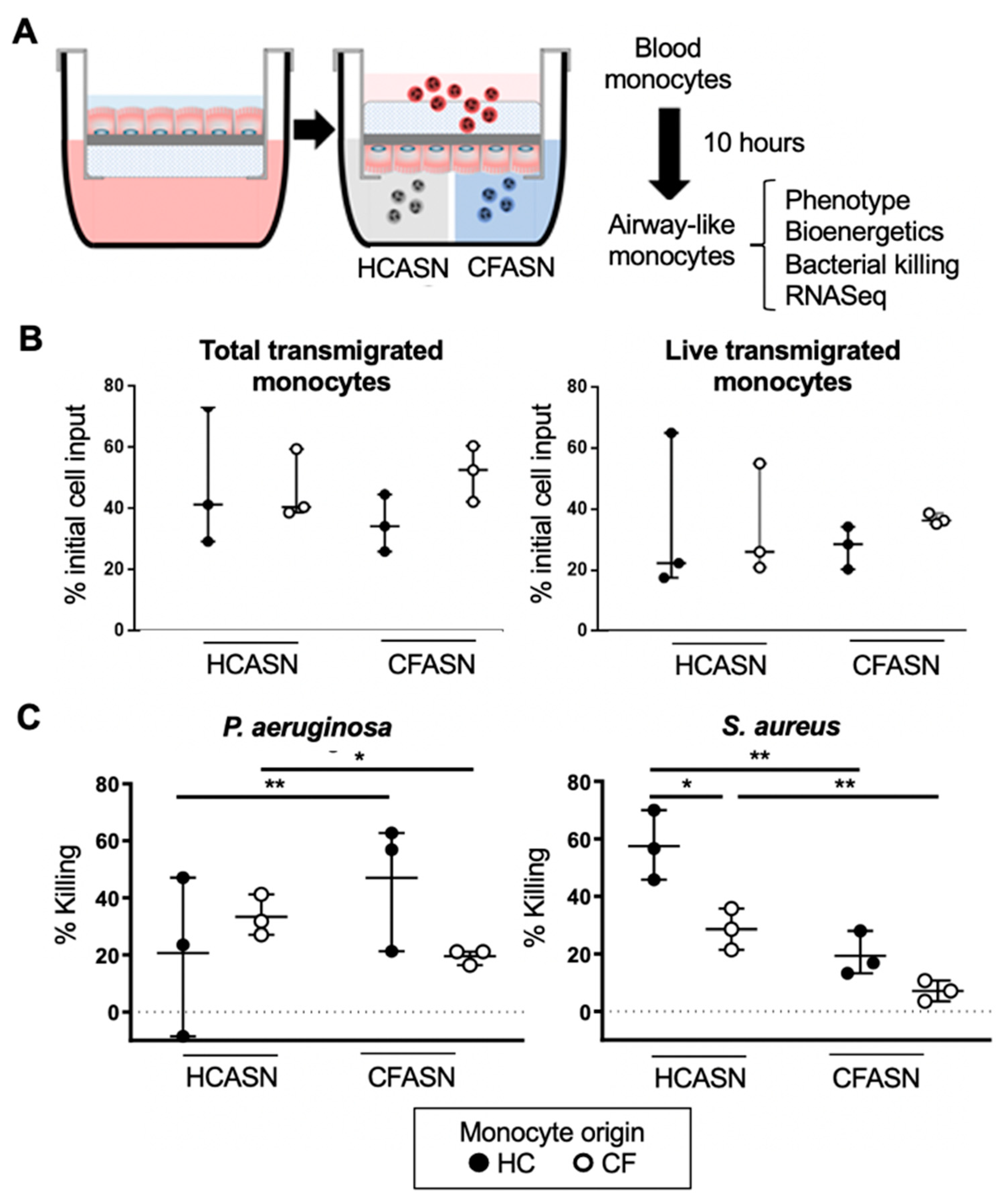
2.1. Blood Monocyte Transmigration into CFASN vs. HCASN Results in Similar Yield and Viability But Different Bactericidal Abilities
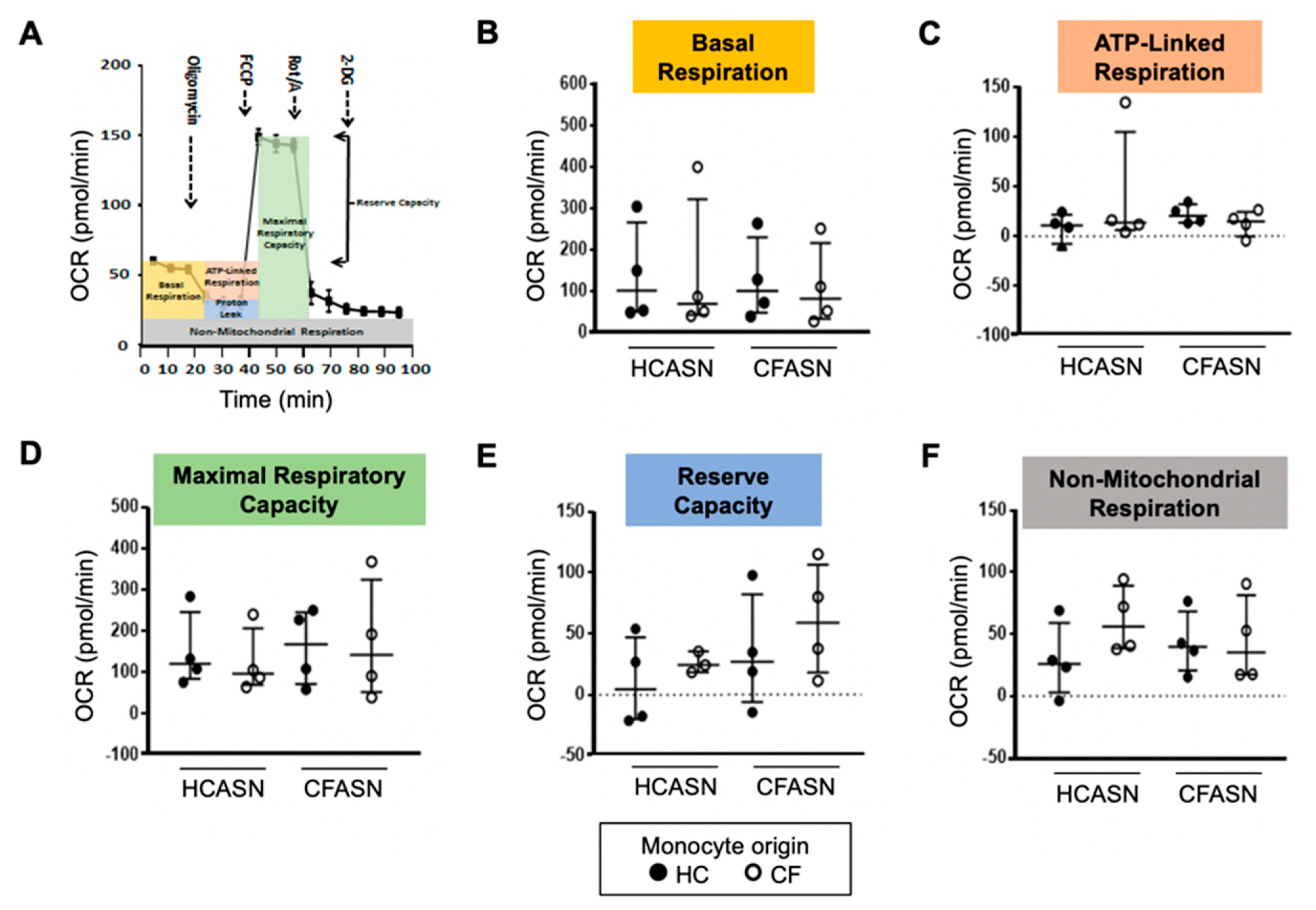
2.2. Blood Monocyte Transmigration into CFASN vs. HCASN Results in Similar Metabolic Activity
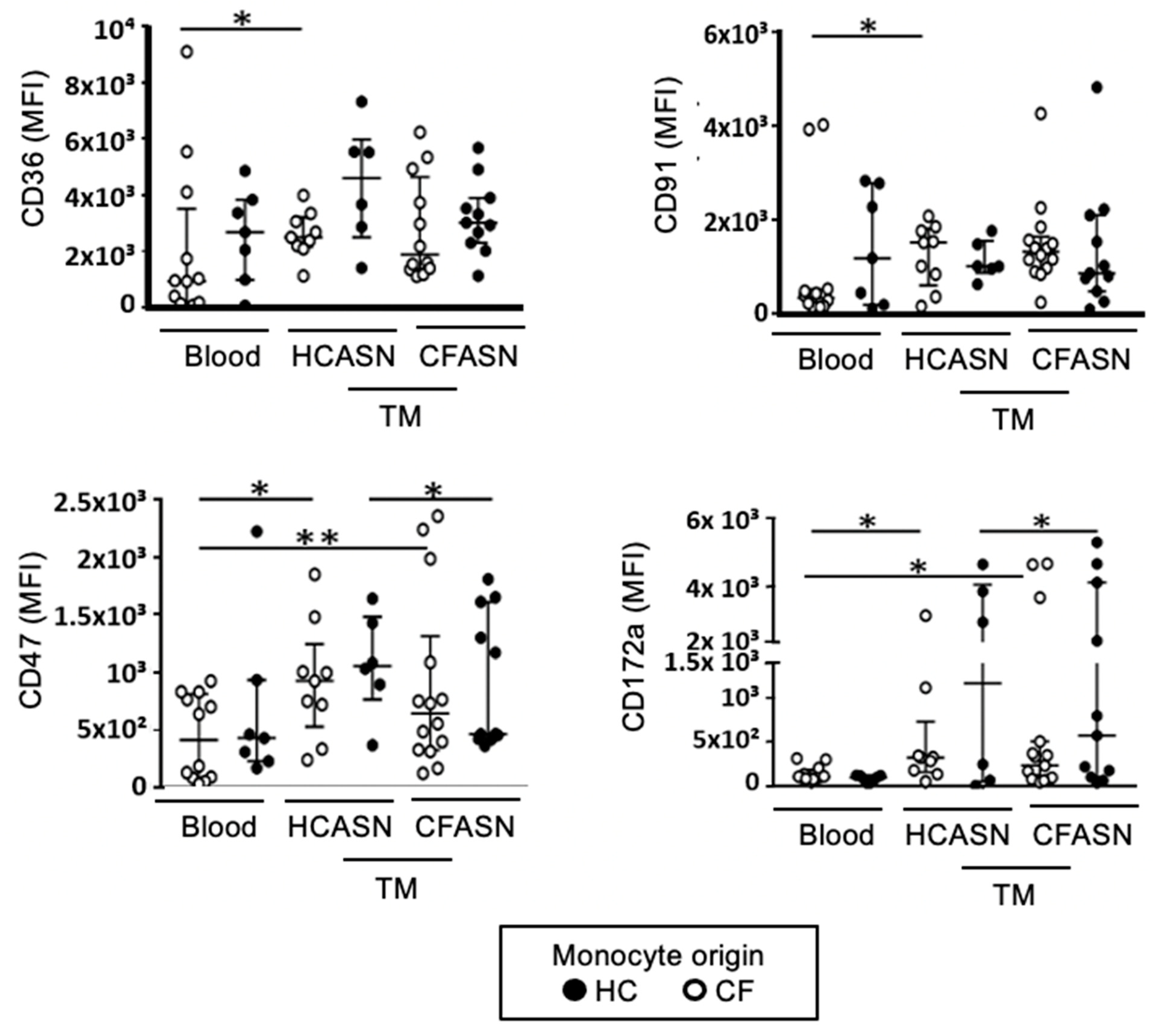
2.3. Blood Monocyte Transmigration into CFASN vs. HCASN Results in Minimal Differences in Expression of Scavenger Receptors and Activation Markers
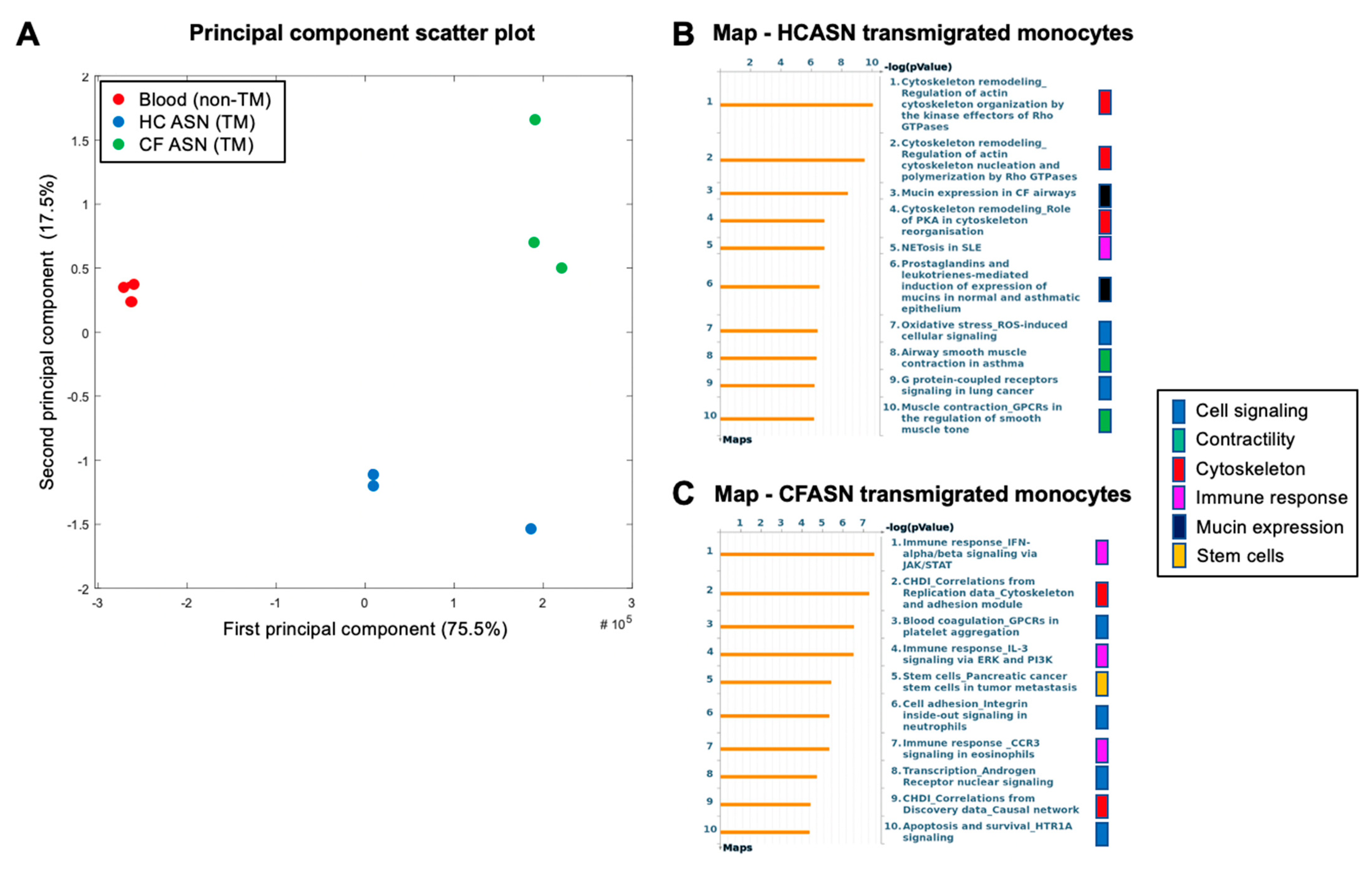
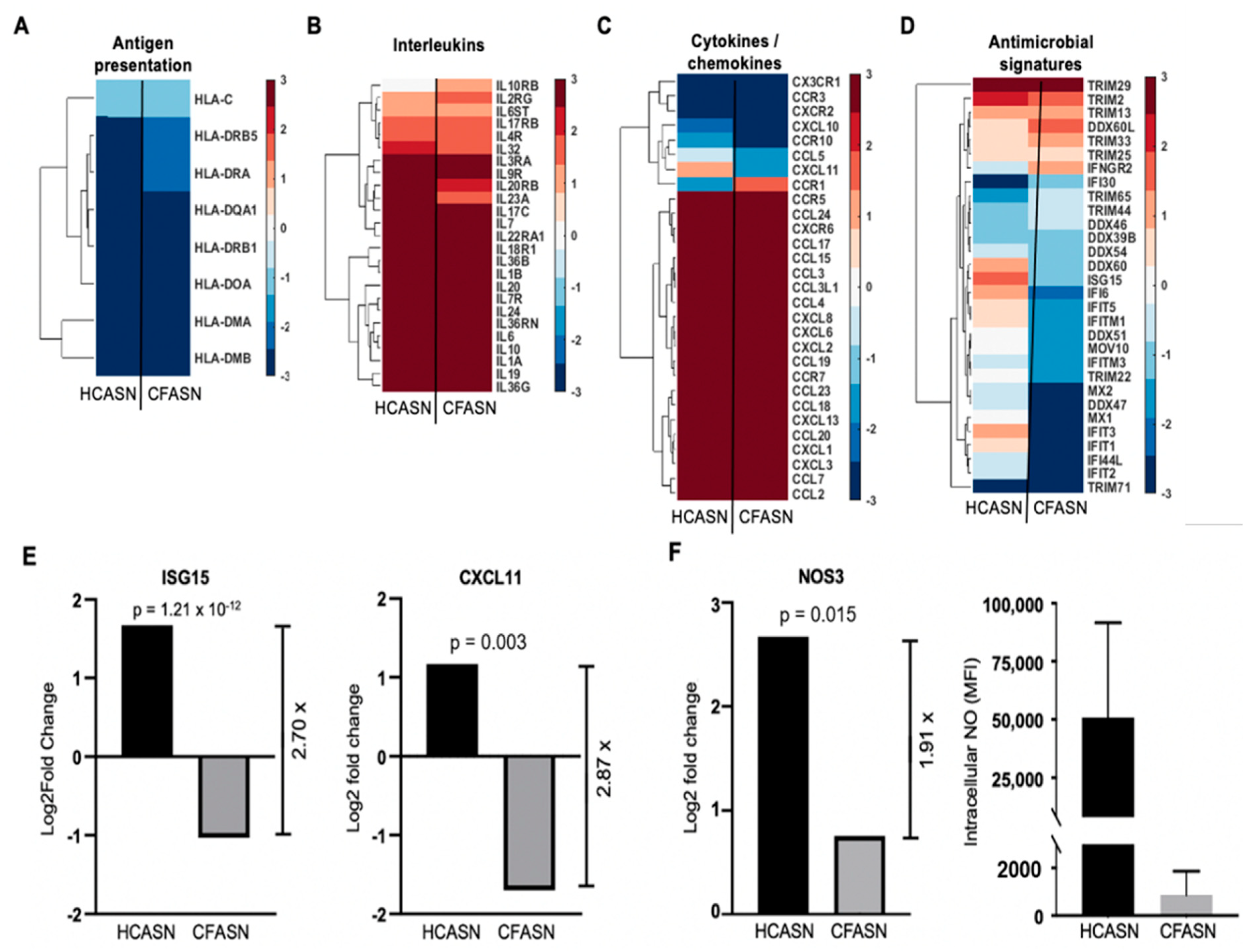
2.4. Blood Monocyte Transmigration into CFASN vs. HCASN Results in Significant Differences in Transcriptional Profile
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Blood Collection and Monocyte Isolation
4.3. Transmigration Model
4.4. Flow Cytometry
4.5. Bacterial Killing
4.6. Metabolic Analysis
4.7. RNA Extraction and Transcriptomic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASN | Airway fluid supernatant |
| CF | Cystic fibrosis |
| COPD | Chronic obstructive pulmonary disease |
| CXCL11 | CXC-motif chemokine 11 |
| HC | Healthy control |
| ISG15 | Interferon-stimulated gene 15 |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
References
- Cutting, G.R. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Fanen, P.; Wohlhuter-Haddad, A.; Hinzpeter, A. Genetics of cystic fibrosis: CFTR mutation classifications toward genotype-based CF therapies. Int. J. Biochem. Cell Biol. 2014, 52, 94–102. [Google Scholar] [CrossRef]
- Hartl, D.; Gaggar, A.; Bruscia, E.; Hector, A.; Marcos, V.; Jung, A.; Greene, C.; McElvaney, G.; Mall, M.; Doring, G. Innate immunity in cystic fibrosis lung disease. J. Cyst. Fibros 2012, 11, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros 2015, 14, 419–430. [Google Scholar] [CrossRef]
- Tirouvanziam, R.; Gernez, Y.; Conrad, C.K.; Moss, R.B.; Schrijver, I.; Dunn, C.E.; Davies, Z.A.; Herzenberg, L.A.; Herzenberg, L.A. Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proc. Natl. Acad. Sci. USA 2008, 105, 4335–4339. [Google Scholar] [CrossRef]
- Makam, M.; Diaz, D.; Laval, J.; Gernez, Y.; Conrad, C.K.; Dunn, C.E.; Davies, Z.A.; Moss, R.B.; Herzenberg, L.A.; Herzenberg, L.A.; et al. Activation of critical, host-induced, metabolic and stress pathways marks neutrophil entry into cystic fibrosis lungs. Proc. Natl. Acad. Sci. USA 2009, 106, 5779–5783. [Google Scholar] [CrossRef] [PubMed]
- Laval, J.; Touhami, J.; Herzenberg, L.A.; Conrad, C.; Taylor, N.; Battini, J.L.; Sitbon, M.; Tirouvanziam, R. Metabolic adaptation of neutrophils in cystic fibrosis airways involves distinct shifts in nutrient transporter expression. J. Immunol. 2013, 190, 6043–6050. [Google Scholar] [CrossRef]
- Forrest, O.A.; Ingersoll, S.A.; Preininger, M.K.; Laval, J.; Limoli, D.H.; Brown, M.R.; Lee, F.E.; Bedi, B.; Sadikot, R.T.; Goldberg, J.B.; et al. Frontline Science: Pathological conditioning of human neutrophils recruited to the airway milieu in cystic fibrosis. J. Leukoc. Biol. 2018, 104, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Garratt, L.W.; Wright, A.K.; Ranganathan, S.C.; Grigg, J.; Sly, P.D.; AREST CF. Small macrophages are present in early childhood respiratory disease. J. Cyst. Fibros 2012, 11, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.K.; Plumb, J.; Gaskell, R.; Mason, S.; Broome, C.S.; Booth, G.; Catley, M.; Vestbo, J.; Singh, D. COPD monocytes demonstrate impaired migratory ability. Respir. Res. 2017, 18, 90. [Google Scholar] [CrossRef]
- Hirose, S.; Lin, Q.; Ohtsuji, M.; Nishimura, H.; Verbeek, J.S. Monocyte subsets involved in the development of systemic lupus erythematosus and rheumatoid arthritis. Int. Immunol. 2019, 31, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Roediger, B.; Weninger, W. Monocyte homeostasis and the plasticity of inflammatory monocytes. Cell Immunol. 2014, 291, 22–31. [Google Scholar] [CrossRef]
- Grunwell, J.R.; Giacalone, V.D.; Stephenson, S.; Margaroli, C.; Dobosh, B.S.; Brown, M.R.; Fitzpatrick, A.M.; Tirouvanziam, R. Neutrophil dysfunction in the airways of children with acute respiratory failure due to lower respiratory tract viral and bacterial coinfections. Sci. Rep. 2019, 9, 2874. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.C. A GRIM fate for human neutrophils in airway disease. J. Leukoc. Biol. 2018, 104, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Assani, K.; Tazi, M.F.; Amer, A.O.; Kopp, B.T. IFN-gamma stimulates autophagy-mediated clearance of Burkholderia cenocepacia in human cystic fibrosis macrophages. PLoS ONE 2014, 9, e96681. [Google Scholar]
- Hisert, K.B.; Birkland, T.P.; Schoenfelt, K.Q.; Long, M.E.; Grogan, B.; Carter, S.; Liles, W.C.; McKone, E.F.; Becker, L.; Manicone, A.M. Ivacaftor decreases monocyte sensitivity to interferon-gamma in people with cystic fibrosis. ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef]
- Leuer, L.; Krill, A.; Wilkens, H.; Wagenpfeil, G.; Bischoff, M.; Meier, C.; Bals, R.; Tschernig, T. The phagocytosis of blood leukocytes from cystic fibrosis patients is not impaired in general. Lung 2020, 198, 235–239. [Google Scholar] [CrossRef]
- Tarique, A.A.; Sly, P.D.; Cardenas, D.G.; Luo, L.; Stow, J.L.; Bell, S.C.; Wainwright, C.E.; Fantino, E. Differential expression of genes and receptors in monocytes from patients with cystic fibrosis. J. Cyst. Fibros 2019, 18, 342–348. [Google Scholar] [CrossRef]
- Champion, T.C.; Partridge, L.J.; Ong, S.M.; Malleret, B.; Wong, S.C.; Monk, P.N. Monocyte subsets have distinct patterns of tetraspanin expression and different capacities to form multinucleate giant cells. Front. Immunol. 2018, 9, 1247. [Google Scholar] [CrossRef]
- Avendano-Ortiz, J.; Llanos-Gonzalez, E.; Toledano, V.; Del Campo, R.; Cubillos-Zapata, C.; Lozano-Rodriguez, R.; Ismail, A.; Prados, C.; Gomez-Campelo, P.; Aguirre, L.A.; et al. Pseudomonas aeruginosa colonization causes PD-L1 overexpression on monocytes, impairing the adaptive immune response in patients with cystic fibrosis. J. Cyst. Fibros 2019, 18, 630–635. [Google Scholar] [CrossRef]
- Ingersoll, S.A.; Laval, J.; Forrest, O.A.; Preininger, M.; Brown, M.R.; Arafat, D.; Gibson, G.; Tangpricha, V.; Tirouvanziam, R. Mature cystic fibrosis airway neutrophils suppress T cell function: Evidence for a role of arginase 1 but not programmed death-ligand 1. J. Immunol. 2015, 194, 5520–5528. [Google Scholar] [CrossRef]
- Marcovecchio, P.M.; Thomas, G.D.; Mikulski, Z.; Ehinger, E.; Mueller, K.A.L.; Blatchley, A.; Wu, R.; Miller, Y.I.; Nguyen, A.T.; Taylor, A.M.; et al. Scavenger receptor CD36 Directs nonclassical monocyte patrolling along the endothelium during early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2043–2052. [Google Scholar] [CrossRef]
- Puente Navazo, M.D.; Daviet, L.; Ninio, E.; McGregor, J.L. Identification on human CD36 of a domain (155–183) implicated in binding oxidized low-density lipoproteins (Ox-LDL). Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1033–1139. [Google Scholar] [CrossRef]
- Binder, R.J.; Han, D.K.; Srivastava, P.K. CD91: A receptor for heat shock protein gp96. Nat. Immunol. 2000, 1, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Gardai, S.J.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-Ullrich, J.E.; Bratton, D.L.; Oldenborg, P.A.; Michalak, M.; Henson, P.M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005, 123, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Bian, Z.; Shi, L.; Niu, S.; Ha, B.; Tremblay, A.; Li, L.; Zhang, X.; Paluszynski, J.; Liu, M.; et al. Loss of cell surface CD47 clustering formation and binding avidity to SIRPalpha facilitate apoptotic cell clearance by macrophages. J. Immunol. 2015, 195, 661–671. [Google Scholar] [CrossRef]
- Gardai, S.J.; Xiao, Y.Q.; Dickinson, M.; Nick, J.A.; Voelker, D.R.; Greene, K.E.; Henson, P.M. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 2003, 115, 13–23. [Google Scholar] [CrossRef]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, J.; Potter, J.L.; Haas, A.L. Conjugation of the 15-kDa interferon-induced ubiquitin homolog is distinct from that of ubiquitin. J. Biol. Chem. 1996, 271, 324–330. [Google Scholar] [CrossRef]
- Ovstebo, R.; Olstad, O.K.; Brusletto, B.; Moller, A.S.; Aase, A.; Haug, K.B.; Brandtzaeg, P.; Kierulf, P. Identification of genes particularly sensitive to lipopolysaccharide (LPS) in human monocytes induced by wild-type versus LPS-deficient Neisseria meningitidis strains. Infect. Immun. 2008, 76, 2685–2695. [Google Scholar] [CrossRef]
- Dos Santos, P.F.; Mansur, D.S. Beyond ISGlylation: Functions of free intracellular and extracellular ISG15. J. Interferon. Cytokine Res. 2017, 37, 246–253. [Google Scholar] [CrossRef]
- Gao, N.; Me, R.; Dai, C.; Yu, F.X. ISG15 acts as a mediator of innate immune response to Pseudomonas aeruginosa infection in C57BL/6J mouse corneas. Investig. Ophthalmol. Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Ganz, T.; Liese, A.M.; Burdick, M.D.; Liu, L.; Strieter, R.M. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J. Immunol. 2001, 167, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Csoma, B.; Bikov, A.; Nagy, L.; Tóth, B.; Tábi, T.; Szűcs, G.; Komlósi, Z.I.; Müller, V.; Losonczy, G.; Lázár, Z. Dysregulation of the endothelial nitric oxide pathway is associated with airway inflammation in COPD. Respir. Res. 2019, 20, 156. [Google Scholar] [CrossRef] [PubMed]
- Reiling, N.; Ulmer, A.J.; Duchrow, M.; Ernst, M.; Flad, H.D.; Hauschildt, S. Nitric oxide synthase: mRNA expression of different isoforms in human monocytes/macrophages. Eur. J. Immunol. 1994, 24, 1941–1944. [Google Scholar] [CrossRef]
- Yang, Z.; Chiou, T.T.; Stossel, T.P.; Kobzik, L. Plasma gelsolin improves lung host defense against pneumonia by enhancing macrophage NOS3 function. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L11–L16. [Google Scholar] [CrossRef]
- Grasemann, H.; Ioannidis, I.; Tomkiewicz, R.P.; de Groot, H.; Rubin, B.K.; Ratjen, F. Nitric oxide metabolites in cystic fibrosis lung disease. Arch. Dis. Child. 1998, 78, 49–53. [Google Scholar] [CrossRef]
- De Winter-de Groot, K.M.; van der Ent, C.K. Nitric oxide in cystic fibrosis. J. Cyst. Fibros 2005, 4 (Suppl. 2), 25–29. [Google Scholar] [CrossRef]
- Chen, K.; Fu, Q.; Liang, S.; Liu, Y.; Qu, W.; Wu, Y.; Wu, X.; Wei, L.; Wang, Y.; Xiong, Y.; et al. Stimulator of interferon genes promotes host resistance against Pseudomonas aeruginosa keratitis. Front. Immunol. 2018, 9, 1225. [Google Scholar] [CrossRef]
- Lee, B.; Robinson, K.M.; McHugh, K.J.; Scheller, E.V.; Mandalapu, S.; Chen, C.; Di, Y.P.; Clay, M.E.; Enelow, R.I.; Dubin, P.J.; et al. Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L158–L167. [Google Scholar] [CrossRef]
- Sasaki, S.; Tagawa, Y.; Iwakura, Y.; Nakane, A. The role of gamma interferon in acquired host resistance against Staphylococcus aureus infection in mice. FEMS Immunol. Med. Microbiol. 2006, 46, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Margaroli, C.; Moncada Giraldo, D.; Arafat Gulick, D.; Dobosh, B.; Giacalone, V.D.; Forrest, O.A.; Sun, F.; Gu, C.; Gaggar, A.; Kissick, H.; et al. Broad transcriptional firing represses bactericidal activity in human airway neutrophils. Cell. Rep. Med. 2021. [Google Scholar] [CrossRef]
- Kim, S.; Becker, J.; Bechheim, M.; Kaiser, V.; Noursadeghi, M.; Fricker, N.; Beier, E.; Klaschik, S.; Boor, P.; Hess, T.; et al. Characterizing the genetic basis of innate immune response in TLR4-activated human monocytes. Nat. Commun. 2014, 5, 5236. [Google Scholar] [CrossRef] [PubMed]
- Law, S.M.; Stanfield, S.J.; Hardisty, G.R.; Dransfield, I.; Campbell, C.J.; Gray, R.D. Human cystic fibrosis monocyte derived macrophages display no defect in acidification of phagolysosomes when measured by optical nanosensors. J. Cyst. Fibros 2020, 19, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Baharom, F.; Rankin, G.; Blomberg, A.; Smed-Sorensen, A. Human lung mononuclear phagocytes in health and disease. Front. Immunol. 2017, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Bonaguro, L.; Gemund, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef]
- Alon, R.; Sportiello, M.; Kozlovski, S.; Kumar, A.; Reilly, E.C.; Zarbock, A.; Garbi, N.; Topham, D.J. Leukocyte trafficking to the lungs and beyond: Lessons from influenza for COVID-19. Nat. Rev. Immunol. 2020, 21, 49–64. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Bertolini, F.; Carriero, V.; Hogman, M. Nitric oxide’s physiologic effects and potential as a therapeutic agent against COVID-19. J. Breath Res. 2020, 15, 14001. [Google Scholar] [CrossRef]
- Swaim, C.D.; Canadeo, L.A.; Monte, K.J.; Khanna, S.; Lenschow, D.J.; Huibregtse, J.M. Modulation of extracellular ISG15 signaling by pathogens and viral effector proteins. Cell. Rep. 2020, 31, 107772. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]

| Participant Group | N | CFTR Genotype | Gender | Age (Years) |
|---|---|---|---|---|
| Healthy Control | 12 | WT | F = 7, M = 5 | 25.9 ± 1.5 |
| CF Patient | 19 | HO = 9, HZ = 7, OT = 3 | F = 8, M = 11 | 31.2 ± 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ford, B.D.; Moncada Giraldo, D.; Margaroli, C.; Giacalone, V.D.; Brown, M.R.; Peng, L.; Tirouvanziam, R. Functional and Transcriptional Adaptations of Blood Monocytes Recruited to the Cystic Fibrosis Airway Microenvironment In Vitro. Int. J. Mol. Sci. 2021, 22, 2530. https://doi.org/10.3390/ijms22052530
Ford BD, Moncada Giraldo D, Margaroli C, Giacalone VD, Brown MR, Peng L, Tirouvanziam R. Functional and Transcriptional Adaptations of Blood Monocytes Recruited to the Cystic Fibrosis Airway Microenvironment In Vitro. International Journal of Molecular Sciences. 2021; 22(5):2530. https://doi.org/10.3390/ijms22052530
Chicago/Turabian StyleFord, Bijean D., Diego Moncada Giraldo, Camilla Margaroli, Vincent D. Giacalone, Milton R. Brown, Limin Peng, and Rabindra Tirouvanziam. 2021. "Functional and Transcriptional Adaptations of Blood Monocytes Recruited to the Cystic Fibrosis Airway Microenvironment In Vitro" International Journal of Molecular Sciences 22, no. 5: 2530. https://doi.org/10.3390/ijms22052530
APA StyleFord, B. D., Moncada Giraldo, D., Margaroli, C., Giacalone, V. D., Brown, M. R., Peng, L., & Tirouvanziam, R. (2021). Functional and Transcriptional Adaptations of Blood Monocytes Recruited to the Cystic Fibrosis Airway Microenvironment In Vitro. International Journal of Molecular Sciences, 22(5), 2530. https://doi.org/10.3390/ijms22052530







