Evaluation of the Effects of Developmental Trauma on Neurotransmitter Systems Using Functional Molecular Imaging
Abstract
1. Introduction
2. Results
2.1. Body Weight and Corticosterone Levels
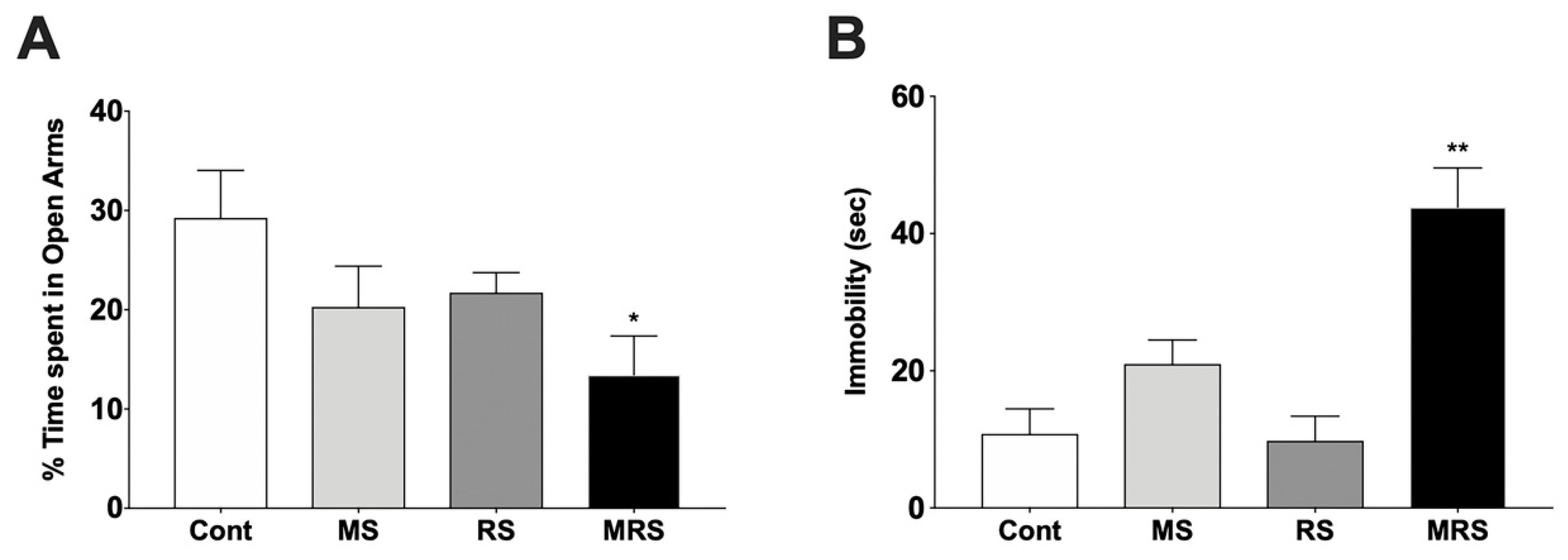
2.2. Behavioral Tests
2.2.1. Elevated Plus Maze
2.2.2. Forced Swim Test
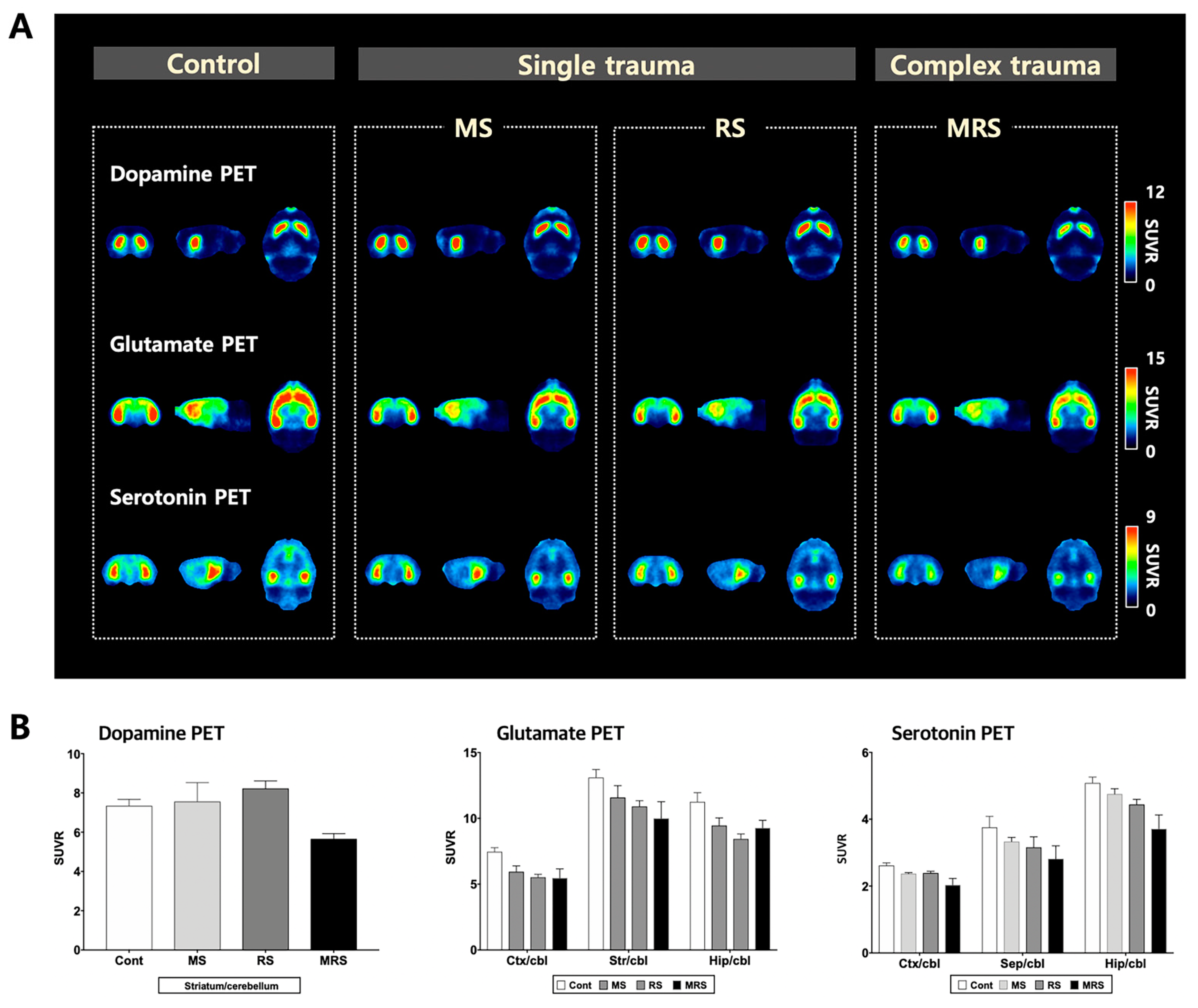
2.3. NeuroPET
2.3.1. Dopaminergic System
2.3.2. Glutamatergic System
2.3.3. Serotoninergic System
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Stress Exposure Protocol
4.3. Behavioral Tests
4.3.1. Elevated Plus Maze
4.3.2. Forced Swim Test
4.4. Molecular Imaging
4.4.1. Preparation of Radiotracers
4.4.2. PET/CT Scan
4.4.3. PET Image Analysis
4.4.4. MR Imaging
4.4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fogelman, N.; Canli, T. Early Life Stress, Physiology, and Genetics: A Review. Front. Psychol. 2019, 10, 1668. [Google Scholar] [CrossRef]
- Hovens, J.G.F.M.; Wiersma, J.E.; Giltay, E.J.; Van Oppen, P.; Spinhoven, P.; Penninx, B.W.J.H.; Zitman, F.G. Childhood life events and childhood trauma in adult patients with depressive, anxiety and comorbid disorders vs. controls. Acta Psychiatr. Scand. 2009, 122, 66–74. [Google Scholar] [CrossRef]
- Jonas, S.; Bebbington, P.; McManus, S.; Meltzer, H.; Jenkins, R.; Kuipers, E.; Cooper, C.; King, M.; Brugha, T. Sexual abuse and psychiatric disorder in England: Results from the 2007 Adult Psychiatric Morbidity Survey. Psychol. Med. 2010, 41, 709–719. [Google Scholar] [CrossRef]
- Leverich, G.S.; McElroy, S.L.; Suppes, T.; Keck, P.E.; Denicoff, K.D.; Nolen, W.A.; Altshuler, L.L.; Rush, A.; Kupka, R.W.; Frye, M.A.; et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol. Psychiatry 2002, 51, 288–297. [Google Scholar] [CrossRef]
- Tyrka, A.R.; Wyche, M.C.; Kelly, M.M.; Price, L.H.; Carpenter, L.L. Childhood maltreatment and adult personality disorder symptoms: Influence of maltreatment type. Psychiatry Res. 2009, 165, 281–287. [Google Scholar] [CrossRef]
- Maniam, J.; Antoniadis, C.; Morris, M.J. Early-Life Stress, HPA Axis Adaptation, and Mechanisms Contributing to Later Health Outcomes. Front. Endocrinol. 2014, 5, 73. [Google Scholar] [CrossRef]
- Kessler, R.C.; McLaughlin, K.A.; Green, J.G.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Aguilar-Gaxiola, S.; Alhamzawi, A.O.; Alonso, J.; Angermeyer, M.; et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry 2010, 197, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Korosia, A.; Naninck, E.F.G.; Oomen, C.A.; Schouten, M.; Krugers, H.; Fitzsimons, C.; Lucassen, P.J. Early-life stress mediated modulation of adult neurogenesis and behavior. Behav. Brain Res. 2012, 227, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Gatt, J.M.; Nemeroff, C.B.; Dobson-Stone, C.; Paul, R.H.; Bryant, R.A.; Schofield, P.R.; Gordon, E.; Kemp, A.; Williams, L.M. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol. Psychiatry 2009, 14, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Pechtel, P.; Pizzagalli, D.A. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology 2010, 214, 55–70. [Google Scholar] [CrossRef]
- Hanson, J.L.; Nacewicz, B.M.; Sutterer, M.J.; Cayo, A.A.; Schaefer, S.M.; Rudolph, K.D.; Shirtcliff, E.A.; Pollak, S.D.; Davidson, R.J. Behavioral Problems After Early Life Stress: Contributions of the Hippocampus and Amygdala. Biol. Psychiatry 2015, 77, 314–323. [Google Scholar] [CrossRef]
- Van Harmelen, A.-L.; Van Tol, M.-J.; Van Der Wee, N.J.A.; Veltman, D.J.; Aleman, A.; Spinhoven, P.; Van Buchem, M.A.; Zitman, F.G.; Penninx, B.W.J.H.; Elzinga, B.M. Reduced Medial Prefrontal Cortex Volume in Adults Reporting Childhood Emotional Maltreatment. Biol. Psychiatry 2010, 68, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Tottenham, N.; Hare, T.A.; Millner, A.; Gilhooly, J.T.; Zevin, J.D.; Casey, B.J. Elevated amygdala response to faces following early deprivation. Dev. Sci. 2011, 14, 190–204. [Google Scholar] [CrossRef]
- Shukla, A.K.; Kumar, U. Positron emission tomography: An overview. J. Med. Phys. 2006, 31, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.A. Positron Emission Tomography as an Imaging Biomarker. J. Clin. Oncol. 2006, 24, 3282–3292. [Google Scholar] [CrossRef]
- Bogdan, R.; Nikolova, Y.S.; Pizzagalli, D.A. Neurogenetics of depression: A focus on reward processing and stress sensitivity. Neurobiol. Dis. 2013, 52, 12–23. [Google Scholar] [CrossRef]
- Hanson, J.L.; Albert, D.; Iselin, A.-M.R.; Carré, J.M.; Dodge, K.A.; Hariri, A.R. Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Soc. Cogn. Affect. Neurosci. 2016, 11, 405–412. [Google Scholar] [CrossRef]
- Derks, N.A.V.; Krugers, H.J.; Hoogenraad, C.C.; Joëls, M.; Sarabdjitsingh, R.A. Effects of Early Life Stress on Synaptic Plasticity in the Developing Hippocampus of Male and Female Rats. PLoS ONE 2016, 11, e0164551. [Google Scholar] [CrossRef]
- Chiba, S.; Numakawa, T.; Ninomiya, M.; Richards, M.C.; Wakabayashi, C.; Kunugi, H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2012, 39, 112–119. [Google Scholar] [CrossRef]
- Huot, R.L.; Thrivikraman, K.V.; Meaney, M.J.; Plotsky, P.M. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology 2001, 158, 366–373. [Google Scholar] [CrossRef]
- Kalinichev, M.; Easterling, K.W.; Plotsky, P.M.; Holtzman, S.G. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long–Evans rats. Pharmacol. Biochem. Behav. 2002, 73, 131–140. [Google Scholar] [CrossRef]
- Jackowski, A.; Perera, T.D.; Abdallah, C.G.; Garrido, G.; Tang, C.Y.; Martinez, J.; Mathew, S.J.; Gorman, J.M.; Rosenblum, L.A.; Smith, E.L.P.; et al. Early-life stress, corpus callosum development, hippocampal volumetrics, and anxious behavior in male nonhuman primates. Psychiatry Res. Neuroimaging 2011, 192, 37–44. [Google Scholar] [CrossRef]
- Walsh, N.D.; Dalgleish, T.; Lombardo, M.V.; Dunn, V.J.; Van Harmelen, A.-L.; Ban, M.; Goodyer, I.M. General and specific effects of early-life psychosocial adversities on adolescent grey matter volume. NeuroImage Clin. 2014, 4, 308–318. [Google Scholar] [CrossRef]
- Spinelli, S.; Chefer, S.; Carson, R.E.; Jagoda, E.; Lang, L.; Heilig, M.; Barr, C.S.; Suomi, S.J.; Higley, J.D.; Stein, E.A. Effects of Early-Life Stress on Serotonin1A Receptors in Juvenile Rhesus Monkeys Measured by Positron Emission Tomography. Biol. Psychiatry 2010, 67, 1146–1153. [Google Scholar] [CrossRef]
- Ichise, M.; Vines, D.C.; Gura, T.; Anderson, G.M.; Suomi, S.J.; Higley, J.D.; Innis, R.B. Effects of Early Life Stress on [11C]DASB Positron Emission Tomography Imaging of Serotonin Transporters in Adolescent Peer- and Mother-Reared Rhesus Monkeys. J. Neurosci. 2006, 26, 4638–4643. [Google Scholar] [CrossRef]
- Murrough, J.W.; Czermak, C.; Henry, S.; Nabulsi, N.; Gallezot, J.-D.; Gueorguieva, R.; Planeta-Wilson, B.; Krystal, J.H.; Neumaier, J.F.; Huang, Y.; et al. The Effect of Early Trauma Exposure on Serotonin Type 1B Receptor Expression Revealed by Reduced Selective Radioligand Binding. Arch. Gen. Psychiatry 2011, 68, 892–900. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Champagne, F.; Meaney, M.J.; Dagher, A. Dopamine Release in Response to a Psychological Stress in Humans and Its Relationship to Early Life Maternal Care: A Positron Emission Tomography Study Using [11C] Raclopride. J. Neurosci. 2004, 24, 2825–2831. [Google Scholar] [CrossRef]
- Parr, L.A.; Boudreau, M.; Hecht, E.; Winslow, J.T.; Nemeroff, C.B.; Sanchez, M.M. Early life stress affects cerebral glucose metabolism in adult rhesus monkeys (Macaca mulatta). Dev. Cogn. Neurosci. 2012, 2, 181–193. [Google Scholar] [CrossRef]
- Corcoran, S.B.E.; Howell, L.L. Impact of early life stress on the reinforcing and behavioral-stimulant effects of psychostimulants in rhesus monkeys. Behav. Pharmacol. 2010, 21, 69–76. [Google Scholar] [CrossRef]
- Nitta, A.; Ohmiya, M.; Sometani, A.; Itoh, M.; Nomoto, H.; Furukawa, Y.; Furukawa, S. Brain-derived neurotrophic factor prevents neuronal cell death induced by corticosterone. J. Neurosci. Res. 1999, 57, 227–235. [Google Scholar] [CrossRef]
- Brummelte, S.; Galea, L. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience 2010, 168, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Cizza, G. Stress-induced changes in brain-derived neurotrophic factor expression are attenuated in aged Fischer 344/N rats. Neurobiol. Aging 1996, 17, 859–864. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Shi, H.J.; Chang, L.; Zhang, C.C.; Si, M.; Li, N.; Zhu, D.Y. nNOS-CAPON blockers produce anxiolytic effects by promoting synaptogenesis in chronic stress-induced animal models of anxiety. Br. J. Pharmacol. 2020, 177, 3674–3690. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, A.C. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef]
- Saigal, N.; Pichika, R.; Easwaramoorthy, B.; Collins, D.; Christian, B.T.; Shi, B.; Narayanan, T.K.; Potkin, S.G.; Mukherjee, J. Synthesis and biologic evaluation of a novel serotonin 5-HT1A receptor radioligand, 18F-labeled mefway, in rodents and imaging by PET in a nonhuman primate. J. Nucl. Med. 2006, 47, 1697–1706. [Google Scholar]
- Lim, K.; Labaree, D.; Li, S.; Huang, Y. Preparation of the Metabotropic Glutamate Receptor 5 (mGluR5) PET Tracer [18F] FPEB for Human Use: An Automated Radiosynthesis and a Novel One-Pot Synthesis of its Radiolabeling Precursor. Appl. Radiat. Isot. 2014, 94, 349–354. [Google Scholar] [CrossRef]
- Moon, B.S.; Park, J.H.; Lee, H.J.; Kim, J.S.; Kil, H.S.; Lee, B.S.; Chi, D.Y.; Lee, B.C.; Kim, Y.K.; Kim, S.E. Highly efficient production of [18F]fallypride using small amounts of base concentration. Appl. Radiat. Isot. 2010, 68, 2279–2284. [Google Scholar] [CrossRef]

| Group | Body Weight (g) | Corticosterone (ng/mL) |
|---|---|---|
| Control | 402.9 ± 36.9 | 196.5 ± 21.3 |
| MS | 388.7 ± 19.4 g | 123.9 ± 16.8 |
| RS | 403.7 ± 39 | 111.7 ± 18.6 |
| MRS | 281 ± 48 ** | 138.8 ± 15.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.; Oh, S.-J.; Park, J.-W.; Nam, K.-R.; Kang, K.-J.; Lee, K.-C.; Lee, Y.-J.; Choi, J.-S.; Seok, J.-H.; Choi, J.-Y. Evaluation of the Effects of Developmental Trauma on Neurotransmitter Systems Using Functional Molecular Imaging. Int. J. Mol. Sci. 2021, 22, 2522. https://doi.org/10.3390/ijms22052522
Lee N, Oh S-J, Park J-W, Nam K-R, Kang K-J, Lee K-C, Lee Y-J, Choi J-S, Seok J-H, Choi J-Y. Evaluation of the Effects of Developmental Trauma on Neurotransmitter Systems Using Functional Molecular Imaging. International Journal of Molecular Sciences. 2021; 22(5):2522. https://doi.org/10.3390/ijms22052522
Chicago/Turabian StyleLee, Namhun, Se-Jong Oh, Jang-Woo Park, Kyung-Rok Nam, Kyung-Jun Kang, Kyo-Chul Lee, Yong-Jin Lee, June-Seek Choi, Jeong-Ho Seok, and Jae-Yong Choi. 2021. "Evaluation of the Effects of Developmental Trauma on Neurotransmitter Systems Using Functional Molecular Imaging" International Journal of Molecular Sciences 22, no. 5: 2522. https://doi.org/10.3390/ijms22052522
APA StyleLee, N., Oh, S.-J., Park, J.-W., Nam, K.-R., Kang, K.-J., Lee, K.-C., Lee, Y.-J., Choi, J.-S., Seok, J.-H., & Choi, J.-Y. (2021). Evaluation of the Effects of Developmental Trauma on Neurotransmitter Systems Using Functional Molecular Imaging. International Journal of Molecular Sciences, 22(5), 2522. https://doi.org/10.3390/ijms22052522






