Calcium-Sensing Receptor (CaSR), Its Impact on Inflammation and the Consequences on Cardiovascular Health
Abstract
1. Introduction
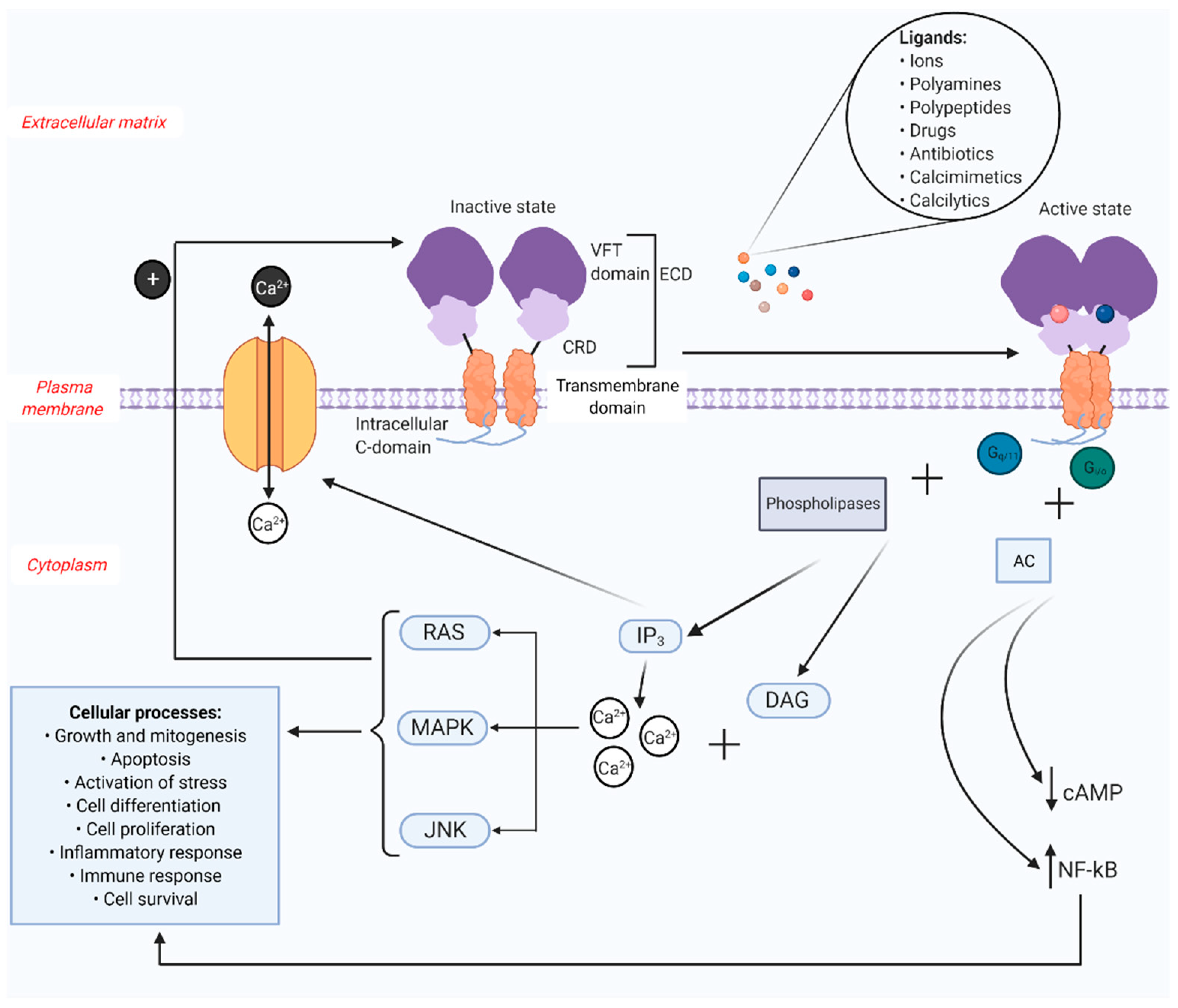
2. CaSR Structure and Regulation
3. CaSR in Cardiovascular System-Related Cells
3.1. CaSR in Non-Hematopoietic Cardiovascular Cells
3.2. CaSR in Hematopoietic Cardiovascular Cells
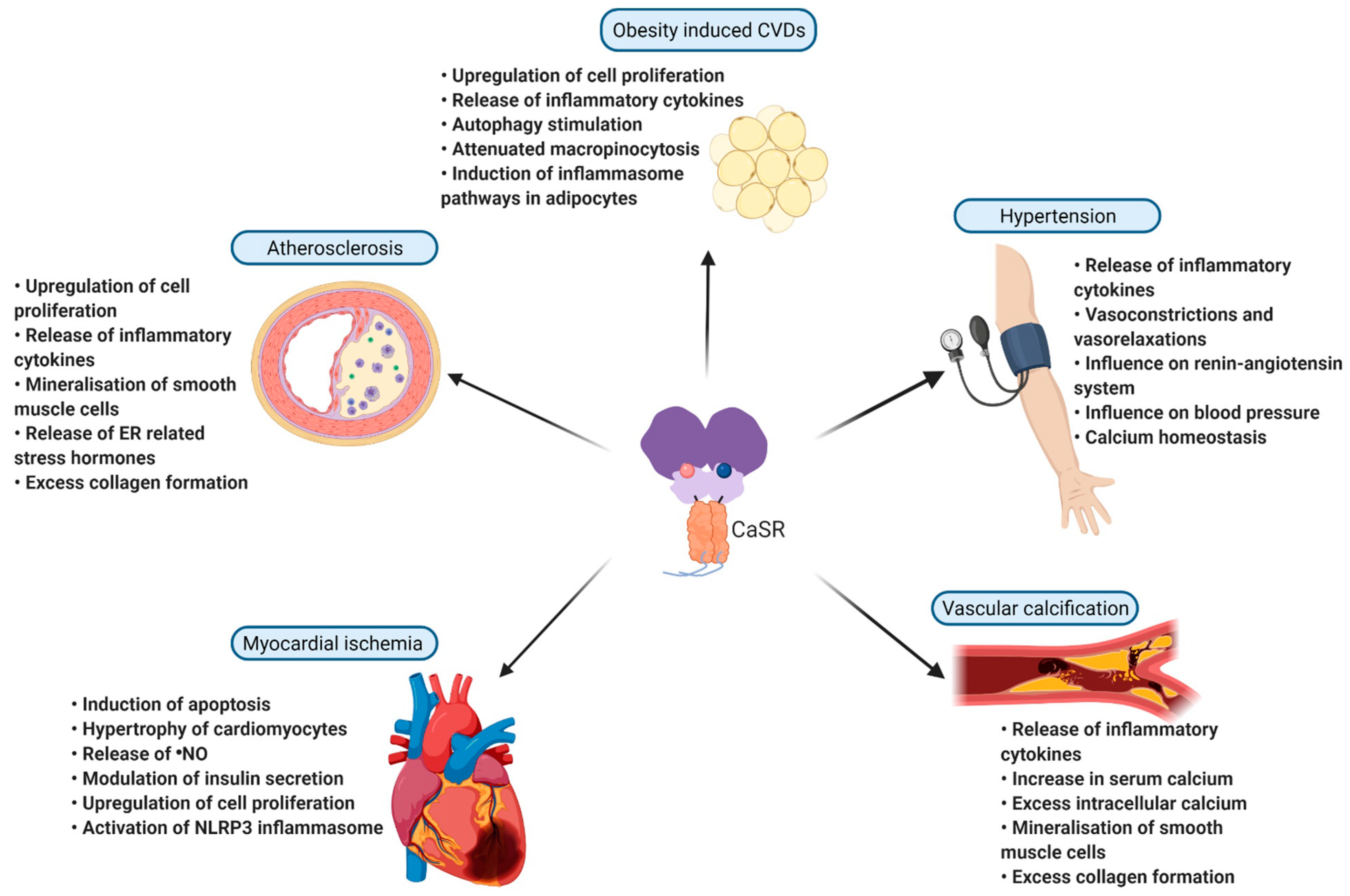
4. Pathological Role of CaSR in Inflammation and Cardiovascular Diseases
4.1. Imbalance in Mineral Homeostasis and Cardiovascular Disease
4.2. Heart Failure
4.3. Atherosclerosis and Vascular Calcification
4.4. Myocardial Infarction
4.5. Hypertension
4.6. Obesity’s Influence
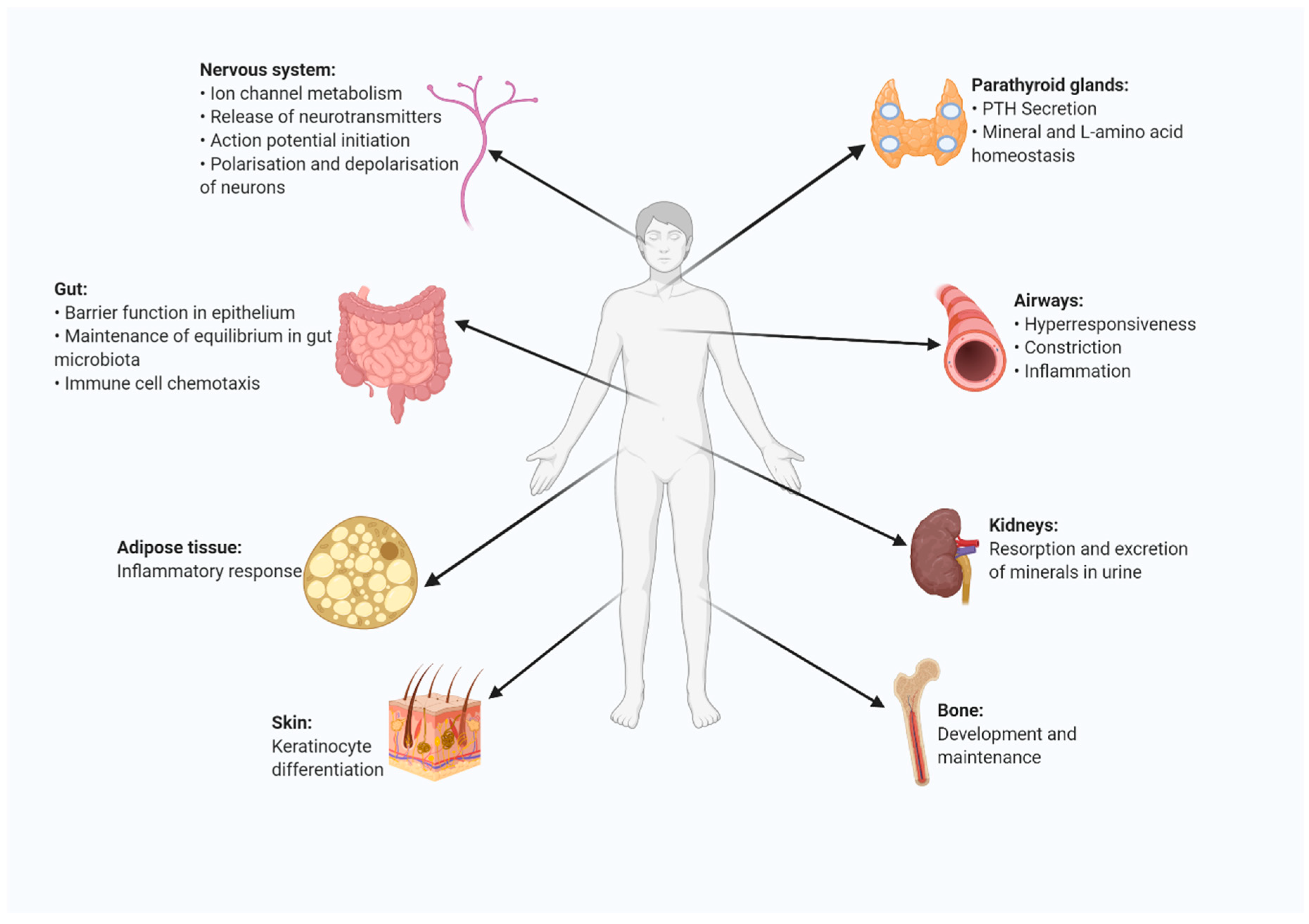
5. Presence and Function of CaSR in Other Organs and Organ Systems
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-SMA | Alpha smooth muscle actin |
| AC | Adenylate cyclase |
| AngII | Angiotensin II |
| CAD | Coronary artery disease |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| cAMP | Cyclic adenosine monophosphate |
| CaSR | Calcium sensing receptor |
| CRD | Cysteine rich domain |
| CVD | Cardiovascular disease |
| DAG | Diacylglycerol |
| EC | Endothelial cell |
| ECD | Extracellular domain |
| ER | Endoplasmic reticulum |
| FHH | Familial hypocalciuric hypercalcemia |
| HUVEC | Human umbilical vein endothelial cell |
| IL-1R | Interleukin-1 receptor |
| IP3 | Inositol triphosphate |
| JNK | C-Jun n-terminal kinases |
| LDL | Low-density lipoproteins |
| LPS | Lipopolysaccharide |
| LTCC | L type Ca2+ channel |
| MAPK | Mitogen-activated protein kinase |
| MMP2 | Matrix metalloproteinase-2 |
| MI | Myocardial infarction |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain containing 3 |
| ●NO | Nitric oxide |
| NOD2 | Nucleotide-binding oligomerization domain-containing protein 2 |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PLC | Phospholipase C |
| PTH | Parathyroid hormone |
| RAAS | Renin-angiotensin-aldosterone system |
| RAS | Rat sarcoma |
| ROC | Receptor-operated channels |
| TLR | Toll-like receptor |
| VFT | Venus fly trap |
| vSMC | Vascular smooth muscle cell |
References
- Hendy, G.N.; Canaff, L. Calcium-sensing receptor, proinflammatory cytokines and calcium homeostasis. Semin. Cell Dev. Biol. 2016, 49, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Brown, E. Cloning and functional characterization of extracellular Ca2+-sensing receptors from parathyroid and kidney. Bone 1995, 17, S7–S11. [Google Scholar] [CrossRef]
- Gerbino, A.; Colella, M. The different facets of extracellular calcium sensors: Old and new concepts in calcium-sensing receptor signalling and pharmacology. Int. J. Mol. Sci. 2018, 19, 999. [Google Scholar] [CrossRef]
- Pollak, M.R. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 1993, 75, 1297–1303. [Google Scholar] [CrossRef]
- Vahe, C. Diseases associated with calcium-sensing receptor. Orphanet J. Rare Dis. 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Geng, Y. Structural mechanism of ligand activation in human calcium-sensing receptor. Elife 2016, 5, e13662. [Google Scholar] [CrossRef]
- Centeno, P.P. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Kallay, E. Physiology and Pathophysiology of the Extracellular Calcium-Sensing Receptor. Am. J. Med. 2018, 9, 413. [Google Scholar]
- Zhang, C. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci. Adv. 2016, 2, e1600241. [Google Scholar] [CrossRef]
- Bai, M.; Trivedi, S.; Brown, E.M. Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J. Biol. Chem. 1998, 273, 23605–23610. [Google Scholar] [CrossRef]
- Zhang, C.; Miller, C.L.; Brown, E.M.; Yang, J.J. The calcium sensing receptor: From calcium sensing to signaling. Sci. China Life Sci. 2015, 58, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Conigrave, A.D.J.B. Mechanisms of multimodal sensing by extracellular Ca2+-sensing receptors: A domain-based survey of requirements for binding and signalling. Br. J. Pharmacol. 2010, 159, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Gorvin, C.M. Molecular and clinical insights from studies of calcium-sensing receptor mutations. J. Mol. Endocrinol. 2019, 63, R1–R16. [Google Scholar] [CrossRef] [PubMed]
- Smajilovic, S.; Tfelt-Hansen, J. Calcium acts as a first messenger through the calcium-sensing receptor in the cardiovascular system. Cardiovasc. Res. 2007, 75, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Hu, J. A region in the seven-transmembrane domain of the human Ca2+ receptor critical for response to Ca2+. J. Biol. Chem. 2005, 280, 5113–5120. [Google Scholar] [CrossRef]
- Leach, K. International Union of Basic and Clinical Pharmacology. CVIII. Calcium-Sensing Receptor Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2020, 72, 558–604. [Google Scholar] [CrossRef]
- Hofer, A.M.; Brown, E.M. Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. 2003, 4, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Calcium and polyamine regulated calcium-sensing receptors in cardiac tissues. Eur. J. Biochem. 2003, 270, 2680–2688. [Google Scholar] [CrossRef]
- Guo, J. Increased expression of calcium-sensing receptors induced by ox-LDL amplifies apoptosis of cardiomyocytes during simulated ischaemia–reperfusion. Clin. Exp. Pharmacol. Physiol. 2010, 37, e128–e135. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.U. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc. Res. 2009, 81, 260–268. [Google Scholar] [CrossRef]
- Chow, J.Y. Calcium-sensing receptor modulates extracellular Ca2+ entry via TRPC-encoded receptor-operated channels in human aortic smooth muscle cells. Am. J. Physiol. Cell Physiol. 2011, 301, C461–C468. [Google Scholar] [CrossRef]
- Schepelmann, M. The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure. Am. J. Physiol. Cell Physiol. 2016, 310, C193–C204. [Google Scholar] [CrossRef]
- Guha, S. Dietary γ-Glutamyl Valine Ameliorates TNF-α-Induced Vascular Inflammation via Endothelial Calcium-Sensing Receptors. J. Agric. Food Chem. 2020, 68, 9139–9149. [Google Scholar] [CrossRef]
- Weston, A.H. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: Studies with calindol and Calhex 231. Circ. Res. 2005, 97, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Ziegelstein, R.C.; Xiong, Y.; He, C.; Hu, Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem. Biophys. Res. Commun. 2006, 342, 153–163. [Google Scholar] [CrossRef]
- Horinouchi, T.; Mazaki, Y.; Terada, K.; Miwa, S. Extracellular Ca2+ promotes nitric oxide production via Ca2+-sensing receptor-Gq/11 protein-endothelial nitric oxide synthase signaling in human vascular endothelial cells. J. Pharmacol. Sci. 2020, 143, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D.; Hendy, G.N. The calcium-sensing receptor in bone—Mechanistic and therapeutic insights. Nat. Rev. Endocrinol. 2015, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- House, M.G. Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J. Bone Miner. Res. 1997, 12, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Li, T. Expression of the calcium sensing receptor in human peripheral blood T lymphocyte and its contribution to cytokine secretion through MAPKs or NF-κB pathways. Mol. Immunol. 2013, 53, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.M.; White, D.; Tomic, J.; Shi, Y.; Spaner, D.E. Extracellular calcium sensing promotes human B-cell activation and function. J. Am. Soc. Hematol. 2007, 110, 3985–3995. [Google Scholar] [CrossRef]
- Klein, G.L.; Castro, S.M.; Garofalo, R.P. The calcium-sensing receptor as a mediator of inflammation. Semin. Cell Dev. Biol. 2016, 49, 52–56. [Google Scholar] [CrossRef]
- Canton, J. Calcium-sensing receptors signal constitutive macropinocytosis and facilitate the uptake of NOD2 ligands in macrophages. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Olszak, I.T. Extracellular calcium elicits a chemokinetic response from monocytes in vitro and in vivo. J. Clin. Investig. 2000, 105, 1299–1305. [Google Scholar] [CrossRef]
- Hannan, F.M.; Babinsky, V.N.; Thakker, R.V. Disorders of the calcium-sensing receptor and partner proteins: Insights into the molecular basis of calcium homeostasis. J. Mol. Endocrinol. 2016, 57, R127. [Google Scholar] [CrossRef] [PubMed]
- März, W. Alanine to serine polymorphism at position 986 of the calcium-sensing receptor associated with coronary heart disease, myocardial infarction, all-cause, and cardiovascular mortality. J. Clin. Endocrinol. Metab. 2007, 92, 2363–2369. [Google Scholar] [CrossRef]
- Shingu, T. Significance of intracellular free calcium and magnesium and calcium-regulating hormones with sodium chloride loading in patients with essential hypertension. J. Hypertens. 1991, 9, 1021–1028. [Google Scholar] [CrossRef]
- Bolland, M.J. Abdominal aortic calcification on vertebral morphometry images predicts incident myocardial infarction. J. Bone Miner. Res. 2010, 25, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Burgess, S.; Michaelsson, K. Association of Genetic Variants Related to Serum Calcium Levels with Coronary Artery Disease and Myocardial Infarction. JAMA 2017, 318, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-H. Calcium-sensing receptor induces rat neonatal ventricular cardiomyocyte apoptosis. Biochem. Biophys. Res. Commun. 2006, 350, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.-H. Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cell. Physiol. Biochem. 2013, 31, 728–743. [Google Scholar] [CrossRef]
- Majumdar, S.K. A Novel Variant in the Calcium-Sensing Receptor Associated with Familial Hypocalciuric Hypercalcemia and Low-to-Normal PTH. Case Rep. Endocrinol. 2020. [Google Scholar] [CrossRef]
- Roszko, K.L.; Bi, R.D.; Mannstadt, M. Autosomal dominant hypocalcemia (hypoparathyroidism) types 1 and 2. Front. Physiol. 2016, 7, 458. [Google Scholar] [CrossRef]
- Liu, K. The G allele of CaSR R990G polymorphism increases susceptibility to urolithiasis and hypercalciuria: Evidences from a comprehensive meta-analysis. Biomed Res. Int. 2015. [Google Scholar] [CrossRef]
- Vezzoli, G. Risk of nephrolithiasis in primary hyperparathyroidism is associated with two polymorphisms of the calcium-sensing receptor gene. J. Nephrol. 2015, 28, 67–72. [Google Scholar] [CrossRef]
- Li, X.M. Downregulation of survival signalling pathways and increased apoptosis in the transition of pressure overload-induced cardiac hypertrophy to heart failure. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1054–1061. [Google Scholar] [CrossRef]
- Lu, M. Calcium sensing receptor-related pathway contributes to cardiac injury and the mechanism of Astragaloside IV on cardioprotection. Front. Pharmacol. 2018, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
- Chi, J. Activation of calcium-sensing receptor-mediated autophagy in angiotensinII-induced cardiac fibrosis in vitro. Biochem. Biophys. Res. Commun. 2018, 497, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Han, G. Relationship between CaSRs and LPS-injured cardiomyocytes. Int. J. Clin. Exp. Pathol. 2018, 11, 1965. [Google Scholar]
- Ren, Z. Calcium-sensing receptor on neutrophil promotes myocardial apoptosis and fibrosis after acute myocardial infarction via NLRP3 inflammasome activation. Can. J. Cardiol. 2020, 36, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Calcium sensing receptor protects high glucose-induced energy metabolism disorder via blocking gp78-ubiquitin proteasome pathway. Cell Death Dis. 2017, 8, e2799. [Google Scholar] [CrossRef] [PubMed]
- Cavalera, M.; Wang, J.; Frangogiannis, N.G. Obesity, metabolic dysfunction, and cardiac fibrosis: Pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl. Res. 2014, 164, 323–335. [Google Scholar] [CrossRef]
- Rybczyńska, A. Activity of the calcium-sensing receptor influences blood glucose and insulin levels in rats. Pharmacol. Rep. 2017, 69, 709–713. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [PubMed]
- Schaftenaar, F.; Frodermann, V.; Kuiper, J.; Lutgens, E. Atherosclerosis: The interplay between lipids and immune cells. Curr. Opin. Lipidolgy 2016, 27, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, J.-H. Involvement of immune cell network in aortic valve stenosis: Communication between valvular interstitial cells and immune cells. Immune Netw. 2016, 16, 26–32. [Google Scholar] [CrossRef]
- Otto, C.M.; O’Brien, K. Why is there discordance between calcific aortic stenosis and coronary artery disease? Heart 2001, 85, 601–602. [Google Scholar] [CrossRef]
- Carabello, B.A.; Paulus, W.J. Aortic stenosis. Lancet 2009, 373, 956–966. [Google Scholar] [CrossRef]
- Molostvov, G.; Hiemstra, T.F.; Fletcher, S.; Bland, R.; Zehnder, D. Arterial Expression of the Calcium-Sensing Receptor Is Maintained by Physiological Pulsation and Protects against Calcification. PLoS ONE 2015, 10, e0138833. [Google Scholar] [CrossRef] [PubMed]
- Rossol, M. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012, 3, 1329. [Google Scholar] [CrossRef]
- Risinger, G.M., Jr.; Hunt, T.S.; Updike, D.L.; Bullen, E.C.; Howard, E.W. Matrix metalloproteinase-2 expression by vascular smooth muscle cells is mediated by both stimulatory and inhibitory signals in response to growth factors. J. Biol. Chem. 2006, 281, 25915–25925. [Google Scholar] [CrossRef]
- Li, H.X. Involvement of calcium-sensing receptor in oxLDL-induced MMP-2 production in vascular smooth muscle cells via PI3K/Akt pathway. Mol. Cell Biochem. 2012, 362, 115–122. [Google Scholar] [CrossRef]
- Molostvov, G.; Fletcher, S.; Bland, R.; Zehnder, D. Extracellular calcium-sensing receptor mediated signalling is involved in human vascular smooth muscle cell proliferation and apoptosis. Cell. Physiol. Biochem. 2008, 22, 413–422. [Google Scholar] [CrossRef]
- Lu, L. Myocardial infarction: Symptoms and treatments. Cell Biochem. Biophys. 2015, 72, 865–867. [Google Scholar] [CrossRef]
- Thygesen, K. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007, 116, 2634–2653. [Google Scholar]
- Wang, Y. Calcium sensing receptor initiating cystathionine-gamma-lyase/hydrogen sulfide pathway to inhibit platelet activation in hyperhomocysteinemia rat. Exp. Cell Res. 2017, 358, 171–181. [Google Scholar] [CrossRef]
- Luo, Y. Activation of the CaR-CSE/H2S pathway confers cardioprotection against ischemia-reperfusion injury. Exp. Cell Res. 2020, 398, 112389. [Google Scholar]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Zhu, T. The role of MCPIP1 in ischemia/reperfusion injury-induced HUVEC migration and apoptosis. Cell. Physiol. Biochem. 2015, 37, 577–591. [Google Scholar] [CrossRef]
- Xie, X. MCPIP1-induced autophagy mediates ischemia/reperfusion injury in endothelial cells via HMGB1 and CaSR. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Xi, Y.-H. The functional expression of calcium-sensing receptor in the differentiated THP-1 cells. Mol. Cell. Biochem. 2010, 342, 233–240. [Google Scholar] [CrossRef]
- Touyz, R.M. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Zhang, X. NLRP3 inflammasome is involved in calcium-sensing receptor-induced aortic remodeling in SHRs. Mediat. Inflamm. 2019, 2019. [Google Scholar] [CrossRef]
- Ward, B.K.; Magno, A.L.; Walsh, J.P.; Ratajczak, T. The role of the calcium-sensing receptor in human disease. Clin. Biochem. 2012, 45, 943–953. [Google Scholar] [CrossRef]
- Rybczynska, A. Blockade of calcium channels and AT1 receptors prevents the hypertensive effect of calcilytic NPS 2143 in rats. J. Physiol. Pharmacol. 2010, 61, 163. [Google Scholar]
- Zhang, T. Calcimimetic R568 improved cardiac remodeling by classic and novel renin-angiotensin system in spontaneously hypertensive rats. Exp. Biol. Med. 2019, 244, 789–801. [Google Scholar] [CrossRef]
- Liu, C. CaSR activates PKCdelta to induce cardiomyocyte apoptosis via ER stressassociated apoptotic pathways during ischemia/reperfusion. Int. J. Mol. Med. 2019, 44, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Hatton, D.C.; McCarron, D.A. Dietary calcium and blood pressure in experimental models of hypertension. A review. Hypertension 1994, 23, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, H.Z. Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced nitric oxide production and vasorelaxation in rabbit mesenteric arteries. Vasc. Pharmacol. 2017, 96, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, H.Z. Stimulation of calcium-sensing receptors induces endothelium-dependent vasorelaxations via nitric oxide production and activation of IKCa channels. Vasc. Pharmacol. 2016, 80, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Vecchié, A. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur. J. Intern. Med. 2018, 48, 6–17. [Google Scholar] [CrossRef]
- Mattar, P. Calcium-Sensing Receptor in Adipose Tissue: Possible Association with Obesity-Related Elevated Autophagy. Int. J. Mol. Sci. 2020, 21, 7617. [Google Scholar] [CrossRef]
- Mattar, P. Autophagy mediates calcium-sensing receptor-induced TNFα production in human preadipocytes. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3585–3594. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; Tinahones, A.; Bekay, R.E.; Malagón, M.M.; Tinahones, F.J. The Role of Autophagy in White Adipose Tissue Function: Implications for Metabolic Health. Metabolites 2020, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- D’Espessailles, A.; Mora, Y.A.; Fuentes, C.; Cifuentes, M. Calcium-sensing receptor activates the NLRP3 inflammasome in LS14 preadipocytes mediated by ERK1/2 signaling. J. Cell. Physiol. 2018, 233, 6232–6240. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef]
- Bravo-Sagua, R.; Mattar, P.; Díaz, X.; Lavandero, S.; Cifuentes, M. Calcium sensing receptor as a novel mediator of adipose tissue dysfunction: Mechanisms and potential clinical implications. Front. Physiol. 2016, 7, 395. [Google Scholar] [CrossRef]
- Hannan, F.M. The calcilytic agent NPS 2143 rectifies hypocalcemia in a mouse model with an activating calcium-sensing receptor (CaSR) mutation: Relevance to autosomal dominant hypocalcemia type 1 (ADH1). Endocrinology 2015, 156, 3114–3121. [Google Scholar] [CrossRef]
- Conigrave, A.D. The calcium-sensing receptor and the parathyroid: Past, present, future. Front. Physiol. 2016, 7, 563. [Google Scholar] [CrossRef]
- Conigrave, A.D. L-amino acids regulate parathyroid hormone secretion. J. Biol. Chem. 2004, 279, 38151–38159. [Google Scholar] [CrossRef]
- Brown, E.M.; MacLeod, R.J. Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 2001, 81, 239–297. [Google Scholar] [CrossRef]
- Riccardi, D. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am. J. Physiol. Ren. Physiol. 1998, 274, F611–F622. [Google Scholar] [CrossRef]
- Graca, J. Comparative expression of the extracellular calcium-sensing receptor in the mouse, rat, and human kidney. Am. J. Physiol. Ren. Physiol. 2016, 310, F518–F533. [Google Scholar] [CrossRef]
- Procino, G. Calcium-sensing receptor and aquaporin 2 interplay in hypercalciuria-associated renal concentrating defect in humans. An in vivo and in vitro study. PLoS ONE 2012, 7, e33145. [Google Scholar] [CrossRef] [PubMed]
- Santa Maria, C. Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 11–23. [Google Scholar]
- Chang, W.; Tu, C.; Chen, T.-H.; Bikle, D.; Shoback, D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci. Signal. 2008, 1, ra1. [Google Scholar] [CrossRef] [PubMed]
- Chang, W. Complex Formation with the Type B γ-Aminobutyric Acid Receptor Affects the Expression and Signal Transduction of the Extracellular Calcium-sensing Receptor: Studies with HEK-293 Cells and Neurons. J. Biol. Chem. 2007, 282, 25030–25040. [Google Scholar] [CrossRef] [PubMed]
- Washburn, D.L.; Anderson, J.W.; Ferguson, A.V. A subthreshold persistent sodium current mediates bursting in rat subfornical organ neurones. J. Physiol. 2000, 529, 359. [Google Scholar] [CrossRef] [PubMed]
- Nakhoul, N.L. Calcium-sensing receptor deletion in the mouse esophagus alters barrier function. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G144–G161. [Google Scholar] [CrossRef]
- Owen, J.L.; Cheng, S.X.; Ge, Y.; Sahay, B.; Mohamadzadeh, M. Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 44–51. [Google Scholar]
- North, M.L. Increased ornithine-derived polyamines cause airway hyperresponsiveness in a mouse model of asthma. Am. J. Respir. Cell Mol. Biol. 2013, 48, 694–702. [Google Scholar] [CrossRef]
- Cifuentes, M.; Albala, C.; Rojas, C. Calcium-sensing receptor expression in human adipocytes. Endocrinology 2005, 146, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- He, Y. Involvement of calcium-sensing receptor in inhibition of lipolysis through intracellular cAMP and calcium pathways in human adipocytes. Biochem. Biophys. Res. Commun. 2011, 404, 393–399. [Google Scholar] [CrossRef]
- Cifuentes, M. Obesity-associated proinflammatory cytokines increase calcium sensing receptor (CaSR) protein expression in primary human adipocytes and LS14 human adipose cell line. Arch. Biochem. Biophys. 2010, 500, 151–156. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundararaman, S.S.; van der Vorst, E.P.C. Calcium-Sensing Receptor (CaSR), Its Impact on Inflammation and the Consequences on Cardiovascular Health. Int. J. Mol. Sci. 2021, 22, 2478. https://doi.org/10.3390/ijms22052478
Sundararaman SS, van der Vorst EPC. Calcium-Sensing Receptor (CaSR), Its Impact on Inflammation and the Consequences on Cardiovascular Health. International Journal of Molecular Sciences. 2021; 22(5):2478. https://doi.org/10.3390/ijms22052478
Chicago/Turabian StyleSundararaman, Sai Sahana, and Emiel P. C. van der Vorst. 2021. "Calcium-Sensing Receptor (CaSR), Its Impact on Inflammation and the Consequences on Cardiovascular Health" International Journal of Molecular Sciences 22, no. 5: 2478. https://doi.org/10.3390/ijms22052478
APA StyleSundararaman, S. S., & van der Vorst, E. P. C. (2021). Calcium-Sensing Receptor (CaSR), Its Impact on Inflammation and the Consequences on Cardiovascular Health. International Journal of Molecular Sciences, 22(5), 2478. https://doi.org/10.3390/ijms22052478






