Effects of Tyrosine and Tryptophan in Rats with Diet-Induced Obesity
Abstract
1. Introduction
2. Results
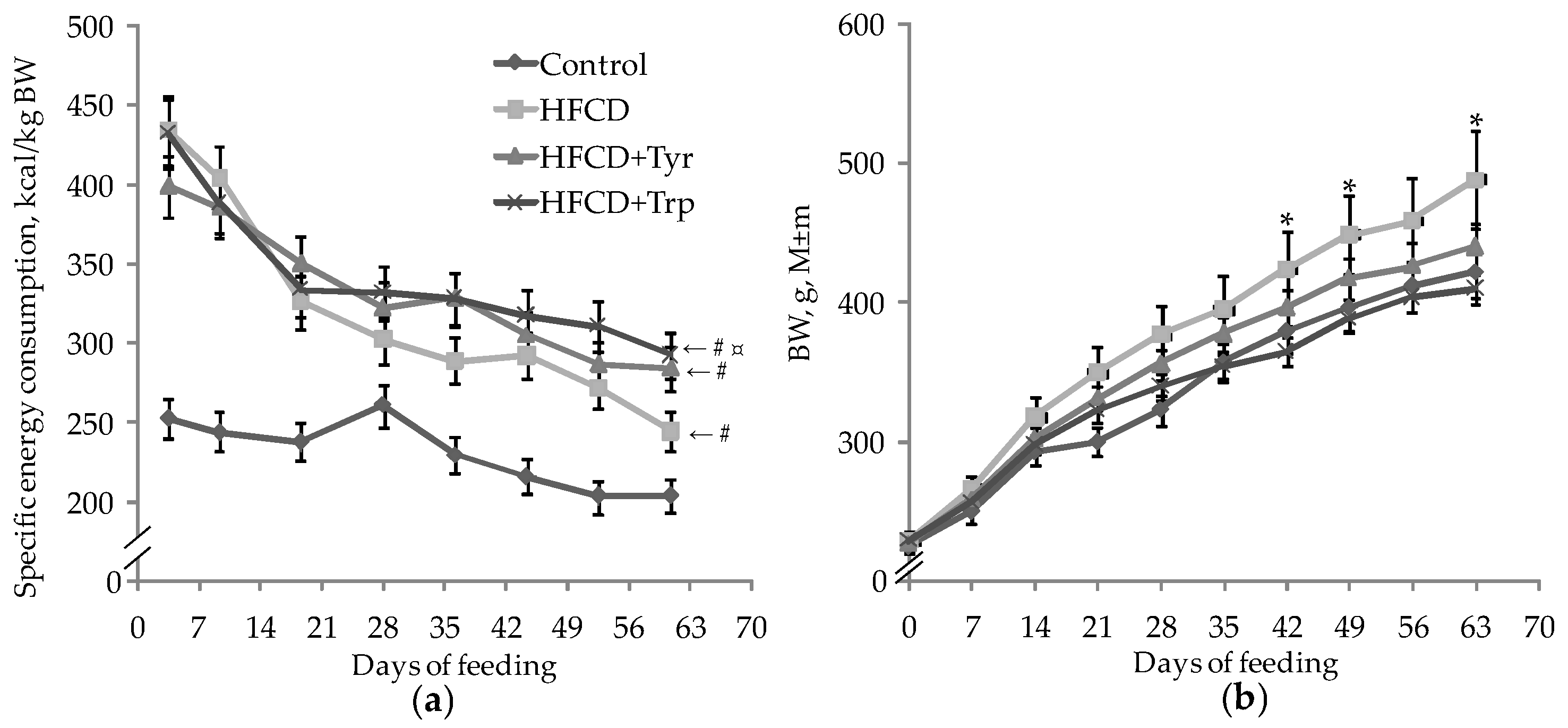
2.1. Integral Indices
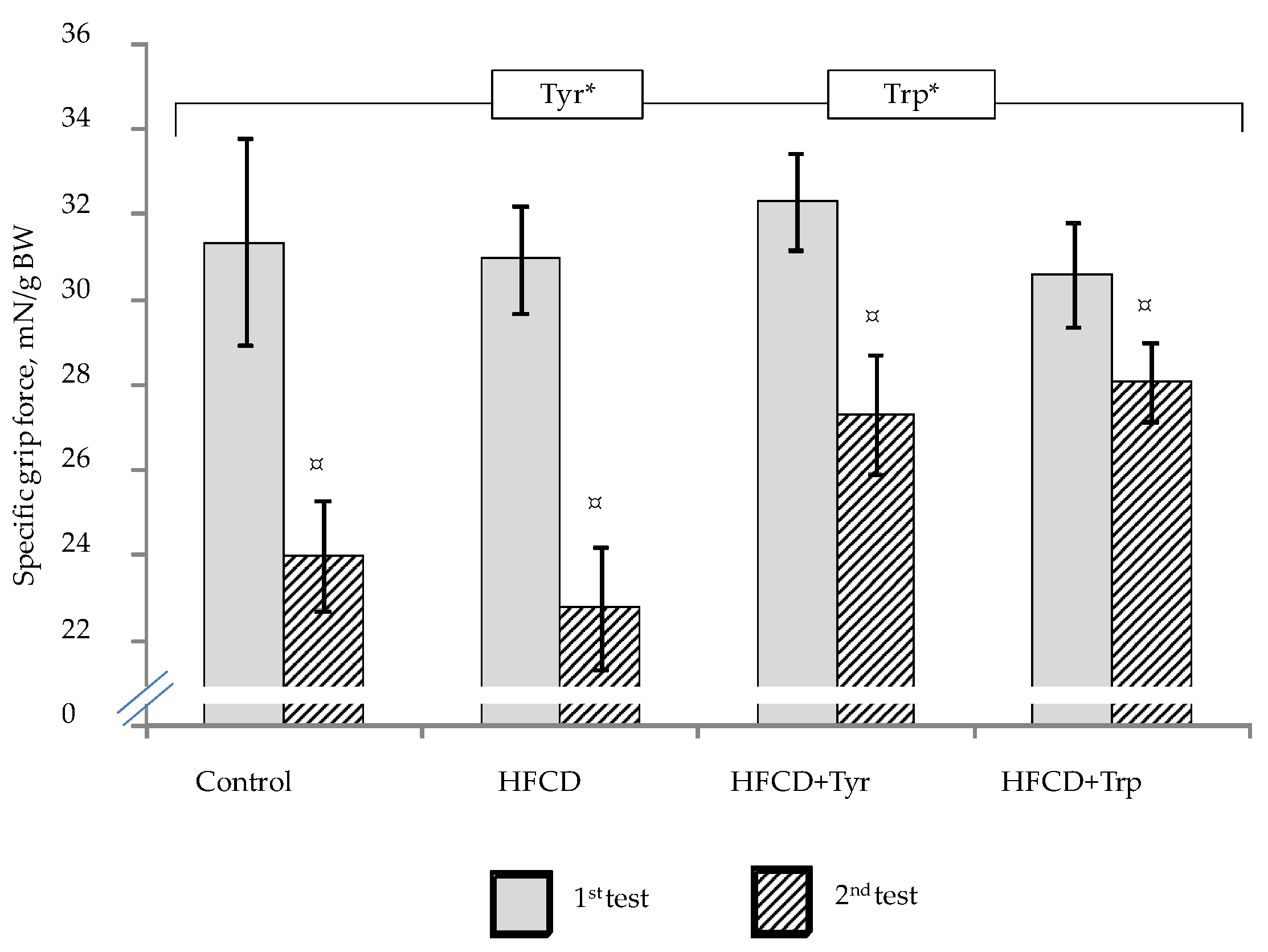
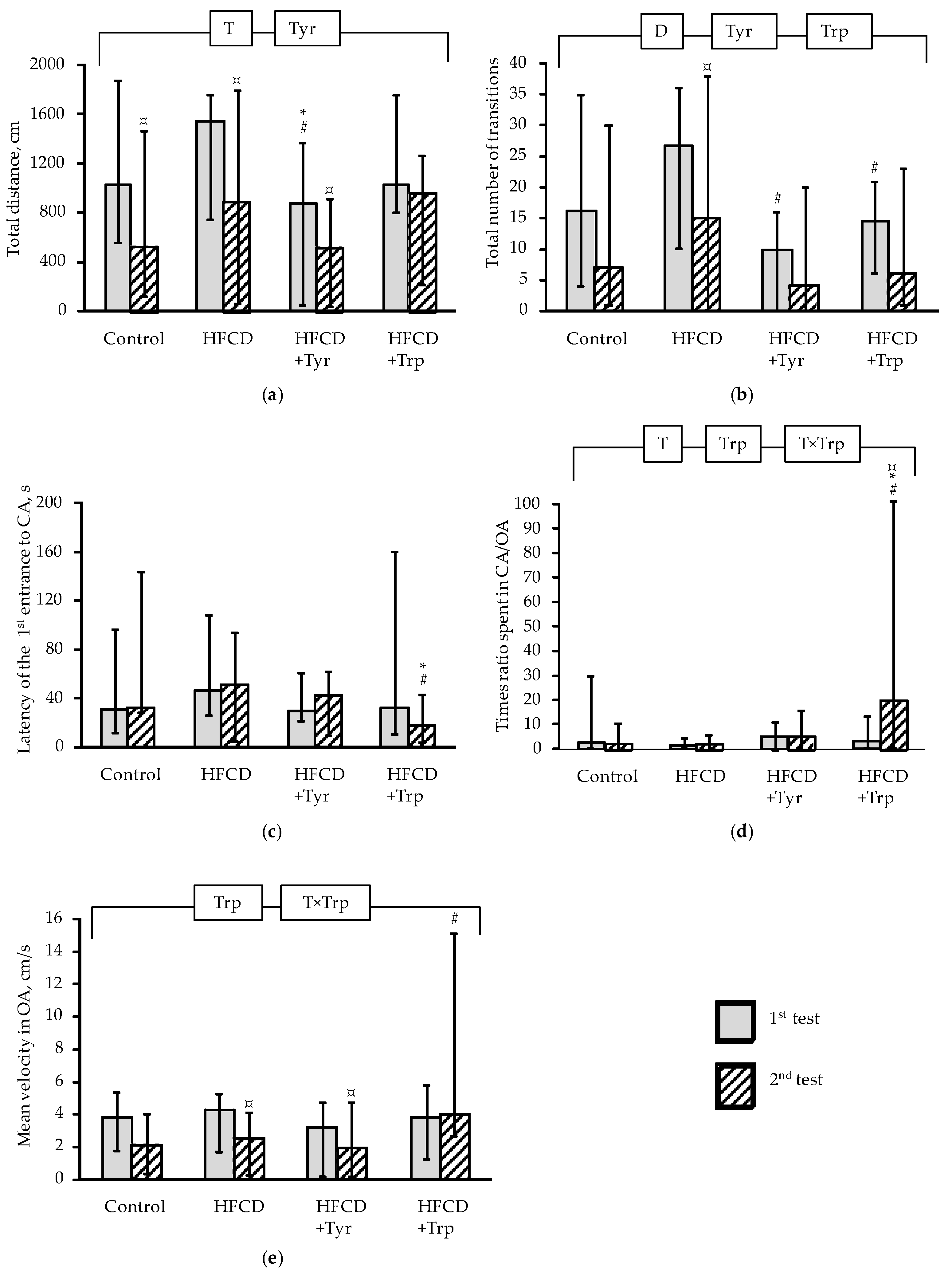
2.2. Muscle Tone, Memory Function and Behavioral Responses
2.3. Biochemical Indices
2.4. Cytokines and Adipokines Levels
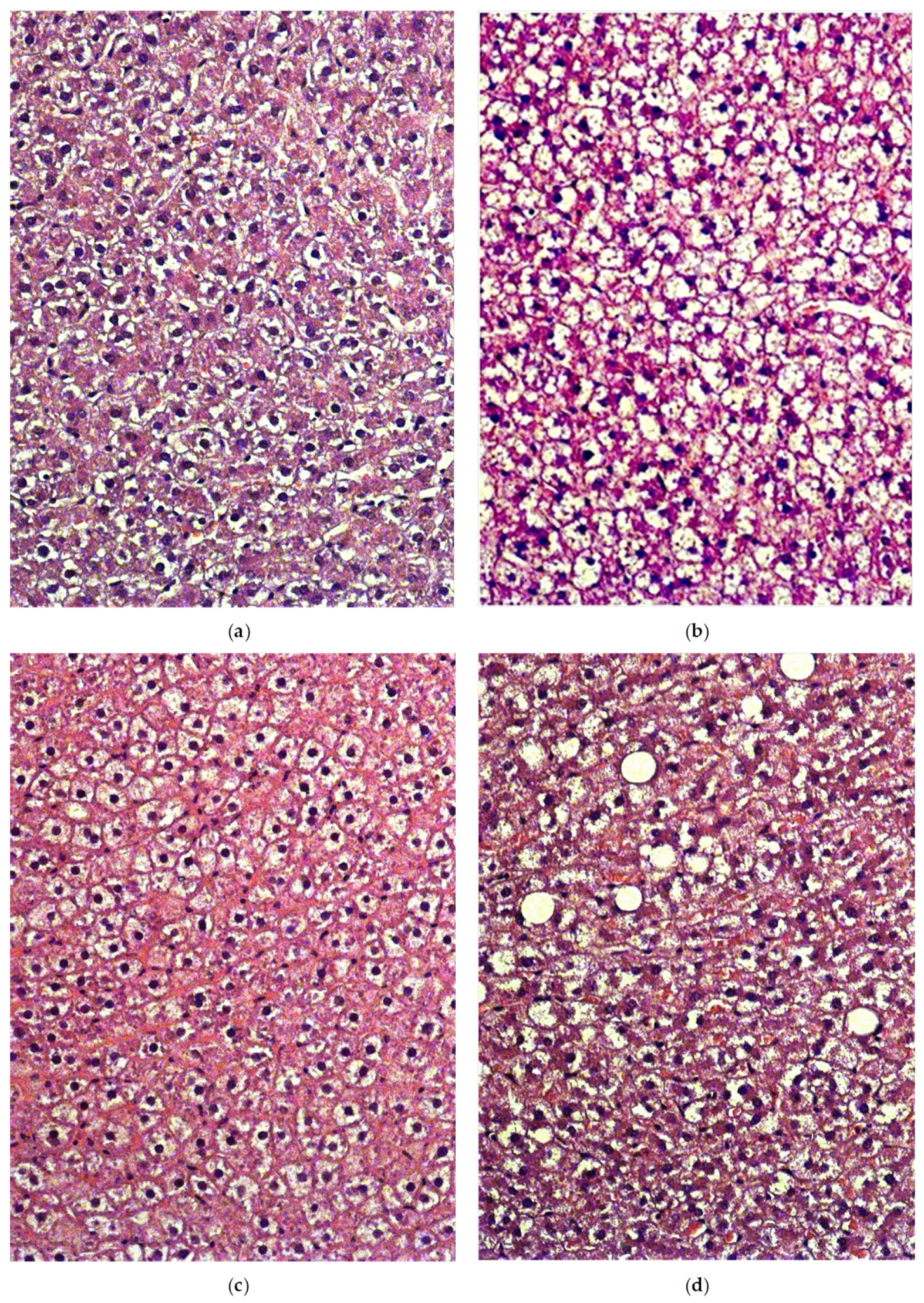
2.5. Liver Histology
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Assessment of Behavioral Responses, Memory Function and Muscle Tone Indices
4.3. Assessment of Integral and Biochemical Indices
4.4. Cytokines and Adipokines Analysis
4.5. Statistical Analysis and Data Availability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bojanowska, E.; Ciosek, J. Can We Selectively Reduce Appetite for Energy-Dense Foods? An Overview of Pharmacological Strategies for Modification of Food Preference Behavior. Curr. Neuropharmacol. 2016, 14, 118–142. [Google Scholar] [CrossRef]
- Kenny, P.J. Reward Mechanisms in Obesity: New Insights and Future Directions. Neuron 2011, 69, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wise, R.A. How can drug addiction help us understand obesity? Nat. Neurosci. 2005, 8, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Rønnestad, I.; Gomes, A.S.; Murashita, K.; Angotzi, R.; Jönsson, E.; Volkoff, H. Appetite-Controlling Endocrine Systems in Teleosts. Front. Endocrinol. 2017, 8, 73. [Google Scholar] [CrossRef]
- Vucetic, Z.; Carlin, J.L.; Totoki, K.; Reyes, T.M. Epigenetic dysregulation of the dopamine system in diet-induced obesity. J. Neurochem. 2012, 120, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.K.; Heisler, L.K. 5-Hydroxytryptamine Medications for the Treatment of Obesity. J. Neuroendocr. 2015, 27, 389–398. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef]
- Hornykiewicz, O. A brief history of levodopa. J. Neurol. 2010, 257, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.P.; Smith, K.; Atkinson, F.; Ruell, P.; Chow, C.M.; O’Connor, H.; Brand-Miller, J. High-glycaemic index and -glycaemic load meals increase the availability of tryptophan in healthy volunteers. Br. J. Nutr. 2011, 105, 1601–1606. [Google Scholar] [CrossRef]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef]
- De Minicis, S.; Seki, E.; Uchinami, H.; Kluwe, J.; Zhang, Y.; Brenner, D.A.; Schwabe, R.F. Gene Expression Profiles During Hepatic Stellate Cell Activation in Culture and In Vivo. Gastroenterology 2007, 132, 1937–1946. [Google Scholar] [CrossRef]
- Boonloh, K.; Kukongviriyapan, V.; Kongyingyoes, B.; Kukongviriyapan, U.; Thawornchinsombut, S.; Pannangpetch, P. Rice Bran Protein Hydrolysates Improve Insulin Resistance and Decrease Pro-inflammatory Cytokine Gene Expression in Rats Fed a High Carbohydrate-High Fat Diet. Nutrients 2015, 7, 6313–6329. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.; Laway, B.A.; Afroze, D.; Wani, A.I. Evaluation of proinflammatory cytokines in obese vs non-obese patients with metabolic syndrome. Indian J. Endocrinol. Metab. 2018, 22, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Gaffen, S.L. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 2010, 21, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Wurtman, J.J. Carbohydrate craving, obesity and brain serotonin. Appetite 1986, 7, 99–103. [Google Scholar] [CrossRef]
- Zhang, L.S.; Davies, S.S. Microbial metabolism of dietary components to bioactive metabolites: Opportunities for new therapeutic interventions. Genome Med. 2016, 8, 1–18. [Google Scholar] [CrossRef]
- Dou, L.; Jourde-Chiche, N.; Faure, V.; Cerini, C.; Berland, Y.; Dignat-George, F.; Brunet, P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 2007, 5, 1302–1308. [Google Scholar] [CrossRef]
- Karbownik, M.; Stasiak, M.; Zygmunt, A.; Zasada, K.; Lewiński, A. Protective effects of melatonin and indole-3-propionic acid against lipid peroxidation, caused by potassium bromate in the rat kidney. Cell Biochem. Funct. 2006, 24, 483–489. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef]
- Tsay, J.C.J.; Wong, T.K.M.; Greenberg, A.K.; Pass, H.; Rom, W.N. Aryl hydrocarbon receptor and lung cancer. Anticancer Res. 2013, 33, 1247–1256. [Google Scholar]
- Zhang, X.; Lu, J.; He, B.; Tang, L.; Liu, X.; Zhu, D.; Cao, H.; Wang, Y.; Li, L. A tryptophan derivative, ITE, enhances liver cell metabolic functions in vitro. Int. J. Mol. Med. 2017, 39, 101–112. [Google Scholar] [CrossRef]
- Apryatin, S.A.; Shipelin, V.A.; Trusov, N.V.; Mzhelskaya, K.V.; Evstratova, V.S.; Kirbaeva, N.V.; Soto, J.S.; Fesenko, Z.S.; Gainetdinov, R.R.; Gmoshinski, I.V. Comparative analysis of the influence of a high-fat/high-carbohydrate diet on the level of anxiety and neuromotor and cognitive functions in Wistar and DAT-KO rats. Physiol. Rep. 2019, 7, e13987. [Google Scholar] [CrossRef] [PubMed]
- Leo, D.; Sukhanov, I.; Zoratto, F.; Illiano, P.; Caffino, L.; Sanna, F.; Messa, G.; Emanuele, M.; Esposito, A.; Dorofeikova, M.; et al. Pronounced Hyperactivity, Cognitive Dysfunctions, and BDNF Dysregulation in Dopamine Transporter Knock-out Rats. J. Neurosci. 2018, 38, 1959–1972. [Google Scholar] [CrossRef]
- Cinque, S.; Zoratto, F.; Poleggi, A.; Leo, D.; Cerniglia, L.; Cimino, S.; Tambelli, R.; Alleva, E.; Gainetdinov, R.R.; Laviola, G.; et al. Behavioral Phenotyping of Dopamine Transporter Knockout Rats: Compulsive Traits, Motor Stereotypies, and Anhedonia. Front. Psychiatry 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Reda, E.; Arrigoni-Martelli, E. Regulation by carnitine of myocardial fatty acid and carbohydrate metabolism under normal and pathological conditions. Basic Res. Cardiol. 2000, 95, 75–83. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Bocarsly, M.E.; Barson, J.R.; Hoebel, B.G.; Leibowitz, S.F. Reduced accumbens dopamine in Sprague–Dawley rats prone to overeating a fat-rich diet. Physiol. Behav. 2010, 101, 394–400. [Google Scholar] [CrossRef]
- Williams, R.L.; Wood, L.G.; Collins, C.E.; Morgan, P.J.; Callister, R. Energy homeostasis and appetite regulating hormones as predictors of weight loss in men and women. Appetite 2016, 101, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Deck, C.A.; Honeycutt, J.L.; Cheung, E.; Reynolds, H.M.; Borski, R.J. Assessing the Functional Role of Leptin in Energy Homeostasis and the Stress Response in Vertebrates. Front. Endocrinol. 2017, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.M.; Hahn, J.D.; Konanur, V.R.; E Noble, E.; Suarez, A.N.; Thai, J.; Nakamoto, E.M.; E Kanoski, S. Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Lee, J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Becattini, B. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol. Metab. 2017, 6, 174–184. [Google Scholar] [CrossRef]
- Mzhelskaya, K.V.; Shipelin, V.A.; Shumakova, A.A.; Musaeva, A.D.; Soto, J.S.; Riger, N.A.; Trusov, N.V.; Kirbaeva, N.V.; Apryatin, S.A.; Gmoshinski, I.V.; et al. Effects of quercetin on the neuromotor function and behavioral responses of Wistar and Zucker rats fed a high-fat and high-carbohydrate diet. Behav. Brain Res. 2020, 378, 2270. [Google Scholar] [CrossRef]
- Lake, B.G. Preparation and characterization of microsomal fractions for studies on xenobiotic metabolism. In Biochemical Toxicology: A Practical Approach; Snell, K., Mullock, B., Eds.; IRL Press: Oxford, UK, 1987; pp. 183–215. [Google Scholar]
- McNally, S.J.; Ross, J.A.; Garden, O.J.; Wigmore, S.J. Optimization of the paired enzyme assay for heme oxygenase activity. Anal. Biochem. 2004, 332, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.M.; Hunkeler, M.J.; Talalay, P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: Possible role in protection against carcinogenesis and toxicity. Proc. Natl. Acad. Sci. USA 1980, 77, 5216–5220. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, W.J.; Jacoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Burchell, B.; Weatherill, P. 4-Nitrophenol UDPglucuronyltransferase (rat liver). Methods Enzymol. 1981, 77, 169–177. [Google Scholar] [PubMed]
- Nakajima, M.; Nakamura, S.; Tokudome, S.; Shimada, N.; Yamazaki, H.; Yokoi, T. Azelastine N-demethylation by cytochrome P-450 (CYP)3A4, CYP2D6, and CYP1A2 in human liver microsomes: Evaluation of approach to predict the contribution of multiple CYPs. Drug Metab. Dispos. 1999, 27, 1381–1391. [Google Scholar] [PubMed]





| Group | Rats Number | Latency, s, M ± s.e.m. | Short-Term Memory, % | Long-Term Memory, % |
|---|---|---|---|---|
| Control | 8 | 19.6 ± 7.9 | 87.5 | 87.5 |
| HFCD | 8 | 17.0 ± 4.8 | 62.5 | 25.0 * |
| HFCD + Tyr | 8 | 25.5 ± 9.2 | 25 * | 25.0 * |
| HFCD + Trp | 8 | 7.3 ± 1.6 *** | 12.5 *,** | 0 *,** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shipelin, V.A.; Trusov, N.V.; Apryatin, S.A.; Shumakova, A.A.; Balakina, A.S.; Riger, N.A.; Gmoshinski, I.V.; Nikityuk, D.B. Effects of Tyrosine and Tryptophan in Rats with Diet-Induced Obesity. Int. J. Mol. Sci. 2021, 22, 2429. https://doi.org/10.3390/ijms22052429
Shipelin VA, Trusov NV, Apryatin SA, Shumakova AA, Balakina AS, Riger NA, Gmoshinski IV, Nikityuk DB. Effects of Tyrosine and Tryptophan in Rats with Diet-Induced Obesity. International Journal of Molecular Sciences. 2021; 22(5):2429. https://doi.org/10.3390/ijms22052429
Chicago/Turabian StyleShipelin, Vladimir A., Nikita V. Trusov, Sergey A. Apryatin, Antonina A. Shumakova, Anastasia S. Balakina, Nikolay A. Riger, Ivan V. Gmoshinski, and Dmitry B. Nikityuk. 2021. "Effects of Tyrosine and Tryptophan in Rats with Diet-Induced Obesity" International Journal of Molecular Sciences 22, no. 5: 2429. https://doi.org/10.3390/ijms22052429
APA StyleShipelin, V. A., Trusov, N. V., Apryatin, S. A., Shumakova, A. A., Balakina, A. S., Riger, N. A., Gmoshinski, I. V., & Nikityuk, D. B. (2021). Effects of Tyrosine and Tryptophan in Rats with Diet-Induced Obesity. International Journal of Molecular Sciences, 22(5), 2429. https://doi.org/10.3390/ijms22052429








