Abstract
Small RNAs are essential to coordinate many cellular processes, including the regulation of gene expression patterns, the prevention of genomic instability, and the suppression of the mutagenic transposon activity. These processes determine the aging, longevity, and sensitivity of cells and an organism to stress factors (particularly, ionizing radiation). The biogenesis and activity of small RNAs are provided by proteins of the Argonaute family. These proteins participate in the processing of small RNA precursors and the formation of an RNA-induced silencing complex. However, the role of Argonaute proteins in regulating lifespan and radioresistance remains poorly explored. We studied the effect of knockdown of Argonaute genes (AGO1, AGO2, AGO3, piwi) in various tissues on the Drosophila melanogaster lifespan and survival after the γ-irradiation at a dose of 700 Gy. In most cases, these parameters are reduced or did not change significantly in flies with tissue-specific RNA interference. Surprisingly, piwi knockdown in both the fat body and the nervous system causes a lifespan increase. But changes in radioresistance depend on the tissue in which the gene was knocked out. In addition, analysis of changes in retrotransposon levels and expression of stress response genes allow us to determine associated molecular mechanisms.
Keywords:
lifespan; aging; radioresistance; ionizing radiation; Agronaute; piwi; Drosophila melanogaster 1. Introduction
Lifespan is determined by the processes that occur at the molecular, cellular, tissue, and organism levels, as well as the influence of damaging environmental factors and other external conditions. Among the molecular mechanisms of lifespan regulation, epigenetic mechanisms have a special place. On the one hand, it provides the implementation of hereditary information embedded in the cells of an organism. On the other hand, it is necessary for fine-tuning the gene expression in accordance with the entering of exogenous stimuli. The well-coordinated work of these two processes maintains the vitality of an organism and ensures its longevity. However, a disturbance of epigenetic regulation can lead to cumulative negative consequences associated with the loss of functionality of cells and an organism, a decrease in its adaptive capabilities [1,2]. This is exactly what happens during the aging of an organism, therefore, epigenetic alterations are one of the basic hallmarks of aging [3]. During aging, there is a change in the structure of chromatin (for example, a loss of nucleosomes and a decrease in the amount of heterochromatin), DNA methylation status, modification of histone marks, changes in the patterns of noncoding RNA activity, epigenetic drift [4]. In addition to the fact that such changes lead to a disturbance of gene expression, they also cause a number of other fatal consequences. For example, the loss of heterochromatin, DNA hypomethylation, and changes in histone labels lead to the activation of the expression of silent transposable elements (or transposons), which increases the accumulation of DNA damages and mutations, and causes genome instability [2,4,5]. Epigenetic dysregulation contributes to the pathogenesis of age-related pathologies, such as cancer, atherosclerosis, type 2 diabetes, mental and neurodegenerative diseases, and a decrease in the immune response [6,7].
Among epigenetic mechanisms, small RNAs are required to coordinate many cellular processes, including post-transcriptional regulation of gene expression, regulation of heterochromatin formation, prevention of genome instability, and suppression of the mutagenic activity of transposons [8,9,10,11]. Small RNAs include three classes and differ in the mechanism of their biogenesis and the type of protein with which they are associated. These are endogenous short interfering RNAs (endo-siRNAs) that are targeted on mRNA and transposons, microRNAs (miRNAs) that regulate mRNA expression, and P-element induced wimpy testis (PIWI)-interacting RNAs (piRNAs) that are essential for the suppression of transposons’ activity. In addition, there are exogenous short interfering RNAs (exo-siRNAs) that are derived from viral double-stranded RNAs (dsRNAs) or artificial dsRNAs, and are aimed to restrict viral and external activity [8,9,10,12]. They are important for coordinating the organism development, forming various organs and tissues, controlling metabolism, and maintaining genome integrity [8,9,10]. Moreover, there is evidence for the important role of small RNAs in regulating lifespan and providing resistance to a range of environmental stressors [2,11,12,13,14,15,16].
The biogenesis and activity of small RNAs are provided by proteins of the Argonaute family. These proteins are involved in the processing of small RNA precursors and the formation of the RNA-induced silence complex (RISC). At the same time, an Argonaute protein loaded with a mature small RNA forms active RISC, which targets a corresponding molecule (mRNA or transposon), carries out its catalytic degradation, and inhibits translation [9,17]. Previous studies have demonstrated the role of some genes of the Argonaute family in the lifespan regulation. For example, it was found that in Caenorhabditis elegans the alg-1 and alg-2 genes conversely regulate lifespan: alg-1 promotes longevity, while alg-2 limits lifespan. This is mediated by their different roles in the regulation of DAF-2/insulin/IGF-1 and DAF-16/FOXO signaling pathways [18]. In Drosophila melanogaster, a mutation in the AGO2 gene leads to a significant reduction in lifespan, which is associated with an increase in transposon expression in the brain and age-dependent memory impairment [19]. In addition, the activity of genes of small RNA biogenesis, including the Argonaute family, mediates beneficial effects of pro-longevity interventions, such as intermittent fasting [20]. However, there is almost no data on the effects of partial downregulation or tissue-specific knockdown of Argonaute.
It should be noted that the range of studied functions of small RNAs and proteins responsible for their biogenesis is currently expanding. In particular, it is known that some miRNAs (as well as lncRNAs) are involved in the response to DNA damage and DNA repair, due to participating in the network of signaling pathways [21,22,23]. At the same time, disruption of the activity of small RNA biogenesis genes and proteins encoded by them (such as AGO2 and PIWIL2) reduces the survival of human cells after exposure to genotoxic impacts (such as UV light, ionizing radiation, and others) as a result of distorted regulation of cell cycle, apoptosis, and DNA repair [24,25,26]. Similar data were obtained in an in vivo model of Caenorhabditis elegans with a mutation in the alg-2 gene [27]. In addition, the role of complexes of double-strand break-induced small RNAs (diRNAs) and AGO2 (diRISCs) in the repair of double-stranded DNA breaks (mainly via homologous recombination) has been described [28,29]. Thus, small RNAs and Argonaute proteins are important for the response to DNA damage, and apparently, play a significant role in the response of cells and an organism to genotoxic agents.
Thus, it is obvious that the Argonaute proteins (as well as the small RNAs associated with them) are involved in regulating lifespan and the organism’s resistance to radiation. However, these functions remain poorly understood and require investigation. The fruit fly Drosophila melanogaster is an appropriate model for this task. Its genome contains five genes encoding proteins of the Argonaute family. Among them, the Argonaute subfamily includes AGO1 (provides maturation and functioning of miRNAs) and AGO2 (performs biogenesis and specifically binds to siRNAs). The PIWI subfamily includes piwi, AGO3, and Aubergine, which are required for piRNA processing and functioning [30,31]. Thus, Argonaute proteins in Drosophila are relatively specific for types of small RNAs, which makes it possible to analyze the contribution of biogenesis and the functioning of each to the studied processes. In addition, fruit fly as a model animal has known advantages, due to their short life cycle, maintenance availability, accessibility of genetic interventions, and evolutionary conservatism of many signaling pathways and genes [32].
In the present work, we studied for the first time the effect of Argonaute genes’ knockdown (AGO1, AGO2, AGO3, piwi) in various tissues on Drosophila melanogaster lifespan and survival after the γ-irradiation at a dose of 700 Gy. In addition, changes in the levels of retrotransposons and expression of stress response genes were analyzed to determine the molecular mechanisms involved. It was previously found that genes of antioxidant defense, DNA damage response, and repair play a critical role in both lifespan regulation and the reaction of cells, tissues, and a whole organism to ionizing irradiation [33,34,35,36,37,38,39,40,41]. In addition, genes involved in different mechanisms of proteostasis demonstrate changes during aging and after irradiation as well [38,39,42,43]. Therefore, we analyses the expression levels of genes from each group.
2. Results
2.1. Effects of Down-Regulation of Argonaute Genes on the Drosophila Lifespan
Tissue-specific knockdown of genes of the Argonaute family in most cases either did not have a statistically significant effect, or led to a decrease in the median lifespan (by 3.0–89.3%, p < 0.05) and the parameter of maximum lifespan (the age of 90% mortality) (by 3.1–62.9%, p < 0.05) in Drosophila males and females (Figure 1 and Figure 2, Table S1). However, in some replicates of the experiment, the studied longevity parameters were increased in flies with RNA interference of the AGO1 and AGO3 genes. Moreover, the median lifespan was reproducibly higher in males and females with piwi knockdown in the nervous system and in the fat body compared with flies without induction of RNA interference (by 2.1–12.5%, p < 0.05) (Figure 2e,f, Table S1).
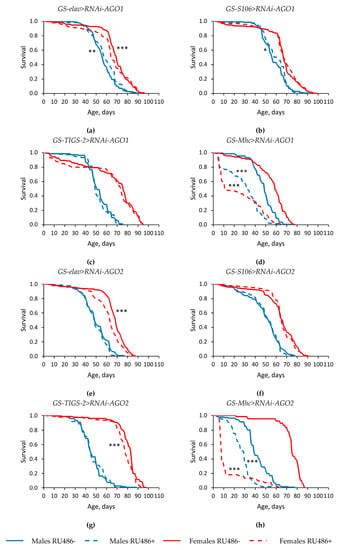
Figure 1.
Influence of AGO1 (a–d) and AGO2 (e–h) knockdown in the nervous system (a,e) (two replicates combined), fat body (b,f) (two replicates combined), guts (c,g), muscles (d,h) on the survival of Drosophila melanogaster. Differences between survival curves of flies with Argonaute genes’ knockdown (RU486+) and without knockdown (RU486-) are statistically significant with * p < 0.05, ** p < 0.01, *** p < 0.001 (Kolmogorov–Smirnov test).
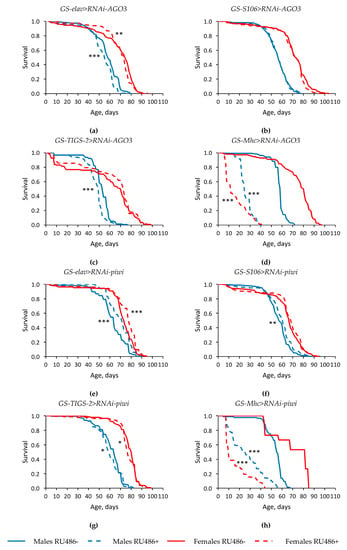
Figure 2.
Influence of AGO3 (a–d) and piwi (e–h) knockdown in the nervous system (a,e) (two replicates combined), fat body (b,f) (two replicates combined), guts (c,g), muscles (d,h) on the survival of Drosophila melanogaster. Differences between survival curves of flies with PIWI genes’ knockdown (RU486+) and without knockdown (RU486-) are statistically significant with * p < 0.05, ** p < 0.01, *** p < 0.001 (Kolmogorov–Smirnov test).
Since the positive effect of knockdown of some of the studied Argonaute genes on longevity was manifested in the case of their RNA interference in the nervous system and the fat body, we carried out further research only with these variants.
2.2. Radioresistance of Drosophila with Knockdown of Argonaute Genes
The exposure to γ-irradiation at a dose of 700 Gy extremely reduced the survival of Drosophila in both sexes, regardless of the tissue-specific expression of the Argonaute family genes. The median survival was decreased by 17.4–76.7% (p < 0.001), and the age of 90% mortality was lower by 18.8–73.8% (p < 0.001) in irradiated flies compared to non-irradiated ones (Figure 3, Table S2).
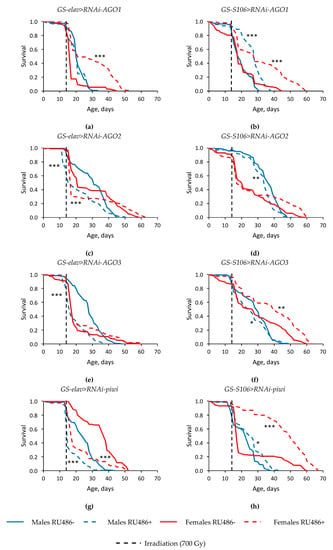
Figure 3.
Influence of AGO1 (a,b), AGO2 (c,d), AGO3 (e, f), piwi (g,h) knockdown in the nervous system (a,c,e,g) and fat body (b,d,f,h) on the survival of Drosophila flies after γ-irradiation at the dose of 700 Gy. Differences between survival of flies curves with Argonaute knockdown (RU486+) and without knockdown (RU486-) are statistically significant with * p < 0.05, ** p < 0.01, *** p < 0.001 (Kolmogorov–Smirnov test).
In the most experimental variants, tissue-specific knockdown of genes of the Argonaute family negatively affected the radioresistance of Drosophila of both sexes, decreased the median survival (by 10.5–55.3%, p < 0.001) and the maximum survival rate (by 21.1–23.5%, p < 0.001) in conditions of γ-irradiation (Figure 3, Table S2). However, flies of both sexes with RNA interference of the AGO1 and piwi genes in the fat body (Figure 3b,h), females (but not males) with RNA interference of AGO2 and AGO3 in the fat body (Figure 3b,h), and females with AGO1 neuronal knockdown showed a high resistance to the radiation exposure (Figure 3a). In these variants of the experiment, the median survival rate was increased by 23.5–200% (p < 0.001), the age of 90% mortality was higher by 17.6–123.8% (p < 0.001) compared with irradiated flies without induction of RNA interference.
It should be noted that an increase in lifespan did not coincide in all cases with an increase in radioresistance. In particular, flies with reduced piwi activity in the nervous system under irradiation conditions had a reduced survival rate compared to variants without induction of RNA interference (Figure 2e and Figure 3g).
2.3. Age-Related Changes in the Expression of Argonaute Genes, Retrotransposons, and Stress Response Genes
In flies of the wild-type Canton-S strain, a slight increase in the expression of genes of the Argonaute family (by 1.6–2.4 times, p < 0.05) and a pronounced activation of retrotransposons (by 1.7–12.0 times, p < 0.05) at the age of 10 weeks was observed (Figure 4a–d, Tables S3 and S4). It should be noted that this tendency was repeated separately in the abdomens of Drosophila, and in part, in the heads (but not in the thoraxes) (Figures S1 and S2, Tables S3 and S4).
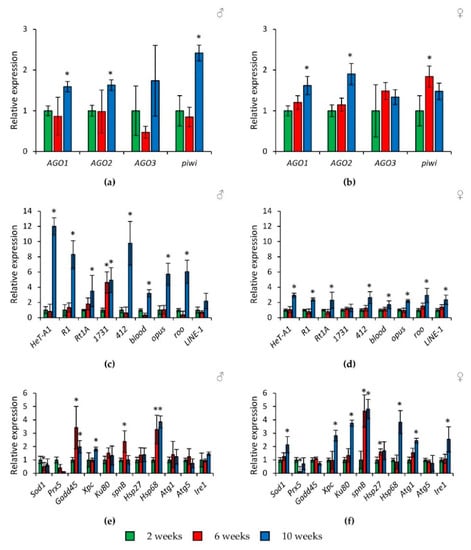
Figure 4.
Age-related changes in the expression of Argonaute genes (a,b), transposable elements (c,d), and stress response genes (e,f) in wild-type Canton-S males (a,c,e) and females (b,d,f). Differences between relative expression levels of the investigated genes at the age of 2 weeks and at the ages of 6 and 10 weeks are statistically significant with * p < 0.05 (Mann-Whitney U-test).
In addition, at the ages of 6 and 10 weeks, flies had increased transcription of some stress response genes both in whole bodies and in individual parts of the body (Figure 4e,f and Figure S3, Tables S3 and S4). In particular, activation (by 1.8–15.2 times, p < 0.05) is shown for genes of response and repair of DNA damages (Gadd45, Xpc, Ku80, spn-B) and proteostasis genes (Hsp27, Hsp68, Atg1, Ire1). At the same time, the activity of the Prx5 gene was decreased.
2.4. Changes in Expression Levels of Retrotransposons Associated with Argonaute Genes’ Knockdown and γ-Irradiation
In Drosophila without the induction of RNA interference of genes of the Argonaute family, γ-irradiation at a dose of 700 Gy caused the activation of retrotransposons (by 1.3–9.3 times, p < 0.05), or did not lead to statistically significant changes (Figure 5 and Figure 6, Tables S5–S8). Exceptions are flies with the genotypes GS-elav > RNAi-AGO2 and GS-elav>RNAi-AGO2, in which radiation exposure suppressed the retrotransposons’ expression in some cases (Figure 5e–h, Table S6).
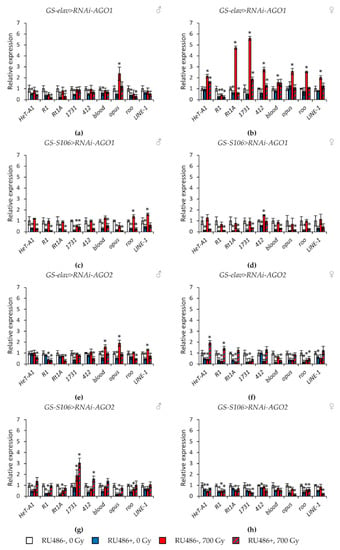
Figure 5.
Changes in the expression of retrotransposons in irradiated and unirradiated males (a,c,e,g) and females (b,d,f,h) with AGO1 (a–d) and AGO2 (e–h) knockdown. Differences between relative expression levels of retrotransposons of unirradiated flies without Argonaute genes’ knockdown (RU486-, 0Gy) and each of other experimental variants are statistically significant with * p < 0.05 (Mann-Whitney U-test).
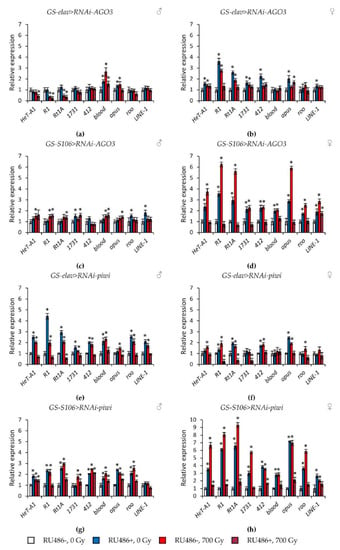
Figure 6.
Changes in the expression of retrotransposons in irradiated and unirradiated males (a,c,e,g) and females (b,d,f,h) with AGO3 (a–d) and piwi (e–h) knockdown. Differences between relative expression levels of retrotransposons of unirradiated flies without PIWI genes’ knockdown (RU486-, 0Gy) and each of other experimental variants are statistically significant with * p < 0.05 (Mann-Whitney U-test).
At the same time, knockdown of genes of the subfamilies Argonaute and PIWI had different effects on the activity of retrotransposons. In flies with knockdown of genes of the Argonaute subfamily (AGO1 and AGO2) in the nervous system and the fat body both under γ-irradiation and without irradiation, the activity of retrotransposons decreased by 1.4–22.1 times (p < 0.05) compared with the variants without induction of RNA interference (Figure 5, Tables S5 and S6). RNA interference of genes of the Piwi subfamily (AGO3 and piwi) significantly increased the activity of retrotransposons (by 1.3–9.3 times, p < 0.05) in unirradiated flies. However, γ-irradiation, on the contrary, reduced the activity of transposable elements in males and females with knockdown of PIWI genes by 1.2–8.2 times (p < 0.05) compared with irradiated flies without induction of RNA interference (Figure 6, Tables S7 and S8).
2.5. Changes in Expression Levels of Stress Response Genes Associated with Argonaute Genes’ Knockdown and γ-Irradiation
Tissue-specific knockdown of the AGO1 gene caused the greatest activation of stress response genes. In particular, the expression of genes of antioxidant defense (Sod1, Prx5), genes of DNA damage response and repair (Gadd45, spn-B), genes of heat shock proteins (Hsp27, Hsp68) was increased by 1.7–14.3 times (p < 0.05) (Figure 7a–d, Table S5). The most pronounced induction of their activity was observed in males with AGO1 RNA interference in the fat body. In addition to these genes, Ku80, Atg1, and Ire1 were also activated in this variant of the experiment (Figure 7c). Similar but less pronounced changes were observed in flies with piwi knockdown in the nervous system and the fat body (Figure 8e–h, Table S8). At the same time, the decreased activity of AGO2 and AGO3 mainly decreased the activity of stress response genes (Figure 7e–h and Figure 8a–d, Tables S6 and S7).
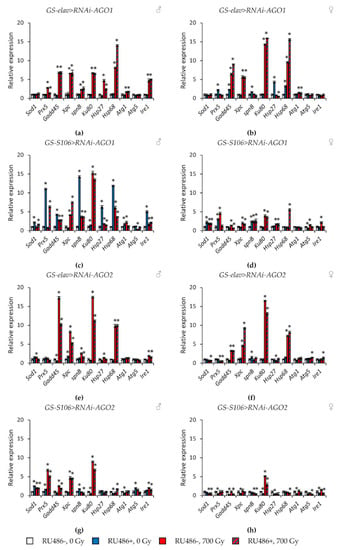
Figure 7.
Changes in the expression of stress response genes in irradiated and unirradiated males (a,c,e,g) and females (b,d,f,h) with AGO1 (a–d) and AGO2 (e–h) knockdown. Differences between relative expression levels of the investigated genes of unirradiated flies without Argonaute genes’ knockdown (RU486-, 0Gy) and each of other experimental variants are statistically significant with * p < 0.05 (Mann-Whitney U-test).
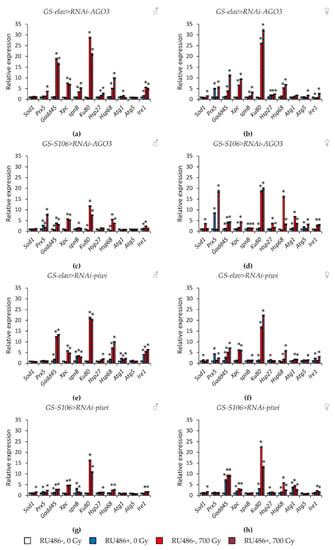
Figure 8.
Changes in the expression of stress response genes in irradiated and unirradiated males (a,c,e,g) and females (b,d,f,h) with AGO3 (a–d) and piwi (e–h) knockdown. Differences between relative expression levels of the investigated genes of unirradiated flies without PIWI genes’ knockdown (RU486-, 0Gy) and each of other experimental variants are statistically significant with * p < 0.05 (Mann-Whitney U-test).
γ-Irradiation led to a significant activation (by 1.4–32.2, p < 0.05) of genes responsible for the response to genotoxic stress (Gadd45, Xpc, Ku80) and proteotoxic stress (Hsp68). This effect was observed both in variants with RNA interference of Argonaute genes and without induction of RNA interference (Figure 7 and Figure 8, Tables S5–S8). Other studied stress response genes were also activated in some variants of the experiment, but to a lesser extent.
3. Discussion
Aging is accompanied by age-related differential changes in the expression of small RNAs, which is closely associated with impaired biogenesis and regulation. Dysregulation of small RNA biogenesis proteins and corresponding changes in the functioning of miRNAs, siRNAs, and piRNAs lead to a global disruption of gene expression and chromatin structure with subsequent negative consequences at the molecular, cellular, tissue, and organismal levels. For example, they include loss of genome integrity and genetic instability, impaired stress response, metabolism, immunity, regenerative abilities, increased inflammatory responses, and others. Such changes significantly deplete the organism’s life support systems, cause age-related disorders and aging [4,44].
During aging, depending on the tissue and physiological state of the organism, both a critical decrease in the expression of small RNA biogenesis proteins and their excessive activation can occur. Predominantly, aging human cell cultures, as well as cells obtained from old donors, are characterized by reduced activity of small RNA biogenesis genes, such as Drosha, Dicer, Exportin 5, and AGO2. Such changes are accompanied by shifts in the expression patterns of miRNAs [45,46,47,48]. Similar data were obtained in studies of age-related changes in various tissues of rodents [47,48,49] and in nematodes [48]. Nevertheless, some data indicate the nonlinear pattern in the dynamics of the activity of genes encoding enzymes of small RNA biogenesis. Thus, in the hearts of rats, AGO1 and AGO2 firstly increase the expression, but at the end of life, they decrease it [50]. In addition, it should be noted that the levels of small RNAs not only depend on the activity of proteins of its biogenesis, but the feedback loop is observed. For example, a miRNA-directed mechanism of age-related changes in the expression of an Argonaute gene has been described using the Caenorhabditis elegans model. In particular, miR-71, which is activated during aging, suppresses alg-1 and limits the lifespan of nematodes [14].
In this work, the analysis of gene expression showed that there is an age-related increase in the expression of the Argonaute family genes in whole Drosophila bodies (total homogenate), in heads and abdomens (but not in thoraxes). At the same time, increased activity was also observed in retrotransposons and particular stress response genes. In other words, despite the fact that aging flies activate mechanisms aimed at the piRNAs, miRNAs, and siRNAs production, which suppress the activity of transposable elements and target mRNAs, we did not observe the corresponding effect.
We assume at least two explanations for the data obtained. First, an increase in the transcriptional activity of the Argonaute genes does not indispensably indicate an increase in the level of the corresponding proteins and their functional activity. Deregulation of their activity may occur at post-transcriptional levels. For example, it was found that a decrease in AGO2 mRNA methylation in human cells during aging takes place, probably leading to deregulation of miRNA expression [45]. Second, an increase in the activity of the Argonaute genes may be a manifestation of a compensatory response to the increasing age-related activity of retrotransposons, disruption of the heterochromatin structure, and cellular stress. This may also be the reason for the activation of stress response genes in old fruit flies. Indeed, the chronic activation of stress-sensitive pathways during aging has been previously described. In several experimental models, the induction of stress response genes was found both in individual organs and throughout the body [51,52,53,54]. In the early stages of aging or in the case of a short period of time after an acute damaging impact, this tendency can provide faster recovery and better survival of an organism. However, the chronic activation and dysregulation of the stress-sensitive pathways during aging causes homeostasis destruction and energy depletion. There is a general decrease in the efficiency of cellular and organismal responses to stressful influences, a decrease in the work of repair systems, an increase in the number of senescent and malfunctioning cells, and other destructive processes [53,55,56,57].
An increase in the activity of retrotransposons (which we observed in the experiment) is both a consequence of age-related deregulation of the mechanisms maintaining the heterochromatin in the condensed state and preserving cellular defense, and the cause of genotoxic stress with the subsequent development of degenerative processes [5,58]. Earlier it has been found that the activity of transposable elements increases in various organs of aging animals. For example, such changes have been shown in the brain [19] and the adipose body of fruit flies [59]. The changes observed are accompanied by an age-related loss of the organs’ functions.
In the present research, we studied the effect of knockdown of the Argonaute genes in various tissues on the Drosophila melanogaster lifespan. Based on the data described above, we assumed two possible consequences of the Argonaute knockdown. Firstly, a decrease in the activity of the Argonaute genes forces up age-related changes in the tissues of flies as a result of small RNA deregulation, and leads to a lifespan shortening. Secondly, suppression of the Argonaute genes partly restores the imbalance in the abundance and functioning of the proteins translated from them and diminishes age-related hyperactivation, at the level of retaining energy resources at least.
We found that decreased activity of genes in the Argonaute family causes changes in the lifespan depending on a gene and the tissue in which a gene was knocked down. In most cases, tissue-specific RNA interference of genes of the Argonaute family either did not have a statistically significant effect, or led to a shortened lifespan, which is consistent with the first hypothesis. It is worth noting that there are few studies where the reduced activity of the Argonaute genes also led to a lifespan reduction in model animals. For example, in Drosophila melanogaster, mutations in the AGO2 gene resulted in a progressive deterioration in the functions of the nervous system and a lifespan decrease [19]. At the same time, piwi mutations lead to opposite effects on the lifespan and health of fruit flies, depending on the allele. Drosophila with a heterozygous piwi2 mutation had a short lifespan, increased sensitivity to starvation, and reduced immunity. In the fat body of flies, the piwi2 mutation caused a decrease in the level of piRNAs, activation of transposable elements, an increase in DNA damages, and a loss of lipid stores [60]. However, the piwic362 mutation led to an increase in lifespan [61]. In our study, RNA interference of the piwi gene in the nervous system and the fat body, as well as knockdown of the AGO1 and AGO3 genes in individual cases, also led to an increase in the lifespan. These data are consistent with the second hypothesis and indicate a critical role in the emerging epigenetic imbalance in the biogenesis mechanisms of miRNAs and piRNAs, but not siRNAs. Activated Argonaute proteins can enhance gene repression (as a response to an increase in the proportion of heterochromatin and activation of transposons), and at the same time, suppress the activity of genes important for survival. Indeed, it has been found that proteins of the Argonaute family in nematodes [18] and piwi in fruit flies [62] affect the DAF-16/FOXO and DAF-2/IGF-1/insulin signaling pathways, which regulate longevity and aging. In our study, knockdown of AGO1 and piwi genes in the nervous system and the fat body caused activation of stress response genes, especially antioxidant defense genes (Sod1, Prx5), genes of DNA damage response and repair (Gadd45, spn-B), and genes encoding heat shock proteins (Hsp27, Hsp68). Several of these genes have been identified previously as pro-longevity genes [33,34,35,42,43]. At the same time, suppression of AGO2 and AGO3 expression mainly reduced the activity of stress response genes.
At the same time, unexpected data were obtained on the effect of RNA interference of Argonaute genes on the activity of retrotransposons. Since the genes of the PIWI subfamily are an important part of the mechanism for controlling the activity of transposons, a disruption of their regulation leads to a surge in the activity of these genetic elements. This effect we observed in flies with tissue-specific knockdown of AGO3 and piwi. Surprisingly, knockdown of genes of the Argonaute subfamily (AGO1 and AGO2), on the contrary, reduced the activity of retrotransposons. The mechanism of siRNAs is also aimed at suppressing the activity of transposable elements; mutations in AGO2 were previously found to increase their expression in the Drosophila brain [19]. Despite the fact that it is not clear how the knockdown of AGO1 and AGO2 reduces the activity of retrotransposons in our experiment, it is obvious that the change in the activity of these genetic elements did not play a key role in the observed effects.
Currently, there are a little data regarding the contribution of the activity of Argonaute genes to age-related changes and lifespan regulation, therefore we can only assume the mechanisms of the lifespan effects of tissue-specific Argonautes’ knockdown. It is known that AGO2 takes over part of the functions of AGO1 in aging fruit flies. Deep sequencing of small RNAs revealed a global increase in miRNAs loaded into AGO2, but not AGO1, with age. This process is mediated by an increase in the level of 2′-O-methylation of miRNAs. Despite the fact that this mechanism is assumed to be associated with age-related events, its violation has even greater negative consequences. Thus, the AGO2 mutation or the disruption of miRNA 2′-O-methylation leads to accelerated neurodegeneration and a reduction in the lifespan of flies [63]. Thus, the age-related activation of AGO2 can be justified in connection with the increasing load on it; therefore, its knockdown caused rather a negative effect on the lifespan. At the same time, AGO1 hyperactivation can enhance the growing imbalance with aging, so we observed the positive effects of its knockdown in some cases.
The piRNAs-PIWI mechanism was initially identified in germline cells. However, the understanding of the functions of piRNAs and proteins of the PIWI subfamily in somatic tissues and their role in regulating lifespan is now expanding [5,64]. For example, the regulation of transposon activity and the functioning of PIWI proteins is important for the maintenance of somatic stem cells and the prevention of aging-related tissue degeneration. Thus, it was shown that piwi is crucial for the suppression of age-related expression of transposons in stem cells of the Drosophila intestine and maintenance of epithelial homeostasis [65]. The piwi activity in the fat body is essential for regulating metabolism and the normal lifespan of flies [60]. A number of studies in rodents have established the role of PIWI proteins and piRNAs in the regeneration of axons of sensory neurons [66] and in the implementation of neuronal functions, for example, memory [67]. It is known that they not only regulate the formation of heterochromatin and break down transposable elements, but can also affect the activity of genes encoding proteins [66,68]. In addition, the activity of genes encoding PIWI proteins affects the fertility of model animals (their defects lead to infertility), determining age-related changes in reproductive abilities and affecting longevity [62,69].
We observed that neuronal knockdown of the piwi gene in males and females, as well as AGO3 in females, increases the lifespan. Recent studies on Drosophila melanogaster have shown that the brain is characterized by genomic heterogeneity, and the mobility of retrotransposons is important for the activity of some parts of the brain. For example, transpositions in αβ neurons of mushroom bodies are important for the implementation of some functions, e.g., for memory. In these neurons, the activity of piRNA biogenesis proteins is reduced [70]. Thus, the elimination of age-related hyperactivation of the piwi and AGO3 genes specifically in the nerve cells could lead to the preservation of the fly’s brain activity and an increase in the lifespan. In addition, piwi knockdown in the fat body of males also increased lifespan. But further research is required to identify possible mechanisms for this effect. Generally, divergency in the lifespan effects of Argonaute genes’ knockdown in different tissues may be associated with significant variations between gene expression profiles and the implementation of inherited information between tissues and cell types, as well as differences in proteome and metabolome composition [71,72,73,74].
Currently, data on the role of small RNA biogenesis proteins in the response of cells and an organism to the action of stress factors, as well as their interaction with proteins and signaling pathways of the stress response, are expanding. Previously, small RNAs called double-strand break-induced RNAs (diRNAs) have been identified. In human cells, they are loaded onto AGO2 (forming diRISC) and are important for triggering the repair of DNA double-strand breaks (mainly homologous recombination) by recruiting repair factors (particularly, Rad51) to target sites [28,29]. There are studies indicating the relationship of the AGO2 protein with isoforms of the transcription factor p53, one of the central regulators of the genotoxic stress response and the anticancer mechanisms. Studies on human cancer cell cultures have shown that p53 interacts (including indirectly through miRNAs) with AGO2 after DNA damage, affecting the biogenesis and activity of specific miRNAs. In turn, they can regulate the activity of p53 targets (such as GADD45A) and determine cellular processes, in particular, cell cycle arrest and apoptosis [75,76]. In addition, there is evidence of Argonaute- and miRNA-dependent mechanisms of regulation of the activity of other DNA damage response proteins, for example, ATM [23] and CDK [24]. It should be noted that proteins of the PIWI subfamily may also be involved in the repair of DNA damage caused by genotoxic agents, in particular, through the regulation of histone acetylation and chromatin relaxation [26].
For the Argonaute proteins’ functioning, they must interact with certain heat shock proteins. In particular, Hsp90 activity is required for the efficient targeting of AGO2 to processing bodies and stress granules, and also affects the production and functional activity of miRNAs and siRNAs [77]. Similarly, the association of the PIWI proteins with chaperones, for example, with the heat shock protein DNAJA1 in planarians, has been shown. Homologs of these proteins also interact in human gastric cancer cells [78]. In addition, the Drosophila organizing protein homolog Hsp70/90 (Hop) interacts with piwi and mediates the maintenance of genome stability in germline cells [79].
As indicated above, in our study, long-lived flies with knockdown of the AGO1 and piwi genes in the nervous system and the fat body were characterized by activation of stress response genes. This effect also indicates the contribution of small RNA biogenesis genes to the stress response.
As a rule, changes in stress resistance, and in particular, radioresistance, corresponds to changes in lifespan. Thus, organisms that are more resistant to the action of negative environmental factors have higher viability and longevity [80]. We compared the survival rate of fruit flies under normal conditions and after acute exposure to γ-radiation. Nevertheless, the obtained data did not always correspond to the described pattern. For example, fruit flies with AGO1 knockdown in the fat body and the nervous system and piwi knockdown in the fat body showed both increased lifespan and radioresistance. However, piwi RNA interference in the nervous system, which had a pro-longevity effect, significantly reduced the survival under irradiation conditions. RNA interference of AGO2 and AGO3 in the fat body of females did not significantly affect the lifespan of females under normal conditions, but increased the survival rate after γ-irradiation.
It should be noted that the relationship between radioresistance and the tissue in which an Argonaute gene was knocked out is more likely than the relationship with a particular gene. In general, flies with neuronal knockdown of the Argonaute genes were sensitive to the action of γ-radiation. It has been established that the brain in Drosophila is a highly sensitive organ to radiation exposure even at lower doses [81,82]. Thus, changes in the expression profile in neural tissues can critically affect radiosensitivity and overall survival. Our studies demonstrate that genes of the Argonaute family are necessary for the stability of the nervous system functioning under stressful conditions. At the same time, females (and to a lesser extent males) with knockdown of the Argonaute genes in the fat body, on the contrary, showed higher resistance to radiation. Previous data do not explain the observed effects. In contrast, piwi mutants exhibit piRNA depletion in the fat body, enhanced transposon mobilization, increased levels of DNA damage, decreased lipid stores, and increased stress sensitivity [60]. Mutations of AGO2 and PIWIL2 in human and rodent cells reduced their survival underexposure to UV light and ionizing radiation, and led to impaired responses to DNA damage [24,25,26]. Similar results were obtained for germ cells in irradiated Caenorhabditis elegans with loss of the alg-2 gene. In this case, increased cell apoptosis associated with MAPK hyperactivation was observed [27]. In addition, studies on non-small cell lung cancer cells indicate no effect of AGO2 gene knockdown on their radiosensitivity [83]. As in the case of differences in lifespan, opposite tissue-specific effects can be, due to significant differences in the profiles of transcriptomes, proteomes, and metabolomes in the tissues. However, pathways that determine the organismal radioresistance have their own specificity.
The data obtained for the activity of transposons and the expression of stress response genes also do not draw conclusions about the mechanisms of the observed effects of tissue-specific Argonaute genes’ knockdown on the radioresistance of Drosophila. γ-Irradiation caused the activation of genes provided the response to genotoxic stress, in particular, its coordination, nucleotide excision repair, and repair of double-strand breaks by non-homologous end joining (Gadd45, Xpc, Ku80), as well as the response to proteotoxic stress (Hsp68). These genes belong to the basic signaling pathways of reaction to acute irradiation, and their activation ensures survival under adverse conditions [80,84]. Indeed, in vivo and in vitro studies established that the activity of DNA damage recognition and repair genes is necessary for the normal reaction of cells and an organism to the action of ionizing radiation, the formation of specific responses [36,37,40,41]. Similarly, genes encoding heat shock proteins are activated under acute radiation and provide an adaptive response to stress [38,39,85]. We also expected changes in the expression of genes of autophagy and ER stress response—since, in studies on rodent and human cell lines, a connection between these mechanisms and the response to radiation has been established [86,87,88,89,90]. However, their changes in our study were small and inconsistent. On the other hand, it is important to note that we observed pronounced changes in the expression of genes of response to genotoxic and proteotoxic stress both in variants with RNA interference of the Argonaute genes and without activation of RNA interference.
It should be noted that in meta-analysis and bioinformatics studies of the radiosensitivity of normal and tumor cells, it was shown that genes of DNA damage response and repair, as well as genes of antioxidant defense, play a key role in the reaction of healthy tissues to the action of ionizing radiation [40]. In our study on the Drosophila model, it was shown that RNA interference of Argonaute genes increases the activity of orthologs of at least some genes important for this reaction (in particular, GADD45A, XPC, XRCC3). At the same time, a normal cellular response is maintained under in vivo irradiation conditions. However, these changes do not always provide benefits for the survival of the whole organism under radiation conditions. Suppression of the activity of some Argonaute genes could be a possible strategy for increasing the stress resistance of healthy tissues of higher organisms, including humans, but additional detailed studies are required to assess this perspective.
Irradiation increased the activity of retrotransposons in experimental variants without activation of RNA interference of the Argonaute genes. Indeed, it is known that damaging environmental factors (including ionizing radiation) can disrupt epigenetic control, and as a consequence, cause the activation of transposable elements. An increase in their activity is characterized by early manifestation and persistence, which makes it possible to use transposons as biomarkers of exposure to environmental stressors [91,92,93]. Surprisingly, irradiated Drosophila with Argonaute genes’ knockdown had lower levels of retrotransposon expression than irradiated animals without knockdown. One of the reasons may be associated with the complex interaction of the different factors involved in transposon activity regulation. As transposition activation factors, radiation-induced disruption of DNA integrity can act, which interacts with radiation-induced blockage of the transcriptional apparatus and epigenetic regulation of retrotransposition process in tissue-specific manner. It seems to be that the final level of transposon activity is determined by the balance of these factors. It should be noted that in this part of the experiment, one biological replication was carried out, and further verification is necessary to confirm the obtained data.
Additionally, it should be noted that we observed a greater sensitivity to irradiation in males than in females. This may be due to the specificity of the epigenome in different sexes. For example, males have more heterochromatic DNA than females, due to the presence of a Y chromosome with a large number of repeats [94]. Consequently, they are more sensitive to changes in the functioning of systems that regulate gene expression and repression of transposable elements.
4. Materials and Methods
4.1. Drosophila Melanogaster Strains and Induction of Argonaute Genes’ Knockdown
The wild-type Canton-S strain was used to assess age-related changes in gene expression.
In experiments to study the influence of tissue-specific knockdown of genes of the Argonaute family (AGO1, AGO2, AGO3, piwi) on the lifespan and the effects of γ-irradiation, the tested flies were obtained based on the GAL4/UAS system [95,96,97]. We used strains carrying double-stranded RNA (dsRNA) for RNA interference of these genes under the control of the UAS promoter (RNAi-AGO1, RNAi-AGO2, RNAi-AGO3, RNAi-piwi, respectively) and strains expressing the conditional (mifepristone-inducible) driver GAL4-GeneSwitch in specific tissues (GS-elav-in the nervous system, GS-S106-in the fat body, GS-TIGS-2-in the digestive system, GS-Mhc-in the muscles) (see Table S9).
The use of GAL4-GeneSwitch controls the expression level of a studied gene, the stage of development (imago), and the age of flies, at which the expression is induced, as well as the localization of suppression of the studied genes (ubiquitous or tissue-specific). First, the choice of tissue-specific expression was associated with the topicality of studying the role of Argonaute genes in various tissues in regulating lifespan and aging. Second, ubiquitous RNA interference in itself reduces the lifespan of Drosophila, while tissue-specific (including the drivers that were used in our study) does not have a negative effect on the lifespan [98]. In addition, the use of conditional GAL4 excludes the influence on the lifespan of an unequal genetic background in experimental and control animals.
To obtain experimental flies of each of the genotypes, virgin females of a line with UAS-construction and males of a line with GAL4-GeneSwitch were crossed. In males and virgin females obtained by crossing, an Argonaute gene knockdown was induced by mifepristone (RU486, Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 3.2 mg/mL in ethanol, which was dripped onto a nutrient medium at 30 μL [99]. Control variants were obtained by the same crosses, but were kept in the medium without mifepristone. The decrease in the activity of Argonaute genes was verified by using RT-PCR analysis (Figure S4).
4.2. Lifespan Assay
Flies were kept at 25 C, 12:12 day-night regimen in climate chamber Binder KBF720-ICH (Binder, Tuttlingen, Germany) on nutrient medium (gram per 1 L): agar agar-5.2, dry yeast-32.1, glucose-136.9, yellow cornmeal-92.0 [100]. To prevent simple fungus and bacteria growth, a 10% solution of methyl 4-hydroxybenzoate (Sigma-Aldrich, St. Louis, MO, USA) and a 50% solution of propionic acid (Sigma-Aldrich, St. Louis, MO, USA) were added.
To silence the target genes, females expressing dsRNA under control of UAS sequences were crossed with GAL4 driver males. The F1 males and virgin females were used. Experimental flies were sorted by sex using CO2 anesthesia and were kept separately, 30 animals per Drosophila vial (Genesee Scientific, San Diego, CA, USA) with 5 mL of nutrient medium (see above) and 30 μL mifepristone solution, which was applied to the surface of the nutrient medium [99]. Control F1 flies were maintained on medium without mifepristone (with 30 μL ethanol).
Flies were transferred to fresh medium without anesthesia twice a week. The number of dead flies was counted daily. Further lifespan parameters (particularly the mean and median lifespan, the age of 90% mortality, the mortality rate doubling time (MRDT)) were calculated. Experiments were done in one-two independent biological replicates (two replicates were used for flies with RNA interference of Argonaute genes in the nervous system and the fat body to confirm the positive lifespan effects).
Statistical analysis was carried out using nonparametric criteria. The survival curves were shaped using a Kaplan–Meier procedure. The comparative analysis of the shape of survival curves was made using the Kolmogorov–Smirnov test [101]. Both the Mantel–Cox [102] and Gehan–Breslow–Wilcoxon tests [103] were used to estimate the statistical differences in the median lifespan. A Wang–Allison test was used to estimate differences in the age of 90% mortality [104]. The statistical analyses of the data were carried out using STATISTICA software, version 6.1 (StatSoft, Tulsa, OK, USA) and R, version 2.15.1 (The R Foundation, Indianapolis, IN, USA).
4.3. Irradiation Conditions
Experimental and control flies were obtained and cultivated in the same manner as for the lifespan assay. At the age of 14 days, experimental flies were irradiated using a Cs-137 γ-source “Issledovatel” (Russia) with a dose rate of 0.74 Gy/min. The radiosensitivity of adult flies to acute γ-irradiation was measured in the preliminary tests. A number of previous experimental data demonstrated that Drosophila adult imagoes are highly resistant to ionizing radiation [39,105,106]. For example, fifty percent lethality 1 h postirradiation has been shown to be approximately 1228 Gy for adult males and 1250 Gy for adult females [106].
To determine the dose of γ-irradiation useful for the current study, male imagoes from the control groups (without induction of RNA interference) were irradiated at a dose of 200–1000 Gy with increments of 200 Gy (Figure S5). According to the survival rate measurements, the radiation dose of 700 Gy (dose between 600 Gy and 800 Gy) was selected for the following experiments. This dose can significantly reduce survival without an acute lethality effect. After the exposure to γ-radiation, experimental and control flies were kept under standard conditions on the medium without mifepristone. Next, their survival was assessed. Statistical analysis was similar to the lifespan assay.
To avoid possible small differences in the accumulated dose, flies of each of the studied variants were placed in vials with 30 individuals. A total of four vials per experimental variant were used, each of which can be considered as a biological replicate. Replicates’ data were statistically processed together.
4.4. Real-Time RT-PCR
The gene expression analyses were carried out using whole Drosophila bodies or their parts (heads, thoraxes, or abdomens). In the case of whole flies, 10 males or 10 females were prepared per variant of the experiment. In other cases, 30 males or 30 females were partitioned into heads, thoraxes, or abdomens, places to separate tubes, and used for further procedures.
RNA was isolated by Aurum Total RNA mini kit (Bio-Rad, Hercules, CA, USA). To determine total RNA concentration was used Quant-iT RNA Assay Kit (Life Technologies, Eugene, OR, USA). Reverse transcription was performed using the iScript cDNA Synthesis Kit (Bio-Rad, USA). The mix for RT-PCR was prepared by iTaq Universal SYBR Green Supermix (Bio-Rad, USA) with primers listed in Table S10. The reaction was carried out on the CFX96 Real-Time PCR Detection System (Bio-Rad, USA) using the following parameters: One cycle of 95 °C for 30 s; 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Expression levels of target genes were calculated relative to the expression of reference genes (β-Tubulin, RpL32, EF1α) using the CFX Manager 3.1 software (Bio-Rad, USA) by the 2−∆∆Ct method [107]. The ∆∆Ct value was calculated as ∆Ct (Experimental sample) − ∆Ct (Control sample), and each value of ∆Ct = Ct (Target gene) − Ct (Reference genes), where Ct—cycle thresholds. Experiments were carried out in two independent biological replicates, with three technical replicates in each.
RNA and cDNA samples were prepared using the equipment of the Molecular Biology Core Facility (IB FRC Komi SC UB RAS, Syktyvkar, Russia).
5. Conclusions
For the first time, we investigated the role of genes of the Argonaute family in regulating lifespan and radioresistance at the level of a whole complex organism using the in vivo model of Drosophila melanogaster. We found that a tissue-specific decrease in the activity of genes of the Argonaute family causes changes in lifespan and resistance to γ-irradiation at a dose of 700 Gy, depending on the gene and tissue in which a gene knockdown was triggered. In most cases, these parameters were reduced or did not change significantly in flies with tissue-specific RNA interference. Surprisingly, piwi knockdown in both the fat body and the nervous system, as well as AGO1 and AGO3 RNA interference in some cases caused a lifespan increase. Such positive changes were associated with increased expression of some stress response genes, but apparently, did not depend on the activity of transposons. At the same time, changes in radioresistance depended on the tissue in which the gene was knocked out. Thus, neuronal RNA interference of the Argonaute genes predominantly reduced the survival of irradiated flies, while RNA interference in the fat body increased the radioresistance of females.
The mechanism of epigenetic control using small RNAs is highly evolutionary conserved and persists through animal phylogeny [13]. Accordingly, in vivo studies in animal models (such as fruit flies or nematodes) suggest the function of small RNA orthologs, as well as proteins of their biogenesis in other animals, including humans. At the same time, epigenetic mechanisms are highly susceptible to external stimuli and affect a wide range of cellular processes, and small RNA biogenesis genes and proteins can be targets for potential geroprotectors and drugs in age-related diseases [1,2]. Indeed, it was found that the dysregulation of their activity is associated with the development of a number of age-related diseases, including cancer, inflammatory, neurodegenerative, cardiovascular, metabolic, and immune disorders [108,109,110,111,112,113,114,115,116]. We have found that suppression of some genes of the Argonaute family can prolong the life of fruit flies or enhance their radioresistance. This indicates the potential for their use as targets for geroprotective or radioprotective interventions (for example, using selective pharmacological drugs). However, a detailed study of the molecular mechanisms associated with the observed effects and possible negative consequences affecting quality of life is required.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/22/5/2396/s1. Figure S1: Age-related changes in the expression of Argonaute genes in heads, thoraxes, abdomens of wild-type Canton-S males and females. Figure S2: Age-related changes in the expression of transposable elements in heads, thoraxes, abdomens of wild-type Canton-S males and females. Figure S3: Age-related changes in the expression of stress response genes in heads, thoraxes, abdominals of wild-type Canton-S males and females. Figure S4: Knockdown of AGO1, AGO2, AGO3, and piwi in investigated flies. Figure S5: Effects of acute γ-irradiation on the survival of Drosophila male imago from the controls for RNAi of AGO1, AGO2, AGO3, piwi in the fat body and nervous system. Table S1: Lifespan parameters of flies with tissue-specific knockdown of the Argonaute genes. Table S2: Survival of flies with tissue-specific knockdown of the Argonaute genes in the condition of γ-irradiation. Table S3: Age-related changes of gene expression in different parts of wild-type Canton-S males. Table S4: Age-related changes of gene expression in different parts of wild-type Canton-S females. Table S5: Mean relative gene expression of flies with tissue-specific AGO1 knockdown in the condition of γ-irradiation. Table S6: Mean relative gene expression of flies with tissue-specific AGO2 knockdown in the condition of γ-irradiation. Table S7: Mean relative gene expression of flies with tissue-specific AGO3 knockdown in the condition of γ-irradiation. Table S8: Mean relative gene expression of flies with tissue-specific piwi knockdown in the condition of γ-irradiation. Table S9: Drosophila melanogaster strains. Table S10: Primers for real-time PCR.
Author Contributions
Conceptualization, E.P., E.Y., M.S., A.M.; investigation, E.P., E.Y., L.K., N.Z., E.S., I.S., D.Y., N.P., N.U.; statistical analysis, E.P., E.Y., M.S.; data curation, E.P., E.Y.; writing—original draft preparation, E.P., E.Y., L.K., M.S.; writing—review and editing, E.P., E.Y., M.S., A.M.; visualization, E.P., E.Y., M.S.; supervision, A.M.; funding acquisition, EP. All authors have read and agreed to the published version of the manuscript.
Funding
E.P.: L.K.: N.Z., E.S., I.S., D.Y. carried out the work within the PRF grant № MK-1229.2019.4 “The role of genes of small RNA biogenesis and regulation in the lifespan control and aging of Drosophila melanogaster”. E.Y., N.P., N.U., M.S., A.M. carried out the work within the framework of the state task on the theme “Molecular-genetic mechanisms of aging, lifespan, and stress resistance of Drosophila melanogaster”, state registration № AAAA-A18-118011120004-5.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Benayoun, B.A.; Pollina, E.A.; Brunet, A. Epigenetic regulation of ageing: Linking environmental inputs to genomic stability. Nat. Rev. Mol. Cell. Biol. 2015, 16, 593–610. [Google Scholar] [CrossRef]
- Yu, G.; Wu, Q.; Gao, Y.; Chen, M.; Yang, M. The Epigenetics of Aging in Invertebrates. Int. J. Mol. Sci. 2019, 20, 4535. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Kane, A.E.; Sinclair, D.A. Epigenetic changes during aging and their reprogramming potential. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 61–83. [Google Scholar] [CrossRef]
- Sturm, Á.; Ivics, Z.; Vellai, T. The mechanism of ageing: Primary role of transposable elements in genome disintegration. Cell Mol. Life Sci. 2015, 72, 1839–1847. [Google Scholar] [CrossRef]
- Pasyukova, E.G.; Vaiserman, A.M. HDAC inhibitors: A new promising drug class in anti-aging research. Mech. Ageing Dev. 2017, 166, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Avrahami, D.; Kaestner, K.H. The dynamic methylome of islets in health and disease. Mol. Metab. 2019, 27s, S25–S32. [Google Scholar] [CrossRef] [PubMed]
- Iatsenko, I.; Sinha, A.; Rödelsperger, C.; Sommer, R.J. New role for DCR-1/dicer in Caenorhabditis elegans innate immunity against the highly virulent bacterium Bacillus thuringiensis DB27. Infect. Immun. 2013, 81, 3942–3957. [Google Scholar] [CrossRef]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef]
- Okamura, K.; Lai, E.C. Endogenous small interfering RNAs in animals. Nat. Rev. Mol. Cell Biol. 2008, 9, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Tsurumi, A.; Li, W. Aging mechanisms—A perspective mostly from Drosophila. Genet. Genom. Next 2020. [Google Scholar] [CrossRef]
- Lai, R.W.; Lu, R.; Danthi, P.S.; Bravo, J.I.; Goumba, A.; Sampathkumar, N.K.; Benayoun, B.A. Multi-level remodeling of transcriptional landscapes in aging and longevity. BMB Rep. 2019, 52, 86–108. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V.; Ruvkun, G. Recent Molecular Genetic Explorations of Caenorhabditis elegans MicroRNAs. Genetics 2018, 209, 651–673. [Google Scholar] [CrossRef]
- Inukai, S.; Pincus, Z.; de Lencastre, A.; Slack, F.J. A microRNA feedback loop regulates global microRNA abundance during aging. RNA 2018, 24, 159–172. [Google Scholar] [CrossRef]
- Kato, M.; Chen, X.; Inukai, S.; Zhao, H.; Slack, F.J. Age-associated changes in expression of small, noncoding RNAs, including microRNAs, in C. elegans. RNA 2011, 17, 1804–1820. [Google Scholar] [CrossRef] [PubMed]
- Gerasymchuk, M.; Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. The Role of microRNAs in Organismal and Skin Aging. Int. J. Mol. Sci. 2020, 21, 5281. [Google Scholar] [CrossRef] [PubMed]
- Meister, G. Argonaute proteins: Functional insights and emerging roles. Nat. Rev. Genet. 2013, 14, 447–459. [Google Scholar] [CrossRef]
- Aalto, A.P.; Nicastro, I.A.; Broughton, J.P.; Chipman, L.B.; Schreiner, W.P.; Chen, J.S.; Pasquinelli, A.E. Opposing roles of microRNA Argonautes during Caenorhabditis elegans aging. PLoS Genet. 2018, 14, e1007379. [Google Scholar] [CrossRef]
- Li, W.; Prazak, L.; Chatterjee, N.; Grüninger, S.; Krug, L.; Theodorou, D.; Dubnau, J. Activation of transposable elements during aging and neuronal decline in Drosophila. Nat. Neurosci. 2013, 16, 529–531. [Google Scholar] [CrossRef]
- Kogure, A.; Uno, M.; Ikeda, T.; Nishida, E. The microRNA machinery regulates fasting-induced changes in gene expression and longevity in Caenorhabditis elegans. J. Biol. Chem. 2017, 292, 11300–11309. [Google Scholar] [CrossRef]
- Wan, G.; Liu, Y.; Han, C.; Zhang, X.; Lu, X. Noncoding RNAs in DNA repair and genome integrity. Antioxid. Redox Signal. 2014, 20, 655–677. [Google Scholar] [CrossRef]
- Yang, Y.G.; Qi, Y. RNA-directed repair of DNA double-strand breaks. DNA Repair 2015, 32, 82–85. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, G.; Berger, F.G.; He, X.; Lu, X. The ATM kinase induces microRNA biogenesis in the DNA damage response. Mol. Cell 2011, 41, 371–383. [Google Scholar] [CrossRef]
- Pothof, J.; Verkaik, N.S.; van, I.W.; Wiemer, E.A.; Ta, V.T.; van der Horst, G.T.; Jaspers, N.G.; van Gent, D.C.; Hoeijmakers, J.H.; Persengiev, S.P. MicroRNA-mediated gene silencing modulates the UV-induced DNA-damage response. Embo J. 2009, 28, 2090–2099. [Google Scholar] [CrossRef]
- Kraemer, A.; Anastasov, N.; Angermeier, M.; Winkler, K.; Atkinson, M.J.; Moertl, S. MicroRNA-mediated processes are essential for the cellular radiation response. Radiat. Res. 2011, 176, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.T.; Wang, Q.; Chen, L.; Liu, M.Y.; Han, C.; Yan, Q.; Shen, R.; He, G.; Duan, W.; Li, J.J.; et al. Germline stem cell gene PIWIL2 mediates DNA repair through relaxation of chromatin. PLoS ONE 2011, 6, e27154. [Google Scholar] [CrossRef]
- Doll, M.A.; Soltanmohammadi, N.; Schumacher, B. ALG-2/AGO-Dependent mir-35 Family Regulates DNA Damage-Induced Apoptosis Through MPK-1/ERK MAPK Signaling Downstream of the Core Apoptotic Machinery in Caenorhabditis elegans. Genetics 2019, 213, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ba, Z.; Gao, M.; Wu, Y.; Ma, Y.; Amiard, S.; White, C.I.; Rendtlew Danielsen, J.M.; Yang, Y.G.; Qi, Y. A role for small RNAs in DNA double-strand break repair. Cell 2012, 149, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wei, W.; Li, M.M.; Wu, Y.S.; Ba, Z.; Jin, K.X.; Li, M.M.; Liao, Y.Q.; Adhikari, S.; Chong, Z.; et al. Ago2 facilitates Rad51 recruitment and DNA double-strand break repair by homologous recombination. Cell Res. 2014, 24, 532–541. [Google Scholar] [CrossRef]
- Rubio, M.; Maestro, J.L.; Piulachs, M.D.; Belles, X. Conserved association of Argonaute 1 and 2 proteins with miRNA and siRNA pathways throughout insect evolution, from cockroaches to flies. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, H.; Siomi, M.C. PIWI-Interacting RNA in Drosophila: Biogenesis, Transposon Regulation, and Beyond. Chem. Rev. 2018, 118, 4404–4421. [Google Scholar] [CrossRef]
- Piper, M.D.W.; Partridge, L. Drosophila as a model for ageing. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2707–2717. [Google Scholar] [CrossRef]
- Petrosyan, A.; Hsieh, I.H.; Phillips, J.P.; Saberi, K. Enhanced tethered-flight duration and locomotor activity by overexpression of the human gene SOD1 in Drosophila motorneurons. Genet. Mol. Biol. 2015, 38, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Radyuk, S.N.; Michalak, K.; Klichko, V.I.; Benes, J.; Rebrin, I.; Sohal, R.S.; Orr, W.C. Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila. Biochem. J. 2009, 419, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Plyusnina, E.N.; Shaposhnikov, M.V.; Moskalev, A.A. Increase of Drosophila melanogaster lifespan due to D-GADD45 overexpression in the nervous system. Biogerontology 2011, 12, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.A.; Plyusnina, E.N.; Shaposhnikov, M.V. Radiation hormesis and radioadaptive response in Drosophila melanogaster flies with different genetic backgrounds: The role of cellular stress-resistance mechanisms. Biogerontology 2011, 12, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.; Plyusnina, E.; Shaposhnikov, M.; Shilova, L.; Kazachenok, A.; Zhavoronkov, A. The role of D-GADD45 in oxidative, thermal and genotoxic stress resistance. Cell Cycle 2012, 11, 4222–4241. [Google Scholar] [CrossRef] [PubMed]
- Landis, G.; Shen, J.; Tower, J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging 2012, 4, 768–789. [Google Scholar] [CrossRef]
- Moskalev, A.; Zhikrivetskaya, S.; Krasnov, G.; Shaposhnikov, M.; Proshkina, E.; Borisoglebsky, D.; Danilov, A.; Peregudova, D.; Sharapova, I.; Dobrovolskaya, E.; et al. A comparison of the transcriptome of Drosophila melanogaster in response to entomopathogenic fungus, ionizing radiation, starvation and cold shock. BMC Genom. 2015, 16, S8. [Google Scholar] [CrossRef]
- Pavlopoulou, A.; Bagos, P.G.; Koutsandrea, V.; Georgakilas, A.G. Molecular determinants of radiosensitivity in normal and tumor tissue: A bioinformatic approach. Cancer Lett. 2017, 403, 37–47. [Google Scholar] [CrossRef]
- Koval, L.; Proshkina, E.; Shaposhnikov, M.; Moskalev, A. The role of DNA repair genes in radiation-induced adaptive response in Drosophila melanogaster is differential and conditional. Biogerontology 2020, 21, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Kazemi-Esfarjani, P.; Benzer, S. Multiple-stress analysis for isolation of Drosophila longevity genes. Proc. Natl. Acad. Sci. USA 2004, 101, 12610–12615. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.C.; Lin, H.Y.; Yuh, C.H.; Yu, L.K.; Wang, H.D. The effect of neuronal expression of heat shock proteins 26 and 27 on lifespan, neurodegeneration, and apoptosis in Drosophila. Biochem. Biophys. Res. Commun. 2008, 376, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, E.; Solovev, I.; Koval, L.; Moskalev, A. The critical impacts of small RNA biogenesis proteins on aging, longevity and age-related diseases. Ageing Res. Rev. 2020, 62, 101087. [Google Scholar] [CrossRef]
- Min, K.-W.; Zealy, R.W.; Davila, S.; Fomin, M.; Cummings, J.C.; Makowsky, D.; McDowell, C.H.; Thigpen, H.; Hafner, M.; Kwon, S.-H.; et al. Profiling of m6A RNA modifications identified an age-associated regulation of AGO2 mRNA stability. Aging Cell 2018, 17, e12753. [Google Scholar] [CrossRef] [PubMed]
- Dellago, H.; Preschitz-Kammerhofer, B.; Terlecki-Zaniewicz, L.; Schreiner, C.; Fortschegger, K.; Chang, M.W.; Hackl, M.; Monteforte, R.; Kühnel, H.; Schosserer, M.; et al. High levels of oncomiR-21 contribute to the senescence-induced growth arrest in normal human cells and its knock-down increases the replicative lifespan. Aging Cell 2013, 12, 446–458. [Google Scholar] [CrossRef]
- Nidadavolu, L.S.; Niedernhofer, L.J.; Khan, S.A. Identification of microRNAs dysregulated in cellular senescence driven by endogenous genotoxic stress. Aging 2013, 5, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.A.; Raghavan, P.; Thomou, T.; Boucher, J.; Robida-Stubbs, S.; Macotela, Y.; Russell, S.J.; Kirkland, J.L.; Blackwell, T.K.; Kahn, C.R. Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab. 2012, 16, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P.; Konovalova, J.; Najam, S.S.; Alter, H.; Piepponen, T.P.; Erfle, H.; Sonntag, K.C.; Schütz, G.; Vinnikov, I.A.; Domanskyi, A. Dicer and microRNAs protect adult dopamine neurons. Cell Death Dis. 2017, 8, e2813. [Google Scholar] [CrossRef]
- Zhang, X.; Azhar, G.; Wei, J.Y. The expression of microRNA and microRNA clusters in the aging heart. PLoS ONE 2012, 7, e34688. [Google Scholar] [CrossRef]
- Bishop, N.A.; Lu, T.; Yankner, B.A. Neural mechanisms of ageing and cognitive decline. Nature 2010, 464, 529–535. [Google Scholar] [CrossRef]
- Yankner, B.A.; Lu, T.; Loerch, P. The aging brain. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Yankner, B.A. The aging stress response. Mol. Cell 2010, 40, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Shimizu, H.; Nagao, T.; Ueda, R.; Ishii, S. ATF-2 regulates fat metabolism in Drosophila. Mol. Biol. Cell 2007, 18, 1519–1529. [Google Scholar] [CrossRef]
- Tomaru, U.; Takahashi, S.; Ishizu, A.; Miyatake, Y.; Gohda, A.; Suzuki, S.; Ono, A.; Ohara, J.; Baba, T.; Murata, S. Decreased proteasomal activity causes age-related phenotypes and promotes the development of metabolic abnormalities. Am. J. Pathol. 2012, 180, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, L.C.D.; Wong, S.; Carney, C.; Shen, B.; Tower, J.; Davies, K.J.A. The age- and sex-specific decline of the 20s proteasome and the Nrf2/CncC signal transduction pathway in adaption and resistance to oxidative stress in Drosophila melanogaster. Aging 2017, 9, 1153–1185. [Google Scholar] [CrossRef]
- Sas, K.; Szabo, E.; Vecsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef]
- Lenart, P.; Novak, J.; Bienertova-Vasku, J. PIWI-piRNA pathway: Setting the pace of aging by reducing DNA damage. Mech. Ageing Dev. 2018, 173, 29–38. [Google Scholar] [CrossRef]
- Wood, J.G.; Jones, B.C.; Jiang, N.; Chang, C.; Hosier, S.; Wickremesinghe, P.; Garcia, M.; Hartnett, D.A.; Burhenn, L.; Neretti, N.; et al. Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila. Proc. Natl. Acad. Sci. USA 2016, 113, 11277–11282. [Google Scholar] [CrossRef]
- Jones, B.C.; Wood, J.G.; Chang, C.; Tam, A.D.; Franklin, M.J.; Siegel, E.R.; Helfand, S.L. A somatic piRNA pathway in the Drosophila fat body ensures metabolic homeostasis and normal lifespan. Nat. Commun. 2016, 7, 13856. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, H.; Cai, Y.; Wang, H.; Niu, K.; Wu, X.; Ma, H.; Yang, Y.; Tong, W.; Liu, F.; et al. Epigenetic drift of H3K27me3 in aging links glycolysis to healthy longevity in Drosophila. Elife 2018, 7. [Google Scholar] [CrossRef]
- Simon, M.; Sarkies, P.; Ikegami, K.; Doebley, A.L.; Goldstein, L.D.; Mitchell, J.; Sakaguchi, A.; Miska, E.A.; Ahmed, S. Reduced insulin/IGF-1 signaling restores germ cell immortality to Caenorhabditis elegans Piwi mutants. Cell Rep. 2014, 7, 762–773. [Google Scholar] [CrossRef]
- Abe, M.; Naqvi, A.; Hendriks, G.J.; Feltzin, V.; Zhu, Y.; Grigoriev, A.; Bonini, N.M. Impact of age-associated increase in 2′-O-methylation of miRNAs on aging and neurodegeneration in Drosophila. Genes Dev. 2014, 28, 44–57. [Google Scholar] [CrossRef]
- Lin, K.Y.; Wang, W.D.; Lin, C.H.; Rastegari, E.; Su, Y.H.; Chang, Y.T.; Liao, Y.F.; Chang, Y.C.; Pi, H.; Yu, B.Y.; et al. Piwi reduction in the aged niche eliminates germline stem cells via Toll-GSK3 signaling. Nat. Commun. 2020, 11, 3147. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; Ayyaz, A.; Hayashi, R.; Qi, Y.; Madden, D.T.; Lunyak, V.V.; Jasper, H. Piwi Is Required to Limit Exhaustion of Aging Somatic Stem Cells. Cell Rep. 2017, 20, 2527–2537. [Google Scholar] [CrossRef]
- Phay, M.; Kim, H.H.; Yoo, S. Analysis of piRNA-Like Small Non-coding RNAs Present in Axons of Adult Sensory Neurons. Mol. Neurobiol. 2018, 55, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Rajasethupathy, P.; Antonov, I.; Sheridan, R.; Frey, S.; Sander, C.; Tuschl, T.; Kandel, E.R. A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell 2012, 149, 693–707. [Google Scholar] [CrossRef]
- Praher, D.; Zimmermann, B.; Genikhovich, G.; Columbus-Shenkar, Y.; Modepalli, V.; Aharoni, R.; Moran, Y.; Technau, U. Characterization of the piRNA pathway during development of the sea anemone Nematostella vectensis. RNA Biol. 2017, 14, 1727–1741. [Google Scholar] [CrossRef]
- Théron, E.; Maupetit-Mehouas, S.; Pouchin, P.; Baudet, L.; Brasset, E.; Vaury, C. The interplay between the Argonaute proteins Piwi and Aub within Drosophila germarium is critical for oogenesis, piRNA biogenesis and TE silencing. Nucleic Acids Res. 2018, 46, 10052–10065. [Google Scholar] [CrossRef]
- Perrat, P.N.; DasGupta, S.; Wang, J.; Theurkauf, W.; Weng, Z.; Rosbash, M.; Waddell, S. Transposition-driven genomic heterogeneity in the Drosophila brain. Science 2013, 340, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Murali, T.; Pacifico, S.; Finley, R.L., Jr. Integrating the interactome and the transcriptome of Drosophila. BMC Bioinform. 2014, 15, 177. [Google Scholar] [CrossRef]
- Chintapalli, V.R.; Al Bratty, M.; Korzekwa, D.; Watson, D.G.; Dow, J.A. Mapping an atlas of tissue-specific Drosophila melanogaster metabolomes by high resolution mass spectrometry. PLoS ONE 2013, 8, e78066. [Google Scholar] [CrossRef]
- Gibilisco, L.; Zhou, Q.; Mahajan, S.; Bachtrog, D. Alternative Splicing within and between Drosophila Species, Sexes, Tissues, and Developmental Stages. PLoS Genet. 2016, 12, e1006464. [Google Scholar] [CrossRef]
- Moskalev, A.; Guvatova, Z.; Shaposhnikov, M.; Lashmanova, E.; Proshkina, E.; Koval, L.; Zhavoronkov, A.; Krasnov, G.; Kudryavtseva, A. The Neuronal Overexpression of Gclc in Drosophila melanogaster Induces Life Extension With Longevity-Associated Transcriptomic Changes in the Thorax. Front. Genet. 2019, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Krell, J.; Stebbing, J.; Carissimi, C.; Dabrowska, A.F.; de Giorgio, A.; Frampton, A.E.; Harding, V.; Fulci, V.; Macino, G.; Colombo, T.; et al. TP53 regulates miRNA association with AGO2 to remodel the miRNA-mRNA interaction network. Genome Res. 2016, 26, 331–341. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.; Li, S.; Xia, W.; Su, X.; Qin, X.; Chen, Y.; Ding, H.; Li, H.; Huang, A.; et al. An alternative microRNA-mediated post-transcriptional regulation of GADD45A by p53 in human non-small-cell lung cancer cells. Sci. Rep. 2017, 7, 7153. [Google Scholar] [CrossRef] [PubMed]
- Pare, J.M.; Tahbaz, N.; López-Orozco, J.; LaPointe, P.; Lasko, P.; Hobman, T.C. Hsp90 regulates the function of argonaute 2 and its recruitment to stress granules and P-bodies. Mol. Biol. Cell 2009, 20, 3273–3284. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Z.Z.; Guo, F.H.; Shi, S.; Han, X.S.; Zeng, A.; Lin, H.; Jing, Q. Heat shock protein DNAJA1 stabilizes PIWI proteins to support regeneration and homeostasis of planarian Schmidtea mediterranea. J. Biol. Chem. 2019, 294, 9873–9887. [Google Scholar] [CrossRef]
- Karam, J.A.; Parikh, R.Y.; Nayak, D.; Rosenkranz, D.; Gangaraju, V.K. Co-chaperone Hsp70/Hsp90-organizing protein (Hop) is required for transposon silencing and Piwi-interacting RNA (piRNA) biogenesis. J. Biol. Chem. 2017, 292, 6039–6046. [Google Scholar] [CrossRef] [PubMed]
- Cortese, F.; Klokov, D.; Osipov, A.; Stefaniak, J.; Moskalev, A.; Schastnaya, J.; Cantor, C.; Aliper, A.; Mamoshina, P.; Ushakov, I.; et al. Vive la radiorésistance!: Converging research in radiobiology and biogerontology to enhance human radioresistance for deep space exploration and colonization. Oncotarget 2018, 9, 14692–14722. [Google Scholar] [CrossRef]
- Sudmeier, L.J.; Howard, S.P.; Ganetzky, B. A Drosophila model to investigate the neurotoxic side effects of radiation exposure. Dis. Models Mech. 2015, 8, 669–677. [Google Scholar] [CrossRef]
- Wagle, R.; Song, Y.H. Ionizing radiation reduces larval brain size by inducing premature differentiation of Drosophila neural stem cells. Biochem. Biophys. Res. Commun. 2020, 523, 555–560. [Google Scholar] [CrossRef]
- Surova, O.; Akbar, N.S.; Zhivotovsky, B. Knock-down of core proteins regulating microRNA biogenesis has no effect on sensitivity of lung cancer cells to ionizing radiation. PLoS ONE 2012, 7, e33134. [Google Scholar] [CrossRef]
- Khalifa, J.; François, S.; Rancoule, C.; Riccobono, D.; Magné, N.; Drouet, M.; Chargari, C. Gene therapy and cell therapy for the management of radiation damages to healthy tissues: Rationale and early results. Cancer/Radiothérapie 2019, 23, 449–465. [Google Scholar] [CrossRef]
- Moskalev, A.; Shaposhnikov, M.; Turysheva, E. Life span alteration after irradiation in Drosophila melanogaster strains with mutations of Hsf and Hsps. Biogerontology 2009, 10, 3–11. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, Y.J.; Kang, S.; Lim, Y.B. Ionizing radiation activates PERK/eIF2α/ATF4 signaling via ER stress-independent pathway in human vascular endothelial cells. Int. J. Radiat. Biol. 2014, 90, 306–312. [Google Scholar] [CrossRef]
- Chatterjee, J.; Nairy, R.K.; Langhnoja, J.; Tripathi, A.; Patil, R.K.; Pillai, P.P.; Mustak, M.S. ER stress and genomic instability induced by gamma radiation in mice primary cultured glial cells. Metab. Brain Dis. 2018, 33, 855–868. [Google Scholar] [CrossRef]
- Chaurasia, M.; Gupta, S.; Das, A.; Dwarakanath, B.S.; Simonsen, A.; Sharma, K. Radiation induces EIF2AK3/PERK and ERN1/IRE1 mediated pro-survival autophagy. Autophagy 2019, 15, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, W.; Ma, J.; Lu, J.; Yang, X.; Wang, J.; Cao, J.; Tian, Y.; Yang, H.; Zhang, L. The role of Atg7-mediated autophagy in ionizing radiation-induced neural stem cell damage. Gene 2020, 738, 144485. [Google Scholar] [CrossRef]
- Huang, R.; Gao, S.; Han, Y.; Ning, H.; Zhou, Y.; Guan, H.; Liu, X.; Yan, S.; Zhou, P.K. BECN1 promotes radiation-induced G2/M arrest through regulation CDK1 activity: A potential role for autophagy in G2/M checkpoint. Cell Death Discov. 2020, 6, 70. [Google Scholar] [CrossRef]
- Miousse, I.R.; Chalbot, M.C.; Lumen, A.; Ferguson, A.; Kavouras, I.G.; Koturbash, I. Response of transposable elements to environmental stressors. Mutat. Res. Rev. Mutat. Res. 2015, 765, 19–39. [Google Scholar] [CrossRef]
- Prior, S.; Miousse, I.R.; Nzabarushimana, E.; Pathak, R.; Skinner, C.; Kutanzi, K.R.; Allen, A.R.; Raber, J.; Tackett, A.J.; Hauer-Jensen, M.; et al. Densely ionizing radiation affects DNA methylation of selective LINE-1 elements. Environ. Res. 2016, 150, 470–481. [Google Scholar] [CrossRef]
- Koturbash, I.; Miousse, I.R.; Sridharan, V.; Nzabarushimana, E.; Skinner, C.M.; Melnyk, S.B.; Pavliv, O.; Hauer-Jensen, M.; Nelson, G.A.; Boerma, M. Radiation-induced changes in DNA methylation of repetitive elements in the mouse heart. Mutat. Res. 2016, 787, 43–53. [Google Scholar] [CrossRef]
- Brown, E.J.; Nguyen, A.H.; Bachtrog, D. The Y chromosome may contribute to sex-specific ageing in Drosophila. Nat. Ecol. Evol. 2020, 4, 853–862. [Google Scholar] [CrossRef]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar]
- Duffy, J.B. GAL4 system in Drosophila: A fly geneticist’s Swiss army knife. Genesis 2002, 34, 1–15. [Google Scholar] [CrossRef]
- Osterwalder, T.; Yoon, K.S.; White, B.H.; Keshishian, H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. USA 2001, 98, 12596. [Google Scholar] [CrossRef]
- Alic, N.; Hoddinott, M.P.; Foley, A.; Slack, C.; Piper, M.D.W.; Partridge, L. Detrimental effects of RNAi: A cautionary note on its use in Drosophila ageing studies. PLoS ONE 2012, 7, e45367. [Google Scholar] [CrossRef]
- Landis, G.N.; Salomon, M.P.; Keroles, D.; Brookes, N.; Sekimura, T.; Tower, J. The progesterone antagonist mifepristone/RU486 blocks the negative effect on life span caused by mating in female Drosophila. Aging 2015, 7, 53–69. [Google Scholar] [CrossRef]
- Xia, B.; de Belle, J.S. Transgenerational programming of longevity and reproduction by post-eclosion dietary manipulation in Drosophila. Aging 2016, 8, 1115–1134. [Google Scholar] [CrossRef]
- Hilton, J.F.; Mehta, C.R.; Patel, N.R. An algorithm for conducting exact Smirnov tests. Comput. Stat. Data Anal. 1994, 17, 351–361. [Google Scholar] [CrossRef]
- Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966, 50, 163–170. [Google Scholar] [PubMed]
- Martinez, R.L.M.C.; Naranjo, J.D. A pretest for choosing between logrank and wilcoxon tests in the two-sample problem. Metron 2010, 68, 111–125. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Redden, D.T.; Weindruch, R.; Allison, D.B. Statistical methods for testing effects on “maximum lifespan”. Mech. Ageing Dev. 2004, 125, 629–632. [Google Scholar] [CrossRef]
- Parashar, V.; Frankel, S.; Lurie, A.G.; Rogina, B. The effects of age on radiation resistance and oxidative stress in adult Drosophila melanogaster. Radiat. Res. 2008, 169, 707–711. [Google Scholar] [CrossRef]
- Paithankar, J.G.; Deeksha, K.; Patil, R.K. Gamma radiation tolerance in different life stages of the fruit fly Drosophila melanogaster. Int. J. Radiat. Biol. 2017, 93, 440–448. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Doss, C.G.P.; Lee, S.S. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Mol. Ther. Nucleic Acids 2017, 8, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Chen, D.; Chen, N. The Regulation of microRNAs in Alzheimer’s Disease. Front. Neurol. 2020, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Dallas, S.L. Exosomes and Extracellular RNA in Muscle and Bone Aging and Crosstalk. Curr. Osteoporos. Rep. 2019, 17, 548–559. [Google Scholar] [CrossRef]
- Ooi, J.Y.Y.; Bernardo, B.C. Translational Potential of Non-coding RNAs for Cardiovascular Disease. Adv. Exp. Med. Biol. 2020, 1229, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, Q.; Jiang, W.; Bian, Y.; Zhou, Y.; Gou, A.; Zhang, W.; Fu, K.; Shi, W. Emerging roles of piRNAs in cancer: Challenges and prospects. Aging 2019, 11, 9932–9946. [Google Scholar] [CrossRef]
- Barnes, P.J. Small airway fibrosis in COPD. Int. J. Biochem. Cell Biol. 2019, 116, 105598. [Google Scholar] [CrossRef]
- Watson, C.N.; Belli, A.; Di Pietro, V. Small Non-coding RNAs: New Class of Biomarkers and Potential Therapeutic Targets in Neurodegenerative Disease. Front. Genet. 2019, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Iswariya, G.T.; Paital, B.; Padma, P.R.; Nirmaladevi, R. microRNAs: Epigenetic players in cancer and aging. Front. Biosci. 2019, 11, 29–55. [Google Scholar]
- McCormick, R.; Goljanek-Whysall, K. MicroRNA Dysregulation in Aging and Pathologies of the Skeletal Muscle. Int. Rev. Cell Mol. Biol. 2017, 334, 265–308. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).