Effects of Omega-3 Polyunsaturated Fatty Acids and Their Metabolites on Haemostasis—Current Perspectives in Cardiovascular Disease
Abstract
1. Introduction
2. Metabolism and Mechanism of Action of Omega-3 PUFAs
3. Omega-3 PUFAs and Blood Platelets
4. Omega-3 PUFAs and Endothelium
5. Epidemiological Studies and Clinical Trials
Adverse Effects
6. Novel Perspectives of Omega-3 PUFAs
7. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.Y.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation and Cardiovascular Diseases. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Academic Press: Cambridge, MA, USA, 2019; pp. 53–117. [Google Scholar]
- Khodadi, E. Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules. Cardiovasc. Toxicol. 2019, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arter. Thromb. Vasc. Biol. 2020, 40, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Adili, R.; Voigt, E.M.; Bormann, J.L.; Foss, K.N.; Hurley, L.J.; Meyer, E.S.; Veldman, A.J.; Mast, K.A.; West, J.L.; Whiteheart, S.W.; et al. In vivo modeling of docosahexaenoic acid and eicosapentaenoic acid-mediated inhibition of both platelet function and accumulation in arterial thrombi. Platelets 2019, 30, 271–279. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Stanger, L.; Freedman, C.J.; Standley, M.; Hoang, T.; Adili, R.; Tsai, W.; Van Hoorebeke, C.; Holman, T.R.; Holinstat, M. DHA 12-LOX-derived oxylipins regulate platelet activation and thrombus formation through a PKA-dependent signaling pathway. J. Thromb. Haemost. 2020. [Google Scholar] [CrossRef]
- Capurso, A.; Capurso, C. Hemostasis and Thrombosis. In Principles of Nutrigenetics and Nutrigenomics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 361–369. [Google Scholar]
- Bowen, K.J.; Harris, W.S.; Kris-Etherton, P.M. Omega-3 Fatty Acids and Cardiovascular Disease: Are There Benefits? Curr. Treat. Options Cardiovasc. Med. 2016, 18, 1–16. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tatsuno, I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020, 27, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Ferguson, J.J.A.; Abbott, K.A.; Dias, C.B.; Garg, M.L. Science behind the cardio-metabolic benefits of omega-3 polyunsaturated fatty acids: Biochemical effectsvs. clinical outcomes. Food Funct. 2018, 9, 3576–3596. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Bassuk, S.S.; Cook, N.R.; Lee, I.-M.; Mora, S.; Albert, C.M.; Buring, J.E.; VITAL Research Group. Vitamin D, Marine n-3 Fatty Acids, and Primary Prevention of Cardiovascular Disease Current Evidence. Circ. Res. 2020, 126, 112–128. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef]
- Osadnik, K.; Jaworska, J. Analysis of ω-3 fatty acid content of polish fish oil drug and dietary supplements. Acta Pol. Pharm. Drug Res. 2016, 73, 875–883. [Google Scholar]
- Vale, F.M.D.; Diógenes, M.J.; Barbacena, H.A. Controversies about the cardiovascular effects of OM3FA. Did inappropriate placebos skew clinical trial results? Pharmacol. Res. 2021, 164, 105368. [Google Scholar] [CrossRef] [PubMed]
- Piper, K.; Garelnabi, M. Eicosanoids: Atherosclerosis and cardiometabolic health. J. Clin. Transl. Endocrinol. 2020, 19, 100216. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef]
- Miller, G.J. Dietary fatty acids and the haemostatic system. Atherosclerosis 2005, 179, 213–227. [Google Scholar] [CrossRef]
- Gao, L.-G.; Cao, J.; Mao, Q.-X.; Lu, X.-C.; Zhou, X.-L.; Fan, L. Influence of omega-3 polyunsaturated fatty acid-supplementation on platelet aggregation in humans: A meta-analysis of randomized controlled trials. Atherosclerosis 2013, 226, 328–334. [Google Scholar] [CrossRef]
- McEwen, B.; Morel-Kopp, M.-C.; Chen, W.; Tofler, G.; Ward, C. Effects of Omega-3 Polyunsaturated Fatty Acids on Platelet Function in Healthy Subjects and Subjects with Cardiovascular Disease. Semin. Thromb. Hemost. 2013, 39, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Paes, A.M.D.A.; Gaspar, R.S.; Fuentes, E.; Wehinger, S.; Palomo, I.; Trostchansky, A. Lipid Metabolism and Signaling in Platelet Function. Single Mol. Single Cell Seq. 2019, 1127, 97–115. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S.; Shido, O. Docosahexaenoic acid but not eicosapentaenoic acid withstands dietary cholesterol-induced decreases in platelet membrane fluidity. Mol. Cell. Biochem. 2006, 293, 1–8. [Google Scholar] [CrossRef]
- Larson, M.K.; Tormoen, G.W.; Weaver, L.J.; Luepke, K.J.; Patel, I.A.; Hjelmen, C.E.; Ensz, N.M.; McComas, L.S.; Mccarty, O.J.T. Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation. Am. J. Physiol. Physiol. 2013, 304, C273–C279. [Google Scholar] [CrossRef]
- McEwen, B.; Morel-Kopp, M.-C.; Tofler, G.; Ward, C. The Effect of Omega-3 Polyunsaturated Fatty Acids on Fibrin and Thrombin Generation in Healthy Subjects and Subjects with Cardiovascular Disease. Semin. Thromb. Hemost. 2015, 41, 315–322. [Google Scholar] [CrossRef]
- Adili, R.; Hawley, M.; Holinstat, M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018, 139, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.Z. Przeciwzapalne „prowygaszeniowe” pochodne wielonienasyconych kwasów tłuszczowych omega 3 i omega 6* Anti-inflammatory pro-resolving derivatives of omega-3 and omega-6 polyunsaturated fatty acids. Postepy Hig. Med. Dosw. 2010, 64, 115–132. [Google Scholar]
- Dona, M.; Fredman, G.; Schwab, J.M.; Chiang, N.; Arita, M.; Goodarzi, A.; Cheng, G.; Von Andrian, U.H.; Serhan, C.N. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood 2008, 112, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Kacik, M.; Olivan-Viguera, A.; Köhler, R. Modulation of KCa3.1 Channels by Eicosanoids, Omega-3 Fatty Acids, and Molecular Determinants. PLoS ONE 2014, 9, e112081. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, A.; Rai, S.N.; Cambon, A.; Miles, R.; Jaffe, A.S.; Moser, A.B.; O Jones, R.; Bolli, R.; Schulman, S.P. Fatty acids and TxA2 generation, in the absence of platelet-COX-1 activity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M. Omega-3 fatty acids in atherosclerosis and coronary artery disease. Futur. Sci. OA 2017, 3, FSO236. [Google Scholar] [CrossRef]
- Sheikh, O.; Hei, A.G.V.; Battisha, A.; Hammad, T.; Pham, S.; Chilton, R. Cardiovascular, electrophysiologic, and hematologic effects of omega-3 fatty acids beyond reducing hypertriglyceridemia: As it pertains to the recently published REDUCE-IT trial. Cardiovasc. Diabetol. 2019, 18, 1–12. [Google Scholar] [CrossRef]
- Fredman, G.; Van Dyke, T.E.; Serhan, C.N. Resolvin E1 Regulates Adenosine Diphosphate Activation of Human Platelets. Arter. Thromb. Vasc. Biol. 2010, 30, 2005–2013. [Google Scholar] [CrossRef]
- Larson, M.K.; Shearer, G.C.; Ashmore, J.H.; Anderson-Daniels, J.M.; Graslie, E.L.; Tholen, J.T.; Vogelaar, J.L.; Korth, A.J.; Nareddy, V.; Sprehe, M.; et al. Omega-3 fatty acids modulate collagen signaling in human platelets. Prostaglandins Leukot. Essent. Fat. Acids 2011, 84, 93–98. [Google Scholar] [CrossRef]
- He, K.; Liu, K.; Daviglus, M.L.; Jenny, N.S.; Mayer-Davis, E.; Jiang, R.; Steffen, L.; Siscovick, D.; Tsai, M.; Herrington, D. Associations of Dietary Long-Chain n-3 Polyunsaturated Fatty Acids and Fish With Biomarkers of Inflammation and Endothelial Activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2009, 103, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Prevention of Endothelial Dysfunction and Cardiovascular Disease by n-3 Fatty Acids-Inhibiting Action on Oxidative Stress and Inflammation. Curr. Pharm. Des. 2020, 26, 3652–3666. [Google Scholar] [CrossRef] [PubMed]
- Łacheta, D.; Olejarz, W.; Włodarczyk, M.; Nowicka, G. Effect of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) on the regulation of vascular endothelial cell function. Postępy Higieny i Medycyny Doświadczalnej 2019, 73, 467–475. [Google Scholar] [CrossRef]
- Yamagata, K. Docosahexaenoic acid regulates vascular endothelial cell function and prevents cardiovascular disease. Lipids Health Dis. 2017, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-M.; Chen, C.-J.; Lee, T.-S.; Chao, H.-Y.; Wu, W.-H.; Hsieh, S.-C.; Sheu, H.-H.; Chiang, A.-N. Docosahexaenoic acid attenuates VCAM-1 expression and NF-κB activation in TNF-α-treated human aortic endothelial cells. J. Nutr. Biochem. 2011, 22, 187–194. [Google Scholar] [CrossRef]
- Liu, K.-L.; Yang, Y.-C.; Yao, H.-T.; Chia, T.-W.; Lu, C.-Y.; Li, C.-C.; Tsai, H.J.; Lii, C.-K.; Chen, H.-W. Docosahexaenoic acid inhibits inflammation via free fatty acid receptor FFA4, disruption of TAB2 interaction with TAK1/TAB1 and downregulation of ERK-dependent Egr-1 expression in EA.hy926 cells. Mol. Nutr. Food Res. 2016, 60, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Din, J.N.; Sarma, J.; Harding, S.A.; Lyall, K.; Newby, D.E.; Flapan, A.D. Effect of ω-3 fatty acid supplementation on endothelial function, endogenous fibrinolysis and platelet activation in patients with a previous myocardial infarction: A randomised controlled trial. BMJ Open 2013, 3, e003054. [Google Scholar] [CrossRef][Green Version]
- Chen, H. EPA and DHA attenuate ox-LDL-induced expression of adhesion molecules in human coronary artery endothelial cells via protein kinase B pathway. J. Mol. Cell. Cardiol. 2003, 35, 769–775. [Google Scholar] [CrossRef]
- Qureshi, A.; AlTamimy, R.; El Habhab, A.; El Itawi, H.; Farooq, M.; Zobairi, F.; Hasan, H.; Amoura, L.; Kassem, M.; Auger, C.; et al. Ageing enhances the shedding of splenocyte microvesicles with endothelial pro-senescent effect that is prevented by a short-term intake of omega-3 PUFA EPA:DHA 6:1. Biochem. Pharmacol. 2020, 173, 113734. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; Mozaffarian, D.; et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin. Cardiol. 2018, 41, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Ascend Study Collaborative Group; Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bo, Y.; Liu, Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: A dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019, 18, 1–14. [Google Scholar] [CrossRef]
- Bernasconi, A.A.; Wiest, M.M.; Lavie, C.J.; Milani, R.V.; Laukkanen, J.A. Effect of Omega-3 Dosage on Cardiovascular Outcomes. Mayo Clin. Proc. 2021, 96, 304–313. [Google Scholar] [CrossRef]
- Dyerberg, J.; Bang, H. Hæmostatic function and platelet polyunsaturated fatty acids in eskimos. Lancet 1979, 314, 433–435. [Google Scholar] [CrossRef]
- Holub, B.J. Dietary fish oils containing eicosapentaenoic acid and the prevention of atherosclerosis and thrombosis. Can. Med Assoc. J. 1988, 139, 377–381. [Google Scholar]
- Li, X.L.; Steiner, M. Dose response of dietary fish oil supplementations on platelet adhesion. Arter. Thromb. A J. Vasc. Biol. 1991, 11, 39–46. [Google Scholar] [CrossRef]
- Mărginean, A.; Bănescu, C.; Scridon, A.; Dobreanu, M. Anti-platelet Therapy Resistance—Concept, Mechanisms and Platelet Function Tests in Intensive Care Facilities. J. Crit. Care Med. 2016, 2, 6–15. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, M.; Piao, L.; Li, X.; Yang, F.; Zhang, J.; Xiao, B.; Zhang, Q.; Song, W.-L.; Yin, H.; et al. Aspirin enhances protective effect of fish oil against thrombosis and injury-induced vascular remodelling. Br. J. Pharmacol. 2014, 172, 5647–5660. [Google Scholar] [CrossRef]
- Vanschoonbeek, K.; Feijge, M.A.; Paquay, M.; Rosing, J.; Saris, W.; Kluft, C.; Giesen, P.L.; De Maat, M.P.; Heemskerk, J.W. Variable Hypocoagulant Effect of Fish Oil Intake in Humans. Arter. Thromb. Vasc. Biol. 2004, 24, 1734–1740. [Google Scholar] [CrossRef]
- Bach, R.; Schmidt, U.; Jung, F.; Kiesewetter, H.; Hennen, B.; Wenzel, E.; Schieffer, H.; Bette, L.; Heyden, S. Effects of Fish Oil Capsules in Two Dosages on Blood Pressure, Platelet Functions, Haemorheological and Clinical Chemistry Parameters in Apparently Healthy Subjects. Ann. Nutr. Metab. 1989, 33, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Carletto, A.; Guarini, P.; Galvani, S.; Biasi, D.; Bellavite, P.; Corrocher, R.; Andrioli, G. Differential Effects of Dietary Supplementation with Fish Oil or Soy Lecithin on Human Platelet Adhesion. Thromb. Haemost. 1999, 82, 1522–1527. [Google Scholar] [CrossRef][Green Version]
- Bhatt, D.L.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Steg, P.G.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. REDUCE-IT USA: Results from the 3146 Patients Randomized in the United States. Circulation 2020, 141, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.J.; Scholz, K.P. Rounding the corner on residual risk: Implications of REDUCE-IT for omega-3 polyunsaturated fatty acids treatment in secondary prevention of atherosclerotic cardiovascular disease. Clin. Cardiol. 2019, 42, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Woodman, R.J.; A Mori, T.; Burke, V.; Puddey, I.B.; Barden, A.; Watts, G.F.; Beilin, L.J. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis 2003, 166, 85–93. [Google Scholar] [CrossRef]
- Candela, C.G.; López, L.M.B.; Kohen, V.L. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health: Nutritional recommendations. Nutrición Hospitalaria 2011, 26, 323–326. [Google Scholar]
- DiNicolantonio, J.J.; Okeefe, J. Importance of maintaining a low omega-6/omega-3 ratio for reducing platelet aggregation, coagulation and thrombosis. Open Hear. 2019, 6, e001011. [Google Scholar] [CrossRef]
- Miśkowiec, D.; Kasprzak, J.D. Postępy farmakoterapii w prewencji chorób serca–skuteczne leki, nieskuteczne suplementy. Dane z ostatnich kongresów AHA i ACC. Folia Cardiol. 2019, 14, 648–654. [Google Scholar]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 3, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Poreba, M.; Mostowik, M.; Siniarski, A.; Golebiowska-Wiatrak, R.; Malinowski, K.P.; Haberka, M.; Konduracka, E.; Nessler, J.; Undas, A.; Gajos, G. Treatment with high-dose n-3 PUFAs has no effect on platelet function, coagulation, metabolic status or inflammation in patients with atherosclerosis and type 2 diabetes. Cardiovasc. Diabetol. 2017, 16, 1–11. [Google Scholar] [CrossRef]
- Bagge, A.; Schött, U.; Kander, T. High-dose omega-3 fatty acids have no effect on platelet aggregation or coagulation measured with static and flow-based aggregation instruments and Sonoclot; an observational study in healthy volunteers. Scand. J. Clin. Lab. Investig. 2018, 78, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Sut, A.; Pytel, M.; Zadrożny, M.; Golański, J.; Rozalski, M. Polyphenol-rich diet is associated with decreased level of inflammatory biomarkers in breast cancer patients. Roczniki Państwowego Zakładu Higieny 2019, 70, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sut, A.; Chizynski, K.; Rozalski, M.; Golanski, J. Dietary intake of omega fatty acids and polyphenols and its relationship with the levels of inflammatory markers in men with chronic coronary syndrome after percutaneous coronary intervention. Kardiol. Pol. 2020, 78, 117–123. [Google Scholar] [CrossRef]
- Song, J.; Hu, M.; Li, C.; Yang, B.; Ding, Q.; Wang, C.; Mao, L. Dose-dependent effects of fish oil on cardio-metabolic biomarkers in healthy middle-aged and elderly Chinese people: A double-blind randomized controlled trial. Food Funct. 2018, 9, 3235–3243. [Google Scholar] [CrossRef]
- Hasturk, H.; Abdallah, R.; Kantarci, A.; Nguyen, D.; Giordano, N.; Hamilton, J.; Van Dyke, T.E. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arter. Thromb. Vasc. Biol. 2015, 35, 1123–1133. [Google Scholar] [CrossRef]
- Mackay, I.; Ford, I.; Thies, F.; Fielding, S.; Bachoo, P.; Brittenden, J. Effect of Omega-3 fatty acid supplementation on markers of platelet and endothelial function in patients with peripheral arterial disease. Atherosclerosis 2012, 221, 514–520. [Google Scholar] [CrossRef]
- Mason, R.P.; Dawoud, H.; Jacob, R.F.; Sherratt, S.C.; Malinski, T. Eicosapentaenoic acid improves endothelial function and nitric oxide bioavailability in a manner that is enhanced in combination with a statin. Biomed. Pharmacother. 2018, 103, 1231–1237. [Google Scholar] [CrossRef]
- Wachira, J.K.; Larson, M.K.; Harris, W.S. n-3 Fatty acids affect haemostasis but do not increase the risk of bleeding: Clinical observations and mechanistic insights. Br. J. Nutr. 2014, 111, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.A. Role of Fish Oil in Post-Cardiotomy Bleeding: A Summary of the Basic Science and Clinical Trials. Ann. Thorac. Surg. 2018, 105, 1563–1567. [Google Scholar] [CrossRef] [PubMed]
- Parish, S.; Mafham, M.; Offer, A.; Barton, J.; Wallendszus, K.; Stevens, W.; Buck, G.; Haynes, R.; Collins, R.; Bowman, L.; et al. Effects of Omega-3 Fatty Acid Supplements on Arrhythmias. Circulation 2020, 141, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Bonutti, P.M.; Sodhi, N.; Patel, Y.H.; Sultan, A.A.; Khlopas, A.; Chughtai, M.; Kolisek, F.R.; Williams, N.; Mont, M.A. Novel venous thromboembolic disease (VTED) prophylaxis for total knee arthroplasty—Aspirin and fish oil. Ann. Transl. Med. 2017, 5, S30. [Google Scholar] [CrossRef]
- Isaksen, T.; Evensen, L.H.; Johnsen, S.H.; Jacobsen, B.K.; Hindberg, K.; Braekkan, S.K.; Hansen, J.-B. Dietary intake of marine n-3 polyunsaturated fatty acids and future risk of venous thromboembolism. Res. Pract. Thromb. Haemost. 2019, 3, 59–69. [Google Scholar] [CrossRef]
- Zheng, X.; Jia, R.; Li, Y.; Liu, T.; Wang, Z. Omega-3 fatty acids reduce post-operative risk of deep vein thrombosis and pulmonary embolism after surgery for elderly patients with proximal femoral fractures: A randomized placebo-controlled, double-blind clinical trial. Int. Orthop. 2020, 44, 2089–2093. [Google Scholar] [CrossRef]
- Isaksen, T.; Evensen, L.H.; Brækkan, S.K.; Hansen, J.-B. Dietary Intake of Marine Polyunsaturated n-3 Fatty Acids and Risk of Recurrent Venous Thromboembolism. Thromb. Haemost. 2019, 119, 2053–2063. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Guo, H.; Liang, J.; Li, Y. Associations of Fish and Omega-3 Fatty Acids Consumption With the Risk of Venous Thromboembolism. A Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2020, 7, 614784. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, T. Letter to the editor regarding “Omega-3 fatty acids reduce post-operative risk of deep vein thrombosis and pulmonary embolism after surgery for elderly patients with proximal femoral fractures: A randomized placebo-controlled, double-blind clinical trial” by Zheng et al. Int. Orthop. 2020, 44, 2809. [Google Scholar] [CrossRef]
- Merritt, R.J.; Bhardwaj, V.; Jami, M.M. Fish oil and COVID-19 thromboses. J. Vasc. Surgery Venous Lymphat. Disord. 2020, 8, 1120. [Google Scholar] [CrossRef]
- Rogero, M.M.; Leão, M.D.C.; Santana, T.M.; Pimentel, M.V.D.M.; Carlini, G.C.; da Silveira, T.F.; Gonçalves, R.C.; Castro, I.A. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic. Biol. Med. 2020, 156, 190–199. [Google Scholar] [CrossRef]
- Darwesh, A.M.; Bassiouni, W.; Sosnowski, D.K.; Seubert, J.M. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharmacol. Ther. 2021, 219, 107703. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Thrombosis and COVID-19: The Potential Role of Nutrition. Front. Nutr. 2020, 7, 583080. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.R.; Marques, R.M.; Gomez, E.A.; Colas, R.A.; De Matteis, R.; Zak, A.; Patel, M.; Collier, D.J.; Dalli, J. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses. Circ. Res. 2020, 126, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Hosogoe, N.; Ishikawa, S.; Yokoyama, N.; Kozuma, K.; Isshiki, T. Add-on Antiplatelet Effects of Eicosapentaenoic Acid with Tailored Dose Setting in Patients on Dual Antiplatelet Therapy. Int. Hear. J. 2017, 58, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Skeaff, C.; Holub, B. The effect of fish oil consumption on platelet aggregation responses in washed human platelet suspensions. Thromb. Res. 1988, 51, 105–115. [Google Scholar] [CrossRef]

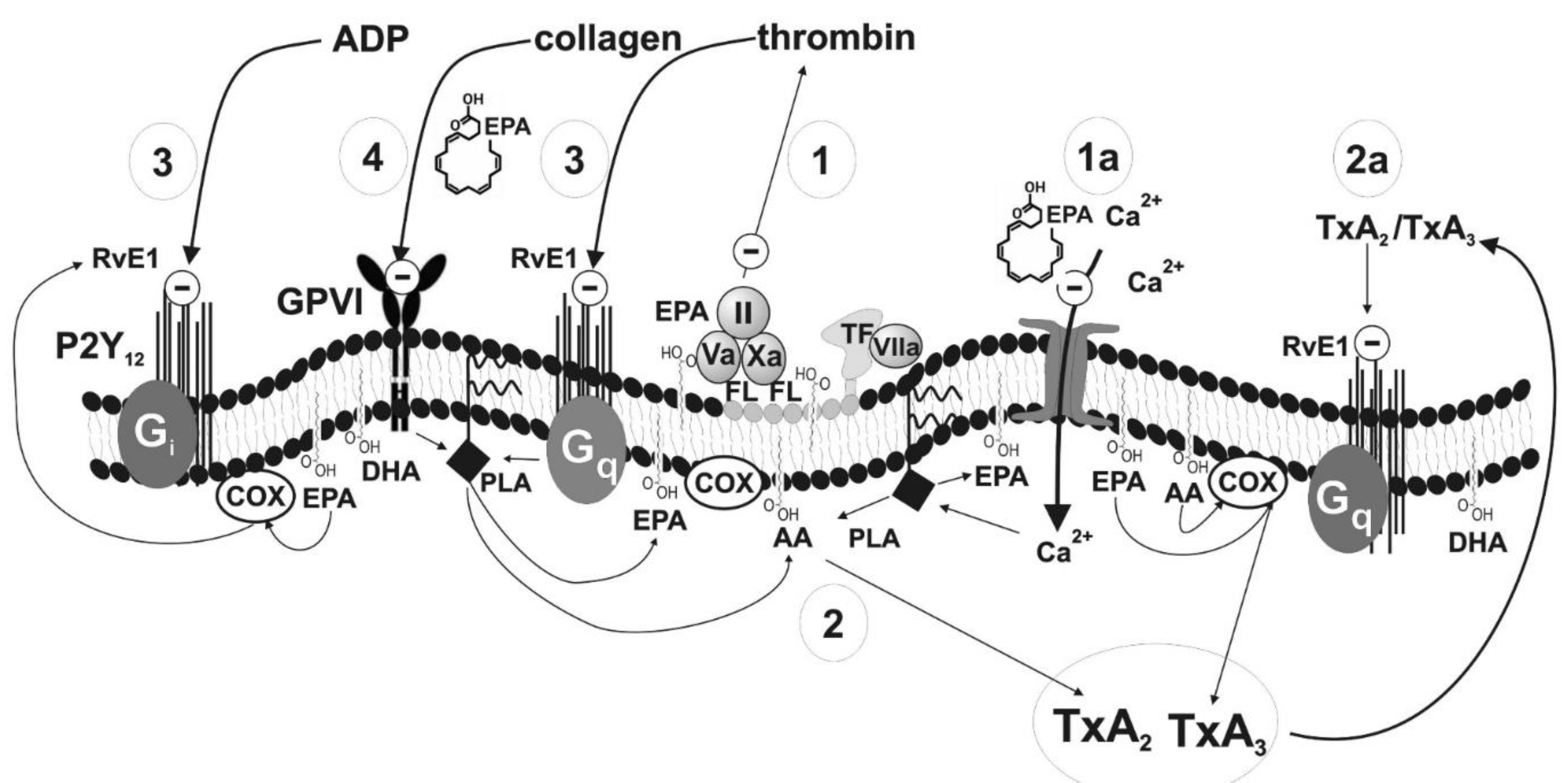
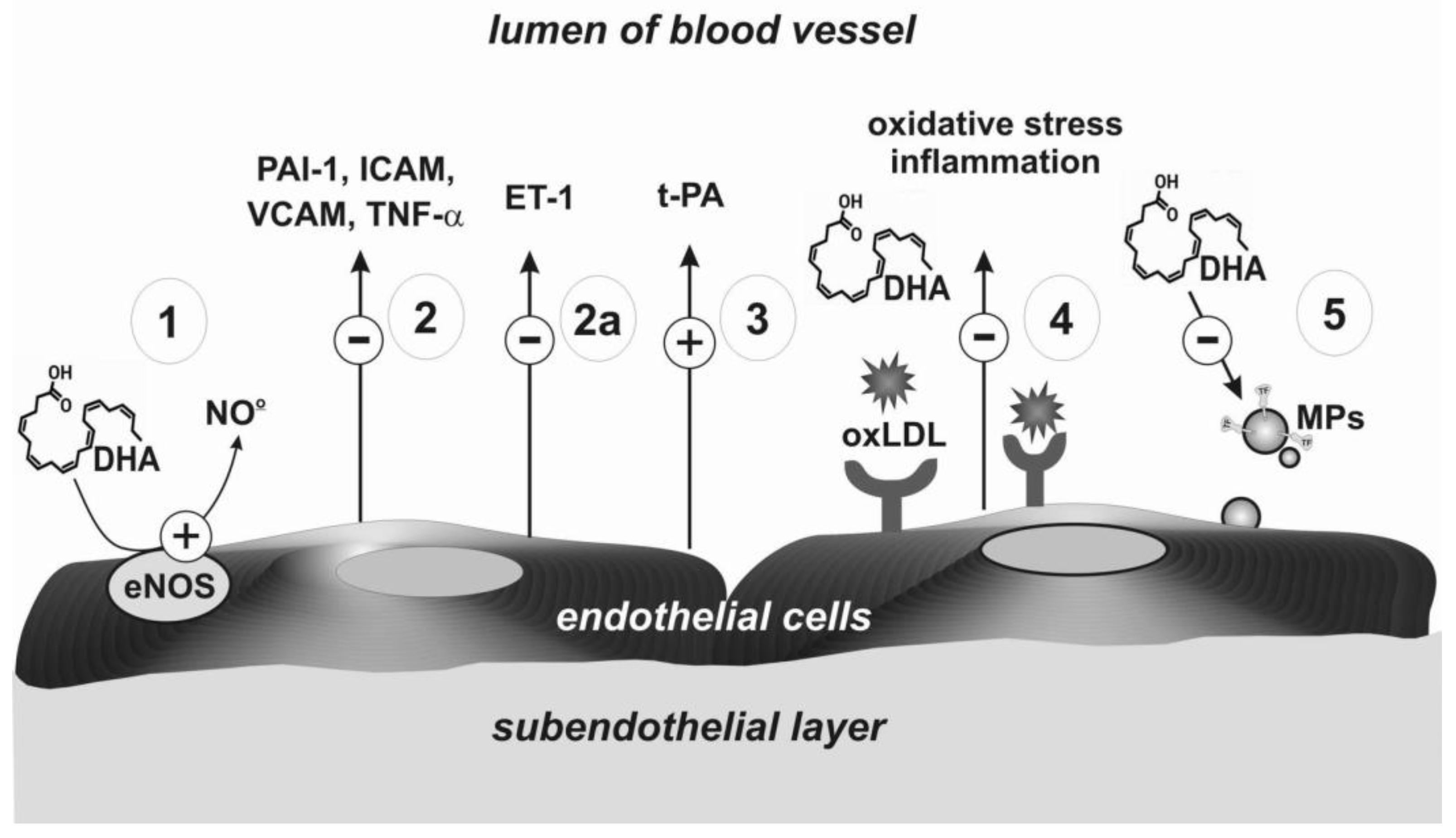
| Number in Figure 2 | Haemostasis Component | Potential Mechanism | Effect | Strength of Evidence | Reference |
|---|---|---|---|---|---|
| 1 | Prothrombinase complex (factors Va, Xa, II) on the surface of blood platelets | Decrease in cell membrane fluidity resulting in poor availability of procoagulant phospholipids (phosphatidylserine) | Slower conversion of prothrombin into thrombin; inhibition of coagulation cascade and platelet aggregation | Low | Larson [26] |
| 1a | Ionotropic calcium channels in plasma membrane | Decrease in cell membrane fluidity resulting in reduced influx of Ca2+ | Inhibition of blood platelets activation | Low | Kacik [31] |
| 2 | Synthesis of TxA2 | Competition between AA and EPA for enzymes essential for further metabolic steps | Inhibition of TxA2 synthesis leading to a production of TxA3 having weak pro-aggregatory properties; inhibition of TXA2-dependent platelet activation | High | DeFilippis Adili, Bäck [28,32,33] |
| 2a | Synthesis of TxA2 | Metabolite of EPA–resolvin binding to TxA2 receptor | Inhibition of TXA2-dependent platelet activation | Moderate | Sheikh, Bäck [33,34] |
| 3 | Receptor for ADP and receptor for thrombin | Metabolite of EPA–resolvin binding to receptors for ADP and thrombin | Inhibition of TXA2-and thrombin-dependent platelet activation | Moderate | Fredman, Dona [30,35] |
| 4 | Receptor GPVI (collagen receptor) | Blocking of the receptor (detailed mechanism unknown) or inhibition of platelet reactivity in a glycoprotein VI-dependent manner via activation of protein kinase A | Inhibition of collagen-dependent platelet activation | Low | Larson [36] Yamaguchi [8] |
| Number in Figure 3 | Haemostasis Component | Potential Mechanism | Effect | Strength of Evidence | Reference |
|---|---|---|---|---|---|
| 1 | Endothelial synthase of nitric oxide (eNOS) | Higher production of NO | Vasodilatation effect | High | Łacheta, Yamagata [39,40] |
| 2 | Adhesive receptors | Decrease of expression of adhesive molecules (VCAM, ICAM); reduced expression of tumor necrosis factor alpha (TNF-α) and PAI-1 | High | Wang, Liu [41,42] | |
| 2a | Protein synthesis | Decreased synthesis of endothelin-1 | Anticoagulation effects | Moderate | Yamagata [40] |
| 3 | Receptor for t-PA | Higher level of t-PA | Fibrinolytic effects | Low | Din [43] |
| 4 | Receptor for oxLDL | Regulation of expression of the receptor for oxLDL | Inhibition of formation of oxidized LDL | High | Chen [44] |
| 5 | Inhibition of microparticles formation | Lower expression of tissue factor | Reduction of oxidative stress, inhibition of thrombin generation | Low | Qureshi [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golanski, J.; Szymanska, P.; Rozalski, M. Effects of Omega-3 Polyunsaturated Fatty Acids and Their Metabolites on Haemostasis—Current Perspectives in Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 2394. https://doi.org/10.3390/ijms22052394
Golanski J, Szymanska P, Rozalski M. Effects of Omega-3 Polyunsaturated Fatty Acids and Their Metabolites on Haemostasis—Current Perspectives in Cardiovascular Disease. International Journal of Molecular Sciences. 2021; 22(5):2394. https://doi.org/10.3390/ijms22052394
Chicago/Turabian StyleGolanski, Jacek, Patrycja Szymanska, and Marcin Rozalski. 2021. "Effects of Omega-3 Polyunsaturated Fatty Acids and Their Metabolites on Haemostasis—Current Perspectives in Cardiovascular Disease" International Journal of Molecular Sciences 22, no. 5: 2394. https://doi.org/10.3390/ijms22052394
APA StyleGolanski, J., Szymanska, P., & Rozalski, M. (2021). Effects of Omega-3 Polyunsaturated Fatty Acids and Their Metabolites on Haemostasis—Current Perspectives in Cardiovascular Disease. International Journal of Molecular Sciences, 22(5), 2394. https://doi.org/10.3390/ijms22052394






