The Effect of Melatonin on Periodontitis
Abstract
:1. Introduction
2. Material and Methods
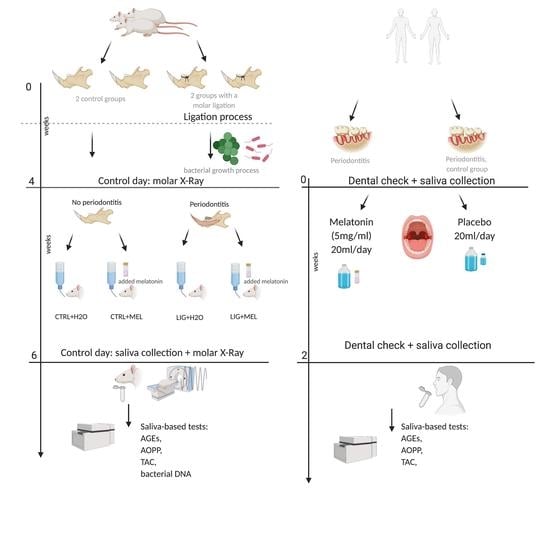
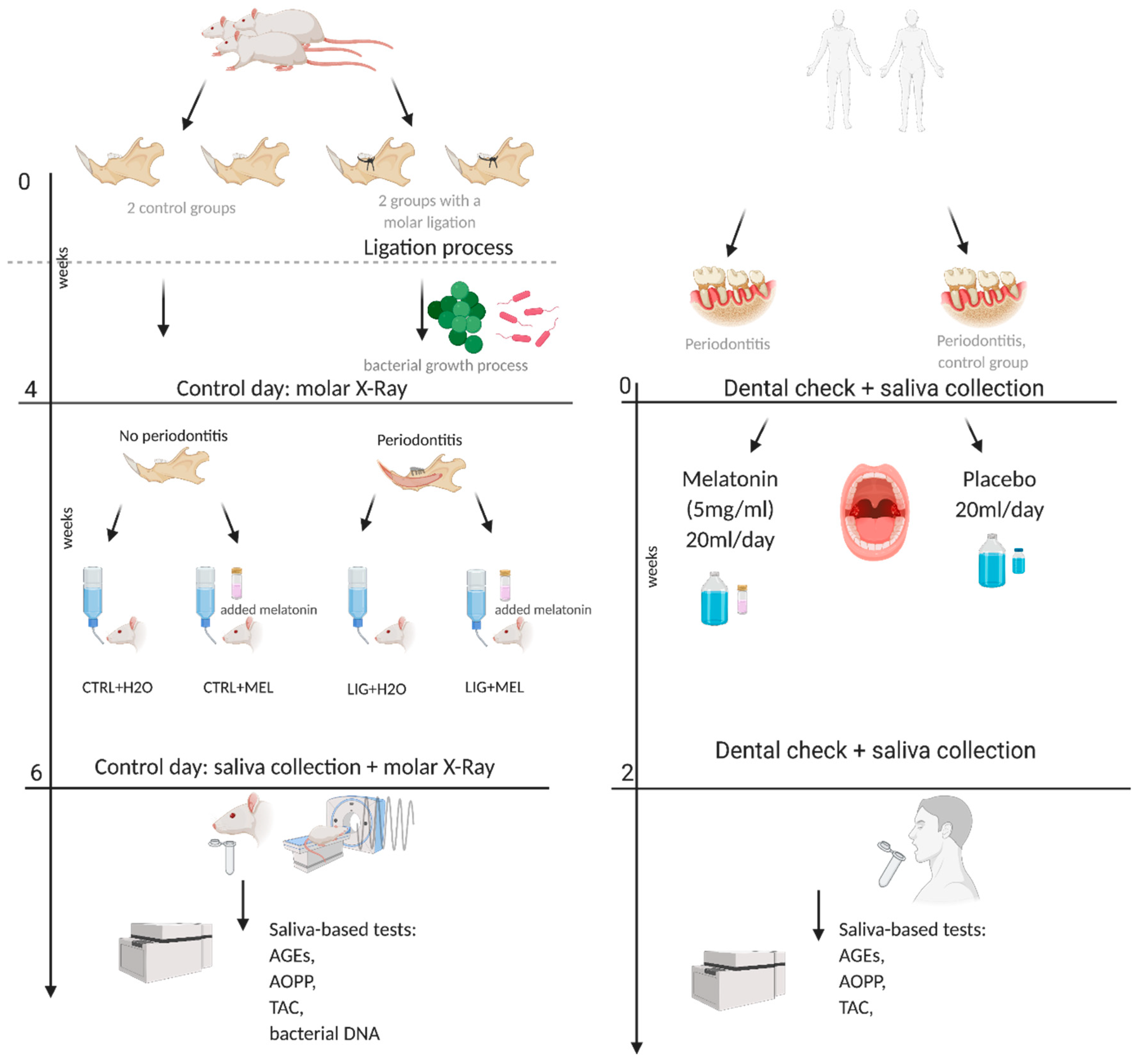
2.1. Animals
2.2. Induction of Periodontitis
2.3. Real-Time PCR Analysis
2.4. Clinical Study
2.5. Sampling
2.6. Melatonin Concentration Measurement
2.7. Oxidative Stress
2.8. Statistical Analysis
3. Results
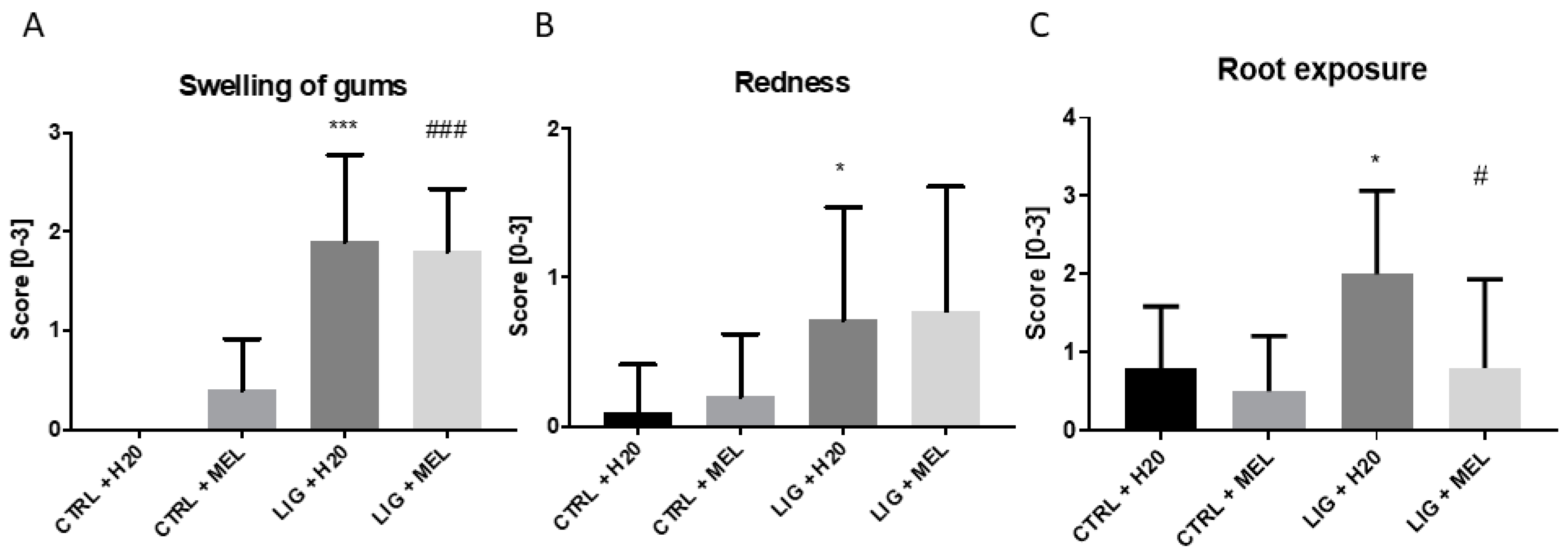
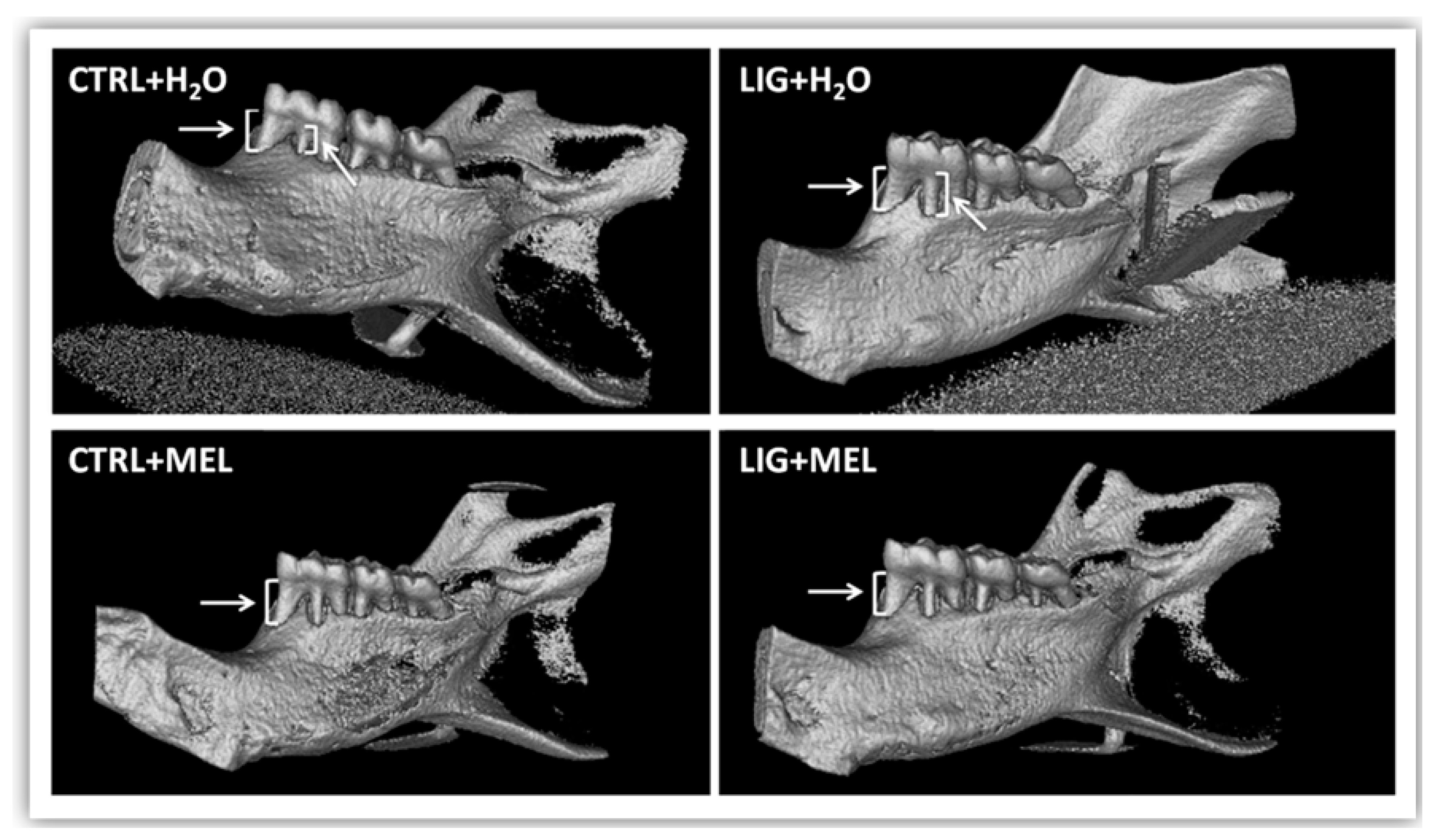
3.1. Morphometric Changes in Rats
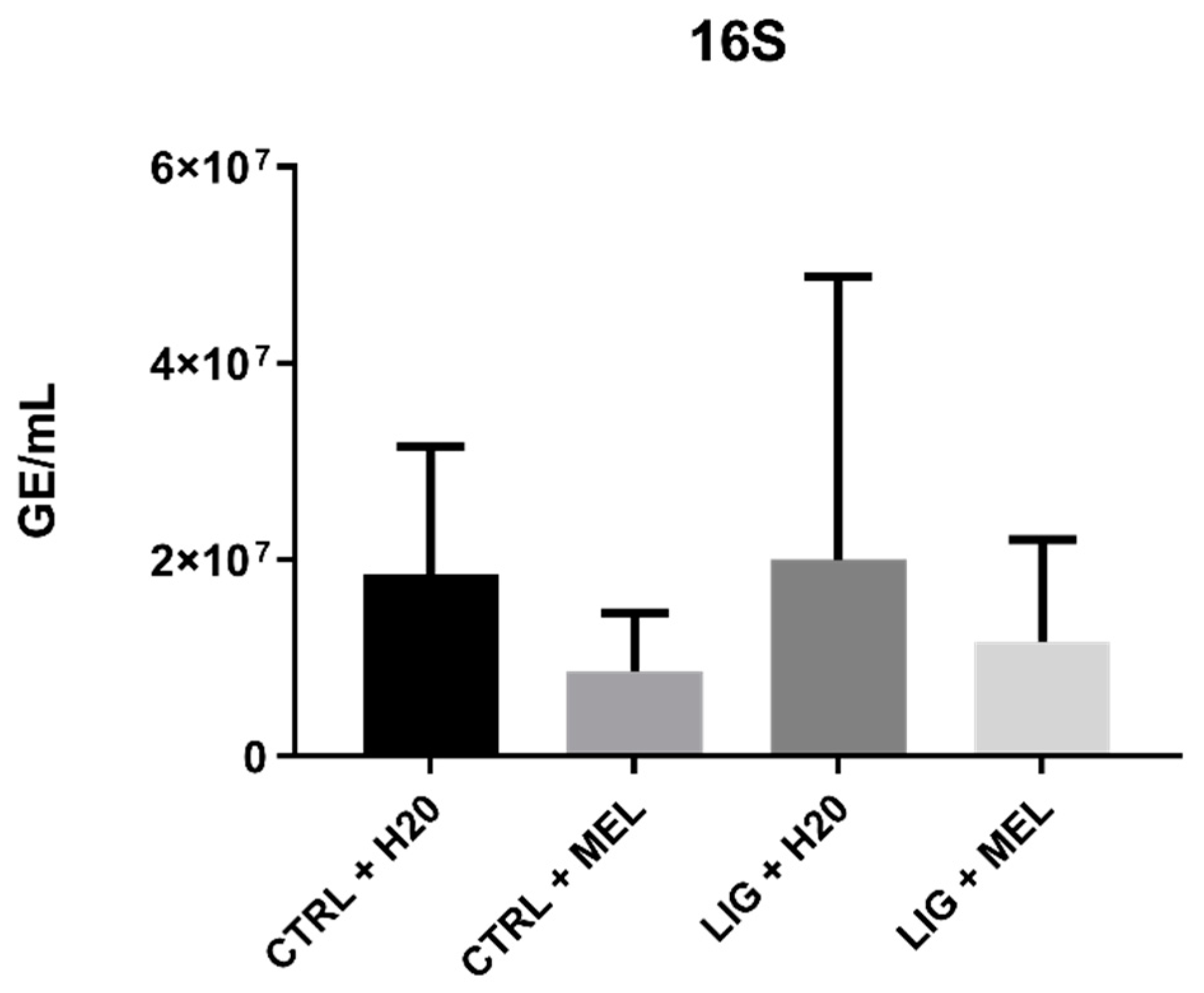
3.2. Bacterial DNA
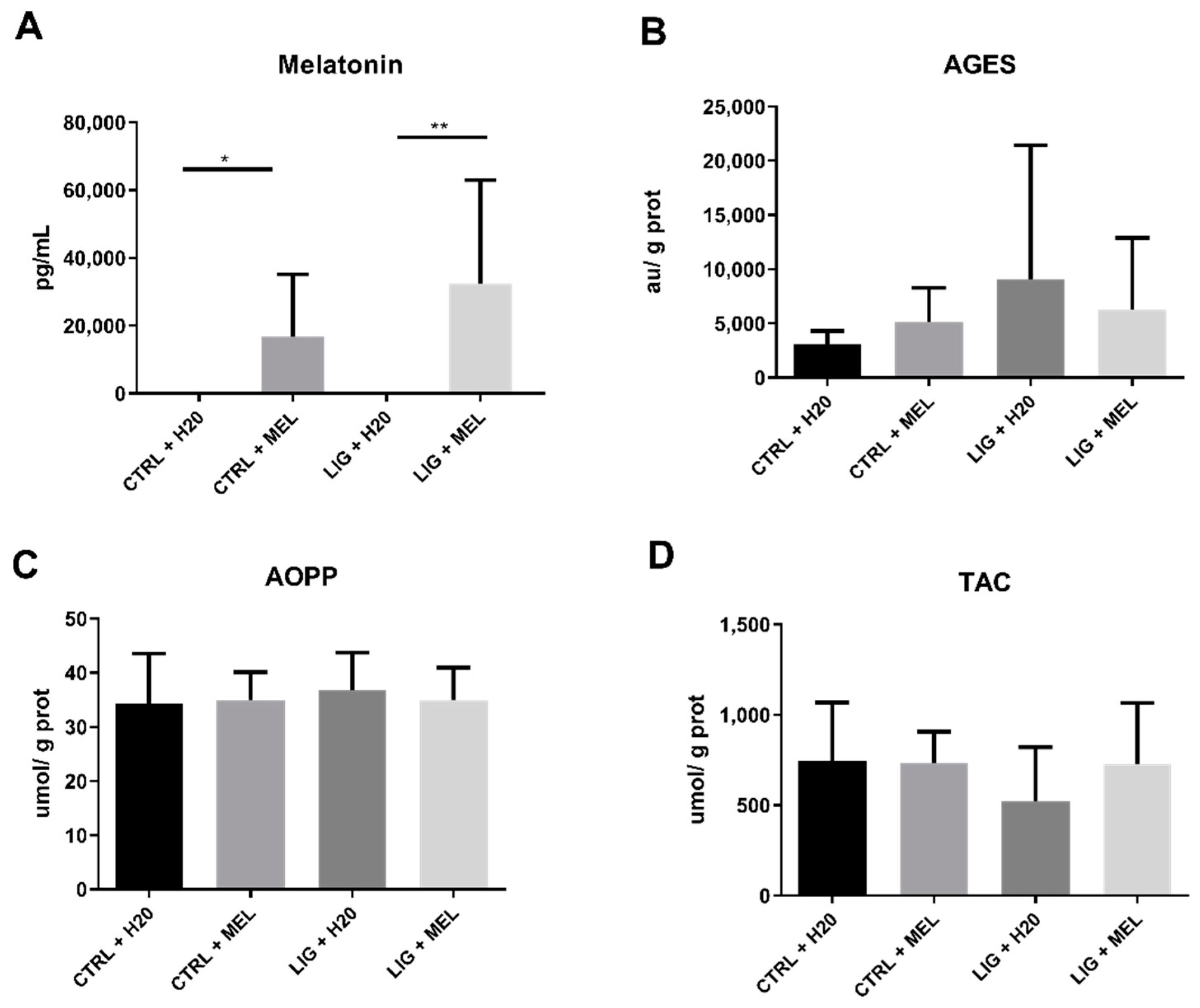
3.3. Melatonin Concentration and Oxidative status in Rats
3.4. Clinical Data of Patients
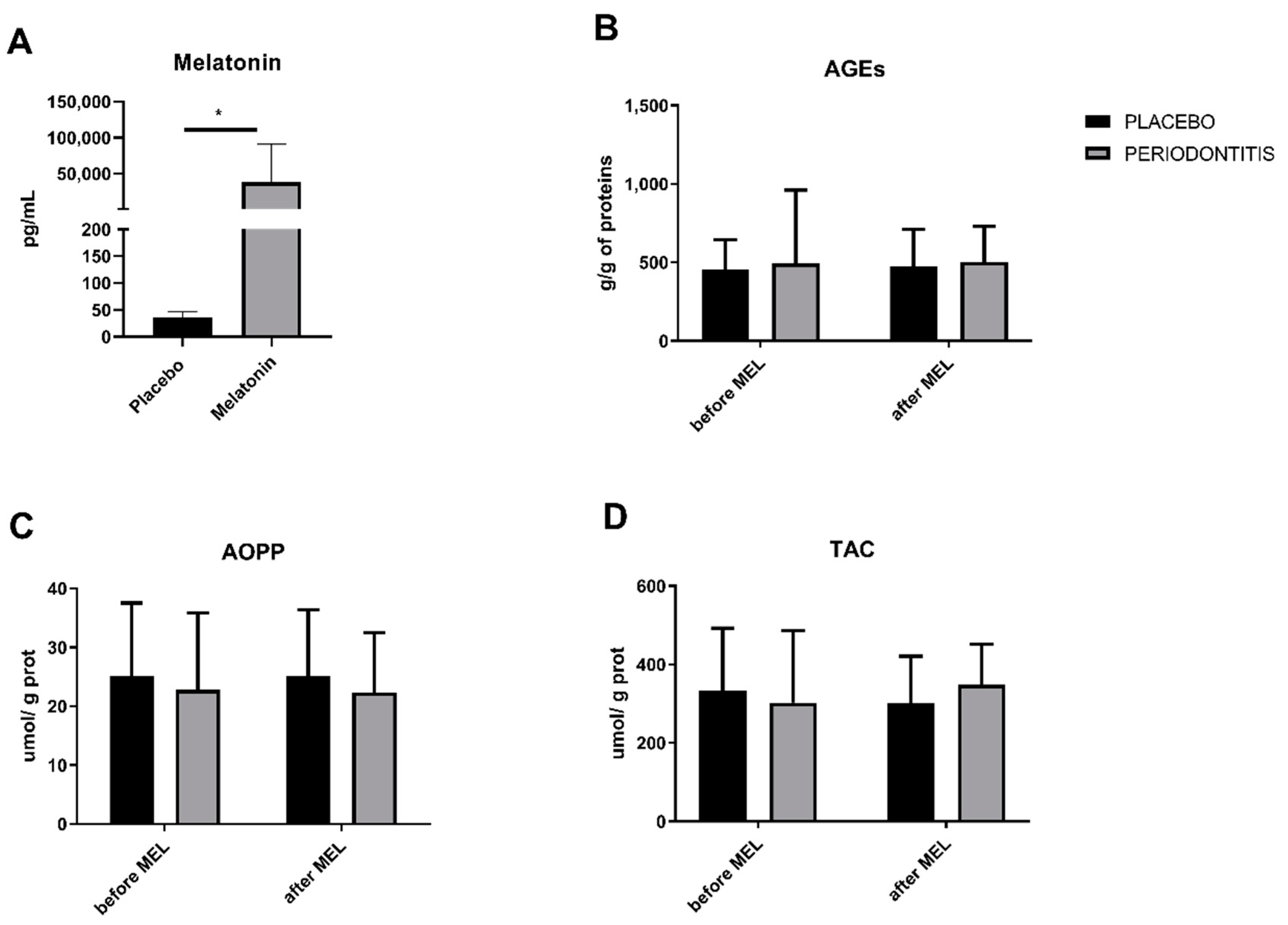
3.5. Melatonin Concentrations and Salivary Markers of Oxidative Status in Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eke, P.I.; Zhang, X.; Lu, H.; Wei, L.; Thornton-Evans, G.; Greenlund, K.J.; Holt, J.B.; Croft, J.B. Predicting Periodontitis at State and Local Levels in the United States. J. Dent. Res. 2016, 95, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers. 2017, 3, 17038. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Leonardi, R. Independent impact of periodontitis and cardiovascular disease on elevated soluble urokinase-type plasminogen activator receptor (suPAR) levels. J. Periodontol. 2020, 10.1002/JPER.20-0242. [Google Scholar] [CrossRef]
- Matarese, G.; Isola, G.; Anastasi, G.P.; Favaloro, A.; Milardi, D.; Vermiglio, G.; Vita, G.; Cordasco, G.; Cutroneo, G. Immunohistochemical analysis of TGF-beta1 and VEGF in gingival and periodontal tissues: A role of these biomarkers in the pathogenesis of scleroderma and periodontal disease. Int. J. Mol. Med. 2012, 30, 502–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J. Health Sci. (Qassim.) 2017, 11, 72–80. [Google Scholar]
- Correa, M.G.; Absy, S.; Tenenbaum, H.; Ribeiro, F.V.; Cirano, F.R.; Casati, M.Z.; Pimentel, S.P. Resveratrol attenuates oxidative stress during experimental periodontitis in rats exposed to cigarette smoke inhalation. J. Periodontal. Res. 2019, 54, 225–232. [Google Scholar] [CrossRef]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Aiuto, F.; Nibali, L.; Parkar, M.; Patel, K.; Suvan, J.; Donos, N. Oxidative stress, systemic inflammation, and severe periodontitis. J. Dent. Res. 2010, 89, 1241–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guentsch, A.; Preshaw, P.M.; Bremer-Streck, S.; Klinger, G.; Glockmann, E.; Sigusch, B.W. Lipid peroxidation and antioxidant activity in saliva of periodontitis patients: Effect of smoking and periodontal treatment. Clin. Oral Investig. 2008, 12, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef]
- Araujo, A.A.; Morais, H.B.; Medeiros, C.; Brito, G.A.C.; Guedes, P.M.M.; Hiyari, S.; Pirih, F.Q.; Araujo Junior, R.F. Gliclazide reduced oxidative stress, inflammation, and bone loss in an experimental periodontal disease model. J. Appl. Oral Sci. 2019, 27, e20180211. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, A.R.; Peralta Lopez, M.E.; Aguilar, J.; Sola, V.M. Melatonin and periodontal tissues: Molecular and clinical perspectives. Pharmacol. Res. 2017, 125, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Bertl, K.; Schoiber, A.; Haririan, H.; Laky, M.; Steiner, I.; Rausch, W.D.; Andrukhov, O.; Rausch-Fan, X. Non-surgical periodontal therapy influences salivary melatonin levels. Clin. Oral Investig. 2013, 17, 1219–1225. [Google Scholar] [CrossRef]
- Gomez-Moreno, G.; Cutando-Soriano, A.; Arana, C.; Galindo, P.; Bolanos, J.; Acuna-Castroviejo, D.; Wang, H.L. Melatonin expression in periodontal disease. J. Periodontal. Res. 2007, 42, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Akman, S.; Ozkanlar, S.; Tozoglu, U.; Kalkan, Y.; Canakci, C.F.; Tozoglu, S. Immune modulatory and antioxidant effects of melatonin in experimental periodontitis in rats. Free Radic. Biol. Med. 2013, 55, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.A.; Liu, X.Y.; Acuna-Castroviejo, D.; Escames, G.; Tan, D.X. Melatonin in the oral cavity: Physiological and pathological implications. J. Periodontal. Res. 2015, 50, 9–17. [Google Scholar] [CrossRef]
- Kamodyova, N.; Tothova, L.; Celec, P. Salivary markers of oxidative stress and antioxidant status: Influence of external factors. Dis. Markers 2013, 34, 313–321. [Google Scholar] [CrossRef]
- Tresguerres, I.F.; Clemente, C.; Blanco, L.; Khraisat, A.; Tamimi, F.; Tresguerres, J.A. Effects of local melatonin application on implant osseointegration. Clin. Implant. Dent. Relat. Res. 2012, 14, 395–399. [Google Scholar] [CrossRef]
- Maria, S.; Samsonraj, R.M.; Munmun, F.; Glas, J.; Silvestros, M.; Kotlarczyk, M.P.; Rylands, R.; Dudakovic, A.; van Wijnen, A.J.; Enderby, L.T.; et al. Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J. Pineal. Res. 2018, 64. [Google Scholar] [CrossRef]
- Shimozuma, M.; Tokuyama, R.; Tatehara, S.; Umeki, H.; Ide, S.; Mishima, K.; Saito, I.; Satomura, K. Expression and cellular localizaion of melatonin-synthesizing enzymes in rat and human salivary glands. Histochem. Cell. Biol. 2011, 135, 389–396. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainamo, J.; Barmes, D.; Beagrie, G.; Cutress, T.; Martin, J.; Sardo-Infirri, J. Development of the World Health Organization (WHO) community periodontal index of treatment needs (CPITN). Int. Dent. J. 1982, 32, 281–291. [Google Scholar]
- Lang, N.P.; Adler, R.; Joss, A.; Nyman, S. Absence of bleeding on probing. An indicator of periodontal stability. J. Clin. Periodontol. 1990, 17, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, H.R.; Son, S. Gingival sulcus bleeding--a leading symptom in initial gingivitis. Helv. Odontol. Acta 1971, 15, 107–113. [Google Scholar]
- Silness, J.; Loe, H. Periodontal Disease in Pregnancy. Ii. Correlation between Oral Hygiene and Periodontal Condtion. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Banasova, L.; Kamodyova, N.; Jansakova, K.; Tothova, L.; Stanko, P.; Turna, J.; Celec, P. Salivary DNA and markers of oxidative stress in patients with chronic periodontitis. Clin. Oral Investig. 2015, 19, 201–207. [Google Scholar] [CrossRef]
- Gyuraszova, M.; Kovalcikova, A.; Jansakova, K.; Sebekova, K.; Celec, P.; Tothova, L. Markers of oxidative stress and antioxidant status in the plasma, urine and saliva of healthy mice. Physiol. Res. 2018, 67, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Isola, M.; Lilliu, M.A. Melatonin localization in human salivary glands. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2016, 45, 510–515. [Google Scholar] [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Tothova, L.; Celecova, V.; Celec, P. Salivary markers of oxidative stress and their relation to periodontal and dental status in children. Dis. Markers 2013, 34, 9–15. [Google Scholar] [CrossRef]
- Kose, O.; Arabaci, T.; Kara, A.; Yemenoglu, H.; Kermen, E.; Kizildag, A.; Gedikli, S.; Ozkanlar, S. Effects of Melatonin on Oxidative Stress Index and Alveolar Bone Loss in Diabetic Rats With Periodontitis. J. Periodontol. 2016, 87, e82–e90. [Google Scholar] [CrossRef]
- Slavish, D.C.; Graham-Engeland, J.E.; Smyth, J.M.; Engeland, C.G. Salivary markers of inflammation in response to acute stress. Brain Behav. Immun. 2015, 44, 253–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, R.A.; Gorjao, R.; Mayer, M.P.A.; Corazza, P.F.L.; Guare, R.O.; Ferreira, A.; Santos, M. Inflammatory markers in the saliva of cerebral palsy individuals with gingivitis after periodontal treatment. Braz. Oral Res. 2019, 33, e033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gooneratne, N.S.; Edwards, A.Y.; Zhou, C.; Cuellar, N.; Grandner, M.A.; Barrett, J.S. Melatonin pharmacokinetics following two different oral surge-sustained release doses in older adults. J. Pineal. Res. 2012, 52, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazyar, H.; Gholinezhad, H.; Moradi, L.; Salehi, P.; Abadi, F.; Ravanbakhsh, M.; Zare Javid, A. The effects of melatonin supplementation in adjunct with non-surgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type 2 diabetes mellitus patients with chronic periodontitis: A double-blind, placebo-controlled trial. Inflammopharmacology 2019, 27, 67–76. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Patini, R.; Ferlito, S.; Alibrandi, A.; Palazzo, G. Association among serum and salivary A. actinomycetemcomitans specific immunoglobulin antibodies and periodontitis. BMC Oral Health 2020, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Aytekin, Z.; Arabaci, T.; Toraman, A.; Bayir, Y.; Albayrak, M.; Ustun, K. Immune modulatory and antioxidant effects of locally administrated vitamin C in experimental periodontitis in rats. Acta Odontol. Scand. 2020, 78, 425–432. [Google Scholar] [CrossRef]
- Cho, J.H.; Bhutani, S.; Kim, C.H.; Irwin, M.R. Anti-inflammatory effects of melatonin: A systematic review and meta-analysis of clinical trials. Brain Behav. Immun. 2021, 10.1016/j.bbi.2021.01.034. [Google Scholar] [CrossRef]
- Skottrup, P.D.; Dahlen, G.; Baelum, V.; Lopez, R. Soluble urokinase-type plasminogen activator receptor is associated with signs of periodontitis in adolescents. Eur J. Oral Sci. 2018, 126, 292–299. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Placebo (Before Treatment) | Placebo (After Treatment) | Melatonin (Before Treatment) | Melatonin (After Treatment) |
|---|---|---|---|---|
| AGE [years] | 47 ± 6 | 44 ± 7 | ||
| BOP | 65.69 ± 11.99 | 60.73 ± 11.50 | 60.15 ± 20.99 | 52.68 ± 21.53 |
| SBI | 3.06 ± 0.52 | 2.821 ± 0.41 | 2.581 ± 0.87 | 2.37 ± 0.88 |
| PI | 1.53 ± 0.94 | 1.38 ± 0.91 | 1.69 ± 1.34 | 1.45 ± 1.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konečná, B.; Chobodová, P.; Janko, J.; Baňasová, L.; Bábíčková, J.; Celec, P.; Tóthová, Ľ. The Effect of Melatonin on Periodontitis. Int. J. Mol. Sci. 2021, 22, 2390. https://doi.org/10.3390/ijms22052390
Konečná B, Chobodová P, Janko J, Baňasová L, Bábíčková J, Celec P, Tóthová Ľ. The Effect of Melatonin on Periodontitis. International Journal of Molecular Sciences. 2021; 22(5):2390. https://doi.org/10.3390/ijms22052390
Chicago/Turabian StyleKonečná, Barbora, Paulína Chobodová, Jakub Janko, Lenka Baňasová, Janka Bábíčková, Peter Celec, and Ľubomíra Tóthová. 2021. "The Effect of Melatonin on Periodontitis" International Journal of Molecular Sciences 22, no. 5: 2390. https://doi.org/10.3390/ijms22052390
APA StyleKonečná, B., Chobodová, P., Janko, J., Baňasová, L., Bábíčková, J., Celec, P., & Tóthová, Ľ. (2021). The Effect of Melatonin on Periodontitis. International Journal of Molecular Sciences, 22(5), 2390. https://doi.org/10.3390/ijms22052390