Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents
Abstract
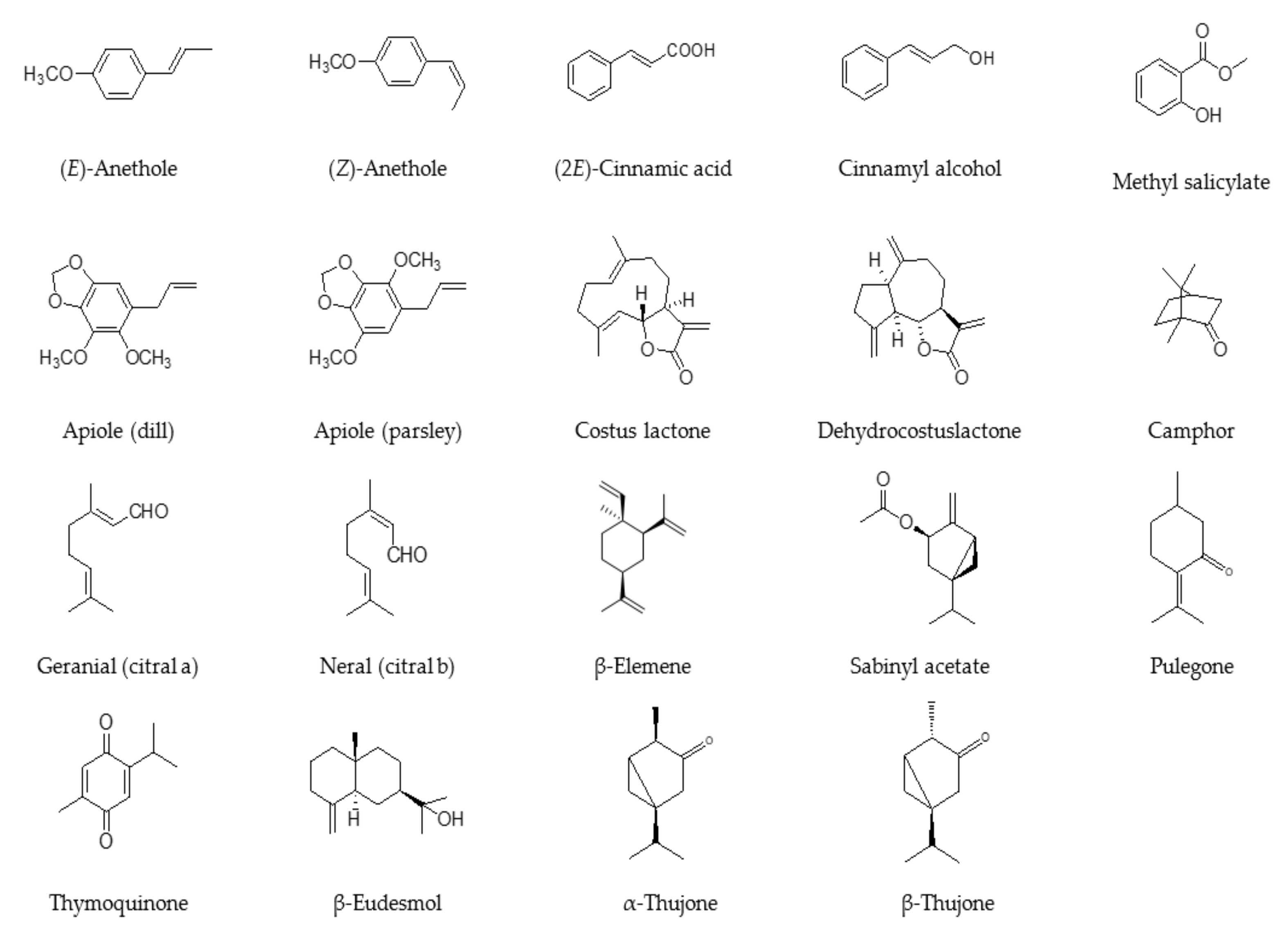
1. Introduction
2. Anethole-Rich Essential Oils
3. Methyl Salicylate-Rich Essential Oils
4. cis-Sabinyl Acetate-Rich Essential Oils
5. Thujone-Rich Essential Oils
6. Apiole-Rich Essential Oils
7. Camphor-Rich Essential Oils
8. Citral-Rich Essential Oils
9. β-Pulegone-Rich Essential Oils
10. Costunolide and Dehydrocostus Lactone-Rich Essential Oils
11. Thymoquinone-Rich Essential Oils
12. β-Elemene- and/or β-Eudesmol-Rich Essential Oils
13. Other Essential Oils
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EO | essential oil |
| CNS | central nervous system |
| GSK-3β | glycogen synthase kinase-3β |
| sc | subcutaneous |
| PMS | premenstrual syndrome |
| GRAS | generally recognized as safe |
| i.p. | intraperitoneal |
| IC50 | median inhibitory concentration |
| NOAEL | no observed adverse effect level |
| ppm | parts per million |
| VEGF | vascular endothelial growth factor |
| GABAA | gamma-aminobutyric acid type A |
References
- Howes, M.-J.R.; Houghton, P.J.; Barlow, D.J.; Pocock, V.J.; Milligan, S.R. Assessment of estrogenic activity in some common essential oil constituents. J. Pharm. Pharmacol. 2002, 54, 1521–1528. [Google Scholar] [CrossRef]
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: A review of the present state of research. Phyther. Res. 2003, 17, 845–869. [Google Scholar] [CrossRef] [PubMed]
- Azaizeh, H.; Saad, B.; Cooper, E.; Said, O. Traditional Arabic and Islamic medicine, a re-emerging health aid. Evidence-Based Complement. Altern. Med. 2010, 7, 419–424. [Google Scholar] [CrossRef]
- Al-Ramahi, R.; Jaradat, N.; Adawi, D. Use of herbal medicines during pregnancy in a group of Palestinian women. J. Ethnopharmacol. 2013, 150, 79–84. [Google Scholar] [CrossRef]
- Mbemya, G.T.; Vieira, L.A.; Canafistula, F.G.; Pessoa, O.D.L.; Rodrigues, A.P.R. Reports on in vivo and in vitro contribution of medicinal plants to improve the female reproductive function. Reprodução Clim. 2017, 32, 109–119. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Jamous, R.M. Plants used during pregnancy, childbirth, postpartum and infant healthcare in Palestine. Complement. Ther. Clin. Pract. 2015, 21, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Macht, D.I. The action of so-called emmenagogue oils on the isolated uterine strip. J. Pharm. Exp. Ther. 1913, 4, 547–553. [Google Scholar]
- Soares, M.C.M.S.; Damiani, C.E.N.; Moreira, C.M.; Stefanon, I.; Vassallo, D.V. Eucalyptol, an essential oil, reduces contractile activity in rat cardiac muscle. Braz. J. Med. Biol. Res. 2005, 38, 453–461. [Google Scholar] [CrossRef]
- Kong, Y.C.; Lau, C.P.; Wat, K.H.; Ng, K.H.; But, P.P.H.; Cheng, K.F.; Waterman, P.G. Antifertility Principle of Ruta graveolens. Planta Med. 1989, 55, 176–178. [Google Scholar] [CrossRef]
- Datnow, M.M. An experimental investigation concerning toxic abortion produced by chemical agents. J. Obs. Gynaecol. 1928, 35, 693–724. [Google Scholar] [CrossRef]
- Diel, P.; Smolnikar, K.; Michna, H. In vitro test systems for the evaluation of the estrogenic activity of natural products. Planta Med. 1999, 65, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Timbrell, J.A. Principles of Biochemical Toxicology, 3rd ed.; Taylor & Francis: Padstow, UK, 2000. [Google Scholar]
- Chaiworapongsa, T.; Romero, R.; Kusanovic, J.P.; Savasan, Z.A.; Kim, S.K.; Mazaki-Tovi, S.; Vaisbuch, E.; Ogge, G.; Madan, I.; Dong, Z.; et al. Unexplained Fetal Death is Associated with Increased Concentrations of Anti-Angiogenic Factors in Amniotic Fluid. J. Matern. Fetal Neonatal. Med. 2010, 23, 794–805. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, K.; Li, J.; Cui, X.; Wang, A.; Huang, S.; Zheng, S.; Lu, Y.; Chen, W. Inhibition of vascular endothelial growth factor-mediated angiogenesis involved in reproductive toxicity induced by sesquiterpenoids of Curcuma zedoaria in rats. Reprod. Toxicol. 2013, 37, 62–69. [Google Scholar] [CrossRef]
- Ogata, A.; Ando, H.; Kubo, Y.; Nagasawa, A.; Ogawa, H.; Yasuda, K.; Aoki, N. Teratogenicity of Thujaplicin in ICR Mice. Food Chem. Toxicol. 1999, 37, 1097–1104. [Google Scholar] [CrossRef]
- Reynolds, J.E.F. Martindale: The Extra Pharmacopoeia; The Pharmaceutical Press: London, UK, 1993. [Google Scholar]
- Maickel, R.P.; Snodgrass, W.R. Physicochemical factors in maternal-fetal distribution of drugs. Toxicol. Appl. Pharmacol. 1973, 26, 218–230. [Google Scholar] [CrossRef]
- Forschmidt, P.; Liban, E.; Abramovici, A. Teratogenic activity of flavour additives. Teratology 1979, 19, 26A. [Google Scholar]
- Jori, A.; Briatico, G. Effect of eucalyptol on microsomal enzyme activity of foetal and newborn rats. Biochem. pharmacol. 1973, 22, 543–544. [Google Scholar] [CrossRef]
- Hausner, H.; Bredie, W.L.; Molgaard, C.; Petersen, M.A.; Moller, P. Differential transfer of dietary flavour compounds into human breast milk. Physiol. Behav. 2008, 95, 118–124. [Google Scholar] [CrossRef]
- Chhabra, S.K.; Rao, A.R. Postnatal modulation of xenobiotic metabolizing enzymes in liver of mouse pups following translactational exposure to sandalwood oil. Nutr. Res. 1993, 13, 1191–1202. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety, 2nd. ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Lawrence, B.M. Essential Oils 1988–1991; Allured Publishing: Wheaton, IL, USA, 1995. [Google Scholar]
- Kubeczka, K.-H.; Formacek, V. Essential Oils Analysis by Capillary Gas Chromatography and Carbon-13NMR Spectroscopy, 2nd ed.; J. Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Brophy, J.J.; Goldsack, R.J.; Fookes, C.J.R.; Forster, P.I. Leaf Oils of the Genus Backhousia (Myrtaceae). J. Essent. Oil Res. 1995, 7, 237–254. [Google Scholar] [CrossRef]
- Raharivelomanana, P.; Cambon, A.; Azzaro, M.; Bianchini, J.P.; Claude-Lafontaine, A.; George, G. Volatile constituents of Neocallitropsis pancheri (Carriere) de Laubenfels heartwood extracts (Cupressaceae). J. Essent. Oil Res. 1993, 5, 587–595. [Google Scholar] [CrossRef]
- Mozaffari, F.S.; Ghorbanli, M.; Babai, A.; Sepehr, M.F. The Effect of Water Stress on the Seed Oil of Nigella sativa L. J. Essent. Oil Res. 2000, 12, 36–38. [Google Scholar] [CrossRef]
- Doimo, L. Azulenes, Costols and γ-Lactones from Cypress-Pines (Callitris columellaris, C. glaucophylla and C. intratropica) Distilled Oils and Methanol Extracts. J. Essent. Oil Res. 2001, 13, 25–29. [Google Scholar] [CrossRef]
- Collins, N.F.; Graven, E.H.; Van Beek, T.A.; Lelyveld, G.P. Chemotaxonomy of Commercial Buchu Species (Agathosma betulina and A. crenulata). J. Essent. Oil Res. 1996, 8, 229–235. [Google Scholar] [CrossRef]
- Posthumus, M.A.; van Beek, T.A.; Collins, N.F.; Graven, E.H. Chemical Composition of the Essential Oils of Agathosma betulina, A. crenulata and an A. betulina x crenulata Hybrid (Buchu). J. Essent. Oil Res. 1996, 8, 223–228. [Google Scholar] [CrossRef]
- Mazzoni, V.; Tomi, F.; Casanova, J. A daucane-type sesquiterpene from Daucus carota seed oil. Flavor Fragr. J. 1999, 14, 268–272. [Google Scholar] [CrossRef]
- Lawrence, B.M. Brogress in essential oils. Perfum. Flavorist 1995, 20, 48–49. [Google Scholar]
- Senatore, F.; Della Porta, G.; Reverchon, E. Constituents of Vitex agnus-castus L. Essential Oil. Flavor Fragr. J. 1996, 11, 179–182. [Google Scholar] [CrossRef]
- Sørensen, J.M.; Katsiotis, S.T. Parameters influencing the yield and composition of the essential oil from Cretan Vitex agnus-castus fruits. Planta Med. 2000, 66, 245–250. [Google Scholar] [CrossRef]
- Mahindru, S.N. Indian Plant Perfumes; Metropolitan: New Delhi, India, 1992. [Google Scholar]
- Lawrence, B.M. Progress in essential oils. Perfum. Flavorist 1998, 23, 50. [Google Scholar]
- Lawrence, B.M. Progress in essential oils. Perfum. Flavorist 1999, 24, 56–59. [Google Scholar]
- Mucciarelli, M.; Caramiello, R.; Maffei, M.; Chialva, F. Essential oils from some Artemisia species growing spontaneously in North-West Italy. Flavor Fragr. J. 1995, 10, 25–32. [Google Scholar] [CrossRef]
- Tucker, A.O.; Maciarello, M.J.; Sturtz, G. The essential oils of Artemisia ‘Powis Castle’ and its Putative Parents, A. absinthium and A. arborescens. J. Essent. Oil Res. 1993, 5, 239–242. [Google Scholar] [CrossRef]
- Pélissier, Y.; Marion, C.; Prunac, S.; Bessière, J.-M. Volatile Components of Leaves, Stems and Bark of Cinnamonum camphora Nees et Ebermaier. J. Essent. Oil Res. 1995, 7, 313–315. [Google Scholar]
- Lawrence, B.M. Essential Oils 1981–1987; Allured Publishing: Wheaton, IL, USA, 1989. [Google Scholar]
- Southwell, I.A.; Russell, M.; Smith, R.L.; Archer, D.W. Backhousia citriodora F. Muell. (Myrtaceae), A Superior Source of Citral. J. Essent. Oil Res. 2000, 12, 735–741. [Google Scholar] [CrossRef]
- Horváth, G.; Szabó, L.G.; Héthelyi, É.; Lemberkovics, É. Essential Oil Composition of Three Cultivated Thymus Chemotypes from Hungary. J. Essent. Oil Res. 2006, 18, 315–317. [Google Scholar]
- Lawrence, B.M. Progress in essential oils: Ocimum gratissimum oil, cinnamon oil, tarragon oil, and palmarosa oil. Perfum. Flavorist 2002, 27, 42–52. [Google Scholar]
- Chagonda, L.S.; Makanda, C.; Chalchat, J.C. Essential oils of cultivated Cymbopogon winterianus (Jowitt) and of C. citratus (DC) (Stapf) from Zimbabwe. J. Essent. Oil Res. 2000, 12, 478–480. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Bean, A.R.; Forster, P.I.; Lepsch, B.J. Leaf essential oils of the genus Leptospermum (Myrtaceae) in eastern Australia, Part 6. Leptospermum polygalifolium and allies. Flav. Fragr. J. 2000, 15, 271–277. [Google Scholar] [CrossRef]
- Ristorcelli, D.; Tomi, F.; Casanova, J. Essential Oils of Calamintha nepeta subsp. nepeta and subsp. glandulosa from Corsica (France). J. Essent. Oil Res. 1996, 8, 363–366. [Google Scholar] [CrossRef]
- Lawrence, B.M. Progress in essential oils. Perfum. Flavorist 1996, 21, 59–60. [Google Scholar]
- Haider, F.; Dwivedi, P.D.; Naqvi, A.A.; Bagchi, G.D. Essential oil composition of Artemisia vulgaris harvested at different growth periods under Indo-Gangetic plain conditions. J. Essent. Oil Res. 2003, 15, 376–378. [Google Scholar] [CrossRef]
- Dekebo, A.; Dagne, E.; Sterner, O. Furanosesquiterpenes from Commiphora sphaerocarpa and related adulterants of true myrrh. Fitoterapia 2002, 73, 48–55. [Google Scholar] [CrossRef]
- Simpson, G.I.; Jackson, Y.A. Comparison of the chemical composition of East Indian, Jamaican and other West Indian essential oils of Myristica fragrans Houtt. J. Essent. Oil Res. 2002, 14, 6–9. [Google Scholar] [CrossRef]
- Kaiser, R. New Volatile Constituents of the Flower Concrete of Michelia champaca L. J. Essent. Oil Res. 1991, 3, 129–146. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Kürkçüoglu, M.; Tümen, G. The Essential Oil of Origanum vulgare subsp. hirtum of Turkish Origin. J. Essent. Oil Res. 1994, 6, 31–36. [Google Scholar] [CrossRef]
- Karpouhtsis, I.; Pardali, E.; Feggou, E.; Kokkini, S.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and genotoxic activities of Oregano essential oils. J. Agric. Food Chem. 1998, 46, 1111–1115. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Tümen, G.; Sezik, E. Composition of the Essential Oils of Turkish Origanum Species with Commercial Importance. J. Essent. Oil Res. 1993, 5, 619–623. [Google Scholar] [CrossRef]
- Lagouri, V.; Blekas, G.; Tsimidou, M.; Kokkini, S.; Boskou, D. Composition and antioxidant activity of essential oils from Oregano plants grown wild in Greece. Zeitschrift für Leb. Forsch. 1993, 197, 20–23. [Google Scholar] [CrossRef]
- Lawrence, B.M. Progress in essential oils. Perfum. Flavorist 2001, 26, 38. [Google Scholar]
- Lamarti, A.; Badoc, A.; Bouriquet, R. A Chemotaxonomic Evaluation of Petroselinum crispum (Mill.) A. W. Hill (Parsley) Marketed in France. J. Essent. Oil Res. 1991, 3, 425–433. [Google Scholar] [CrossRef]
- Fournier, G.; Paris, M.; Dumitresco, S.M.; Pages, N.; Boudene, C. Contribution to the study of Plectranthus fruticosus leaf essential oil. Planta Med. 1986, 6, 486–488. [Google Scholar] [CrossRef] [PubMed]
- De Feo, V.; De Simone, F.; Senatore, F. Potential allelochemicals from the essential oil of Ruta graveolens. Phytochemistry 2002, 61, 573–578. [Google Scholar] [CrossRef]
- 61. SCCP Opinion on furocoumarins in cosmetic products. Scientific committee on consumer products. SCCP/0942/05. 2005. Available online: https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_036.pdf (accessed on 29 January 2021).
- Yatagai, M.; Sato, T.; Yakahashi, T. Terpenes of leaf oils from Cupressaceae. Biochem. Syst. Ecol. 1985, 13, 377–385. [Google Scholar] [CrossRef]
- Mathela, C.S.; Kharkwal, H.; Shah, G.C. Essential Oil Composition of Some Himalayan Artemisia Species. J. Essent Oil Res. 1994, 6, 345–348. [Google Scholar] [CrossRef]
- Ristorcelli, D.; Tomi, F.; Casanova, J. 13C-NMR as a tool for identification and enantiomeric differentiation of major terpenes exemplified by the essential oil of Lavandula stoechas L. ssp. stoechas. Flavor Fragr. J. 1998, 13, 154–158. [Google Scholar] [CrossRef]
- Usano-Alemany, J.; Herraiz-Peñalver, D.; Cuadrado, J.; Díaz, S.; Santa-Cruz, M.; Palá-Paúl, J. Seasonal Variation of the Essential Oils of Salvia lavandulifolia: Antibacterial Activity. J. Essent. Oil Bear. Plants 2012, 15, 195–203. [Google Scholar] [CrossRef]
- Singh, G.; Singh, O.P.; Maurya, S. Chemical and biocidal investigations on essential oils of some Indian curcuma species. Prog. Cryst. Growth Charact. Mater. 2002, 45, 75–81. [Google Scholar] [CrossRef]
- Santos-Gomes, P.C.; Fernandes-Ferreira, M.; Vicente, A.M.S. Composition of the Essential Oils from Flowers and Leaves of Vervain (Aloysia triphylla (L’Herit.) Britton) Grown in Portugal. J. Essent. Oil Res. 2005, 17, 73–78. [Google Scholar] [CrossRef]
- Von Rudloff, E.; Lapp, M.S.; Yeh, F.C. Chemosystematic study of Thuja plicata: Multivariate analysis of leaf oil terpene composition. Biochem. Syst. Ecol. 1988, 16, 119–125. [Google Scholar] [CrossRef]
- Ghanmi, M.; Satrani, B.; Aafi, A.; Isamili, M.R.; Houti, H.; El Monfalouti, H.; Benchakroun, K.H.; Aberchane, M.; Harki, L.; Boukir, A. Effect of harvest date on yield, chemical composition and bioactivity of essential oils of sagebrush (Artemisia herbaalba) in the region of Guercif (eastern Morocco). Phytothérapie 2010, 8, 295–301. [Google Scholar] [CrossRef]
- Angel, G.R.; Menon, N.; Vimala, B.; Nambisan, B. Essential oil composition of eight starchy Curcuma species. Ind. Crops Prod. 2014, 60, 233–238. [Google Scholar] [CrossRef]
- Singh, P.; Singh, S.; Kapoor, I.P.S.; Singh, G.; Isidorov, V.; Szczepaniak, L. Chemical composition and antioxidant activities of essential oil and oleoresins from Curcuma zedoaria rhizomes, part-74. Food Biosci. 2013, 3, 42–48. [Google Scholar] [CrossRef]
- Purkayastha, J.; Nath, S.C.; Klinkby, N. Essential oil of the rhizome of Curcuma zedoaria (Christm.) Rosc. Native to northeast India. J. Essent. Oil Res. 2006, 18, 154–155. [Google Scholar] [CrossRef]
- Herath, H.M.I.C.; Wiyasiriwardene, T.D.C.M.K.; Premakumara, G.A.S. Comparative GC-MS analysis of all Curcuma species grown in Sri Lanka by multivariate test. Ruhunu J. Sci. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Boissier, J.R.; Simon, P.; Le Bourhis, B. Experimental psychotropic action of cis and trans isomers of anethol. Therapie 1967, 22, 309–323. [Google Scholar] [PubMed]
- Ritter, A.M.V.; Domiciano, T.P.; Verri, W.A.; Zarpelon, A.C.; Da Silva, L.G.; Barbosa, C.P.; Natali, M.R.M.; Cuman, R.K.N.; Bersani-Amado, C.A. Antihypernociceptive activity of anethole in experimental inflammatory pain. Inflammopharmacology 2013, 21, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Shahbazian, S.; Akbarzadeh, A.; Torabi, S.; Omidi, M. Anti-cancer activity of pegylated liposomal trans-anethole on breast cancer cell lines MCF-7 and T47D. Biotechnol. Lett. 2015, 37, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Domiciano, T.P.; Dalalio, M.M.H.D.O.; Silva, E.L.; Ritter, A.M.V.; Estevão-Silva, C.F.; Ramos, F.S.; Caparroz-Assef, S.M.; Cuman, R.K.N.; Bersani-Amado, C.A. Inhibitory effect of anethole in nonimmune acute inflammation. Naunyn. Schmiedebergs. Arch. Pharmacol. 2013, 386, 331–338. [Google Scholar] [CrossRef]
- Albert-Puleo, M. Fennel and anise as estrogenic agents. J. Ethnopharmacol. 1980, 2, 337–344. [Google Scholar] [CrossRef]
- Türkyilmaz, Z.; Karabulut, R.; Sönmez, K.; Başaklar, K.A. A striking and frequent cause of premature thelarche in children: Foeniculum vulgare. J. Pediatr. Surg. 2008, 43, 2109–2111. [Google Scholar]
- Dhar, S.K. Anti-fertility activity and hormonal profile of trans-anethole in rats. Indian J. Physiol. Pharmacol. 1995, 39, 63–67. [Google Scholar] [PubMed]
- Newberne, P.M.; Carlton, W.W.; Brown, W.R. Histopathological evaluation of proliferative liver lesions in rats fed trans-anethole in chronic studies. Food Chem. Toxicol. 1989, 27, 21–26. [Google Scholar] [CrossRef]
- Kim, S.G.; Liem, A.; Stewart, B.C.; Miller, J.A. New studies on trans-anethole oxide and trans-asarone oxide. Carcinogenesis 1999, 20, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.D.; Caldwell, J. Influence of modulators of epoxide metabolism on the cytotoxicity of trans-anethole in freshly isolated rat hepatocytes. Food Chem. Toxicol. 1992, 30, 467–473. [Google Scholar] [CrossRef]
- Ostad, S.N.; Khakinegad, B.; Sabzevari, O. Evaluation of the teratogenicity of fennel essential oil (FEO) on the rat embryo limb buds culture. Toxicol. In Vitro 2004, 18, 623–627. [Google Scholar] [CrossRef]
- Bilia, A.R.; Flamini, G.; Taglioli, V.; Morelli, I.; Vincieri, F.F. GC–MS analysis of essential oil of some commercial Fennel teas. Food Chem. 2002, 76, 307–310. [Google Scholar] [CrossRef]
- Ostad, S.N.; Soodi, M.; Shariffzadeh, M.; Khorshidi, N.; Marzban, H. The effect of fennel essential oil on uterine contraction as a model for dysmenorrhea, pharmacology and toxicology study. J. Ethnopharmacol. 2001, 76, 299–304. [Google Scholar] [CrossRef]
- Blumenthal, M.; Goldberg, A.; Brinckmann, J. Herbal Medicine: Expanded Commission E Monographs; Integrative Medicine Communications: Boston, MA, USA, 2000. [Google Scholar]
- Blumenthal, M.; Busse, W.R.; Goldberg, A.; Gruenwald, J.; Hall, T.; Riggins, C.W.; Rister, R.S. The Complete German Commission E Monographs. Therapeutic Guide to Herbal Medicines; American Botanical Council: Austin, TX, USA, 1998. [Google Scholar]
- Rietjens, I.M.; Boersma, M.G.; van der Woude, H.; Jeurissen, S.M.; Schutte, M.E.; Alink, G.M. Flavonoids and alkenylbenzenes: Mechanisms of mutagenic action and carcinogenic risk. Mutat. Res. 2005, 574, 124–138. [Google Scholar] [CrossRef]
- Yancu, D.; Vaillancourt, C.; Sanderson, J.T. Evaluating the effects on steroidogenesis of estragole and trans-anethole in a feto-placental co-culture model. Mol. Cell. Endocrinol. 2019, 498, 110583. [Google Scholar] [CrossRef] [PubMed]
- Yancu, D.; Sanderson, T. Essential oils disrupt steroidogenesis in a feto-placental co-culture model. Reprod. Toxicol. 2019, 90, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Marković, T.; Mojović, M.; Pejin, B.; Savić, A.; Perić, T.; Marković, D.; Stević, T.; Soković, M. Chemical composition and biological activity of Gaultheria procumbens L. essential oil. Ind. Crop. Prod. 2013, 49, 561–567. [Google Scholar] [CrossRef]
- Lapczynski, A.; Jones, L.; McGinty, D.; Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on methyl salicylate. Food Chem. Toxicol. 2007, 45, 428–452. [Google Scholar] [CrossRef] [PubMed]
- Heng, M.C. Local necrosis and interstitial nephritis due to topical methyl salicylate and menthol. Cutis 1987, 39, 442–444. [Google Scholar]
- Cross, S.E.; Anderson, C.; Thompson, M.J.; Roberts, M.S. Is there tissue penetration after application of topical salicylate formulations? Lancet 1997, 350, P636. [Google Scholar] [CrossRef]
- Davison, C.; Zimmermann, E.F.; Smith, P.K. On the metabolism and toxicity of methyl salicylate. J. Pharmacol. Exp. Ther. 1961, 132, 207–211. [Google Scholar]
- Ojiambo, H.P. Methylsalicylate myopathy in man. East Afr. Med. J. 1971, 48, 735–740. [Google Scholar] [PubMed]
- Opdyke, D.L.J. Monographs on fragrance raw materials. Food Cosmet. Toxicol. 1978, 16, 56–59. [Google Scholar]
- Adams, J.T.; Bigler, J.A.; Green, O.C. A case of methyl salicylate intoxication treated by exchange transfusion. J. Am. Med. Assoc. 1957, 165, 1563–1565. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, C.; Hu, Y.; Sun, L.; Jiao, J.; Zhao, L.; Zhu, D.; Li, J.; Tian, Y.; Bai, H.; et al. The estrogenic potential of salicylate esters and their possible risks in foods and cosmetics. Toxicol. Lett. 2012, 209, 146–153. [Google Scholar] [CrossRef]
- Ellenhorn, M.J.; Barceloux, D.G. Medical Toxicology: Diagnosis and Treatment of Human Poisoning; Elsevier Science: New York, NY, USA, 1988. [Google Scholar]
- Wilson, J.G. Present status of drugs as teatogens in man. Teratology 1973, 7, 3–15. [Google Scholar] [CrossRef]
- Karabulut, A.K.; Ulger, H.; Pratten, M. Protection by free oxygen radical scavenging enzymes against salicylate-induced embryonic malformations in vitro. Toxicol. In Vitro 2000, 14, 297–307. [Google Scholar] [CrossRef]
- Kavlock, R.J.; Chernoff, N.; Rogers, E.; Whitehouse, D.; Carver, B.; Gray, J.; Robinson, K. An analysis of fetotoxicity using biochemical endpoints of organ differentiation. Teratology 1982, 26, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.C.; Hoar, R.M. “Apparent hydronephrosis” as a normal aspect of renal development in late gestation of rats: The effect of methyl salicylate. Teratology 1972, 6, 191–196. [Google Scholar] [CrossRef]
- Pyun, J.S. Effect of methyl salicylate on developing rat embryos. Ch’oesin Uihak 1970, 13, 63–72. [Google Scholar]
- Daston, G.P.; Rehnberg, B.F.; Carver, B.; Rogers, E.H.; Kavlock, R.J. Functional Teratogens of the Rat Kidney: I. Colchicine, Dinoseb, and Methyl Salicylate. Toxicol. Sci. 1988, 11, 381–400. [Google Scholar] [CrossRef]
- Warkany, J.; Takacs, E. Experimental Production of Congenital Malformations in Rats by Salicylate Poisoning. Am. J. Pathol. 1959, 35, 315–331. [Google Scholar] [PubMed]
- Saito, H.; Yokoyama, A.; Takeno, S.; Sakai, T.; Ueno, K.; Masumura, H.; Kitagawa, H. Fetal Toxicity and Hypocalcemia Induced by Acetylsalicylic Acid Analogues. Res. Commun. Chem. Pathol. Pharmacol. 1982, 38, 209–220. [Google Scholar]
- Overman, D.O.; White, J.A. Comparative Teratogenic Effects of Methyl Salicylate Applied Orally or Topically to Hamsters. Teratology 1983, 28, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Pages, N.; Fournier, G.; Baduel, C.; Tur, N.; Rusnac, M. Sabinyl Acetate, the Main Component of Juniperus sabina L’Hérit. Essential Oil, is Responsible for Antiimplantation Effect. Phyther. Res. 1996, 10, 438–440. [Google Scholar] [CrossRef]
- Chamorro, G.; Salazar, M.; Fournier, G.; Pages, N. Anti-implantation effects of various extracts of Plectranthus fruticosus on pregnant rats. Planta Med. 1991, 57, 81. [Google Scholar] [CrossRef] [PubMed]
- Pages, N.; Salazar, M.; Chamorro, G.; Fournier, G.; Paris, M.; Dumitresco, S.M.; Boudene, C. Teratological evaluation of Plectranthus fruticosus leaf essential oil. Planta Med. 1988, 54, 296–298. [Google Scholar] [CrossRef]
- Pages, N.; Fournier, G.; Chamorro, G.; Salazar, M. Teratogenic effects of Plectranthus fruticosus essential oil in mice. Phyther. Res. 1991, 5, 94–96. [Google Scholar] [CrossRef]
- Pages, N.; Fournier, G.; Velut, V.; Imbert, C. Potential teratogenicity in mice of the essential oil of Salvia lavandulifolia Vahl. Study of a fraction rich in sabinyl acetate. Phyther. Res. 1992, 6, 80–83. [Google Scholar] [CrossRef]
- Pages, N.; Fournier, G.; Chamorro, G.; Salazar, M.; Paris, M.; Boudene, C. Teratological evaluation of Juniperus sabina essential oil in mice. Planta Med. 1989, 55, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Grieve, M. A Modern Herbal; Penguin books: Harmondsworth, UK, 1978. [Google Scholar]
- Chamorro, G.; Salazar, M.; Fournier, G.; Pages, N. The anti-implantation effects of various savine extracts on the pregnant rat. J. Toxicol. Clin. Exp. 1990, 10, 157–160. [Google Scholar]
- Agrawal, O.P.; Bharadwaj, S.; Mathur, R. Antifertility Effects of Fruits of Juniperus Communis. Planta Med. 1980, 39, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.S.; Houghton, P.J.; Sampson, J.; Theobald, A.E.; Hart, S.; Lis-Balchin, M.; Hoult, J.R.; Evans, P.; Jenner, P.; Milligan, S.; et al. In-vitro activity of S. lavandulaefolia (Spanish sage) relevant to treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2001, 53, 1347–1356. [Google Scholar] [CrossRef]
- Millet, Y.; Jouglard, J.; Steinmetz, M.D.; Tognetti, P.; Joanny, P.; Arditti, J. Toxicity of some essential plant oils. Clinical and experimental study. Clin. Toxicol. 1981, 18, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Küpeli Akkol, E.; Ilhan, M.; Ayşe Demirel, M.; Keleş, H.; Tümen, I.; Süntar, I. Thuja occidentalis L. and its active compound, α-thujone: Promising effects in the treatment of polycystic ovary syndrome without inducing osteoporosis. J. Ethnopharmacol. 2015, 168, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.Y.; Foster, S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Gerarde, H.W. Toxicology and Biochemistry of Aromatic Hydrocarbons; Elsevier: London, UK, 1960. [Google Scholar]
- Budavari, S. The Merck Index, 11th ed.; Merck: Branchburg, NJ, USA, 1989. [Google Scholar]
- Sampson, W.K.; Fernandez, G. Experimental convulsions in the rat. J. Pharm. Exp. Ther. 1939, 65, 275–280. [Google Scholar]
- Hall, A.C.; Turcotte, C.M.; Betts, B.A.; Yeung, W.-Y.; Agyeman, A.S.; Burk, L.A. Modulation of human GABAA and glycine receptor currents by menthol and related monoterpenoids. Eur. J. Pharmacol. 2004, 506, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Szczot, M.; Czyzewska, M.M.; Appendino, G.; Mozrzymas, J.W. Modulation of GABAergic Synaptic Currents and Current Responses by α-thujone and Dihydroumbellulone. J. Nat. Prod. 2012, 75, 622–629. [Google Scholar] [CrossRef]
- Czyzewska, M.M.; Mozrzymas, J.W. Monoterpene α-thujone exerts a differential inhibitory action on GABA A receptors implicated in phasic and tonic GABAergic inhibition. Eur. J. Pharmacol. 2013, 702, 38–43. [Google Scholar] [CrossRef]
- Pelkonen, O.; Abass, K.; Wiesner, J. Thujone and thujone-containing herbal medicinal and botanical products: Toxicological assessment. Regul. Toxicol. Pharmacol. 2013, 65, 100–107. [Google Scholar] [CrossRef]
- Margaria, R. Acute and Sub-acute Toxicity Study on Thujone. Unpublished report of the Istituto di Fisiologia, Università di Milano (cited from CoE Datasheet RD4.2/14-44, 1999); Istituto di Fisiologia, Università di Milano: Milano, Italy, 1963. [Google Scholar]
- Abass, K.; Reponen, P.; Mattila, S.; Pelkonen, O. Metabolism of α-thujone in Human Hepatic Preparations in Vitro. Xenobiotica 2011, 41, 101–111. [Google Scholar] [CrossRef]
- Burkhard, P.R.; Burkhardt, K.; Haenggeli, C.A.; Landis, T. Plant-induced Seizures: Reappearance of an Old Problem. J. Neurol. 1999, 246, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Laadraoui, J.; Aboufatima, R.; El Gabbas, Z.; Ferehan, H.; Bezza, K.; Ait Laaradia, M.; Marhoume, F.; Wakrim, E.M.; Chait, A. Effect of Artemisia herba-alba consumption during pregnancy on fertility, morphological and behaviors of mice offspring. J. Ethnopharmacol. 2018, 226, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Almasad, M.M.; Qazan, W.S.; Daradka, H. Reproductive toxic effects of Artemisia herba alba ingestion in female Spague-Dawley rats. Pak. J. Biol. Sci. 2007, 10, 3158–3161. [Google Scholar] [PubMed]
- Ciganda, C.; Laborde, A. Herbal infusions used for induced abortion. J. Toxicol. Clin. Toxicol. 2003, 41, 235–239. [Google Scholar] [CrossRef]
- Lowenstein, L.; Ballew, D.H. Fatal acute haemolytic anaemia, thrombocytopenic purpura, nephrosis and hepatitis resulting from ingestion of a compound containing apiol. Can. Med. Assoc. J. 1958, 78, 195–198. [Google Scholar] [PubMed]
- Laederich, L.; Mamou, H.; Arager, M. Fatal intoxication from apiol. Bull. Soc. Med. Hosp. Paris 1932, 48, 746–751. [Google Scholar]
- Amerio, A.; De Benedictis, G.; Leondeff, J.; Mastrangelo, F.; Coratelli, P. Nephropathy due to apiol. Minerva Nefrol. 1968, 15, 49–70. (In Italian) [Google Scholar] [PubMed]
- Patoir, A.; Patoir, G.; Bedrine, H. Le role abortif de l’apiol. Paris Med. 1936, 3, 442–446. [Google Scholar]
- Riggs, J.; Hamilton, R.; Homel, S.; McCabe, J. Camphorated oil intoxication during pregnancy: Report of a case. Obs. Gynecol. 1965, 25, 255–258. [Google Scholar]
- Phelan, W.J.I. Camphor poisoning: Over-the-counter dangers. Pediatrics 1976, 57, 428–431. [Google Scholar]
- Banerjee, S.; Welsch, C.W.; Rao, A.R. Modulatory Influence of Camphor on the Activities of Hepatic Carcinogen Metabolizing Enzymes and the Levels of Hepatic and Extrahepatic Reduced Glutathione in Mice. Cancer Lett. 1995, 88, 163–169. [Google Scholar] [CrossRef]
- Spector, W.S. Handbook of Toxicology. Vol 1. Acute Toxicities; WB Saunders: Philadelphia, PA, USA, 1956. [Google Scholar]
- Siegel, E.; Wason, S. Camphor toxicity. Pediatr. Clin. N. Am. 1986, 33, 375–379. [Google Scholar] [CrossRef]
- Smith, A.G.; Margolis, G. Camphor Poisoning; Anatomical and Pharmacologic Study; Report of a Fatal Case; Experimental Investigation of Protective Action of Barbiturate. Am. J. Pathol. 1954, 30, 857–869. [Google Scholar]
- Merkulova, O.S. Reflex Mechanism of camphor and pyramidone experimental epilepsy. Dokl. Akad. Nauk. USSR 1957, 112, 968–971. [Google Scholar]
- Wenzel, D.G.; Ross, C.R. Central Stimulating Properties of Some Terpenones. J. Am. Pharm. Assoc. 1957, 46, 77–82. [Google Scholar] [CrossRef]
- Committee on Drugs Camphor revisited: Focus on toxicity (RE9422). Pediatrics 1994, 94, 127–128.
- Rahimi, M.; Shokri, F.; Hassanian-Moghaddam, H.; Zamani, N.; Pajoumand, A.; Shadnia, S. Severe camphor poisoning, a seven-year observational study. Environ. Toxicol. Pharmacol. 2017, 52, 8–13. [Google Scholar] [CrossRef]
- Rabl, W.; Katzgraber, F.; Steinlechner, M. Camphor ingestion for abortion (case report). Forensic Sci. Int. 1997, 89, 137–140. [Google Scholar] [CrossRef]
- Leuschner, J. Reproductive toxicity studies of d-camphor in rats and rabbits. Arzneimmittel-Forschung 1997, 47, 124–128. [Google Scholar]
- Stojanović, N.M.; Randjelović, P.J.; Mladenović, M.Z.; Ilić, I.R.; Petrović, V.; Stojiljković, N.; Ilić, S.; Radulović, N.S. Toxic essential oils, part VI: Acute oral toxicity of lemon balm (Melissa officinalis L.) essential oil in BALB/c mice. Food Chem. Toxicol. 2019, 113, 110794. [Google Scholar] [CrossRef] [PubMed]
- Opdyke, D.L.J. Citral. Food Chem. Toxicol. 1979, 17, 259–266. [Google Scholar]
- Feron, V.J.; TiI, H.P.; de Vrijer, F.; Woutersen, R.A.; Cassee, F.R.; van Bladeren, P.J. Aldehydes: Occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat. Res. 1991, 259, 363–385. [Google Scholar] [CrossRef]
- Toaff, M.E.; Abramovici, A.; Spom, J.; Liban, E. Selective oocyte degeneration and impaired fertility in rats treated with the ahphatic monoterpene, Citral. J. Reprod. Fertil. 1979, 55, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-J.; Shin, J.-O.; Lee, J.-M.; Cho, K.-W.; Lee, M.-J.; Cho, S.-W.; Jung, H.-S. Retinoic acid modulates chondrogenesis in the developing mouse cranial base. J. Exp. Zool. B Mol. Dev. Evol. 2011, 316, 574–583. [Google Scholar] [CrossRef]
- Koussoulakou, D.S.; Margaritis, L.H.; Koussoulakos, S.L. Antagonists of retinoic acid and BMP4 affect fetal mouse osteogenesis and odontoblast differentiation. Pathophysiology 2011, 18, 103–109. [Google Scholar] [CrossRef]
- Tanaka, M.; Tamura, K.; Ide, H. Citral, an inhibitor of retinoic acid synthesis, modifies chick limb development. Dev. Biol. 1996, 175, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, F.; Broccia, M.L.; Giavini, E.; Menegola, E. Citral, an inhibitor of retinoic acid synthesis, attenuates the frequency and severity of branchial arch abnormalities induced by triazole-derivative fluconazole in rat embryos cultured in vitro. Reprod. Toxicol. 2007, 24, 326–332. [Google Scholar] [CrossRef]
- Connor, M.J. Oxidation of retinol to retinoic acid as a requirement for biological activity in mouse epidermis. Cancer Res. 1988, 48, 7038–7040. [Google Scholar] [PubMed]
- Connor, M.J. Modulation of tumor promotion in mouse skin by the food additive citral (3,7-dimethyl-2,6-octadienal). Cancer Lett. 1991, 56, 25–28. [Google Scholar] [CrossRef]
- Abramovici, A. Teratogenic effect of cosmetic eonstituents on the chick embryo. Adv. Exp. Med. Biol. 1972, 27, 161–174. [Google Scholar]
- Abramovici, A.; Kam, J.; Liban, E.; Barishak, R.Y. Incipient histological lesions in citral-induced microphthalmos in chick embryos. Dev. Neurosci. 1978, 1, 177–185. [Google Scholar] [CrossRef]
- Abramovici, A.; Rachmuth-Forschmidt, P.; Liban, E.; Andbank, U. Experimental limb dysmorphogenesis as a model of chemical injury response in undifferentiated embryonic tissues: A light and electron microscopical study. J. Pathol. 1980, 131, 289–308. [Google Scholar] [CrossRef]
- Le Bouffant, R.; Guerquin, M.J.; Duquenne, C.; Frydman, N.; Coffigny, H.; Rouiller-Fabre, V.; Frydman, R.; Habert, R.; Livera, G. Meiosis initiation in the human ovary requires intrinsic retinoic acid synthesis. Hum. Reprod. 2010, 25, 2579–2590. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.C.M.A.; Carvalho, R.R.; Souza, C.A.M.; Chahoud, I.; Paumgartten, F.J. Study on the embryofeto-toxicity of citral in the rat. Toxicology 1995, 96, 105–113. [Google Scholar] [CrossRef]
- Gaworski, C.L.; Vollmuth, T.A.; York, R.G.; Heck, J.D.; Aranyi, C. Developmental toxicity evaluation of inhaled citral in Sprague-Dawley rats. Food Chem. Toxicol. 1992, 30, 269–275. [Google Scholar] [CrossRef]
- Griffith, M.; Zile, M.H. Retinoic acid, midkine, and defects of secondary neurulation. Teratology 2000, 62, 123–133. [Google Scholar] [CrossRef]
- Schuh, T.J.; Hall, B.L.; Kraft, J.C.; Privalsky, M.L.; Kimelman, D. v-erbA and citral reduce the teratogenic effects of all-trans retinoic acid and retinol, respectively, in Xenopus embryogenesis. Development 1993, 119, 785–798. [Google Scholar] [PubMed]
- Kronmiller, J.E.; Beeman, C.S.; Nguyen, T.; Berndt, W. Blockade of the initiation of murine odontogenesis in vitro by citral, an inhibitor of endogenous retinoic acid synthesis. Arch. Oral Biol. 1995, 40, 645–652. [Google Scholar] [CrossRef]
- Geldof, A.A.; Engel, C.; Rao, B.R. Estrogenic action of commonly used fragrant agent citral induces prostatic hyperplasia. Urol. Res. 1992, 20, 139–144. [Google Scholar] [CrossRef]
- Thomassen, D.; Knebel, N.; Slattery, J.T.; McClanahan, R.H.; Nelson, S.D. Reactive intermediates in the oxidation of menthofuran by cytochromes P-450. Chem. Res. Toxicol. 1992, 5, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, Y.; Gao, M.; Wu, J.; Wang, A.; Shi, R. Anti-angiogenesis effect of essential oil from Curcuma zedoaria in vitro and in vivo. J. Ethnopharmacol. 2011, 133, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.P.; Huitric, A.C.; Seth, C.L.; McClanahan, R.H.; Nelson, S.D. The metabolism of the abortifacient terpene, (R)-(+)-pulegone, to a proximate toxin, menthofuran. Drug Metab. Dispos. 1987, 15, 589–594. [Google Scholar]
- Chen, X.W.; Serag, E.S.; Sneed, K.B.; Zhou, S.F. Herbal bioactivation, molecular targets and the toxicity relevance. Chem. Biol. Interact. 2011, 192, 161–176. [Google Scholar] [CrossRef]
- Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Bastaki, M.; Davidsen, J.M.; Harman, C.L.; et al. FEMA GRAS assessment of natural flavor complexes: Mint, buchu, dill and caraway derived flavoring ingredients. Food Chem. Toxicol. 2020, 135, 110870. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.; Madyastha, P.; Madyastha, K.M. Hepatotoxicity of pulegone in rats: Its effects on microsomal enzymes, in vivo. Toxicology 1989, 55, 327–337. [Google Scholar] [CrossRef]
- Gordon, W.P.; Forte, A.J.; McMurtry, R.J.; Gal, J.; Nelson, S.D. Hepatotoxicity and pulmonary toxicity of pennyroyal oil and its constituent terpenes in the mouse. Toxicol. Appl. Pharmacol. 1982, 65, 413–424. [Google Scholar] [CrossRef]
- Moorthy, B.; Vijayasarathi, S.K.; Basu, A.; Madyastha, K.M. Biochemical, histopathological and ultrastructural changes in rat liver induced by (R)-(+)-pulegone, a monoterpene ketone. Toxicol. Environ. Chem. 1991, 33, 121–131. [Google Scholar] [CrossRef]
- Madyastha, P.; Moorthy, B. Pulegone-mediated hepatotoxicity: Evidence for covalent binding of (R)-(+)-14C-pulegone to microsomal proteins in vitro. Chem. Biol. Interact. 1989, 72, 325–333. [Google Scholar] [CrossRef]
- Madyastha, P.; Moorthy, B.; Vaidyanathan, C.S.; Madyastha, K.M. In vivo and in vitro destruction of rat liver cytochrome P450 by a monoterpene ketone, pulegone. Biochem. Biophys. Res. Commun. 1985, 128, 921–927. [Google Scholar] [CrossRef]
- Nair, B. Final report on the safety assessment of Mentha piperita (peppermint) oil, Mentha piperita (peppermint) leaf extract, Mentha piperita (peppermint) leaf, and Mentha piperita (peppermint) leaf water. Int. J. Toxicol. 2001, 20, 61–73. [Google Scholar] [PubMed]
- Anderson, I.B.; Mullen, W.H.; Meeker, J.E.; Khojasteh-Bakht, S.C.; Oishi, S.; Nelson, S.D.; Blanc, P.D. Pennyroyal toxicity: Measurement of toxic metabolite levels in two cases and review of the literature. Ann. Intern. Med. 1996, 124, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.B.J.; Rumack, B.H.; Thomas, H.J.; Peterson, R.G.; Bryson, P. Pennyroyal oil poisoning and hepatotoxicity. J. Am. Med. Assoc. 1979, 242, 2873–2874. [Google Scholar] [CrossRef]
- Bradley, P.R. British Herbal Compendium; British Herbal Medicine Association: Bournemouth, UK, 1992. [Google Scholar]
- Benezra, C.; Epstein, W.L. Molecular recognition patterns of sesquiterpene lactones in costus-sensitive patients. Contact Dermatitis 1986, 15, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Itokawa, T.; Shibuya, M.; Kuwano, M.; Ono, M.; Higuchi, R.; Miyamoto, T. Costunolide, a sesquiterpene lactone from Saussurea lappa, inhibits the VEGFR KDR/Flk-1 signaling pathway. Cancer Lett. 2002, 187, 129–133. [Google Scholar] [CrossRef]
- Wang, C.Y.; Tsai, A.C.; Peng, C.Y.; Chang, Y.L.; Lee, K.H.; Teng, C.M.; Pan, S.L. Dehydrocostuslactone suppresses angiogenesis in vitro and in vivo through inhibition of Akt/GSK-3β and mTOR signaling pathways. PLoS ONE 2012, 7, e31195. [Google Scholar] [CrossRef] [PubMed]
- Bamosa, A.O.; Ali, B.A.; Al-Hawsawi, Z.A. The effect of thymoquinone on blood lipids in rats. Indian J. Physiol. Pharmacol. 2002, 46, 195–201. [Google Scholar] [PubMed]
- Yi, T.; Cho, S.G.; Yi, Z.; Pang, X.; Rodriguez, M.; Wang, Y.; Sethi, G.; Aggarwal, B.B.; Liu, M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Ther. 2008, 7, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- AbuKhader, M.M. Thymoquinone in the clinical treatment of cancer: Fact or fiction? Pharmacogn. Rev. 2013, 7, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Al-Enazi, M.M. Effect of thymoquinone on malformations and oxidative stress-induced diabetic mice. Pak. J. Biol. Sci. 2007, 10, 3115–3119. [Google Scholar]
- Chen, W.; Lu, Y.; Wu, J.; Gao, M.; Wang, A.; Xu, B. Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemother. Pharmacol. 2011, 67, 799–808. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Gao, J.; Xie, J.; Yang, L.; Hu, S. Downregulation effects of betaelemene on the levels of plasma endotoxin, serum TNF-alpha, and hepatic CD14 expression in rats with liver fibrosis. Front. Med. 2011, 5, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhou, Y.; Feng, S.; Lv, C.; Xiu, L.; Zhang, Y.; Shi, J.; Li, Y.; Wei, P.; Qin, Z. β-Elemene-Attenuated Tumor Angiogenesis by Targeting Notch-1 in Gastric Cancer Stem-Like Cells. Evid. Based Complement. Altern. Med. 2013, 2013, 268468. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, G.; Huang, F.; Banda, M.; Reed, E. Antineoplastic effect of beta-elemene on prostate cancer cells and other types of solid tumour cells. J. Pharm. Pharmacol. 2010, 62, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, X.P.; Yu, L.L.; Zheng, S. The antitumor activity of elemene is associated with apoptosis. Zhonghua Zhongliu Zazhi 1996, 18, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Zhong, W.; Cai, W. Clinical study on treatment of 40 cases of malignant brain tumor by elemene emulsion injection. Zhongguo Zhong Xi Yi Jie He Za Zhi 2000, 20, 645–648. [Google Scholar]
- Zhong, Z.F.; Hoi, P.M.; Wu, G.S.; Xu, Z.T.; Tan, W.; Chen, X.P.; Cui, L.; Wu, T.; Wang, Y.T. Anti-angiogenic effect of furanodiene on HUVECs in vitro and on zebrafish in vivo. J. Ethnopharmacol. 2012, 141, 721–727. [Google Scholar] [CrossRef]
- Tsuneki, H.; Ma, E.-L.; Kobayashi, S.; Sekizaki, N.; Maekawa, K.; Sasaoka, T.; Wang, M.-W.; Kimura, I. Antiangiogenic activity of beta-eudesmol in vitro and in vivo. Eur. J. Pharmacol. 2005, 512, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Domaracký, M.; Rehák, P.; Juhás, S.; Koppel, J. Effects of selected plant essential oils on the growth and development of mouse preimplantation embryos in vivo. Physiol. Res. 2007, 56, 97–104. [Google Scholar]
- Dante, G.; Facchinetti, F. Herbal treatments for alleviating premenstrual symptoms: A systematic review. J. Psychosom. Obs. Gynecol. 2011, 32, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Chopin Lucks, B. Vitex agnus-castus essential oil and menopausal balance: A self-care survey. Complement. Ther. Nurs. Midwifery 2002, 8, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Chopin Lucks, B. Vitex agnus castus essential oil and menopausal balance: A research update. Complement Ther Nurs Midwifery 2003, 9, 157–160. [Google Scholar] [CrossRef]
- van Die, M.D.; Burger, H.G.; Teede, H.J.; Bone, K. Vitex agnus-castus (Chaste-Tree = Berry) in the Treatment of Menopause-Related Complaints. J. Alt. Compl. Med. 2009, 15, 853–862. [Google Scholar] [CrossRef] [PubMed]
- O’Mullane, N.M.; Joyce, P.; Kamath, S.V.; Tham, M.K.; Knass, D. Adverse CNS effects of menthol-containing olbas oil. Lancet 1982, 1, 1121. [Google Scholar]
- Höld, K.M.; Sirisoma, N.S.; Sparks, S.E.; Casida, J.E. Metabolism and mode of action of cis- and trans-3-pinanones (the active ingredients of hyssop oil). Xenobiotica 2002, 32, 251–265. [Google Scholar] [CrossRef]
- Sarici, S.U.; Kul, M.; Candemir, G.; Aydin, H.İ.; Alpay, F.; Gökçay, E. Neonatal convulsion after accidental ingestion of sage oil: A case report. Gulhane Med. J. 2004, 46, 161–162. [Google Scholar]
- Halicioglu, O.; Astarcioglu, G.; Yaprak, I.; Aydinlioglu, H. Toxicity of Salvia officinalis in a newborn and a child: An alarming report. Pediatr Neurol. 2011, 45, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Harazono, A.; Fujii, S.; Kawashima, K. Evaluation of developmental toxicity of β-thujaplicin (hinokitiol) following oral administration during organogenesis in rats. Food Chem. Toxicol. 2004, 42, 465–470. [Google Scholar] [CrossRef]
- Dong, J.Y.; Xue, L.Q.; Zhu, X.W.; Zhou, Y. Antifertility agents from seeds of Daucus carota. Zhongcaoyao 1981, 12, 61. [Google Scholar]
- Chu, Y.H.; Zhou, M.H.; Li, Q.; Bao, Y.M. Antifertility effect of volatile oil of Daucus carota seeds. Reprod. Contracept. 1985, 5, 37–40. [Google Scholar]
- Zhou, N.N.; Mao, X.J.; Zhang, J.; Yang, S.W.; Zhang, J.M. Pharmacological investigation on contraindication of Curcuma zedoaria. Chin. Arch. Tradit. Chin. Med. 2004, 22, 2291–2294. [Google Scholar]
- Kong, Y.C.; Xie, J.X.; But, P.P.H. Fertility regulating agents from traditional Chinese medicine. J. Ethnopharmacol. 1986, 15, 1–44. [Google Scholar] [CrossRef]
- Farnsworth, N.R.; Bingel, A.S.; Cordell, G.A.; Crane, F.A.; Fong, H.H. Potential Value of Plants as Sources of New Antifertility Agents I. J. Pharm. Sci. 1975, 64, 535–598. [Google Scholar] [CrossRef] [PubMed]
| EO | Botanical Name | Family | Part Used | Hazard(s) | Toxic Component(s) | Oil Composition | Maximum Oral Dose in Pregnancy [22] | Ref. |
|---|---|---|---|---|---|---|---|---|
| Anise or aniseed | Pimpinella anisum L. | Apiaceae | Seeds | Reproductive hormone modulation | (E)-Anethole | (E)-anethole (75.2–96.1%), d-limonene (tr–4.9%), and estragole (0.5–5.0%) | - | [23,24] |
| Aniseed Myrtle | Syzygium anisatum (Vickery) Craven and Biffin | Myrtaceae | Leaves | Reproductive hormone modulation | (E)-Anethole | (E)-anethole (95.0%), and estragole (4.4%) | - | [25] |
| Araucaria | Neocallitropsis pancheri (Carriere) de Laub. (synonym: Callitropsis araucarioides Compton, and Neocallitropsis araucarioides (Compton) Florin) | Cupressaceae | Wood | Fetotoxic, anti-angiogenic | β-Eudesmol | β-eudesmol (25.9%), γ-eudesmol (19.0%), α-eudesmol (13.3%), guaiol (6.0%), elemol (5.0%), and β-bisabolenol (4.9%) | - | [26] |
| Atractylis (Cang-zhu atractylodes) | Atractylodes lancea (Thunb.) DC | Asteraceae | Roots | Anti-angiogenic, fetotoxic | β-Elemene and β-eudesmol | β-eudesmol (26.0%), β-elemene (18.0%), hinesol (10.0%), and elemol (6.0%) | - | [22] |
| Australian Lemon balm (lemon-scented ironbark) | Eucalyptus staigeriana F. v. Muell. ex F. M. Bailey | Myrtaceae | Leaves | Teratogenicity | Citral | d-limonene+ β-phellandrene (30.5%), geranial (9.9%), neral (7.7%), α-phellandrene (7.1%), and terpinolene (6.6%) | 238 mg/day based on 17.6% citral content | [22] |
| Basil oil (estragole chemotype) | Ocimum basilicum L. | Lamiaceae | Leaves | Potentially carcinogenic | Estragole and methyleugenol | estragole (73.4–87.4%), linalool (tr–8.6%), and 1,8-cineole (0.6–6.0%) | - | [23] |
| Bitter Fennel | Foeniculum vulgare Mill. subsp. capillaceum Gilib. | Apiaceae | Seeds | Reproductive hormone modulation | (E)-anethole | (E)-anethole (52.5–84.3%), fenchone (4.0–24.0%), α-pinene (tr-10.4%), d-limonene (0.5–9.4%), and estragole (2.8–6.5%). | - | [23] |
| Black seed (black cumin or black caraway) | Nigella sativa L. | Ranunculaceae | Seeds | Fetotoxic | Thymoquinone | thymoquinone (26.8–54.8%), p-cymene (14.7–38.0%), longifolene (1.2–10.2%), and α-thujene (1.3–10.1%) as the main constituents. | - | [27] |
| Blue Cypress (Northern cypress pine) | Callitris intratropica R.T. Baker and H.B. Sm. | Cupressaceae | Wood | Fetotoxic, anti-angiogenic | β-Eudesmol | β-eudesmol (14.4%), dihydrocolumellarin (14.0%), guaiol (13.7%), γ-eudesmol (9.1%), α-eudesmol (7.6%), guaiazulene (6.2%), chamazulene (5.6%) | - | [28] |
| Buchu (diosphenol chemotype) | Agathosma betulina Bergius | Rutaceae | Leaves | Abortifacient; hepatotoxicity | Pulegone | isomenthone (4.6–29.1%), limonene (11.6–28.2%), disophenol (12.0–26.3%), menthone (2.5–25.0%), c-diosphenol (10.3–23.3%), and 8-mercapto-p-menthan-3-one(cis-trans) (0.7–6.6%) | - | [29] |
| Buchu (pulegone chemotype) | Agathosma crenulata L. | Rutaceae | Leaves | abortifacient | Pulegone | (1R)-(+)-β-pulegone (31.6–73.2%), isomenthone (3.6–27.6%), limonene (2.1–17.2%), (E)-8-acetylthio-p-menthan-3-one (0.4–10.4%), and menthone (1.3–7.0%) | - | [29,30] |
| Carrot seed | Daucus carota L. subsp. sativus Hoffm. | Apiaceae | Seeds | antigestational effects | carotol (36.1–73.1%), α-pinene (0.9–11.2%), dauca-4,8-diene (1.6–5.9%), and β-caryophyllene (0.7–5.6%) | - | [31] | |
| Cassia (Chinese or false cinnamon) | Cinnamomum cassia (L.) J. Presl (synonym: Cinnamomum aromaticum Nees) | Lauraceae | Leaves, terminal branches and bark | Embryotoxicity, reproductive toxicity | Methyleugenol and cinnamaldehyde | (E)-cinnamaldehyde (73.2–89.4%), (Z)-cinnamaldehyde (0.8–12.3%), and (E)-cinnamyl acetate (0.1–5.4%) while in the leaf oil (E)-cinnamaldehyde (54.6–90.1%), (E)-cinnamyl acetate (1.4–12.5%), (Z)-cinnamaldehyde (0.4–10.5%), and benzaldehyde (1.1–6.3%) | - | [32] |
| Chaste tree (Monk’s pepper) | Vitex agnus-castus L. | Verbenaceae | Leaves | Reproductive hormone modulation | The oil may contain methyleugenol | Leaf EO: 1,8-cineole (15.6–35.2%), sabinene (6.9–17.1%), α-pinene (1.0–13.9%), α-terpineol (1.4–9.2%), γ-elemene (0–9.1%), β-selinene (0–9.0%), β-caryophyllene (2.3–8.9%), (Z)-β-farnesene (0–8.6%), citronellyl acetate (0.3–7.8%), and citronellic acid (0–6.6%). Seed EO: sabinene (7.1–44.1%), 1,8-cineole (8.4–23.3%), α-pinene (1.2–23.1%), γ-elemene (0–17.0%), (E)-β-farnesene (0–10.3%), β-caryophyllene (0.8–9.3%), α-terpineol (0.2–9.3%), limonene (0.5–7.4%), (Z)-β-farnesene (0–6.9%), citronellyl acetate (0.2–6.0%), β-selinene (0–6.0%), and β-myrcene (0–5.6%). | - | [33,34] |
| Cinnamon bark | Cinnamomum verum J. Presl. (Synonym: Cinnamomum zeylanicum Blume) | Lauraceae | Dried inner bark of young trees | Embryotoxicity | (E)-Cinnamaldehyde | (E)-cinnamaldehyde (63.1–75.7%), eugenol (2.0–13.3%), (E)-cinnamyl acetate (0.3–10.6%), linalool (0.2–7.0%), and β-caryophyllene (1.3–5.8%) | - | [23,24] |
| Costus | Saussurea costus (Falc.) Lipsch. (synonym: Aplotaxis lappa Decne., Aucklandia costus Falc., Saussurea lappa (Decne) C.B. Clarke) | Asteraceae | Dried roots | Fetotoxicity, anti-angiogenicity | Costunolide and dehydrocostus lactone | aplotaxene (20.0%), dihydrocostus lactone (15.0%), costusic acid (14.0%), costunolide (11.0%), dehydrocostus lactone (6.0%), and dihydrodehydrocostus lactone (6.0%) | - | [35] |
| Dalmatian Sage | Salvia officinalis L. | Lamiaceae | Leaves | embryotoxic | Camphor, thujones | camphor (7.3–50.2%), α-thujone (13.1–48.5%), borneol (1.5–23.9%), 1,8-cineole (1.8–21.7%), β-thujone (3.9–19.1%), β-caryophyllene (0.2–9.7%), camphene (0–8.6%), α-pinene (0–8.0%) and bornyl acetate (0.3–5.7%) | - | [36] |
| Feverfew (nosebleed or midsummer daisy) | Tanacetum parthenium (L.) Sch. Bip. (synonym: Chrysanthemum parthenium (L.) Bernh.) | Asteraceae | Leaves | Unsafe, moderately neurotoxic | Camphor | camphor (28.0–44.2%), (E)-chrysanthenyl acetate (22.9–30.2%), camphene (5.4–7.7%), and germacrene D (0.7–4.6%) | - | [37] |
| Genipi (Genepi) | Artemisia genepi Weber. (synonym: A. spicata Wulfen, and A. mutellina Vill.) | Asteraceae | Aerial parts | Neurotoxic | Thujone | α-thujone (79.8%) and β-thujone (10.4%) | - | [38] |
| Great Mugwort | Artemisia arborescens L. | Asteraceae | Aerial parts | Neurotoxic | Thujone | β-thujone (34.0%), chamazulene (22.4%), and camphor (11.8%) | - | [39] |
| Green Yarrow (Ligurian yarrow) | Achillea nobilis L. (synonym: A. ligustica Vis. ex Nym.) | Asteraceae | Aerial parts of the flowering plant | Abortifacient | Sabinyl acetate; camphor | camphor (13.7%) artemisia alcohol (9.2%), germacrene D (8.8%), artemisia ketone (8.7%) and viridiflorol (5.7%) | - | [22] |
| Ho leaf (camphor chemotype) | Cinnamomum camphora (L.) J.Presl | Lauraceae | Leaves | Neurotoxic | Camphor | camphor (37.8–84.1%), 1,8-cineole (1.0–12.0%), and terpinen-4-ol (0.9–6.3%) | - | [40] |
| Honey Myrtle (Marsh honey myrtle) | Melaleuca teretifolia Endl. | Myrtaceae | Leaves | Teratogenicity | Citral | geranial (37.5%), neral (29.0%) and β-myrcene (10.9%) | 63 mg/day based on 66.5% citral content | [22] |
| Hyssop (pinocamphone chemotype) | Hyssopus officinalis L. | Lamiaceae | Leaves and flowering tops | Neurotoxicity; carcinogen | Pinocamphone, methyleugenol | pinocamphone (31.2–42.7%), isopinocamphone (30.9–39.2%) and β-pinene (4.0–8.8%) | - | [37,41] |
| Indian dill seed (Sowa) | Anethum sowa Roxb. ex Flem. | Apiaceae | Seeds | hepatotoxic, nephrotoxic, Abortifacient | Dill apiole | dill apiole (20.7–52.5%), d-limonene (5.9–45.0%), (+)-carvone (17.4–23.1%), (E)-dihydrocarvone (4.2–16.6%), α-phellandrene (tr–6.5%), and (Z)-dihydrocarvone (0.8–5.2%) | - | [32] |
| Lanyana (African wormwood) | Artemisia afra Jacq. ex Willd. | Asteraceae | Leaves and stems | neurotoxic | Thujone | α-thujone (22.5%), (E)-chrysanthenyl acetate (19.2%), 1,8-cineole (19.1%), camphor (11.0%), and β-thujone (8.9%) | - | [22] |
| Lemon basil | Ocimum × africanum Lour. | Lamiaceae | Leaves | Teratogenicity | Citral | geranial (23.3–25.1%), neral (16.0–17.1%), nerol (13.0–15.3%), linalool (5.0–7.8%), and (E)-α-bisabolene (5.3–6.2%) | 99 mg/day based on 42.2% citral content | [22] |
| Lemon leaf (lemon petitgrain) | Citrus × limon L. (synonym: Citrus limonum Risso) | Rutaceae | Leaves | Teratogenicity | Citral | geranial (10.9–39.0%), limonene (8.1–30.7%), neral (6.5–25.3%), geraniol (0.5–15.0%), β-pinene (3.5–13.6%), neryl acetate (3.7–7.4%), nerol (1.3–7.4%), α-terpinyl acetate (tr–7.3%), and linalyl acetate (tr–6.5%) | 84 mg based on 50% citral content | [32] |
| Lemon Myrtle (lemon ironwood or sweet verbena tree) | Backhousia citriodora F. Muell. | Myrtaceae | Leaves | Teratogenicity | Citral | geranial (46.1–60.7%) and neral (32.0–40.9%) | 46 mg/day | [42] |
| Lemon Thyme | Thymus citriodorus (Pers.) Schreb. (Synonyms: Thymus lanuginosus Mill. var. citriodorum Pers., Thymus serpyllum var. citriodorus (Hort.), Thymus serpyllum L. var. vulgaris Benth.); a cross between Thymus vulgaris and Thymus pulegioides. | Lamiaceae | Aerial parts | Teratogenicity | Citral | geraniol (39.2%), carvacrol (15.4%), geranial (9.2%) and neral (7.1%) | 258 mg/day based on 16.3% citral content | [43] |
| Lemongrass | Cymbopogon flexuosus Nees ex Steud. (synonym.: Andropogon flexuosus Nees ex Steud.) (East Indian) and Cymbopogon citratus DC (synonym: Andropogon citratus DC) (West Indian) | Poaceae | Leaves | Teratogenicity | Citral |
| 46 mg/day based on 90% citral content | [41,44,45] |
| Lemon-scented tea tree (lemon tea tree) | Leptospermum petersonii F. M. Bailey (synonym: Leptospermum citratum Chall., Cheel and Penf.; Leptospermum liversidgei R.T. Baker and H. G. Smith) | Myrtaceae | Aerial parts | Teratogenicity | Citral | geranial (45.4%), neral (31.3%), α-pinene (12.3%), and citronellal (6.8%) | 54 mg/day based on 77% citral content | [46] |
| Lesser Calamint (Cuckoo flower, field balm, and nepitella) | Calamintha nepeta L. subsp. glandulosa Req. (synonym: Calamintha officinalis Moench.) | Lamiaceae | Aerial parts | Abortifacient; hepatotoxicity | Pulegone | (1R)-(+)-β-pulegone (17.6–76.1%), menthone (7.0–55.8%), piperitone oxide (0–12.4%), piperitone (0–7.4%), piperitenone (0–7.3%), limonene (0.6–7.2%), and terpinen-4-ol (0–6.8%). | - | [47] |
| May chang (Pheasant pepper tree) | Litsea cubeba (Lour.) Pers. (synonyms: Litsea citrata Blume, Laurus cubeba Lour.) | Lauraceae | Fruits | Teratogenicity | Citral | geranial (37.9–40.6%), neral (25.5–33.8%), limonene (8.4–22.6%), and methyl heptenone (0.5–4.4%) | 56 mg/day based on 74% citral content | [41,48] |
| Melissa (lemon balm) | Melissa officinalis L. | Lamiaceae | Fresh aerial parts | Teratogenicity | Citral | geranial (12.5–38.3%), neral (9.7–26.1%), β-caryophyllene (0.3–19.1%), citronellal (4.5–13.3%), germacrene D (0–13.0%), caryophyllene oxide (0.8–10.0%), and geraniol (1.0–8.1%) | 65 mg/day based on 64% citral content | [37,48] |
| Mugwort (chrysanthenyl acetate CT) | Artemisia vulgaris L. | Asteraceae | Aerial parts | slightly neurotoxic | Thujone | chrysanthenyl acetate (31.7–32.8%) and germacrene D (12.1–15.9%) | - | [22] |
| Mugwort or Indian wormwood oil (camphor/thujone CT) | Artemisia vulgaris L. | Asteraceae | Aerial parts of flowering plant | Slightly neurotoxic | Thujone | camphor (20.8%), artemisia alcohol (15.3%), α-thujone (11.4%), β-caryophyllene (10.6%), isoborneol (9.3%), 1,8-cineole (9.0%), and sabinene (6.1%) | - | [49] |
| Myrrh (Somalian myrrh) | Commiphora myrrha (Nees) Engl. (synonym: Commiphora molmol Engl.) | Burseraceae | Dried gum oleoresin | Fetotoxic, anti-angiogenic | β-Elemene and furanodiene | furanoeudesma-1,3-diene (34.0%), furanodiene (19.7%), lindestrene (12.0%), and β-elemene (8.7%) | - | [50] |
| Nasturtium (Indian cress) absolute | Tropaeolum majus L. | Tropaeolaceae | Flowers | fetal toxicity | Benzyl cyanide, benzyl isothiocyanate | benzyl isothiocyanate (72.3%), unidentified nitrogen compound (16.0%), and benzyl cyanide (2.0%) | - | [22] |
| Nutmeg | Myristica fragrans Houtt (Synonyms: Myristica officinalis L. fil., Myristica moschata Thunb., Myristica aromatica O. Schwartz, and Myristica amboinensis Gand.) | Myristicaceae | Kernels | Potentially carcinogenic; reduced fertility | Safrole, methyleugenol, myristicin |
| - | [32,51] |
| Orange Champaca (golden champa, champak) absolute | Michelia champaca L. | Magnoliaceae | Flowers | Toxic | 2-Phenylethanol | 2-phenylethanol (25.0–34.0%), methyl linoleate (10.0–18.0%), indole (2.9–12.0%), methyl anthranilate (2.1–9.0%), and methyl benzoate (1.0–5.0%) | - | [52] |
| Oregano | Origanum onites L. (synonym: Origanum smyrnaeum L.); Origanum vulgare L. subsp. hirtum (Link) Ietswaart (synonym: Origanum compactum, Origanum hirtum Link); and Thymbra capitata (L.) Cav. (synonym: Thymus capitatus L., Coridothymus capitatus L., Satureja capitata L.) | Lamiaceae | Dried aerial parts of flowering plant | embryotoxic | Not identified |
| - | [53,54,55,56] |
| Parsley leaf | Petroselinum crispum Mill (synonym: P. sativum Hoffm., and P. hortenseauct) | Apiaceae | Leaves | abortifacient | Parsley apiole | Egyptian parsley: p-mentha-1,3,8-triene (6.2–45.2%), β-myrcene (7.8–23.8%), β-phellandrene (6.7–19.5%), myristicin (1.9–8.8%), α-pinene (6.9–7.6%), terpinolene (2.8–6.6%), limonene (3.3–5.4%), α-p-dimethylstyrene (2.7–5.4%), and dill apiole (0.2–5.2%) | - | [22,57] |
| Parsley seed | Petroselinum crispum Mill | Apiaceae | Seeds | abortifacient | Parsley apiole | parsley apiole (11.3–67.5%), myristicin (0.7–37.9%), allyltetramethoxybenzene (0.6–29.0%), α-pinene (8.3–16.9%), β-pinene (5.4–10.7%), and elemicin (0–8.8%) | - | [58] |
| Pennyroyal | Hedeoma pulegioides L. (N. American); Mentha pulegium L. (European) and Micromeria fruticosa L. (Turkish) | Lamiaceae | Fresh aerial parts | abortifacient | Pulegone | Hedeoma pulegioides: (1R)-(+)-β-pulegone (61.3–82.3%) and isomenthone (0.8–31.0%). Mentha pulegium: (1R)-(+)-β-pulegone (67.6–86.7%), menthone (1.5–16.0%) and isomenthone (0.8–8.6%). Micromeria fruticosa: (1R)-(+)-β-pulegone (66.7%) and isomenthone (11.1%). | - | [23] |
| Plectranthus | Plectranthus fruticosus L’Hérit | Lamiaceae | Leaves | embryotoxic, fetotoxic, teratogenic and abortifacient | Sabinyl acetate | sabinyl acetate (> 60.0%) | - | [59] |
| Rue | Ruta graveolens L. and Ruta montana Mill | Rutaceae | Aerial parts | abortifacient | Not identified |
| - | [60,61,62] |
| Savin | Juniperus sabina L. | Cupressaceae | Leaves and terminal branches | embryo-fetotoxic, abortifacient and hepatotoxic | trans-Sabinyl acetate | trans-sabinyl acetate (19.1–53.1%), sabinene (18.3–40.8%), and elemol (tr–7.0%) | - | [23] |
| Sea Wormwood | Artemisia maritima L. (synonyms: Artemisia contra Willd. ex Spreng., Artemisia lercheana Kar. and Kir., Artemisia salina Willd., Seriphidium maritimum (L.) Poljakov) | Asteraceae | Leaves and flowering tops | neurotoxic | Thujone | α-thujone (63.3%), sabinene (7.8%) and 1,8-cineole (6.5%) | - | [63] |
| Spanish Lavender (French lavender or maritime lavender) | Lavandula stoechas L. ssp. stoechas | Lamiaceae | Flowering tops | neurotoxic | Camphor | camphor (16.4–56.2%), (+)-fenchone (14.9–49.1%), 1,8-cineole (3.6–14.5%), α-pinene (3.4–4.5%), and camphene (2.8–5.5%) | - | [64] |
| Spanish Sage (lavender sage) | Salvia lavandulifolia Vahl (synonym: Salvia hispanorum Lag) | Lamiaceae | Flowering tops | abortifacient | Sabinyl acetate |
| - | [23,65] |
| Star anise | Illicium verum J.D. Hook. | Illiciaceae | Fruits | reproductive hormone modulation | (E)-Anethole | (E)-anethole (71.2–91.8%), foeniculin (0.5–14.6%), estragole (0.3–6.6%), and d-limonene (0.7–5.0%) | - | [23] |
| Sweet Birch (black birch or southern birch) | Betula lenta L. | Betulaceae | Bark | reproductively toxic | Methyl salicylate and ethyl salicylate | methyl salicylate (90.4%) and ethyl salicylate (5.5%) | - | [22] |
| Sweet Fennel | Foeniculum vulgare Mill. | Apiaceae | Seeds | Reproductive hormone modulation | (E)-Anethole | (E)-anethole (58.1–92.5%), d-limonene (0.2–21.0%), fenchone (0.2–8.0%), and estragole (1.1–4.8%) | - | [23,24] |
| Tansy | Tanacetum vulgare L. (synonyms: Chrysanthemum tanacetum Karsch, and Chrysanthemum vulgare L.) | Asteraceae | Aerial parts | neurotoxic | Thujone | β-thujone (45.2%), artemisia ketone (10.5%), borneol (7.8%), and bornyl acetate (7.7%) | - | [22] |
| Thuja (cedar leaf, white cedar, eastern white cedar, eastern arborvitae, or swamp cedar) | Thuja occidentalis L. | Cupressaceae | Fresh leaves and terminal branches | neurotoxic | Thujone | α-thujone (48.7–51.5%), fenchone (12.2–12.8%) and β-thujone (7.9–9.9%) | - | [22,66] |
| Verbena (lemon verbena) | Aloysia triphylla L’Hérit (Synonyms: Aloysia citriodora Ortega ex Pers., Lippia citriodora Ortega ex Pers., and Lippia triphylla L’Hérit) | Verbenaceae | Leaves | Teratogenicity | Citral | geranial (29.5–38.3%), neral (22.9–29.6%), and limonene (5.7–15.4%) | 61 mg/day based on 68% citral content | [67] |
| Western red cedar (pacific thuja or western arborvitae) | Thuja plicata Donn ex D. Don | Cupressaceae | Needles (leaves) | neurotoxic | Thujones | α-thujone (63.5–84.0%), β-thujone (4.9–15.2%), and sabinene (1.1–8.8%) | - | [68] |
| White Wormwood (armoise or desert wormwood) α-thujone/camphor chemotype | Artemisia herba-alba Asso | Asteraceae | Leaves and flowering tops | neurotoxic | Thujones; camphor | camphor (34.0–55.0%), α-thujone (25.7–36.8%), β-thujone (2.0–9.0%), camphene (0.5–9.0%), and 1,8-cineole (1.5–8.0%) | - | [32,41,69] |
| Wintergreen | Gaultheria fragrantissima Wall. and Gaultheria procumbens L. | Ericaceae | Leaves | high doses are teratogenic | Methyl salicylate |
| - | [22] |
| Wormwood (Absinthe) | Artemisia absinthium L. | Asteraceae | Leaves and flowering tops | embryo-fetotoxicity; abortifacient | Sabinyl acetate; Thujone |
| - | [23,39] |
| Zedoary (white turmeric, hidden ginger) | Curcuma zedoaria Roscoe | Zingiberaceae | Rhizome | antifertility; embryotoxicity, antigestational and abortifacient | Not identified | epicurzerene (19.0–46.6%), curzerene (10.4%), curdione (7.0–19.6%), curzerenone (22.3–31.6%), debromofiliforminol (31.5%), 1,8-cineole (18.5–40.8%), β-sesquiphellandrene (21.5%), p-cymene (18.4%), curcumenene (18.7%), and α-phellandrene (14.9%) | - | [66,70,71,72,73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosoky, N.S.; Setzer, W.N. Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents. Int. J. Mol. Sci. 2021, 22, 2380. https://doi.org/10.3390/ijms22052380
Dosoky NS, Setzer WN. Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents. International Journal of Molecular Sciences. 2021; 22(5):2380. https://doi.org/10.3390/ijms22052380
Chicago/Turabian StyleDosoky, Noura S., and William N. Setzer. 2021. "Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents" International Journal of Molecular Sciences 22, no. 5: 2380. https://doi.org/10.3390/ijms22052380
APA StyleDosoky, N. S., & Setzer, W. N. (2021). Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents. International Journal of Molecular Sciences, 22(5), 2380. https://doi.org/10.3390/ijms22052380







